Submitted:
26 June 2023
Posted:
27 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
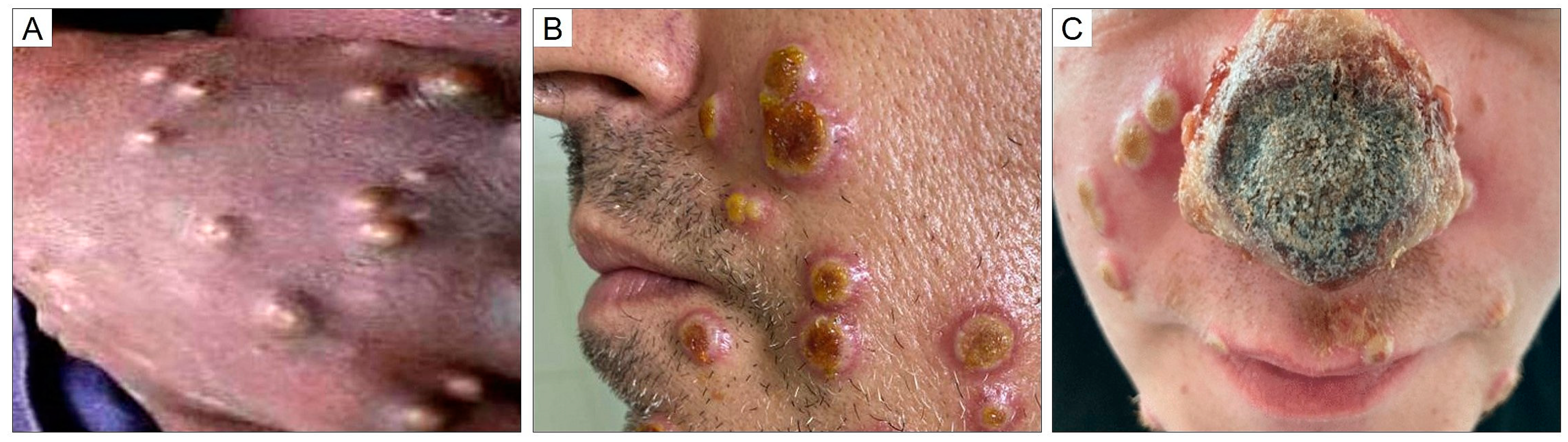
2. What is Mpox?
3. When did Mpox make its debut?
4. Where did Mpox spread to?
5. Who was at risk of getting Mpox?
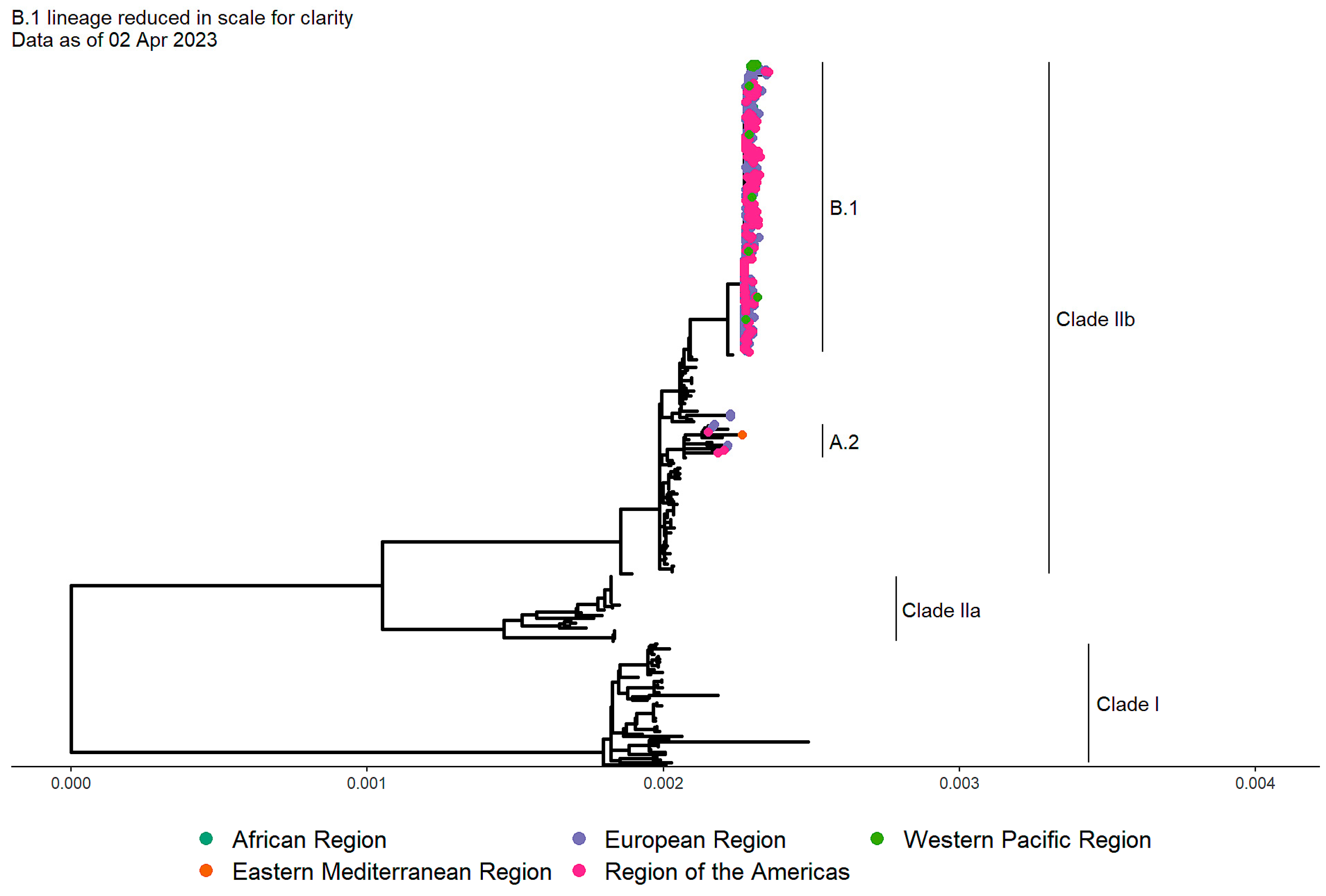
6. Why did Mpox reemerged?
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The Challenge of Emerging and Re-Emerging Infectious Diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K. The Mother of All Pandemics Is 100 Years Old (and Going Strong)! Am J Public Health 2018, 108, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, R.; Anderson, C.; Blomquist, P.; Balasegaram, S.; Bell, A.; Bishop, L.; Brown, C.S.; Chow, Y.; Edeghere, O.; Florence, I.; et al. Community Transmission of Monkeypox in the United Kingdom, April to May 2022. Euro Surveill 2022, 27, 2200422. [Google Scholar] [CrossRef] [PubMed]
- Duque, M.P.; Ribeiro, S.; Martins, J.V.; Casaca, P.; Leite, P.P.; Tavares, M.; Mansinho, K.; Duque, L.M.; Fernandes, C.; Cordeiro, R.; et al. Ongoing Monkeypox Virus Outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill 2022, 27, 2200424. [Google Scholar] [CrossRef]
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. Epidemiological, Clinical and Virological Characteristics of Four Cases of Monkeypox Support Transmission through Sexual Contact, Italy, May 2022. Euro Surveill 2022, 27, 2200421. [Google Scholar] [CrossRef]
- WHO. WHO Director-General’s Statement at the Press Conference Following IHR Emergency Committee Regarding the Multi-Country Outbreak of Monkeypox - 23 July 2022 Available online:. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022 (accessed on 9 May 2023).
- Lewis, R.F.; Kuppalli, K.; Hoxha, A.; Doherty, M.C. Emergency Committee Recommendations on Mpox – What’s next? Bull World Health Organ 2023, 101, 300–300A. [Google Scholar] [CrossRef]
- Roper, R.L.; Garzino-Demo, A.; Del Rio, C.; Bréchot, C.; Gallo, R.; Hall, W.; Esparza, J.; Reitz, M.; Schinazi, R.F.; Parrington, M.; et al. Monkeypox (Mpox) Requires Continued Surveillance, Vaccines, Therapeutics and Mitigating Strategies. Vaccine 2023, S0264-410X(23)00393-6. [Google Scholar] [CrossRef]
- Wadman, M. WHO Ends Mpox Emergency Available online:. Available online: https://www.science.org/content/article/who-ends-mpox-emergency (accessed on 15 May 2023).
- WHO 2022-23 Mpox Outbreak: Global Trends. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 7 June 2023).
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.-J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef]
- Pauli, G.; Blümel, J.; Burger, R.; Drosten, C.; Gröner, A.; Gürtler, L.; Heiden, M.; Hildebrandt, M.; Jansen, B.; Montag-Lessing, T.; et al. Orthopox Viruses: Infections in Humans. Transfus Med Hemother 2010, 37, 351–364. [Google Scholar] [CrossRef]
- Buller, R.M.; Palumbo, G.J. Poxvirus Pathogenesis. Microbiological Reviews 1991, 55, 80–122. [Google Scholar] [CrossRef]
- Lewis-Jones, S. Zoonotic Poxvirus Infections in Humans. Curr Opin Infect Dis 2004, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.M.; Anderson, S.A. Qualitative Assessment of Risk for Monkeypox Associated with Domestic Trade in Certain Animal Species, United States. Emerg Infect Dis 2006, 12, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yuan, Y.; Jiang, L.; Liu, Y.; Liu, Y.; Zhang, L. Animal Host Range of Mpox Virus. J Med Virol 2023, 95, e28513. [Google Scholar] [CrossRef] [PubMed]
- WHO Monkeypox: Experts Give Virus Variants New Names Available online:. Available online: https://www.who.int/news/item/12-08-2022-monkeypox--experts-give-virus-variants-new-names (accessed on 7 June 2023).
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N Engl J Med 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Carroll, D.S.; Olson, V.A.; Hughes, C.; Galley, J.; Likos, A.; Montgomery, J.M.; Suu-Ire, R.; Kwasi, M.O.; Jeffrey Root, J.; et al. A Silent Enzootic of an Orthopoxvirus in Ghana, West Africa: Evidence for Multi-Species Involvement in the Absence of Widespread Human Disease. Am J Trop Med Hyg 2010, 82, 746–754. [Google Scholar] [CrossRef]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence Differences between Monkeypox Virus Isolates from West Africa and the Congo Basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A Tale of Two Clades: Monkeypox Viruses. J Gen Virol 2005, 86, 2661–2672. [Google Scholar] [CrossRef]
- Americo, J.L.; Earl, P.L.; Moss, B. Virulence Differences of Mpox (Monkeypox) Virus Clades I, IIa, and IIb.1 in a Small Animal Model. PNAS 2023, 120, e2220415120. [Google Scholar] [CrossRef]
- Colavita, F.; Antinori, A.; Nicastri, E.; Focosi, D.; Girardi, E.; Vaia, F.; Maggi, F. Monkeypox Virus in Human Body Sites and Fluids: Evidence for Transmission. Lancet Infect Dis 2023, 23, 6–8. [Google Scholar] [CrossRef]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Kalemba, L.; Malekani, J.; Kabamba, J.; et al. Introduction of Monkeypox into a Community and Household: Risk Factors and Zoonotic Reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg 2015, 93, 410–415. [Google Scholar] [CrossRef]
- Besombes, C.; Gonofio, E.; Konamna, X.; Selekon, B.; Gessain, A.; Berthet, N.; Manuguerra, J.-C.; Fontanet, A.; Nakouné, E. Intrafamily Transmission of Monkeypox Virus, Central African Republic, 2018. Emerg Infect Dis 2019, 25, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Lee, K.N.; Abel, J.; Carroll, D.S.; Montgomery, J.M.; Olson, V.A.; Li, Y.; Davidson, W.; Hughes, C.; Dillon, M.; et al. Monkeypox Zoonotic Associations: Insights from Laboratory Evaluation of Animals Associated with the Multi-State US Outbreak. Am J Trop Med Hyg 2007, 76, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Jezek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human Monkeypox: Clinical Features of 282 Patients. J Infect Dis 1987, 156, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Núñez, I.; García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Toledo-Salinas, C.; Carbajal-Sandoval, G.; Sosa-Laso, L.; García-Rodríguez, G.; Cortés-Alcalá, R.; Torre, A. de la; Fragoso-Saavedra, S.; et al. Epidemiological and Clinical Characteristics of Patients with Human Monkeypox Infection in Mexico: A Nationwide Observational Study. Lancet Reg Health Am 2023, 17, 100392. [Google Scholar] [CrossRef] [PubMed]
- Rasizadeh, R.; Shamekh, A.; Shiri Aghbash, P.; Bannazadeh Baghi, H. Comparison of Human Monkeypox, Chickenpox and Smallpox: A Comprehensive Review of Pathology and Dermatological Manifestations. Curr Med Res Opin 2023, 1–10. [Google Scholar] [CrossRef]
- McCollum, A.M.; Damon, I.K. Human Monkeypox. Clinical Infectious Diseases 2014, 58, 260–267. [Google Scholar] [CrossRef]
- Al-Tammemi, A.B.; Albakri, R.; Alabsi, S. The Outbreak of Human Monkeypox in 2022: A Changing Epidemiology or an Impending After Effect of Smallpox Eradication? Front Trop Dis 2022, 3. [Google Scholar] [CrossRef]
- Fink, D.L.; Callaby, H.; Luintel, A.; Beynon, W.; Bond, H.; Lim, E.Y.; Gkrania-Klotsas, E.; Heskin, J.; Bracchi, M.; Rathish, B.; et al. Clinical Features and Management of Individuals Admitted to Hospital with Monkeypox and Associated Complications across the UK: A Retrospective Cohort Study. Lancet Infect Dis 2023, 23, 589–597. [Google Scholar] [CrossRef]
- Magnus, P. von; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A Pox-Like Disease in Cynomolgus Monkeys. Acta Pathologica Microbiologica Scandinavica 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Prier, J.E.; Sauer, R.M.; Malsberger, R.G.; Sillaman, J.M. Studies on a Pox Disease of Monkeys. II. Isolation of the Etiologic Agent. Am J Vet Res 1960, 21, 381–384. [Google Scholar]
- Cho, C.T.; Wenner, H.A. Monkeypox Virus. Bacteriol Rev 1973, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Arita, I.; Henderson, D.A. Smallpox and Monkeypox in Non-Human Primates. Bull World Health Organ 1968, 39, 277–283. [Google Scholar]
- Parker, S.; Buller, R.M. A Review of Experimental and Natural Infections of Animals with Monkeypox Virus between 1958 and 2012. Future Virol 2013, 8, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Doty, J.B.; Malekani, J.M.; Kalemba, L.N.; Stanley, W.T.; Monroe, B.P.; Nakazawa, Y.U.; Mauldin, M.R.; Bakambana, T.L.; Liyandja Dja Liyandja, T.; Braden, Z.H.; et al. Assessing Monkeypox Virus Prevalence in Small Mammals at the Human–Animal Interface in the Democratic Republic of the Congo. Viruses 2017, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of Human Monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerging infectious diseases 2001, 7, 434–438. [Google Scholar] [CrossRef]
- Khodakevich, L.; Jezek, Z.; Kinzanzka, K. Isolation of Monkeypox Virus from Wild Squirrel Infected in Nature. Lancet 1986, 1, 98–99. [Google Scholar] [CrossRef]
- Radonić, A.; Metzger, S.; Dabrowski, P.W.; Couacy-Hymann, E.; Schuenadel, L.; Kurth, A.; Mätz-Rensing, K.; Boesch, C.; Leendertz, F.H.; Nitsche, A. Fatal Monkeypox in Wild-Living Sooty Mangabey, Côte d’Ivoire, 2012. Emerg Infect Dis 2014, 20, 1009–1011. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ 1972, 46, 593–597. [Google Scholar]
- Lourie, B.; Bingham, P.G.; Evans, H.H.; Foster, S.O.; Nakano, J.H.; Herrmann, K.L. Human Infection with Monkeypox Virus: Laboratory Investigation of Six Cases in West Africa. Bull World Health Organ 1972, 46, 633–639. [Google Scholar]
- Durski, K.N.; McCollum, A.M.; Nakazawa, Y.; Petersen, B.W.; Reynolds, M.G.; Briand, S.; Djingarey, M.H.; Olson, V.; Damon, I.K.; Khalakdina, A. Emergence of Monkeypox - West and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep 2018, 67, 306–310. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Negl Trop Dis 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- CDC Update: Multistate Outbreak of Monkeypox --- Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003 Available online:. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5227a5.htm (accessed on 23 May 2023).
- Sale, T.A.; Melski, J.W.; Stratman, E.J. Monkeypox: An Epidemiologic and Clinical Comparison of African and US Disease. J Am Acad Dermatol 2006, 55, 478–481. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.M.; Shelus, V.; Hill, A.; Traore, T.; Onoja, B.; Nakazawa, Y.; Doty, J.B.; Yinka-Ogunleye, A.; Petersen, B.W.; Hutson, C.L.; et al. Epidemiology of Human Mpox - Worldwide, 2018-2021. MMWR Morb Mortal Wkly Rep 2023, 72, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Safronov, P.F.; Ryazankina, O.I.; Petrov, N.A.; Gutorov, V.V.; Uvarova, E.A.; Mikheev, M.V.; Sisler, J.R.; et al. Human Monkeypox and Smallpox Viruses: Genomic Comparison. FEBS Lett 2001, 509, 66–70. [Google Scholar] [CrossRef]
- Vaughan, A.; Aarons, E.; Astbury, J.; Balasegaram, S.; Beadsworth, M.; Beck, C.R.; Chand, M.; O’Connor, C.; Dunning, J.; Ghebrehewet, S.; et al. Two Cases of Monkeypox Imported to the United Kingdom, September 2018. Euro Surveill 2018, 23, 1800509. [Google Scholar] [CrossRef]
- Ng, O.T.; Lee, V.; Marimuthu, K.; Vasoo, S.; Chan, G.; Lin, R.T.P.; Leo, Y.S. A Case of Imported Monkeypox in Singapore. Lancet Infect Dis 2019, 19, 1166. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Singh, S.K. A Systematic Review of 5110 Cases of Monkeypox: What Has Changed Between 1970 and 2022? Cureus 2022, 14, e30841. [Google Scholar] [CrossRef]
- ECDC Communicable Disease Threats Report, 8-14 May 2022, Week 19 Available online:. Available online: https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-8-14-may-2022-week-19 (accessed on 1 June 2023).
- ECDC Epidemiological Update: Monkeypox Multi-Country Outbreak Available online:. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-monkeypox-multi-country-outbreak-0 (accessed on 1 June 2023).
- WHO. WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern (accessed on 24 July 2022).
- 56. WHO 2022-23 Mpox (Monkeypox) Outbreak: Global Trends. https://worldhealthorg.shinyapps.io/mpx_global/ (retrieved 30 April 2023), 30 April.
- Reynolds, M.G.; Damon, I.K. Outbreaks of Human Monkeypox after Cessation of Smallpox Vaccination. Trends Microbiol 2012, 20, 80–87. [Google Scholar] [CrossRef]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major Increase in Human Monkeypox Incidence 30 Years after Smallpox Vaccination Campaigns Cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A 2010, 107, 16262–16267. [Google Scholar] [CrossRef]
- Pinto, P.; Costa, M.A.; Gonçalves, M.F.M.; Rodrigues, A.G.; Lisboa, C. Mpox Person-to-Person Transmission-Where Have We Got So Far? A Systematic Review. Viruses 2023, 15, 1074. [Google Scholar] [CrossRef]
- Choi, Y.; Jeon, E.; Kim, T.; Choi, S.J.; Moon, S.M.; Song, K.-H.; Kim, H.B.; Kim, E.S. Case Report and Literature Review of Occupational Transmission of Monkeypox Virus to Healthcare Workers, South Korea. Emerg Infect Dis 2023, 29, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Angelo, K.M.; Smith, T.; Camprubí-Ferrer, D.; Balerdi-Sarasola, L.; Menéndez, M.D.; Servera-Negre, G.; Barkati, S.; Duvignaud, A.; Huber, K.L.B.; Chakravarti, A.; et al. Epidemiological and Clinical Characteristics of Patients with Monkeypox in the GeoSentinel Network: A Cross-Sectional Study. The Lancet Infectious Diseases 2023, 23, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries — April–June 2022. N Engl J Med 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain: A Prospective Observational Cohort Study. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Iroezindu, M.; James, H.I.; Oladokun, R.; Yinka-Ogunleye, A.; Wakama, P.; Otike-Odibi, B.; Usman, L.M.; Obazee, E.; Aruna, O.; et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin Infect Dis 2020, 71, e210–e214. [Google Scholar] [CrossRef]
- Miller, M.J. Severe Monkeypox in Hospitalized Patients — United States, August 10–October 10, 2022. MMWR Morb Mortal Wkly Rep 2022, 71. [Google Scholar] [CrossRef]
- Mitjà, O.; Alemany, A.; Marks, M.; Mora, J.I.L.; Rodríguez-Aldama, J.C.; Silva, M.S.T.; Herrera, E.A.C.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. Mpox in People with Advanced HIV Infection: A Global Case Series. The Lancet 2023, 0. [Google Scholar] [CrossRef]
- Boesecke, C.; Monin, M.B.; van Bremen, K.; Schlabe, S.; Hoffmann, C. Severe Monkeypox-Virus Infection in Undiagnosed Advanced HIV Infection. Infection 2022, 50, 1633–1634. [Google Scholar] [CrossRef]
- Uysal, F. Detection of Monkeypox Disease from Human Skin Images with a Hybrid Deep Learning Model. Diagnostics (Basel) 2023, 13, 1772. [Google Scholar] [CrossRef]
- Melgosa Ramos, F.J.; Parra Civera, M.; Pons Fuster, J.J. Skin Lesions Due to Monkeypox Virus in a Well-Controlled HIV Patient. Med Clin (Engl Ed) 2022, 159, e87–e88. [Google Scholar] [CrossRef]
- Coltart, C.E.M.; Lindsey, B.; Ghinai, I.; Johnson, A.M.; Heymann, D.L. The Ebola Outbreak, 2013–2016: Old Lessons for New Epidemics. Philos Trans R Soc Lond B Biol Sci 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Tomori, O.; Ogoina, D. Monkeypox: The Consequences of Neglecting a Disease, Anywhere. Science 2022, 377, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, M.A. The Global Eradication of Smallpox. Am J Infect Control 1982, 10, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Vusirikala, A.; Charles, H.; Balasegaram, S.; Macdonald, N.; Kumar, D.; Barker-Burnside, C.; Cumiskey, K.; Dickinson, M.; Watson, M.; Olufon, O.; et al. Epidemiology of Early Monkeypox Virus Transmission in Sexual Networks of Gay and Bisexual Men, England, 2022. Emerg Infect Dis 2022, 28, 2082–2086. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Q.; Dong, X.; Wang, M.; Liu, Z.; Li, Z. Mpox, Caused by the MPXV of the Clade IIb Lineage, Goes Global. Trop Med Infect Dis 2023, 8, 76. [Google Scholar] [CrossRef]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic Characterization and Signs of Microevolution in the 2022 Multi-Country Outbreak of Monkeypox Virus. Nat Med 2022, 28, 1569–1572. [Google Scholar] [CrossRef]
- Falendysz, E.A.; Lopera, J.G.; Rocke, T.E.; Osorio, J.E. Monkeypox Virus in Animals: Current Knowledge of Viral Transmission and Pathogenesis in Wild Animal Reservoirs and Captive Animal Models. Viruses 2023, 15, 905. [Google Scholar] [CrossRef]
- Gao, G.F. From “A”IV to “Z”IKV: Attacks from Emerging and Re-Emerging Pathogens. Cell 2018, 172, 1157–1159. [Google Scholar] [CrossRef]
- Fauci, A.S. It Ain’t Over Till It’s Over…but It’s Never Over — Emerging and Reemerging Infectious Diseases. N Engl J Med 2022, 387, 2009–2011. [Google Scholar] [CrossRef]
- Kirby, T. From Early Alarm to Gradual Control of Monkeypox. Lancet HIV 2022. [Google Scholar] [CrossRef]
- Vairo, F.; Leone, S.; Mazzotta, V.; Piselli, P.; Carli, G.D.; Lanini, S.; Maggi, F.; Nicastri, E.; Gagliardini, R.; Vita, S.; et al. The Possible Effect of Sociobehavioral Factors and Public Health Actions on the Mpox Epidemic Slowdown. International Journal of Infectious Diseases 2023, 130, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, S.A.; Crago, A.-L.; Shannon, K. Harm Reduction and Rights-Based Approaches to Reduce Monkeypox Transmission among Sex Workers. Lancet Infect Dis 2023, 23, e43–e46. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).