Submitted:
27 June 2023
Posted:
28 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
2.1. Bacteria isolation
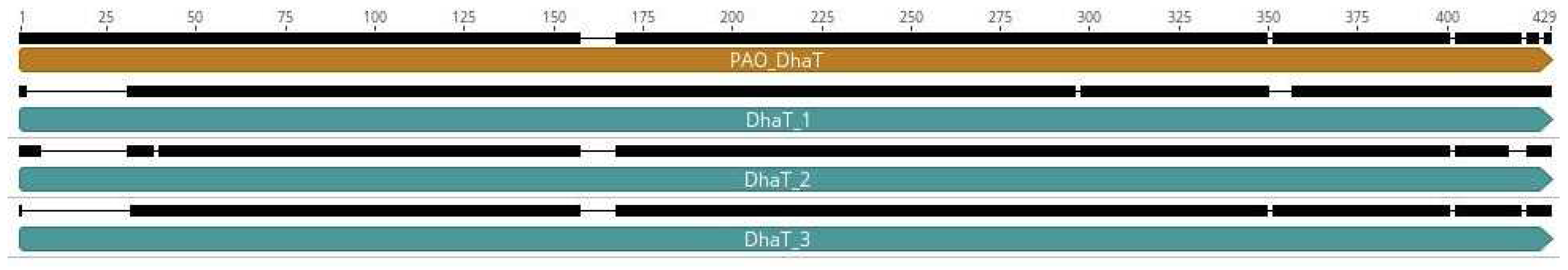
2.2. Genome sequencing and identification of 1,3-PDO-associated genes
2.3. Cultivation of the isolate and culture medium
2.4. Microbial fuel cell design and operation
2.5. Fermentation system
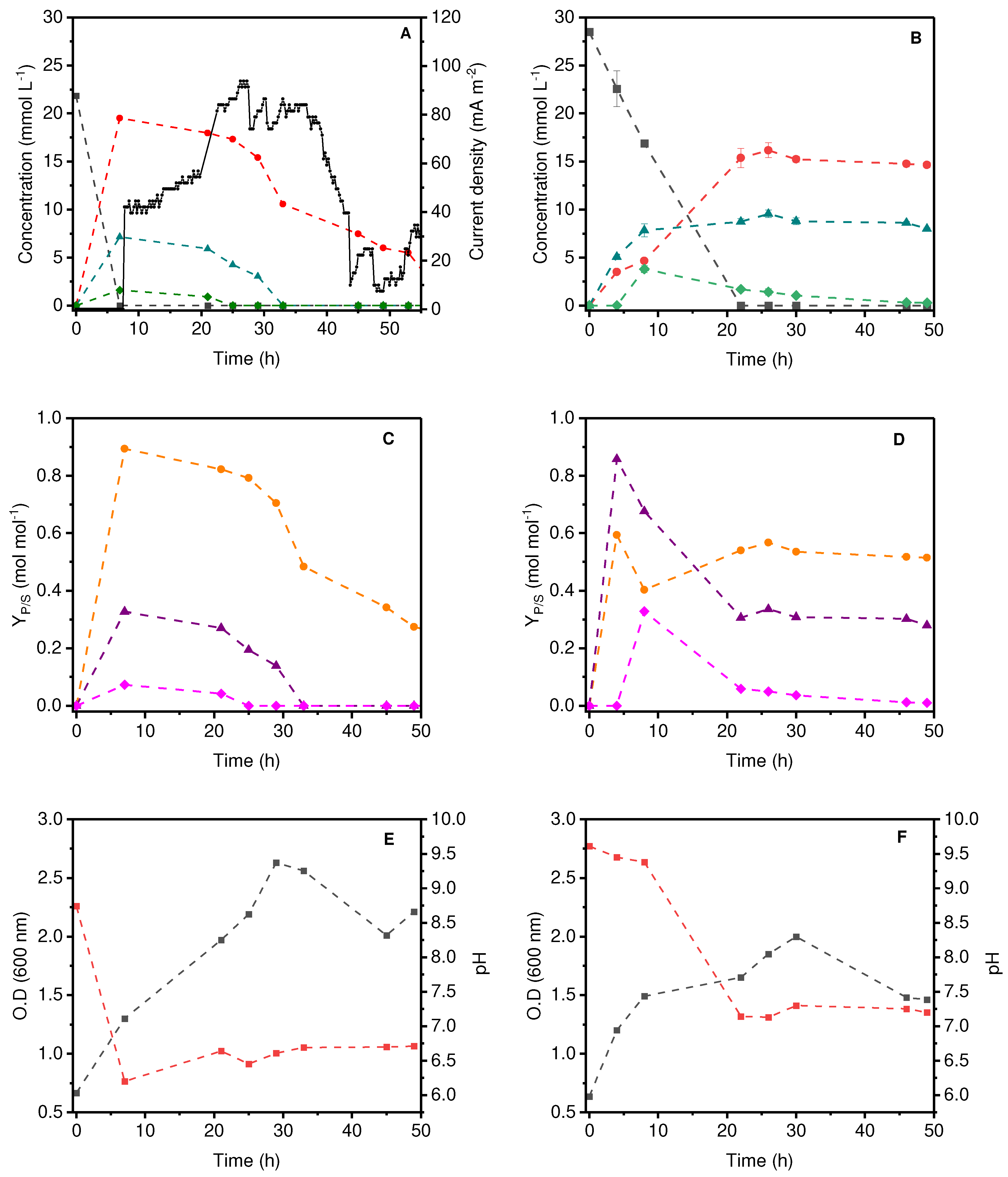
2.6. Substrate and by-product analysis
2.7. Conversion factor (YP/S)
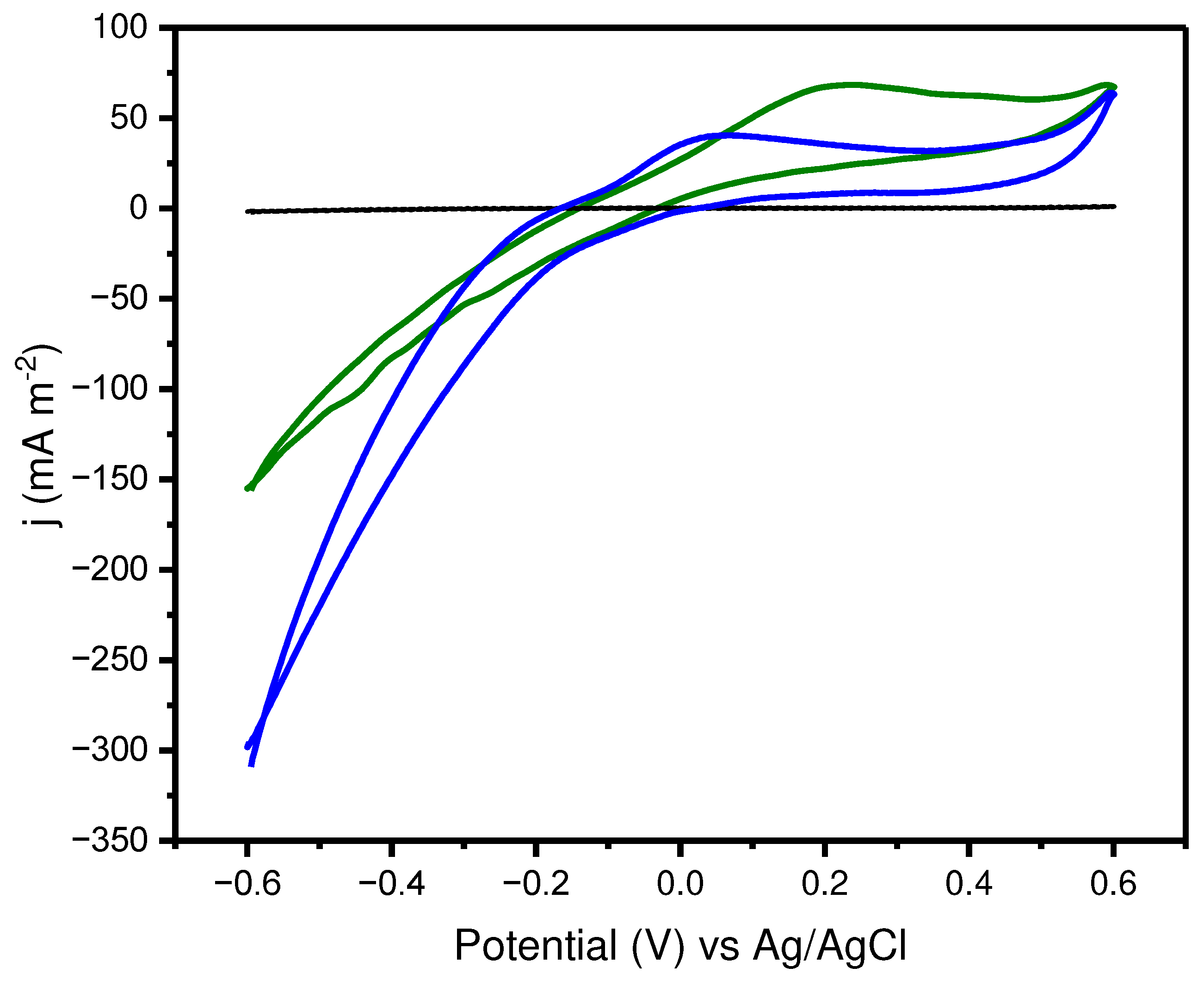
2.8. Electrochemical measurements
2.9. Coulombic Efficiency (CE)
3. Results and discussion
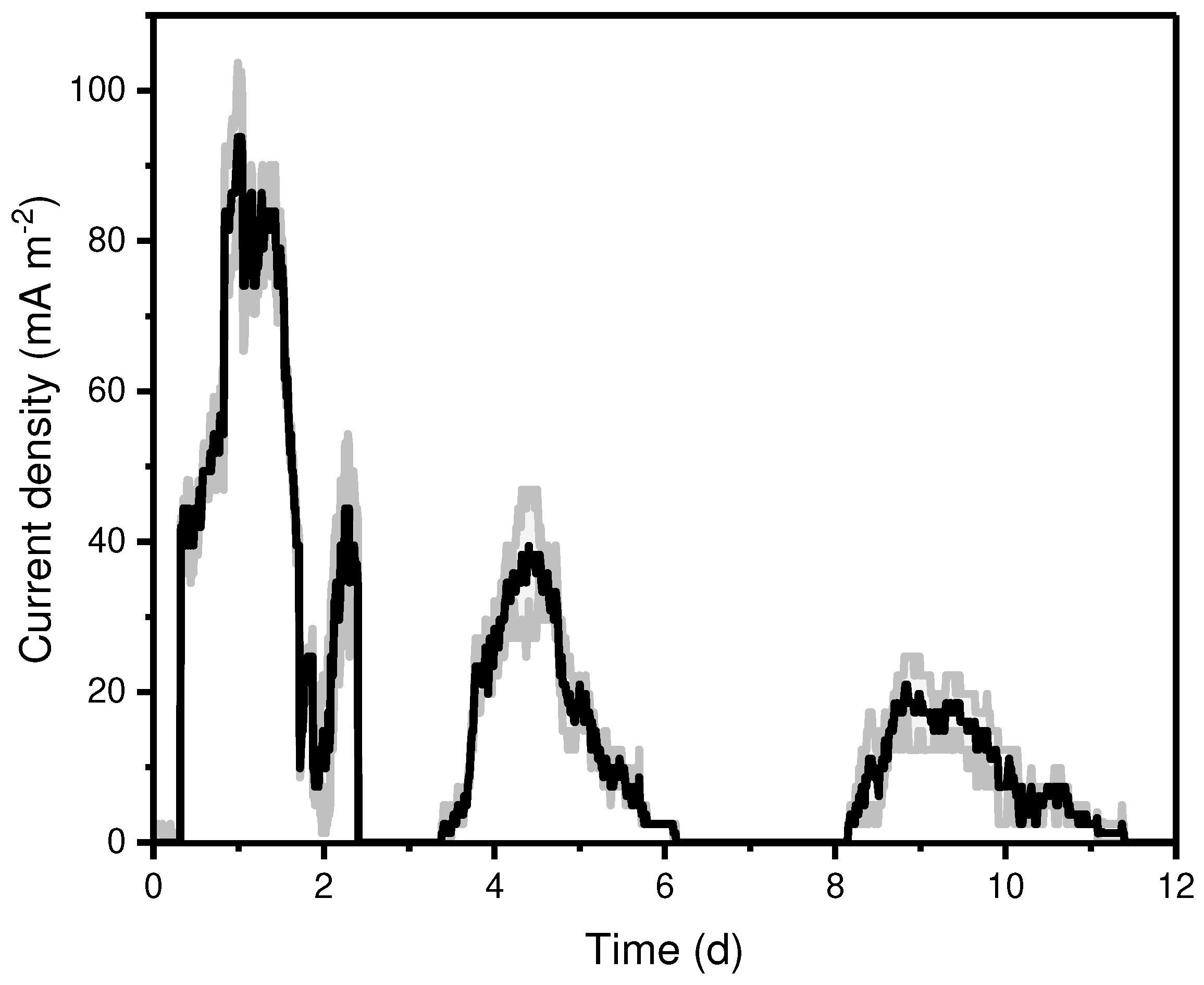
3.1. Electrochemical activity of the isolate
3.2. Glycerol oxidation and by-products formation during MFC and fermentative system operation
3.3. Identification of the genes responsible for the production of 1,3-PDO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial Fuel Cells: An Overview of Current Technology. Renew and Sustain Energy Rev 2019, 101, 60–81. [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.P.; Sutterlin, W.R.; Suppes, G.J. Low-Pressure Hydrogenolysis of Glycerol to Propylene Glycol. Appl Catal A Gen 2005, 281, 225–231. [CrossRef]
- Attarbachi, T.; Kingsley, M.D.; Spallina, V. New Trends on Crude Glycerol Purification: A Review. Fuel 2023, 340, 127485. [CrossRef]
- Zhu, F.; Liu, D.; Chen, Z. Recent Advances in Biological Production of 1,3-Propanediol: New Routes and Engineering Strategies. Green Chemistry 2022, 24, 1390–1403. [CrossRef]
- Przystałowska, H.; Lipiński, D.; Słomski, R. Biotechnological Conversion of Glycerol from Biofuels to 1,3-Propanediol Using Escherichia coli. Acta Biochim Pol 2015, 62, 23–34. [CrossRef]
- Forage, R.G.; Lin, E.C.C. DHA System Mediating Aerobic and Anaerobic Dissimilation of Glycerol in Klebsiella pneumoniae NCIB 418. J Bacteriol 1982, 151, 591–599. [CrossRef]
- da Silva Ruy, A.D.; de Brito Alves, R.M.; Reis Hewer, T.L.; de Aguiar Pontes, D.; Gomes Teixeira, L.S.; Magalhães Pontes, L.A. Catalysts for Glycerol Hydrogenolysis to 1,3-Propanediol: A Review of Chemical Routes and Market. Catal Today 2021, 381, 243–253. [CrossRef]
- Marone, A.; Ayala-Campos, O.R.; Trably, E.; Carmona-Martínez, A.A.; Moscoviz, R.; Latrille, E.; Steyer, J.P.; Alcaraz-Gonzalez, V.; Bernet, N. Coupling Dark Fermentation and Microbial Electrolysis to Enhance Bio-Hydrogen Production from Agro-Industrial Wastewaters and by-Products in a Bio-Refinery Framework. Int J Hydrogen Energy 2017, 42, 1609–1621. [CrossRef]
- Li, Z.; Dong, Y.; Liu, Y.; Cen, X.; Liu, D.; Chen, Z. Systems Metabolic Engineering of Corynebacterium glutamicum for High-Level Production of 1,3-Propanediol from Glucose and Xylose. Metab Eng 2022, 70, 79–88. [CrossRef]
- Zhou, S.; Lama, S.; Sankaranarayanan, M.; Park, S. Metabolic Engineering of Pseudomonas denitrificans for the 1,3-Propanediol Production from Glycerol. Bioresour Technol 2019, 292, 121933. [CrossRef]
- Halfeld, G.G.; de Almeida, E.J.R.; Reginatto, V.; de Andrade, A.R. Acclimatization of a Microbial Consortium into a Stable Biofilm to Produce Energy and 1,3-Propanediol from Glycerol in a Microbial Fuel Cell. Int J Hydrogen Energy 2022, 47, 21241–21252. [CrossRef]
- Allam, F.; Elnouby, M.; Sabry, S.A.; El-Khatib, K.M.; El-Badan, D.E. Optimization of Factors Affecting Current Generation, Biofilm Formation and Rhamnolipid Production by Electroactive Pseudomonas aeruginosa FA17. Int J Hydrogen Energy 2021, 46, 11419–11432. [CrossRef]
- Yong, X.Y.; Yan, Z.Y.; Shen, H.B.; Zhou, J.; Wu, X.Y.; Zhang, L.J.; Zheng, T.; Jiang, M.; Wei, P.; Jia, H.H.; et al. An Integrated Aerobic-Anaerobic Strategy for Performance Enhancement of Pseudomonas aeruginosa-Inoculated Microbial Fuel Cell. Bioresour Technol 2017, 241, 1191–1196. [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Novel Mode of Microbial Energy Metabolism: Organic Carbon Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese. Appl Environ Microbiol 1988, 54, 1472–1480. [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nature Communications 2019 10:1 2019, 10, 1–10. [CrossRef]
- Egoburo, D.E.; Diaz Peña, R.; Kolender, A.; Pettinari, M.J. Optimization and Validation of a GC–FID Method for Quantitative Determination of 1,3-Propanediol in Bacterial Culture Aqueous Supernatants Containing Glycerol. Chromatographia 2017, 80, 1121–1127. [CrossRef]
- Willibaldo Schmidell, Urgel de Almeida Lima, Walter Borzani, E.A. Biotecnologia Industrial - Vol. 2: Engenharia Bioquímica, Volume 2. 8 2001, 2, 1689–1699.
- Liu, H.; Logan, B.E. Electricity Generation Using an Air-Cathode Single Chamber Microbial Fuel Cell in the Presence and Absence of a Proton Exchange Membrane. Environ Sci Technol 2004, 38, 4040–4046. [CrossRef]
- Gomes, A.S.; La Rotta, C.E.; Nitschke, M.; González, E.R. Evaluation of Current Output in Pseudomonas aeruginosa Microbial Fuel Cells Using Glycerol as Susbtrate and Nafion 117 as Proton Exchange Membrane. ECS Trans 2011, 41, 2011–2017. [CrossRef]
- Dantas, P. V.; Peres, S.; Campos-Takaki, G.M.; La Rotta, C.E. Utilization of Raw Glycerol for Pyocyanin Production from Pseudomonas aeruginosa in Half-Microbial Fuel Cells: Evaluation of Two Electrochemical Approaches . J Electrochem Soc 2013, 160, G142–G148. [CrossRef]
- Zani, A.C.B.; Almeida, É.J.R. de; Furlan, J.P.R.; Pedrino, M.; Guazzaroni, M.E.; Stehling, E.G.; Andrade, A.R. de; Reginatto, V. Electrobiochemical Skills of Pseudomonas aeruginosa Species That Produce Pyocyanin or Pyoverdine for Glycerol Oxidation in a Microbial Fuel Cell. Chemosphere 2023, 335, 139073. [CrossRef]
- dos Passos, V.F.; Marcilio, R.; Aquino-Neto, S.; Santana, F.B.; Dias, A.C.F.; Andreote, F.D.; de Andrade, A.R.; Reginatto, V. Hydrogen and Electrical Energy Co-Generation by a Cooperative Fermentation System Comprising Clostridium and Microbial Fuel Cell Inoculated with Port Drainage Sediment. Bioresour Technol 2019, 277, 94–103. [CrossRef]
- Dwivedi, K.A.; Huang, S.J.; Wang, C.T. Integration of Various Technology-Based Approaches for Enhancing the Performance of Microbial Fuel Cell Technology: A Review. Chemosphere 2022, 287, 132248. [CrossRef]
- Bosire, E.M.; Rosenbaum, M.A. Electrochemical Potential Influences Phenazine Production, Electron Transfer and Consequently Electric Current Generation by Pseudomonas aeruginosa. Front Microbiol 2017, 8, 260405. [CrossRef]
- Gandouzi, I.; Tertis, M.; Cernat, A.; Saidane-Mosbahi, D.; Ilea, A.; Cristea, C. A Nanocomposite Based on Reduced Graphene and Gold Nanoparticles for Highly Sensitive Electrochemical Detection of Pseudomonas aeruginosa through Its Virulence Factors. Materials 2019, Vol. 12, Page 1180 2019, 12, 1180. [CrossRef]
- Jia, R.; Yang, D.; Xu, D.; Gu, T. Anaerobic Corrosion of 304 Stainless Steel Caused by the Pseudomonas aeruginosa Biofilm. Front Microbiol 2017, 8, 298487. [CrossRef]
- Lovley, D.R. Electromicrobiology. https://doi.org/10.1146/annurev-micro-092611-150104 2012, 66, 391–409. [CrossRef]
- Murray, D.B.; Haynes, K.; Tomita, M. Redox Regulation in Respiring Saccharomyces cerevisiae. Biochimica et Biophysica Acta (BBA) - General Subjects 2011, 1810, 945–958. [CrossRef]
- Vemuri, G.N.; Altman, E.; Sangurdekar, D.P.; Khodursky, A.B.; Eiteman, M.A. Overflow Metabolism in Escherichia coli during Steady-State Growth: Transcriptional Regulation and Effect of the Redox Ratio. Appl Environ Microbiol 2006, 72, 3653–3661. [CrossRef]
- Mason, J.T.; Kim, S.K.; Knaff, D.B.; Wood, M.J. Thermodynamic Basis for Redox Regulation of the Yap1 Signal Transduction Pathway. Biochemistry 2006, 45, 13409–13417. [CrossRef]
- Riondet, C.; Cachon, R.; Waché, Y.; Alcaraz, G.; Diviès, C. Extracellular Oxidoreduction Potential Modifies Carbon and Electron Flow in Escherichia coli. J Bacteriol 2000, 182, 620–626. [CrossRef]
- Vassilev, I.; Gießelmann, G.; Schwechheimer, S.K.; Wittmann, C.; Virdis, B.; Krömer, J.O. Anodic Electro-Fermentation: Anaerobic Production of L-Lysine by Recombinant Corynebacterium glutamicum. Biotechnol Bioeng 2018, 115, 1499–1508. [CrossRef]
- Williams, H.D.; Zlosnik, J.E.A.; Ryall, B. Oxygen, Cyanide and Energy Generation in the Cystic Fibrosis Pathogen Pseudomonas aeruginosa. Adv Microb Physiol 2006, 52, 1–71. [CrossRef]
- Price-Whelan, A.; Dietrich, L.E.P.; Newman, D.K. Pyocyanin Alters Redox Homeostasis and Carbon Flux through Central Metabolic Pathways in Pseudomonas aeruginosa PA14. J Bacteriol 2007, 189, 6372–6381. [CrossRef]
- Eschbach, M.; Schreiber, K.; Trunk, K.; Buer, J.; Jahn, D.; Schobert, M. Long-Term Anaerobic Survival of the Opportunistic Pathogen Pseudomonas aeruginosa via Pyruvate Fermentation. J Bacteriol 2004, 186, 4596–4604. [CrossRef]
- Poblete-Castro, I.; Wittmann, C.; Nikel, P.I. Biochemistry, Genetics and Biotechnology of Glycerol Utilization in Pseudomonas Species. Microb Biotechnol 2020, 13, 32–53. [CrossRef]
- Head, N.E.; Yu, H. Cross-Sectional Analysis of Clinical and Environmental Isolates of Pseudomonas aeruginosa: Biofilm Formation, Virulence, and Genome Diversity. Infect Immun 2004, 72, 133–144. [CrossRef]
- Malinowski, J.J. Evaluation of Liquid Extraction Potentials for Downstream Separation of 1,3-Propanediol. Biotechnology Techniques 1999, 13, 127–130. [CrossRef]
- Biebl, H.; Zeng, A.P.; Menzel, K.; Deckwer, W.D. Fermentation of Glycerol to 1,3-Propanediol and 2,3-Butanediol by Klebsiella pneumoniae. Appl Microbiol Biotechnol 1998, 50, 24–29. [CrossRef]
- Homann, T.; Tag, C.; Biebl, H.; Deckwer, W.D.; Schink, B. Fermentation of Glycerol to 1,3-Propanediol by Klebsiella and Citrobacter Strains. Appl Microbiol Biotechnol 1990, 33, 121–126. [CrossRef]
- Cheng, K.K.; Zhang, J.A.; Liu, D.H.; Sun, Y.; Liu, H.J.; Yang, M. De; Xu, J.M. Pilot-Scale Production of 1,3-Propanediol Using Klebsiella pneumoniae. Process Biochemistry 2007, 42, 740–744. [CrossRef]
- Nakas, J.P.; Schaedle, M.; Parkinson, C.M.; Coonley, C.E.; Tanenbaum, S.W.; Tertiolecta, D.; Primolecta, D.; Parva, D.; Bardawil, D.; Salina, D. System Development for Linked-Fermentation Production of Solvents from Algal Biomass. Appl Environ Microbiol 1983, 46, 1017–1023. [CrossRef]
- Biebl, H. Fermentation of Glycerol by Clostridium pasteurianum — Batch and Continuous Culture Studies. J Ind Microbiol Biotechnol 2001, 27, 18–26. [CrossRef]
- Papanikolaou, S.; Fick, M.; Aggelis, G. The Effect of Raw Glycerol Concentration on the Production of 1,3-Propanediol by Clostridium butyricum. Journal of Chemical Technology & Biotechnology 2004, 79, 1189–1196. [CrossRef]
- Ahrens, K.; Menzel, K.; Zeng, A.-P.; Deckwer, W.-D. Kinetic, Dynamic, and Pathway Studies of Glycerol Metabolism by Klebsiella pneumoniae in Anaerobic Continuous Culture: III. Enzymes and Fluxes of Glycerol Dissimilation and 1,3-Propanediol Formation. 1998. [CrossRef]
- Skraly, F.A.; Lytle, B.L.; Cameron, D.C. Construction and Characterization of a 1,3-Propanediol Operon. Appl Environ Microbiol 1998, 64, 98–105. [CrossRef]
- Liu, H.; Xu, Y.; Zheng, Z.; Liu, D. 1,3-Propanediol and Its Copolymers: Research, Development and Industrialization. Biotechnol J 2010, 5, 1137–1148. [CrossRef]
| Strain | iMAX (mA m-2) |
CE (%) |
Glycerol (mM)* |
REXT (ohms) | Anode | Salt bridge + cathode |
Reference |
|---|---|---|---|---|---|---|---|
| EW819 | 91.3 | 1.49 | 11 | 1000 | Carbon cloth | PEM + Pt-Carbon cloth (air) | [21] |
| ATCC27853 | 110 | --- | 101 | 500 | Carbon cloth | PEM + Pt-Carbon black (air) | [19] |
| EW603 | 141.2 | 5.16 | 11 | 1000 | Carbon cloth | PEM + Pt-Carbon cloth (air) | [21] |
| ATCC27853 | 153 | --- | 101 | 500 | Carbon cloth | PEM + Pt-Carbon black (air) | [19] |
| ATCC27853 | 399.0 | 46.70 | 271.5 | 1000 | Carbon felt | SB + Carbon felt (ferrocyanide) | [20] |
| ATCC27853 | 418.3 | 48.63 | 271.5 | 1000 | Carbon felt | PEM + Pt-Black on a carbon felt with carbon vulcan powder and Teflon (air) | [20] |
| EL14 | 82.4 | 4.54 | 22 | 2200 | Carbon cloth | PEM + Pt-Carbon cloth (air) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





