1. Introduction
Extracorporeal membrane oxygenation (ECMO) is a well-established therapy utilized in neonates and children. According to the Extracorporeal Life Support Organization (ELSO), the survival rates for patients undergoing ECMO treatment vary depending on the age group and the underlying condition. In pediatric patients who undergo extracorporeal cardiopulmonary resuscitation (eCPR) the survival rate stands at 55%, whereas for neonates receiving ECMO for respiratory issues, the survival rate reaches 84% [
1].
The outcome of children and neonates on ECMO are influenced by various factors, including the presence of a congenital heart defect (CHD). The reported survival rate for neonates with CHD is 40%, while for pediatric patients, it is 48% [
1]. Within the group of patients with CHD, those with single ventricle physiology exhibit poorer outcomes compared to those with biventricular CHD [
2]. Studies have reported survival rates ranging from 31% to 50% for this subgroup of children [
3,
4].
The objective of this study is to provide a comprehensive description of the specific group of children affected by CHD who require ECMO support. Additionally, the study aims to identify the risk factors and complications associated with ECMO utilization in these patients and evaluate the overall outcome following ECMO implantation. By examining these aspects, we hope to enhance our understanding of CHD management in conjunction with ECMO therapy and improve patient care in this population.
2. Materials and Methods
This study is a retrospective, single-center investigation that aimed to evaluate the outcomes of children diagnosed with CHD who required ECMO support between the years 2009 and 2019. The study encompassed children up to the age of 16 and categorized them as either “neonates” if they were up to 30 days old or “pediatric” if they exceeded that age. To ensure the accuracy of the data, exclusion criteria were applied, which involved excluding patients without a CHD diagnosis and those with missing baseline or follow-up information. Notably, patients who underwent a second ECMO run during the same hospital admission were considered separately and labeled as "second ECMO run," with no patient undergoing more than two ECMO runs.
The patients were categorized into two groups based on the underlying anatomical classification of their CHD: those with biventricular CHD and those with single ventricle CHD. The functional single ventricle heart diagnosis encompassed conditions such as hypoplastic left heart syndrome, tricuspid valve atresia, pulmonary atresia, and borderline left ventricle.
The study identified five main categories of factors leading to the need for ECMO support: respiratory failure, cardiac failure, sepsis, postcardiotomy ECMO, and eCPR (
Table S1). Post-cardiotomy ECMO was defined as the inability to wean from cardiopulmonary bypass (CPB) or the requirement for ECMO within the initial 48 hours following a CPB-assisted operation.
Due to the complexity of patient cases, it was often challenging to attribute ECMO support to a single factor, as multiple relevant factors were concurrently present, influencing the decision to initiate ECMO support. In instances where multiple factors were identified, the patient was classified into the categories considered more relevant by two independent senior consultants. Any discrepancies were resolved through discussion until a consensus was reached. Consequently, for the majority of patients, it was established that more than one factor contributed to the need for ECMO support.
The study investigated factors leading to ECMO support, one-year survival rates, and complication rates. A series of complications was defined and encompassed various scenarios. The occurrence of reoperation due to bleeding was used as the primary criterion for defining bleeding as a complication. Specifically, any instance of bleeding, whether occurring within or outside the area of the index operation, was considered. Additionally, bleeding directly associated with ECMO was also taken into account.
Furthermore, the study took into account various other complications, such as the repositioning of a cannula. This adjustment may be required either due to accidental displacement or to optimize the ECMO flow. Moreover, the cleaning of cannulas or tubes to address thrombus formation was also considered a complication. Disseminated intravascular coagulation (DIC) was evaluated based on laboratory and clinical assessments, constituting another complication category. The study also accounted for circuit changes prompted by the depletion of the oxygenator, as well as verified cerebrovascular insults (CVI). CVI, as defined in the study, encompassed any thromboembolic event resulting in a cerebral insult during ECMO, which was confirmed through magnetic resonance imaging (MRI) or computed tomography (CT). These imaging modalities were interpreted by pediatric radiologists or neuroradiologists, ensuring accurate identification and confirmation of CVI cases.
In addition to the primary heart defect, some patients also had additional non-cardiac genetic diseases, which were documented separately as "other diagnoses" (
Table S2).
All data were securely stored in an Excel sheet protected by a password. The document containing personalized information linked to patient IDs was stored separately from the Excel sheet to ensure privacy and confidentiality. The study received ethical approval from the Cantonal Ethics Committee Zurich (BASEC-Nr. 2020-01147).
2.1. Statistical analysis
Data pertaining to patients and ECMO were gathered from the clinical information system "Phoenix®" provided by CGM Clinical (CompuGroup Medical Schweiz AG, Bern) at the University Children's Hospital Zurich. A comprehensive set of variables was selected for analysis, including date of birth, gender, weight, classification as neonate or pediatric, presence of single ventricle or biventricular heart, specific congenital heart defect, concomitant diseases, factors leading to ECMO initiation, occurrence of cardiopulmonary resuscitation (CPR) prior to ECMO, previous cardiotomy, last recorded pH and lactate levels before ECMO, type of ECMO implantation, cannulation site, timing of ECMO initiation and removal, occurrence of a second ECMO run, complications experienced while on ECMO, conversion to a Ventricular Assist Device (VAD), length of stay in the intensive care unit (ICU), survival while on ECMO, and survival after a 1-year follow-up. To ensure the accuracy and consistency of the collected data, an Excel sheet was utilized for data collection. Subsequent statistical analysis and evaluation were conducted using the software "R-Studio" [RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA]. The survival times of the two groups were compared using a log-rank test, with statistical significance assumed at p < 0.05.
3. Results
3.1. Study group
A total of 88 patients underwent ECMO therapy, comprising 42 neonates and 43 females. The mean age at ECMO implantation was 1.4 years, with a standard deviation of 3.6 years. Furthermore, the average weight of the patients was 6.9 kg, with a standard deviation of 1.0 kg. Detailed baseline characteristics for both groups can be found in
Table 1.
Among the 88 patients, 36 (41%) individuals were diagnosed with a single ventricle heart defect, with hypoplastic left heart syndrome (HLHS) being the most common condition within this subgroup, accounting for 23 cases (26%). Conversely, 52 patients (59%) exhibited a congenital heart defect with biventricular physiology, as indicated in
Table 2, showcasing the distribution of CHD types within the cohort.
Cardiac failure emerged as the predominant factor leading to ECMO in both the single ventricle and biventricular groups. Of the 36 patients with a single ventricle defect, 33 (91%) were diagnosed with cardiac failure, while in the biventricular group, this diagnosis was present in 48 patients (92%). Notably, the majority of single ventricle patients (70%) required ECMO support postcardiotomy, compared to 55% of the biventricular patients. Respiratory failure was a contributing factor in 44% of the single ventricle cases and in 32% of the biventricular cases. Conversely, sepsis was relatively infrequent as a leading factor for ECMO, accounting for 5% of the single ventricle group and 2% of the biventricular group.
Further details regarding the ECMO-related data for the two groups can be found in
Table 3:
3.2. Outcomes
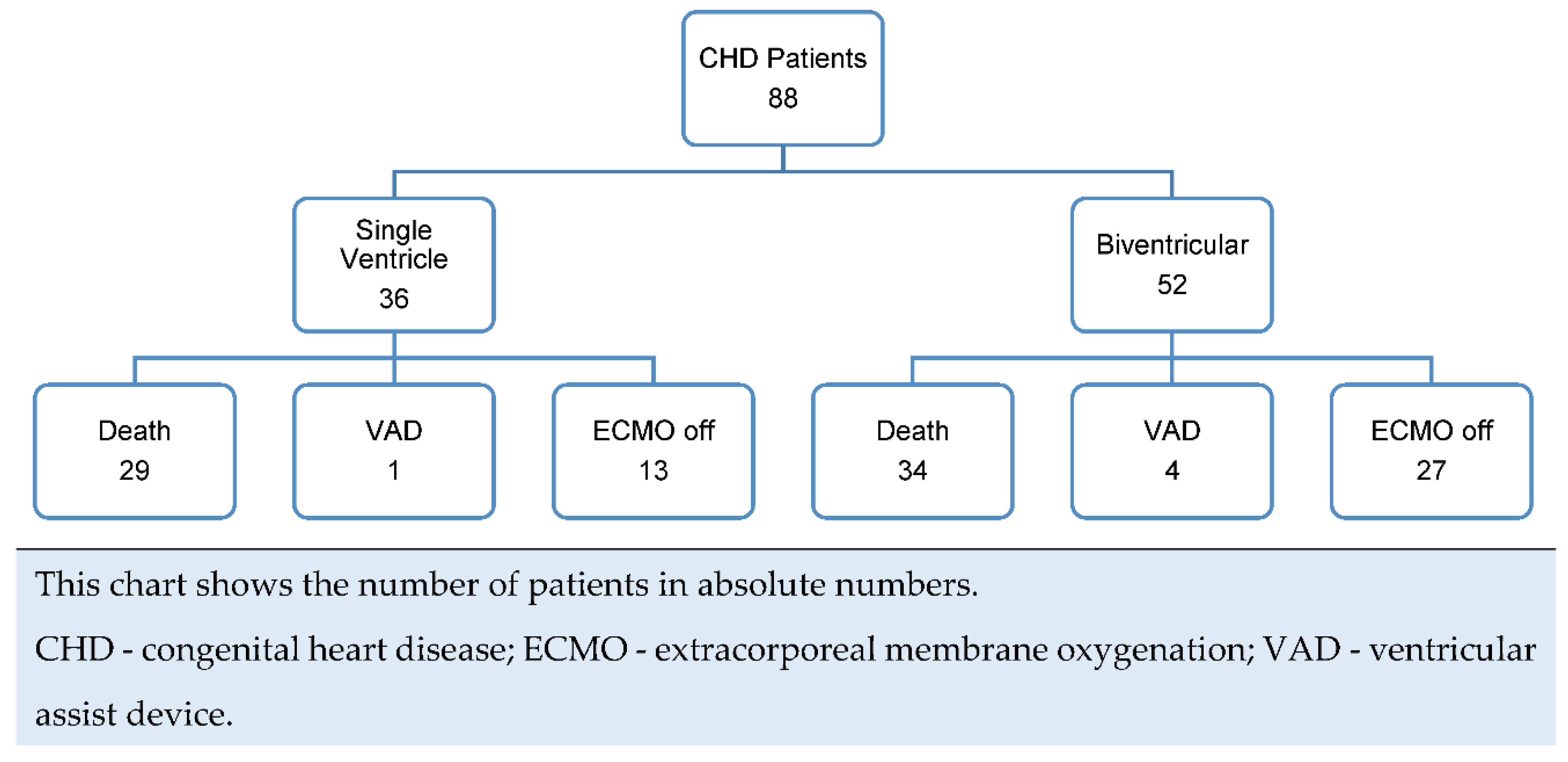
The overall survival rate for children with CHD receiving ECMO support was found to be poor. Out of the 88 patients included in the study, 54% did not survive while on ECMO support. However, 45% of the patients were successfully weaned off ECMO. Additionally, 6% of the patients required VAD support, and 28% of the patients were discharged from the hospital.
Figure 1 presents a schematic representation of the main outcomes in absolute numbers:
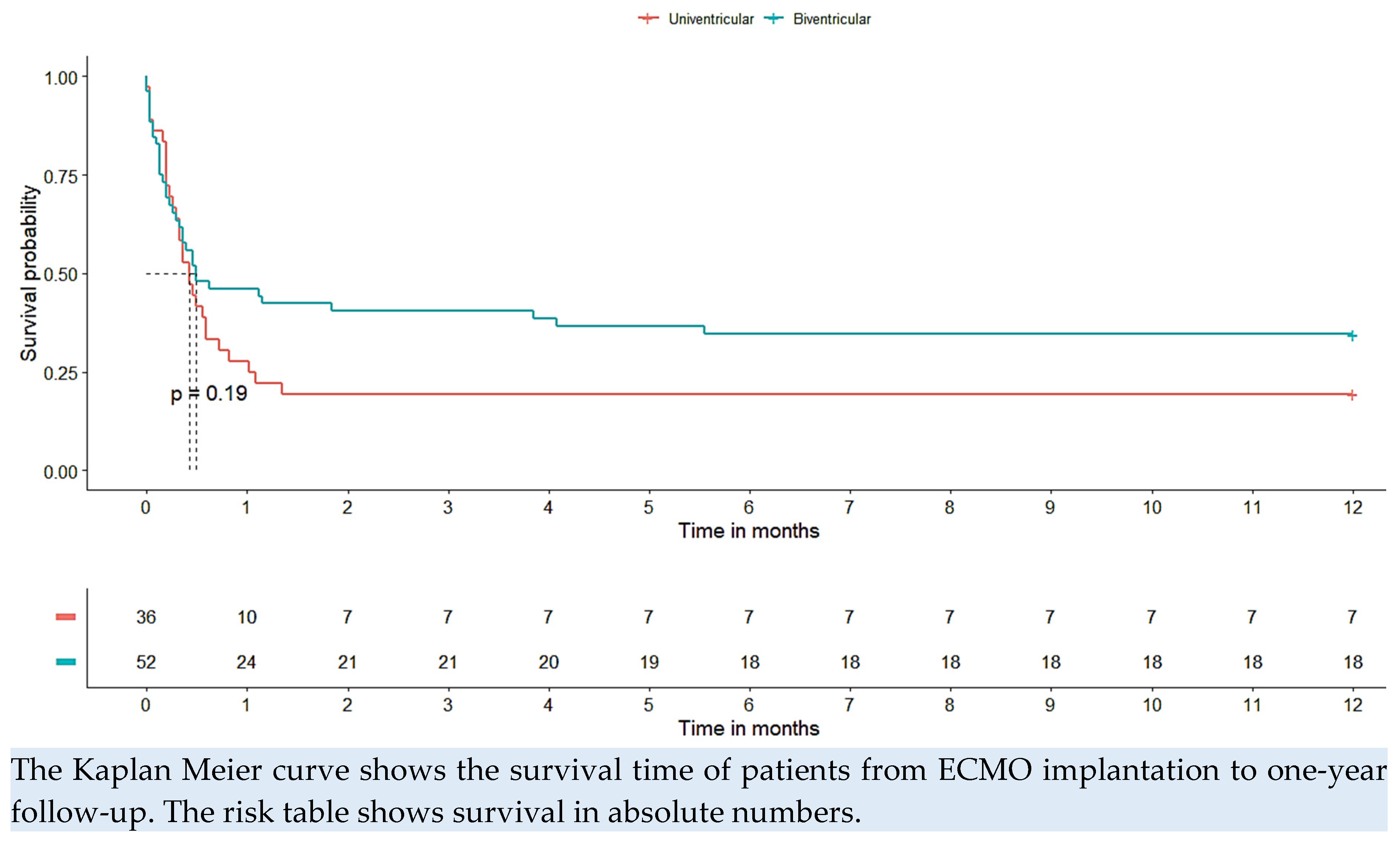
Among the 33 patients who underwent eCPR prior to ECMO implantation, 30% survived to hospital discharge and were still alive at one year. The average duration of CPR before ECMO implantation was consistent at 49 minutes for both groups. However, among the patients who successfully survived, the mean duration of CPR was notably shorter, averaging at 23 minutes. At the time of hospital discharge and one year after, 28% of the patients included in the study were still alive. Among these survivors, seven individuals (28%) had a univentricular heart, while the remaining 18 individuals (72%) exhibited biventricular physiology. Notably, the type of underlying heart disease, whether univentricular or biventricular, did not demonstrate a significant impact on survival outcomes (p = 0.19). The survival curve corresponding to these findings is visually represented in
Figure 2, providing a graphical representation of the observed survival rates over time.
The average duration of ECMO support for the children was 5.4 days, with a standard deviation of 4.4 days. It is worth noting that children with biventricular hearts had longer stays in the intensive care unit (ICU), while their duration of ECMO support was comparatively shorter when compared to children with univentricular hearts. However, the difference in ICU stays between the two groups was not found to be statistically significant (p-value: 0.99), as indicated by the analysis. The outcome data are listed in
Table 4.
Complications arising during the period of ECMO support were found to be common, affecting approximately 49% of the patients. A detailed breakdown of these complications can be found below and in
Table 5. Importantly, patients with univentricular hearts demonstrated a significantly higher incidence of overall complications (odds ratio: 1.57, 95% confidence interval: 0.67 - 3.7). Among these complications, bleeding emerged as the most prevalent issue observed in both groups.
3.3. Subgroup second ECMO run
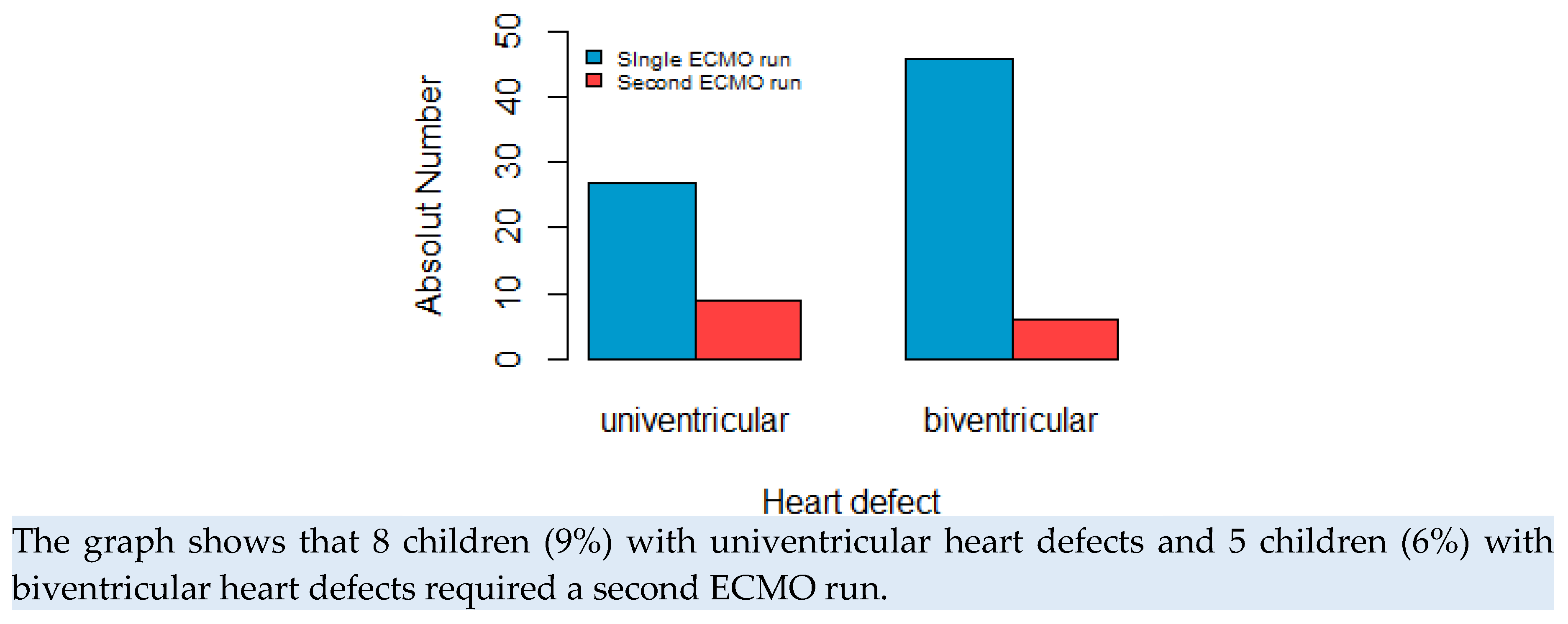
An analysis was conducted specifically on the subset of patients who underwent a second ECMO run, comprising a total of 13 individuals (15% of the total). Among this group, the majority (8 patients) exhibited a single ventricle heart defect, as visually depicted in
Figure 3. Hypoplastic left heart syndrome (HLHS) emerged as the most prevalent specific defect, observed in 7 patients.
The second ECMO run was implemented, on average, 1.9 days after the completion of the first run, with cardiac failure being the predominant factor leading to the need for ECMO in this group, accounting for 85% of cases.
The children who required a second ECMO run received support for an average duration of 7.3 days, with a standard deviation of 1.2 days. Out of the 13 patients, only two individuals survived the second ECMO run. Both of these survivors exhibited a single ventricle heart defect and were pediatric patients.
Complications were observed in 46% of the cases within the subset of patients who underwent a second ECMO run. Among the patients with a univentricular heart defect, complications occurred in four children, representing 50% of this subgroup. Similarly, among the patients with a biventricular heart defect, complications were observed in two individuals, accounting for 40% of this subgroup. Reoperation due to bleeding emerged as the most common complication within this cohort. It is worth highlighting that the two children who survived the second ECMO run did not experience any complications during their ECMO support. Additionally, none of the children in this group required the implantation of a ventricular assist device.
4. Discussion
The use of ECMO in patients with congenital heart disease (CHD) is increasing globally [
5,
6,
7,
8,
9,
10]. In our study, we found that cardiac failure, not surprisingly, was the most common factor leading to the need for ECMO, followed by post-cardiotomy failure. Almost half of the patients (45%) were successfully weaned from ECMO, while a small but substantial number of patients (n=6; 7%) required long-term mechanical circulatory support in the form of a ventricular assist device. For those patients who were successfully discharged, the survival rate at one year was consistent with the rate at hospital discharge, with a survival rate of 22% for neonates and 35% for pediatric patients. When analyzing the underlying anatomy of the entire ECMO population, we observed a relatively high percentage (41%) of patients with single ventricle hearts. Published survival rates for children on ECMO with biventricular heart defects range between 18% and 67%, while those with single ventricle hearts range between 10% and 53% [
11,
12,
13,
14,
15,
16]. The wide range of survival rates reported in the literature is noteworthy and can be attributed to variations in inclusion and exclusion criteria, center size, study magnitude (single-center vs. multicenter), and the timing and setting of ECMO deployment (daytime vs. nighttime), although the latter variable was found in literature to be irrelevant in adults [
17]. Most of the studies found in the literature primarily included children who underwent ECMO after cardiac surgery. In contrast, our study aimed to include all children requiring ECMO, regardless of their correction status or specific heart defect, as long as there was an indication for ECMO that was subsequently implemented. This approach allowed us to avoid selection bias.
Our results, with a survival rate of 35% for biventricular hearts and 19% for single ventricle hearts, align with the published survival rate ranges mentioned above. Importantly, we did not observe a significant difference in survival based on the underlying anatomy, whether the patient had a biventricular or univentricular defect. In the literature, there are conflicting reports, with some studies showing no significant difference in outcome as we found [
12,
18,
19] or showing a better outcome for children with biventricular CHD when ECMO is needed [
20]. It should be noted that dividing patients based on underlying anatomy creates groups (biventricular and single ventricle CHD) that inherently contain a heterogeneous patient population.
Factors other than anatomy may also impact survival probability. These factors, described in the literature, include a high inotrope score, pre-ECMO acidosis, elevated pre-ECMO lactate, failure to clear lactate within 24 hours, bleeding while on ECMO, fluid overload, peripheral cannulation, renal failure, initiation of ECMO in the intensive care unit, and duration of ECMO support [
21,
22,
23,
24]. Bleeding during ECMO is one of the most significant risk factors reported in the literature, contributing to reduced survival rates [
25,
26]. The incidence of bleeding in cardiac patients on ECMO ranges from 25% to 78%, with children having a structural heart defect being at an increased risk of bleeding [
27,
28,
29,
30]. A study by Nardell et al. [
31] investigating risk factors in postcardiotomy patients requiring ECMO reported a 34.4% survival probability without bleeding complications. Our study confirms a high rate of bleeding complications during ECMO and demonstrates that this complication is associated with worse outcomes. The prevalence of reoperation due to bleeding did not differ significantly between single ventricle and biventricular children (25% vs. 29%). However, the mortality rate for single ventricle children with this complication was 100%, compared to 80% for children with biventricular CHD. In our presented cohort, the majority of patients underwent central cannulation, which is expected considering the percentage of post-cardiotomy cases or failures to wean from cardiopulmonary bypass (CPB) as indications for ECMO. It can be speculated that the slightly better survival rate observed in patients with central cannulation (27.3% vs. 22.2%) may be attributed to the use of larger cannulas and increased flow [
32]. On the other hand, peripheral ECMO carries the advantage of a lower risk of bleeding [
33]. Regarding pH and lactate levels, we found lower mean pH values in the group of children with biventricular hearts, along with higher lactate values (pH 7.06 vs. 7.23, lactate 6.93 vs. 6.05). Nevertheless, the survival probability for this group was higher than that for children with a single ventricle heart (35% vs. 19%). Another risk factor can be identified in the presence of genetic abnormalities other than the ones strictly related to CHD. The incidence of non-cardiac and genetic diseases in children with a congenital heart defect is between 15-30% [
34,
35], which aligns with the findings in our cohort (24%). The aforementioned study by Alsoufi et al. demonstrated that the presence of genetic abnormalities is a significant risk factor for mortality, and we also observed a lower survival rate for children with non-cardiac genetic diseases compared to those without (9.5% vs. 26.1%). A well-known and well described risk factor is eCPR. In our study population, 38% of children with ECMO received eCPR. Among these, 10 patients (11%) survived to hospital discharge and are still alive after one year. It is worth noting that 2 patients (2.3%) had an univentricular heart, while 8 patients (9%) had a biventricular circulation. According to the ELSO report, neonates and pediatric patients who undergo ECMO after CPR have survival rates of 40% and 41%, respectively [
1]. A study by Tajik et al. also reported a survival probability similar to ours, with 39.6% until hospital discharge [
36], which is consistent with our findings. This study also observed that a CPR duration exceeding 30 minutes is associated with increased mortality prior to discharge. Furthermore, it is known from this study and others [
37] that neurological complications are common in this subgroup of patients, so studying their long term effects should be one objective of future studies. In our analysis, the mean duration of CPR before ECMO implantation was 49 minutes in both groups. Similar to Tajik et al., the 10 survivors had 23 minutes of CPR.
Furthermore, in our analysis, the survival of children who required a second ECMO run was worse compared to those who had only one run (15% vs. 28% survival). The literature reports survival rates of around 30% for neonates and 35% to 40% for pediatric patients following a second ECMO run [
38,
39]. In our cohort, 13 patients (15%) required a second ECMO run, of which approximately half (n=6) were neonates. The outcomes were poor, with only two children surviving the second ECMO run. Both survivors were pediatric patients with hypoplastic left heart syndrome. Although the additional ECMO run was associated with worse outcomes in terms of survival, no significant differences were observed in the incidence of complications (45.9% vs. 46.1%).
4.1. Limitations
Several limitations should be considered in the interpretation of our findings, given that this study was retrospective and conducted at a single center. The relatively small sample size of 88 patients and the long observation period of 10 years may have implications for the generalizability of the results.
The indication for ECMO implantation was determined retrospectively based on available data, and it is important to note that the decision-making process was often influenced by multiple factors rather than a single determinant. The factors leading to ECMO described in this study represent the most relevant considerations at the time of decision-making.
We acknowledge that we did not report the ultimate cause of death for the patients in our cohort. While failure of cardiac function recovery could be attributed as a cause of death, it is essential to recognize that there may be other contributing factors, such as sepsis, intracranial hemorrhage, or multi-organ failure, which could have played a significant role. Future studies should aim to provide a more comprehensive analysis of the ultimate causes of mortality in this patient population.
It is important to highlight that our study focused on descriptive representation rather than conducting statistical analyses to establish significant differences between the subgroups of patients who underwent eCPR versus those who did not receive CPR. Due to incomplete data, (unfortunately, CPR duration data were only available for 18 of the 23 non-survivors) and limitations inherent to a retrospective single-center study, we were unable to perform comprehensive statistical analyses for this specific comparison. Therefore, our findings regarding the differences between these two variables should be interpreted cautiously. The descriptive representation provides insights into the characteristics and outcomes of these subgroups, but further research is warranted to determine any significant differences in survival or other outcomes between patients who received eCPR and those who did not.
5. Conclusions
Our findings suggest that neonates and children with congenital heart defects can be successfully treated with ECMO, even in the presence of single ventricle heart defects. However, bleeding remains a significant complication associated with poorer outcomes. Additionally, the survival rate for patients requiring a second ECMO run within 30 days remains low. Further research is warranted to explore strategies for improving outcomes in this patient population and to address the identified limitations of this study.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Table S1: Factors leading to ECMO implantation; Table S2: other diagnosis.
Author Contributions
Conceptualization, M.S. (Martin Schweiger), and A.A.; methodology, M.S. (Martin Schweiger) and A.A.; software, A.A. and M.S. (Milena Stojanovic); validation, M.S. (Martin Schweiger), O.K. and R.C.; formal analysis, A.A. M.S. (Milena Stojanovic) and M.S. (Martin Schweiger); investigation, M.S. (Milena Stojanovic), T.E. and A.A.; resources, H.D. and S.P. and M.S. (Martin Schweiger); data curation, M.S. (Milena Stojanovic), T.E., and A.A.; writing—original draft preparation, A.A. and M.S. (Milena Stojanovic); writing—review and editing, A.A., M.S. (Milena Stojanovic), R.C., and M.M.; visualization, A.A, and M.S. (Martin Schweiger); supervision, M.S. (Martin Schweiger) and R.S.; project administration, M.S. (Martin Schweiger) and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external founding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “Cantonal Ethic Commission” of Zurich (protocol code 2020-01147; date: 20 May 2020).
Informed Consent Statement
General informed consent was obtained from all of the parents of the subjects involved in this study.
Data Availability Statement
All of the data of this study are in the Hospital-Database, available with the software “Phoenix®” of CGM Clinical (CompuGroup Medical Schweiz AG, Bern).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; McMullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L.; centers, E.m. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017, 63, 60–67. [Google Scholar] [CrossRef]
- Griselli, M.; Sinha, R.; Jang, S.; Perri, G.; Adachi, I. Mechanical Circulatory Support for Single Ventricle Failure. Front Cardiovasc Med 2018, 5, 115. [Google Scholar] [CrossRef]
- Sherwin, E.D.; Gauvreau, K.; Scheurer, M.A.; Rycus, P.T.; Salvin, J.W.; Almodovar, M.C.; Fynn-Thompson, F.; Thiagarajan, R.R. Extracorporeal membrane oxygenation after stage 1 palliation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2012, 144, 1337–1343. [Google Scholar] [CrossRef]
- Pizarro, C.; Davis, D.A.; Healy, R.M.; Kerins, P.J.; Norwood, W.I. Is there a role for extracorporeal life support after stage I Norwood? Eur J Cardiothorac Surg 2001, 19, 294–301. [Google Scholar] [CrossRef]
- Costello, J.M.; Cooper, D.S.; Jacobs, J.P.; Chai, P.J.; Kirsch, R.; Rosenthal, T.; Dalton, H.J.; Graziano, J.N.; Quintessenza, J.A. Intermediate-term outcomes after paediatric cardiac extracorporeal membrane oxygenation--what is known (and unknown). Cardiol Young 2011, 21 (Suppl. S2), 118–123. [Google Scholar] [CrossRef]
- Haines, N.M.; Rycus, P.T.; Zwischenberger, J.B.; Bartlett, R.H.; Undar, A. Extracorporeal Life Support Registry Report 2008: Neonatal and pediatric cardiac cases. ASAIO J 2009, 55, 111–116. [Google Scholar] [CrossRef]
- Delmo Walter, E.M.; Alexi-Meskishvili, V.; Huebler, M.; Loforte, A.; Stiller, B.; Weng, Y.; Berger, F.; Hetzer, R. Extracorporeal membrane oxygenation for intraoperative cardiac support in children with congenital heart disease. Interact Cardiovasc Thorac Surg 2010, 10, 753–758. [Google Scholar] [CrossRef]
- Erdil, T.; Lemme, F.; Konetzka, A.; Cavigelli-Brunner, A.; Niesse, O.; Dave, H.; Hasenclever, P.; Hubler, M.; Schweiger, M. Extracorporeal membrane oxygenation support in pediatrics. Ann Cardiothorac Surg 2019, 8, 109–115. [Google Scholar] [CrossRef]
- Allen, K.Y.; Allan, C.K.; Su, L.; McBride, M.E. Extracorporeal membrane oxygenation in congenital heart disease. Semin Perinatol 2018, 42, 104–110. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, T.; Li, Y.; Wu, S.; Wu, C.; Wei, G. Use of Extracorporeal Membrane Oxygenation After Congenital Heart Disease Repair: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2020, 7, 583289. [Google Scholar] [CrossRef]
- Chaturvedi, R.R.; Macrae, D.; Brown, K.L.; Schindler, M.; Smith, E.C.; Davis, K.B.; Cohen, G.; Tsang, V.; Elliott, M.; de Leval, M.; et al. Cardiac ECMO for biventricular hearts after paediatric open heart surgery. Heart 2004, 90, 545–551. [Google Scholar] [CrossRef]
- ElMahrouk, A.F.; Ismail, M.F.; Hamouda, T.; Shaikh, R.; Mahmoud, A.; Shihata, M.S.; Alradi, O.; Jamjoom, A. Extracorporeal Membrane Oxygenation in Postcardiotomy Pediatric Patients-15 Years of Experience Outside Europe and North America. Thorac Cardiovasc Surg 2019, 67, 28–36. [Google Scholar] [CrossRef]
- Florez, C.X.; Bermon, A.; Castillo, V.R.; Salazar, L. Setting Up an ECMO Program in a South American Country: Outcomes of the First 104 Pediatric Patients. World J Pediatr Congenit Heart Surg 2015, 6, 374–381. [Google Scholar] [CrossRef]
- Alsoufi, B.; Al-Radi, O.O.; Gruenwald, C.; Lean, L.; Williams, W.G.; McCrindle, B.W.; Caldarone, C.A.; Van Arsdell, G.S. Extra-corporeal life support following cardiac surgery in children: Analysis of risk factors and survival in a single institution. Eur J Cardiothorac Surg 2009, 35, 1004–1011. [Google Scholar] [CrossRef]
- Sasaki, T.; Asou, T.; Takeda, Y.; Onakatomi, Y.; Tominaga, T.; Yamamoto, Y. Extracorporeal life support after cardiac surgery in children: Outcomes from a single institution. Artif Organs 2014, 38, 34–40. [Google Scholar] [CrossRef]
- Merrill, E.D.; Schoeneberg, L.; Sandesara, P.; Molitor-Kirsch, E.; O'Brien, J., Jr.; Dai, H.; Raghuveer, G. Outcomes after prolonged extracorporeal membrane oxygenation support in children with cardiac disease--Extracorporeal Life Support Organization registry study. J Thorac Cardiovasc Surg 2014, 148, 582–588. [Google Scholar] [CrossRef]
- Gomez-Sanchez, R.; Garcia-Carreno, J.; Martinez-Solano, J.; Sousa-Casasnovas, I.; Juarez-Fernandez, M.; Devesa-Cordero, C.; Sanz-Ruiz, R.; Gutierrez-Ibanes, E.; Elizaga, J.; Fernandez-Aviles, F.; et al. Off-Hours versus Regular-Hours Implantation of Peripheral Venoarterial Extracorporeal Membrane Oxygenation in Patients with Cardiogenic Shock. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Morris, M.C.; Ittenbach, R.F.; Godinez, R.I.; Portnoy, J.D.; Tabbutt, S.; Hanna, B.D.; Hoffman, T.M.; Gaynor, J.W.; Connelly, J.T.; Helfaer, M.A.; et al. Risk factors for mortality in 137 pediatric cardiac intensive care unit patients managed with extracorporeal membrane oxygenation. Crit Care Med 2004, 32, 1061–1069. [Google Scholar] [CrossRef]
- Melvan, J.N.; Davis, J.; Heard, M.; Trivedi, J.R.; Wolf, M.; Kanter, K.R.; Deshpande, S.R.; Alsoufi, B. Factors Associated With Survival Following Extracorporeal Cardiopulmonary Resuscitation in Children. World J Pediatr Congenit Heart Surg 2020, 11, 265–274. [Google Scholar] [CrossRef]
- Dohain, A.M.; Abdelmohsen, G.; Elassal, A.A.; ElMahrouk, A.F.; Al-Radi, O.O. Factors affecting the outcome of extracorporeal membrane oxygenation following paediatric cardiac surgery. Cardiol Young 2019, 29, 1501–1509. [Google Scholar] [CrossRef]
- Roeleveld, P.P.; Mendonca, M. Neonatal Cardiac ECMO in 2019 and Beyond. Front Pediatr 2019, 7, 327. [Google Scholar] [CrossRef]
- Amodeo, A.; Erdil, T.; Vanetta, C.; Steigmiller, K.; Schmiady, M.; Schweiger, M.; Pretre, R.; Dave, H. Serum lactate at 24 hours is associated with outcome in children requiring extracorporeal membrane oxygenation for pulmonary causes - a retrospective, observational study. Swiss Med Wkly 2020, 150, w20358. [Google Scholar] [CrossRef]
- Ren, Y.Q.; Zhang, Y.C.; Shi, J.Y.; Shan, Y.J.; Sun, T.; Zhou, Y.P.; Cui, Y. [Analysis of risk factors of central nervous system complications supported on extracorporeal membrane oxygenation]. Zhonghua Er Ke Za Zhi 2022, 60, 1059–1065. [Google Scholar] [CrossRef]
- AbuHassan, H.R.; Arafat, A.A.; Albabtain, M.A.; Alwadai, A.H.; AlArwan, K.M.; Ali, A.A.; Rasheed, S.; Babikr, N.B.; Shaikh, S.F. Postcardiotomy extracorporeal membrane oxygenation in patients with congenital heart disease; the effect of place of initiation. Perfusion 2023, 2676591231177898. [Google Scholar] [CrossRef]
- Carmona, C.; Nellis, M.E.; Karam, O. Anticoagulation and hemostasis on extracorporeal membrane oxygenation. Curr Opin Pediatr 2023, 35, 297–302. [Google Scholar] [CrossRef]
- Saini, A.; Spinella, P.C. Management of anticoagulation and hemostasis for pediatric extracorporeal membrane oxygenation. Clin Lab Med 2014, 34, 655–673. [Google Scholar] [CrossRef]
- Dalton, H.J.; Reeder, R.; Garcia-Filion, P.; Holubkov, R.; Berg, R.A.; Zuppa, A.; Moler, F.W.; Shanley, T.; Pollack, M.M.; Newth, C.; et al. Factors Associated with Bleeding and Thrombosis in Children Receiving Extracorporeal Membrane Oxygenation. Am J Respir Crit Care Med 2017, 196, 762–771. [Google Scholar] [CrossRef]
- Drop, J.G.; Erdem, O.; Wildschut, E.D.; van Rosmalen, J.; de Maat, M.P.M.; Kuiper, J.W.; Houmes, R.J.M.; van Ommen, C.H. Use of rotational thromboelastometry to predict hemostatic complications in pediatric patients undergoing extracorporeal membrane oxygenation: A retrospective cohort study. Res Pract Thromb Haemost 2021, 5, e12553. [Google Scholar] [CrossRef]
- Werho, D.K.; Pasquali, S.K.; Yu, S.; Donohue, J.; Annich, G.M.; Thiagarajan, R.R.; Hirsch-Romano, J.C.; Gaies, M.G.; Extracorporeal Life Support Organization Member, C. Hemorrhagic complications in pediatric cardiac patients on extracorporeal membrane oxygenation: An analysis of the Extracorporeal Life Support Organization Registry. Pediatr Crit Care Med 2015, 16, 276–288. [Google Scholar] [CrossRef]
- Ankola, A.A.; Bailly, D.K.; Reeder, R.W.; Cashen, K.; Dalton, H.J.; Dolgner, S.J.; Federman, M.; Ghassemzadeh, R.; Himebauch, A.S.; Kamerkar, A.; et al. Risk Factors Associated With Bleeding in Children With Cardiac Disease Receiving Extracorporeal Membrane Oxygenation: A Multi-Center Data Linkage Analysis. Front Cardiovasc Med 2021, 8, 812881. [Google Scholar] [CrossRef]
- Nardell, K.; Annich, G.M.; Hirsch, J.C.; Fahrner, C.; Brownlee, P.; King, K.; Fleming, G.M.; Gajarski, R.J. Risk factors for bleeding in pediatric post-cardiotomy patients requiring ECLS. Perfusion 2009, 24, 191–197. [Google Scholar] [CrossRef]
- Harvey, C. Cannulation for Neonatal and Pediatric Extracorporeal Membrane Oxygenation for Cardiac Support. Front Pediatr 2018, 6, 17. [Google Scholar] [CrossRef]
- Kanji, H.D.; Schulze, C.J.; Oreopoulos, A.; Lehr, E.J.; Wang, W.; MacArthur, R.M. Peripheral versus central cannulation for extracorporeal membrane oxygenation: A comparison of limb ischemia and transfusion requirements. Thorac Cardiovasc Surg 2010, 58, 459–462. [Google Scholar] [CrossRef]
- Alsoufi, B.; Gillespie, S.; Mahle, W.T.; Deshpande, S.; Kogon, B.; Maher, K.; Kanter, K. The Effect of Noncardiac and Genetic Abnormalities on Outcomes Following Neonatal Congenital Heart Surgery. Semin Thorac Cardiovasc Surg 2016, 28, 105–114. [Google Scholar] [CrossRef]
- Furlong-Dillard, J.; Bailly, D.; Amula, V.; Wilkes, J.; Bratton, S. Resource Use and Morbidities in Pediatric Cardiac Surgery Patients with Genetic Conditions. J Pediatr 2018, 193, 139–146. [Google Scholar] [CrossRef]
- Tajik, M.; Cardarelli, M.G. Extracorporeal membrane oxygenation after cardiac arrest in children: What do we know? Eur J Cardiothorac Surg 2008, 33, 409–417. [Google Scholar] [CrossRef]
- Speth, M.; Munch, F.; Purbojo, A.; Glockler, M.; Toka, O.; Cesnjevar, R.A.; Ruffer, A. Pediatric Extracorporeal Life Support Using a Third Generation Diagonal Pump. ASAIO J 2016, 62, 482–490. [Google Scholar] [CrossRef]
- Fisher, J.C.; Stolar, C.J.; Cowles, R.A. Extracorporeal membrane oxygenation for cardiopulmonary failure in pediatric patients: Is a second course justified? J Surg Res 2008, 148, 100–108. [Google Scholar] [CrossRef]
- Meehan, J.J.; Haney, B.M.; Snyder, C.L.; Sharp, R.J.; Acosta, J.M.; Holcomb, G.W., 3rd. Outcome after recannulation and a second course of extracorporeal membrane oxygenation. J Pediatr Surg 2002, 37, 845–850. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).