Submitted:
28 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
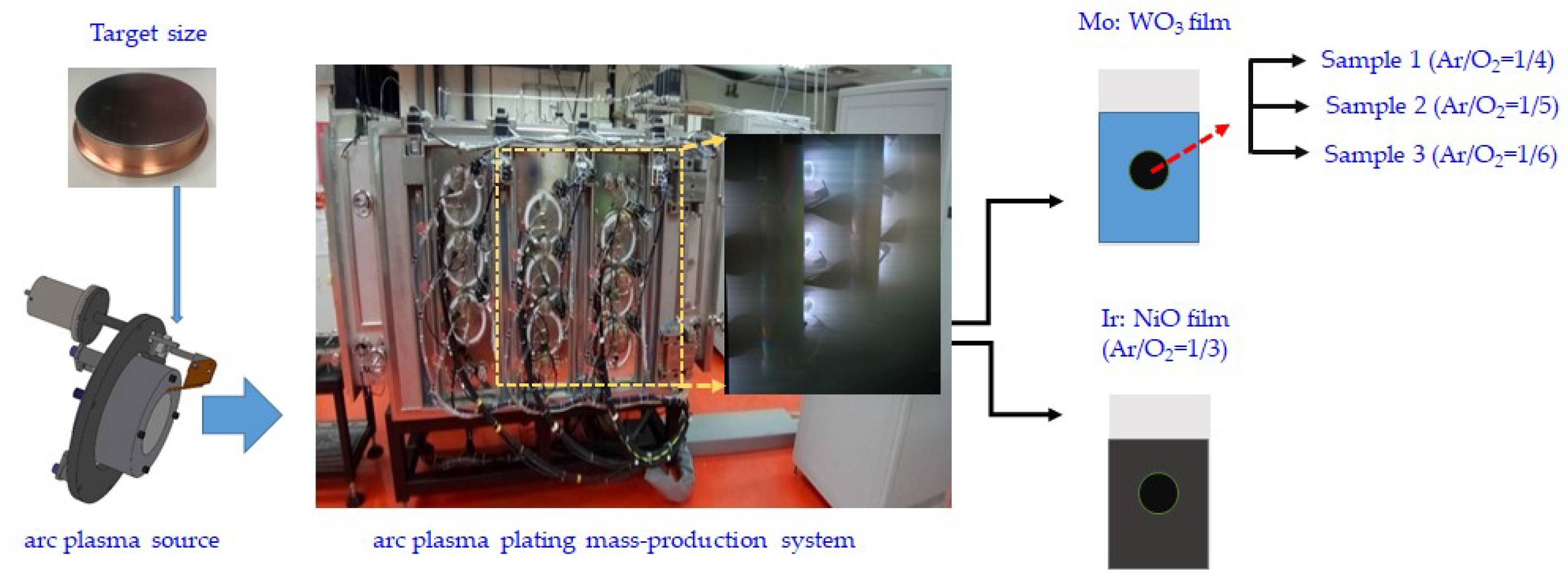
2.1. Synthesis of Mo: WO3 (Electrochromic layer) working and the transparent electrode
2.2. Deposition of IrO2 and NiO Counter Electrode
2.3. Gel Polymer Electrolyte Preparation
2.4. Experimental Details
3. Results
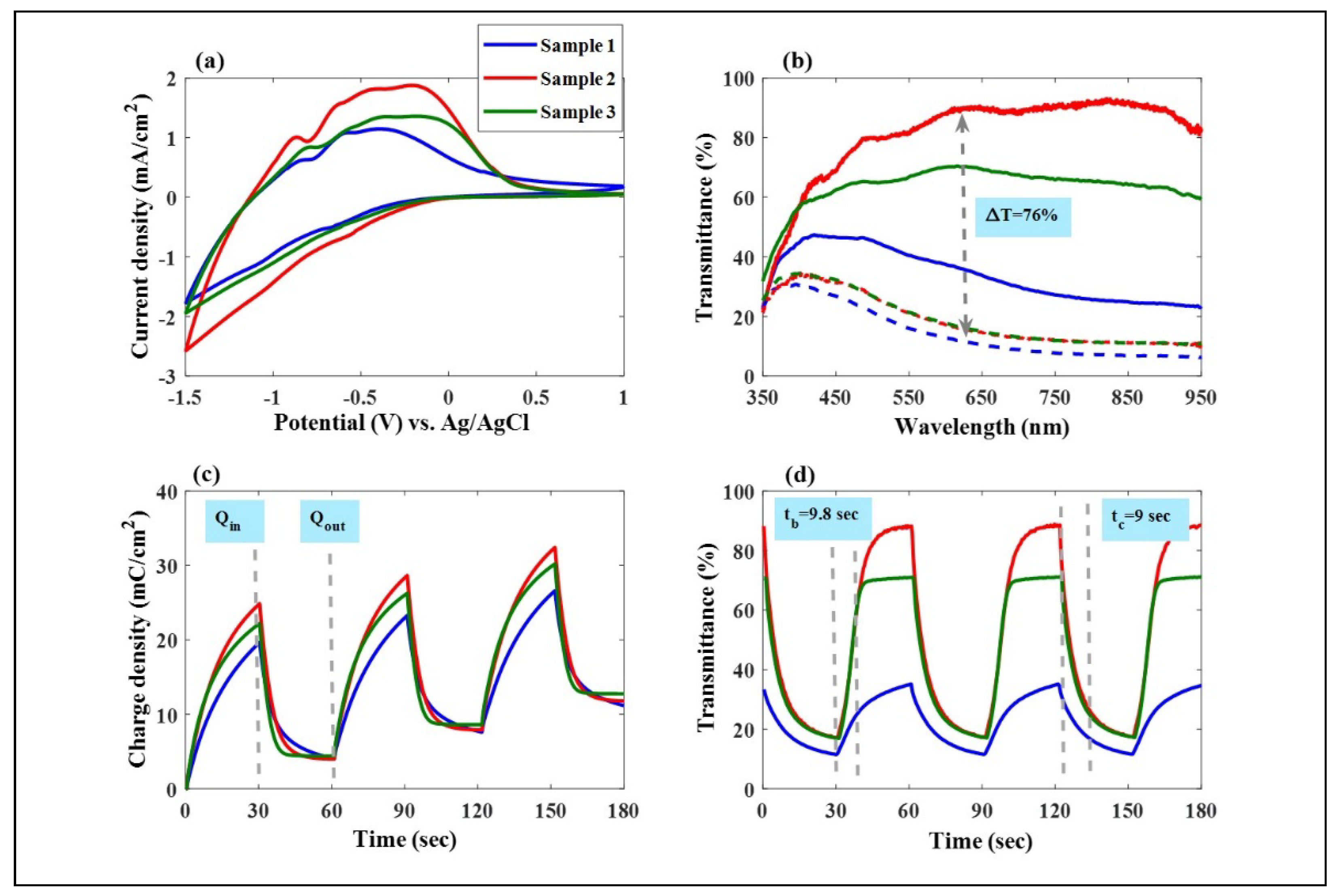
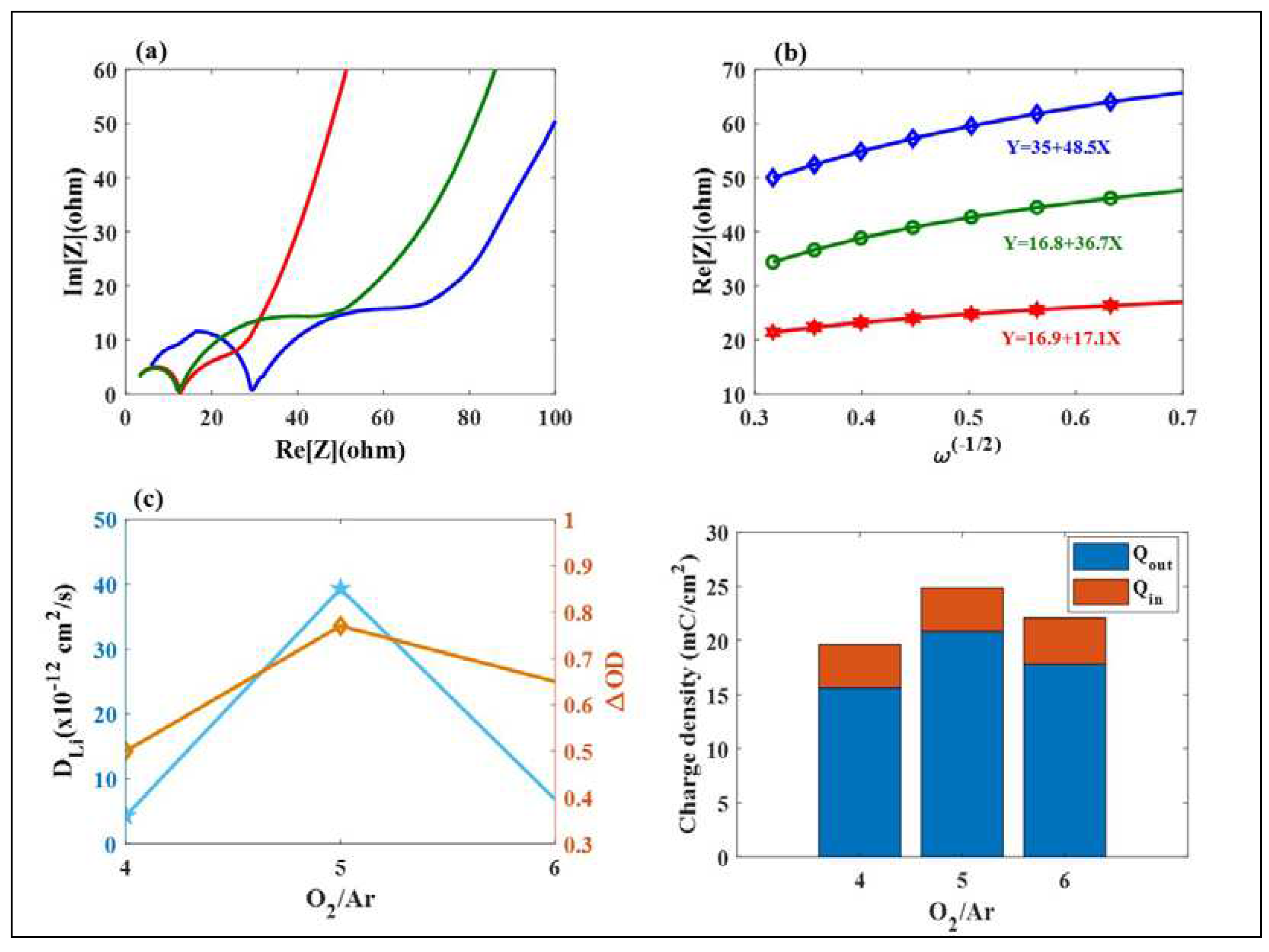
3.1. Mo: WO3 /ITO Films: Electrochromic and Capacitive Performance
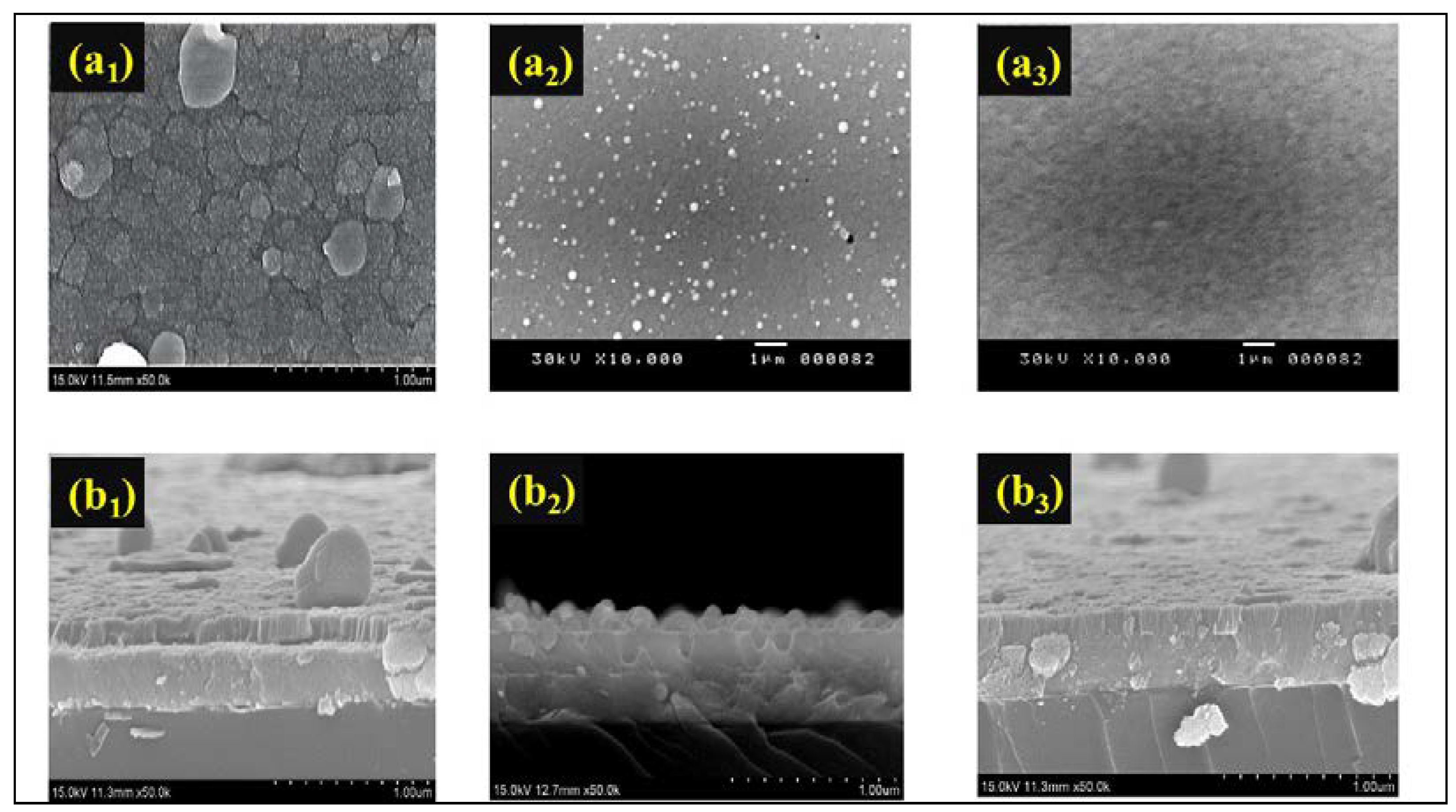
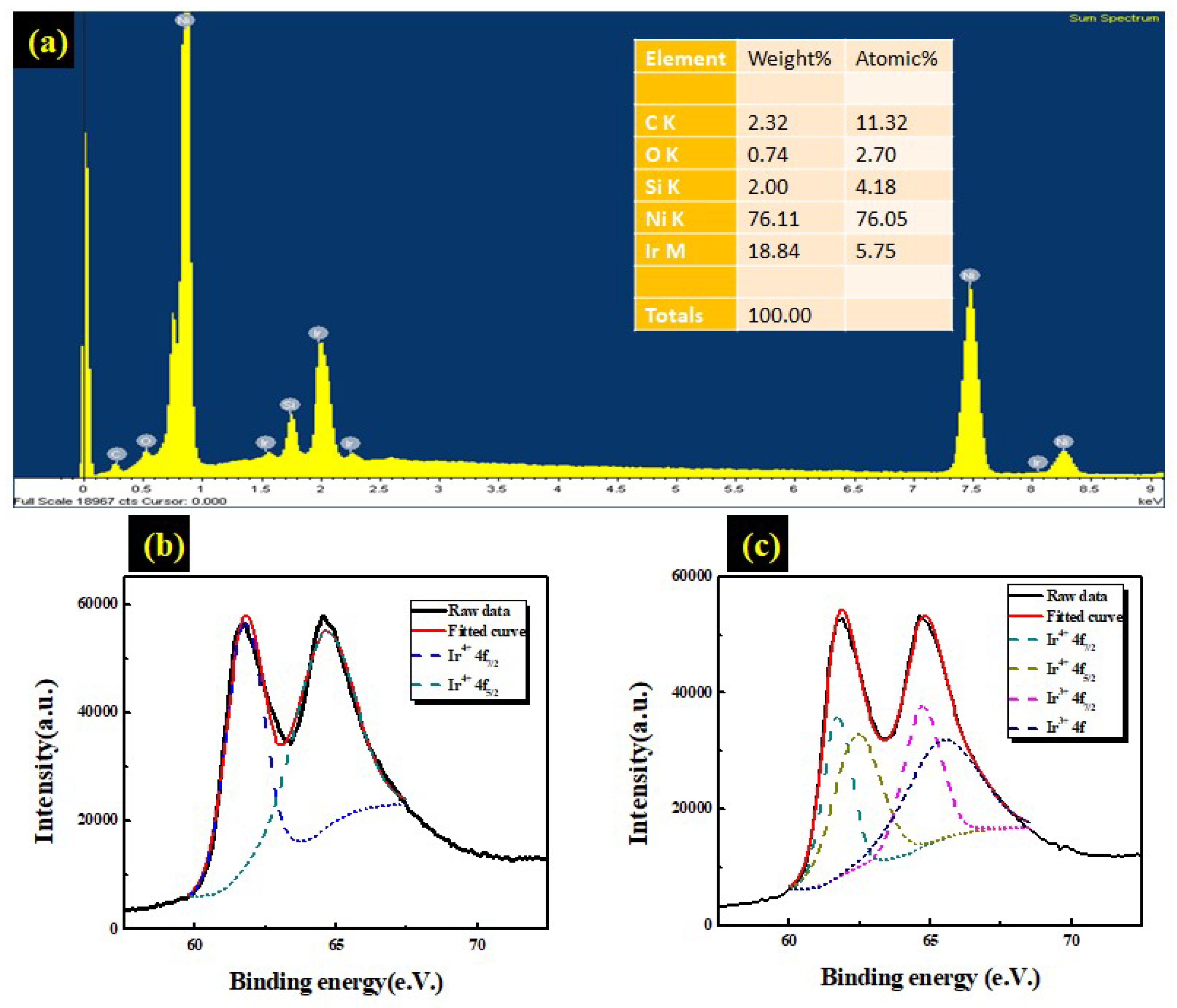
3.2. Characteristic of Ir: NiO/ITO films
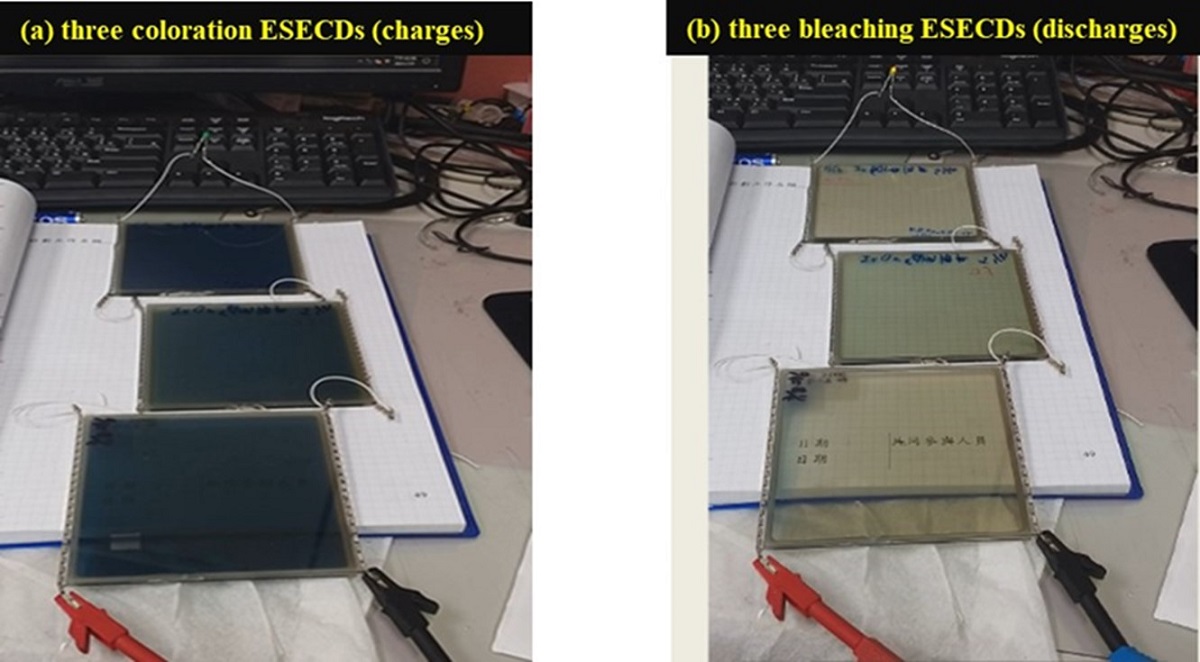
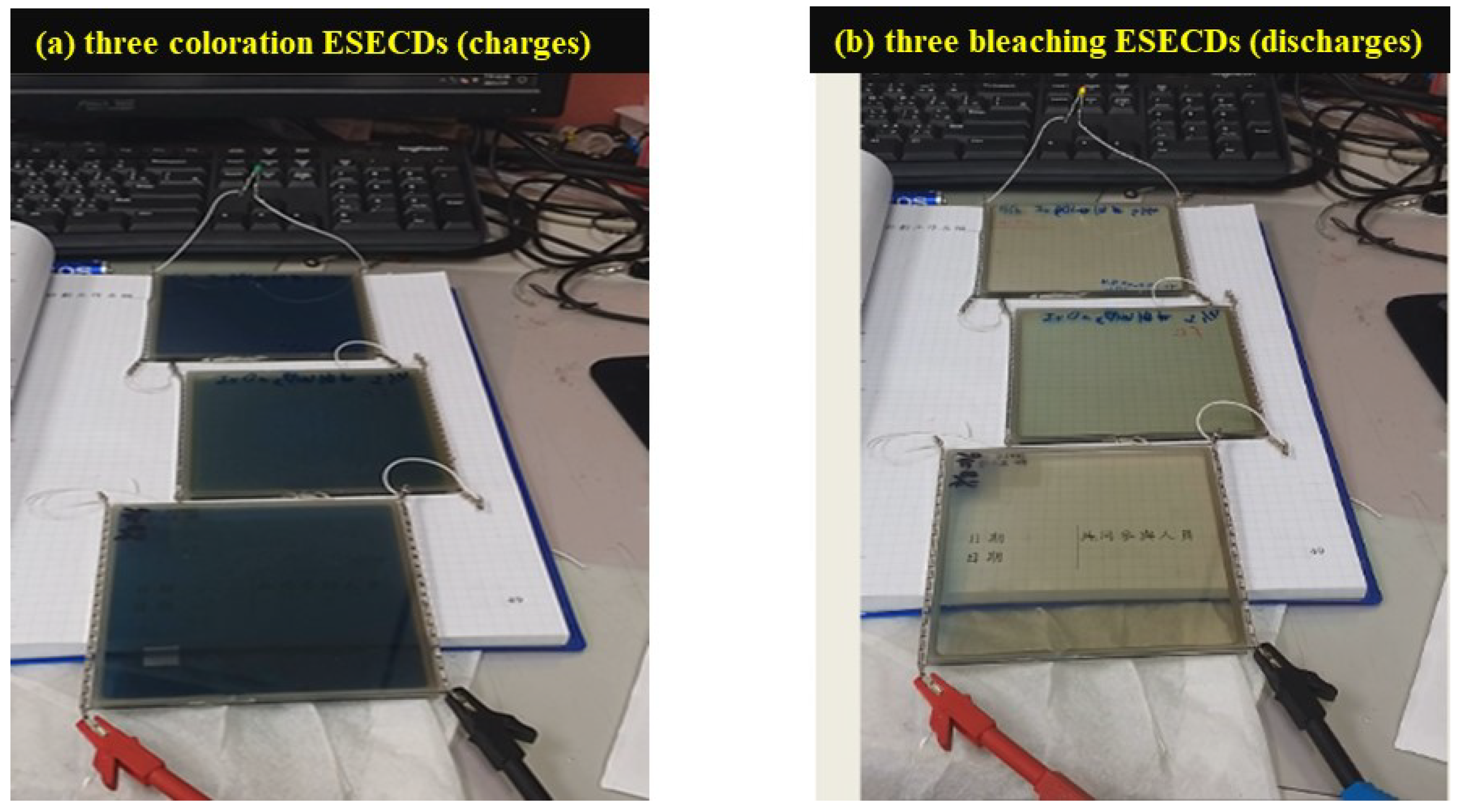
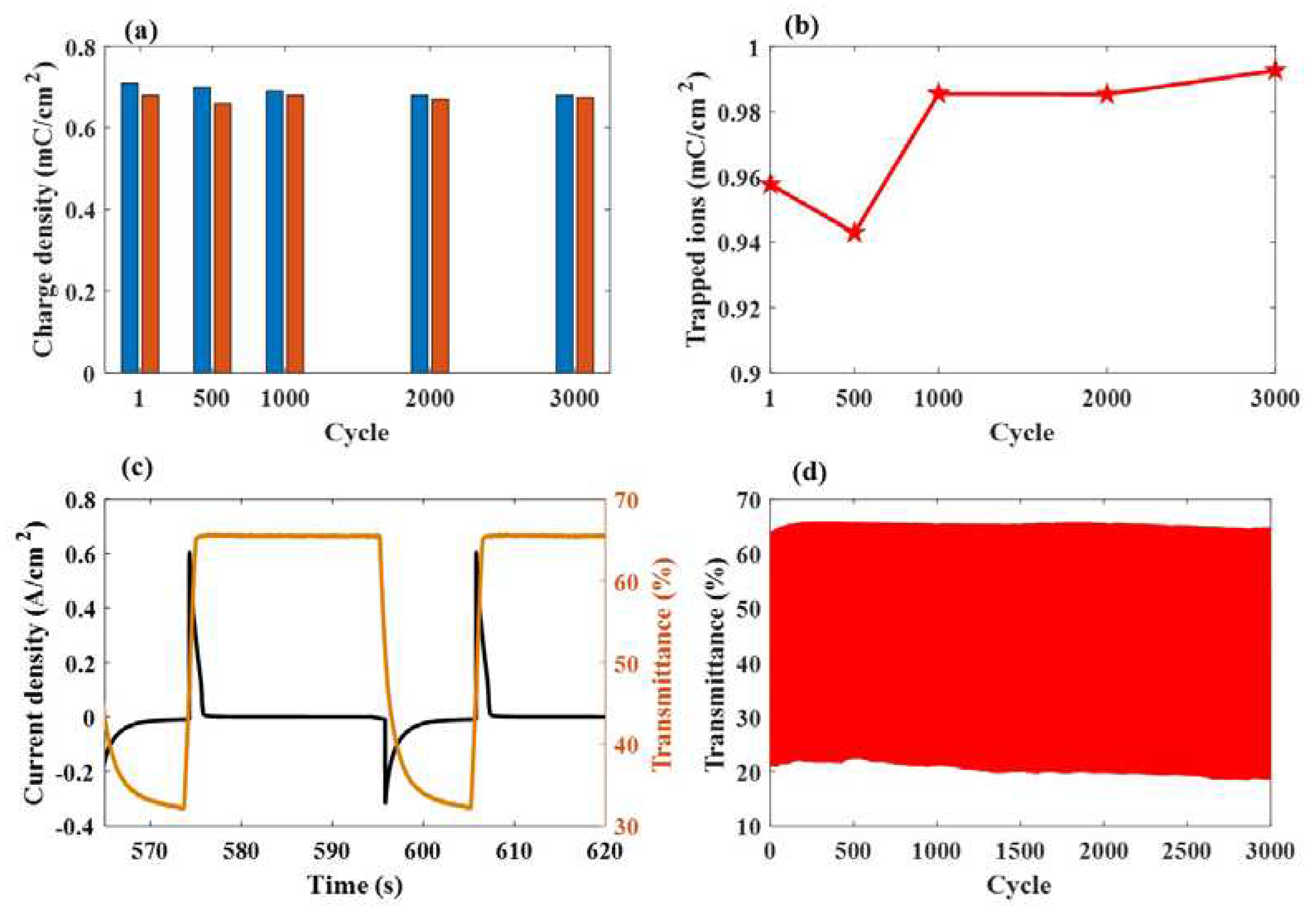
3.3. Bi-Functional ESECDs: Electrochromic and Energy-Storge Performance
4. Conclusions
References
- Wang, J.; Zhang, L.; Yu, L.; Jiao, Z.; Xie, H.; Lou, X.W.; Sun, X.W. A bi-functional device for self-powered electrochromic window and self-rechargeable transparent battery applications. Nat. Commun. 2014, 5, 4921. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wang, X.; Cui, M.; Darmawan, P.; Wang, J.; Eh, A.L.-S.; Lee, P.S. Electrochromo-supercapacitor based on direct growth of NiO nanoparticles. Nano Energy 2015, 12, 258–267. [Google Scholar] [CrossRef]
- Ghosh, A.; Norton, B. Advances in switchable and highly insulating autonomous (self-powered) glazing systems for adaptive low energy buildings. Renew. Energy 2018, 126, 1003–1031. [Google Scholar] [CrossRef]
- Kou, Z.; Wang, J.; Tong, X.; Lei, P.; Gao, Y.; Zhang, S.; Cui, X.; Wu, S.; Cai, G. Multi-functional electrochromic energy storage smart window powered by CZTSSe solar cell for intelligent managing solar radiation of building. Solar Energy Materials & Solar Cells 2023, 254, 112273. [Google Scholar]
- Baetens, R.; Jelle, B.P. Gustavsen, Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energ. Mat. Sol. C. 2010, 94, 87–105. [Google Scholar] [CrossRef]
- Eh, A.L.-S.; Tan AW, M.; Cheng, X.; Magdassi, S.; Lee, P.S. Recent Advances in Flexible Electrochromic Devices: Prerequisites, Challenges, and Prospects. Energy Technol. 2017, 6, 33. [Google Scholar] [CrossRef]
- Zhao, S.-Q.; Liu, Y.-H.; Ming, Z.; Chen, C.; Xu, W.-W.; Chen, L.; Huang, W. Highly Flexible Electrochromic Devices Enabled by Electroplated Nickel Grid Electrodes and Multifunctional Hydrogels. Opt. Express 2019, 27, 29547. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Z.; Qiu, W.; Hou, C.; Wang, Y.; Yao, P.; Yu, R.; Guo, W.; Liu, X.Y. Ultraflexible, Stretchable and Fast-Switching Electrochromic Devices with Enhanced Cycling Stability. RSC Adv. 2018, 8, 18690. [Google Scholar] [CrossRef]
- Rozman, M.; Zener, B.; Matoh, L.; Godec, R.F.; Mourtzikou, A.; Stathatos, E.; Bren, U.; Lukšic, M. Flexible Electrochromic Tape Using Steel Foil with WO3 Thin Film. Electrochim. Acta 2020, 330, 135329. [Google Scholar] [CrossRef]
- Ko, T.F.; Chen, P.W.; Li, K.M.; Young, H.T.; Chang, C.T.; Shu, S.C. High Performance Complementary Electrochromic Device Based on Iridium Oxide as a Counter Electrode. Materials 2021, 14, 1591. [Google Scholar] [CrossRef]
- Chen, P.W.; Chang, C.T.; Ali, M.; Wu, J.-Y.; Li, Y.-C.; Chen, M.-H.; Jan, D.-J.; Yuan, C.-T. Tantalum oxide film deposited by vacuum cathodic arc plasma with improved electrochromic performance. Sol. Energy Mater. Sol. Cells 2018, 182, 188–195. [Google Scholar] [CrossRef]
- Chen, P.W.; Chang, C.T.; Ko, T.F.; Hsu, S.C.; Li, K.F.; Wu, J.Y. Fast response of complementary electrochromic device based on WO3/NiO electrodes. Sci. Rep. 2020, 10, 8430. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Wu, T.H.; Huang, J.W.; Young BLi Jian, W.B.; Lin, Y.L.; Chen, J.T.; Hsu, C.S.; Ma, Y.R.; Tsukagoshi, K. Nanoparticulate films of WO3 and MoO3 composites for enhancing UV light electrochromic transmittance variation and energy storage applications Electrochim. Acta 2023, 442, 141897. [Google Scholar]
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chen, Y.C.; Wang, C.M.; Wang, W.N.; Wen, C.Y.; Lin, J.M. Electrochromic properties of Lithium-doped tungsten oxide prepared by electron beam evaporation. Coatings 2019, 9, 191. [Google Scholar] [CrossRef]
- Kim, K.H.; Koo, B.R.; Ahn, H.J. Title of the chapter Sheet resistance dependence of fluorine-doped tin oxide films for high-performance electrochromic devices. Ceram. Int. 2018, 44, 9408–9413. [Google Scholar] [CrossRef]
- Jiang, B.; Lou, B.; Li, J.; Peng, P.; Chen, J.W.; Chu, L.H.; Li, Y.F.; Li, M.C. Electrochemical effect of graphite fluoride modification on Li-rich cathode material in lithium ion battery. Ceram. Int. 2019, 45, 160–167. [Google Scholar] [CrossRef]
- Runnerstrom, E.L.; Llorde´s, A.; Lounisac, S.D.; Milliron, D.J. Nanostructured electrochromic smart windows:traditional materials and NIR-selective plasmonic nanocrystals. Chem. Commun. 2014, 50, 10555–10572. [Google Scholar] [CrossRef]
- Li, H.; McRaea, L.; Firby, C.J.; Hussein, M.A.; Elezzabi, A.Y. Nanohybridization of molybdenum oxide with tungsten molybdenum oxide nanowires for solution-processed fully reversible switching of energy storing smart windows. Nano. Energy. 2020, 47, 130–139. [Google Scholar] [CrossRef]
- Lang, F.; Liu, J.; Wang, H.; Yan, H. NiO nanocrystalline/reduced graphene oxide composite film with enhanced electrochromic properties. Nano. 2017, 12, 1750058. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Chen, J.; Wang, X.; Eh, A.L.S.; Magdassi, S.; Lee, P.S. , Inkjet-printed all solid-state electrochromic devices based on NiO/WO3. Nanoscale. 2016, 8, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.F.; Chen, P.W.; Li, K.M.; Young, H.T. ; Applied IrO2 Buffer Layer as a Great Promoter on Ti-Doping V2O5 Electrode to Enhance Electrochromic Device Properties. Materials 2022, 15, 1579. [Google Scholar] [CrossRef] [PubMed]
- Li, K.D.; Chen, P.W.; Chang, K.S. Low-Temperature Deposition of Transparent Conducting Films Applied to Flexible Electrochromic Devices. Materials 2021, 14, 4959. [Google Scholar]
- Madhavi, V.; Kumar, P.J.; Kondaiah, P.; Hussain, O.M.; Uthanna, S. Efect of molybdenum doping on the electrochromic properties of tungsten oxide thin fms by RF magnetron sputtering. Ionics. 2014. 20, 1737–1745.
- Pooyodying. Pattarapon.; Son, Y.H.; Sung, Y.M.; Ok, J.W. The effect of sputtering Ar gas pressure on optical and electrical properties of flexible ECD device with WO3 electrode deposited by RF magnetron sputtering on ITO/PET substrate. Opt. Mater. 2022, 123, 111829. [Google Scholar] [CrossRef]
- Panagopoulou, M.; Vernardou, D.; Koudoumas, E.; Tsoukalas, D.; Raptis, Y.S. Oxygen and temperature effects on the electrochemical and electrochromic properties of rf-sputtered V2O5 thin films. Electrochim. Acta. 2017, 232, 54–63. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, C.; Wan, Z.; Weng, X.; Xie, J.; Deng, L. Electrochemical and electrochromic properties of novel nanoporous NiO/ V2O5 hybrid film. Sol. Energy Mater Sol. Cells. 2015, 132, 467–475. [Google Scholar] [CrossRef]
- Najdosk, M.; Koleva, V.; Samet, A. ; Effect of deposition conditions on the electrochromic properties of nanostructured thin films of ammonium intercalated vanadium pentoxide xerogel. J. Phys. Chem. C. 2014, 118, 9636–9646. [Google Scholar] [CrossRef]
- Yu, D.M.; Zhang, S.T.; Liu, D.W.; Zhou, X.Y.; Xie, S.H.; Zhang, Q.F.; Liu, Y.Y.; Cao, G.Z. Effect of manganese doping on Li-ion intercalation properties of V2O5 films. J. Mater. Chem. 2010, 20, 10841–10846. [Google Scholar] [CrossRef]
- Loi, M.R.; Moura, E.A.; Westphal, T.M.; Balboni, R.D.C.; Gündelc, A.; Flores, W.H.; Pereira., M.B.; Santos, M.J.L.; Santos., J.F.L.; Pawlicka, A.; Avellaneda, C.O. Impact of Zr precursor on the electrochemical properties of V2O5 sol-gel films. J. Electroanal. Chem. 2019, 839, 67–74. [Google Scholar] [CrossRef]
- Moura, E.A.; Cholan, C.M.; Balboni, R.D.C.; Westphal, T.M.; Lemos, R.M.J.; Azevedo, C.F.; Gündel, A.; Flores, W.H.; Gomez, J.A.; Ely, F.; Pawlicka, A.; Avellaneda, C.O. Electrochemical properties of thin films of V2O5 doped with TiO2. J. Phys. Chem. Solids. 2018, 119, 1–8. [Google Scholar] [CrossRef]
- Vasanth, R.D.; Ponpandian, N.; Mangalaraj, D.; Viswanathan, C. Effect of annealing and electrochemical properties of sol–gel dip coated nanocrystalline V2O5 thin films. Mater. Sci. Semicond. Process. 2013, 16, 256–262. [Google Scholar] [CrossRef]
- Sinha, S.K. Effect of temperature on structural, optical and electrical properties of pulsed-laser deposited W-doped V2O5 thin films. Superlattices Microstruct. 2019, 25, 88–94. [Google Scholar] [CrossRef]
- Qiu, D.; Wu, J.; Liang, L.; Zhang, H.; Cao, H.; Yong, W.; Tian, T.; Gao, J.; Zhuge, F. Structural and electrochromic properties of undoped and Mo-doped V2O5 thin films by two-electrode electrodeposition. J. Nanosci. Nanotechnol. 2018, 18, 7502–7507. [Google Scholar] [CrossRef]
- Cai, Y.; Hernandez, T.S.; et al. Gel polymer electrolyte for reversible metal electrodeposition dynamic windows enables dual-working electrodes for faster switching and reflectivity control. Front. Nanotechnol. 2022, 4, 1083247. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Q.; Zhang, Q.; Dong, G.; Zhong, X.; Xiao, Yu.; Delplancke-Ogletree, M.P.; Reniers, F.; Diao, X. Dynamic behaviors of inorganic all-solid-state electrochromic device: Role of potential. Electrochim. Acta. 2018, 269, 617–623. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, H.; Zhang, Y.Z.; Liu, J.B.; Yan, H. Electroanalysis 2017, 29, 1573. [CrossRef]
- Zhou, K.; Wang, H.; Liu, J.; Yan, H. Int. J. Electrochem. Sci. 2018, 13, 7335–7346. [CrossRef]
- Zhou, K.L.; Wang, H.; Zhang, Y.Z.; Liu, J.B.; Yan, H. J. Electrochem.Soc. 2016, 163, 1033. [CrossRef]

| No. | Film | Ar/O2 (Ar =150 sccm) |
W.P. (Torr) |
DC Power (W) |
Deposition Temp. (°C) |
Deposition Time (s) |
Thickness (nm) |
|---|---|---|---|---|---|---|---|
| Sample 1 | Mo: WO3 | 1/4 | 1 × 10−2 | 1400 | 50 | 1450 | 200 |
| Sample 2 | Mo: WO3 | 1/5 | 1 × 10−2 | 1400 | 50 | 1500 | 200 |
| Sample 3 | Mo: WO3 | 1/6 | 1 × 10−2 | 1400 | 50 | 1550 | 200 |
| ITO | Ar=150 | 3.5 × 10−3 | 650 | 200 | 3600 | 300 |
| Target | Film | Ar/O2 (Ar =150 sccm) |
W.P. (Torr) |
DC power (W) |
Deposition Time (sec) |
Deposition Rate (nm/sec) |
Deposition Temp °C |
Thickness (nm) |
|---|---|---|---|---|---|---|---|---|
| Ir/Ni Metal | Ir: NiO | 1/3 | 8 × 10−3 | 1500 | 100 | 1 | 50 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




