Submitted:
22 June 2023
Posted:
23 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Metastasis and recurrence throughout medulloblastoma subgroups
3. Routes of metastatic dissemination in medulloblastoma
4. Pathological lymphangiogenesis and cancer
4.1. The VEGFC/VEGFR axis in the tumor lymphatic network
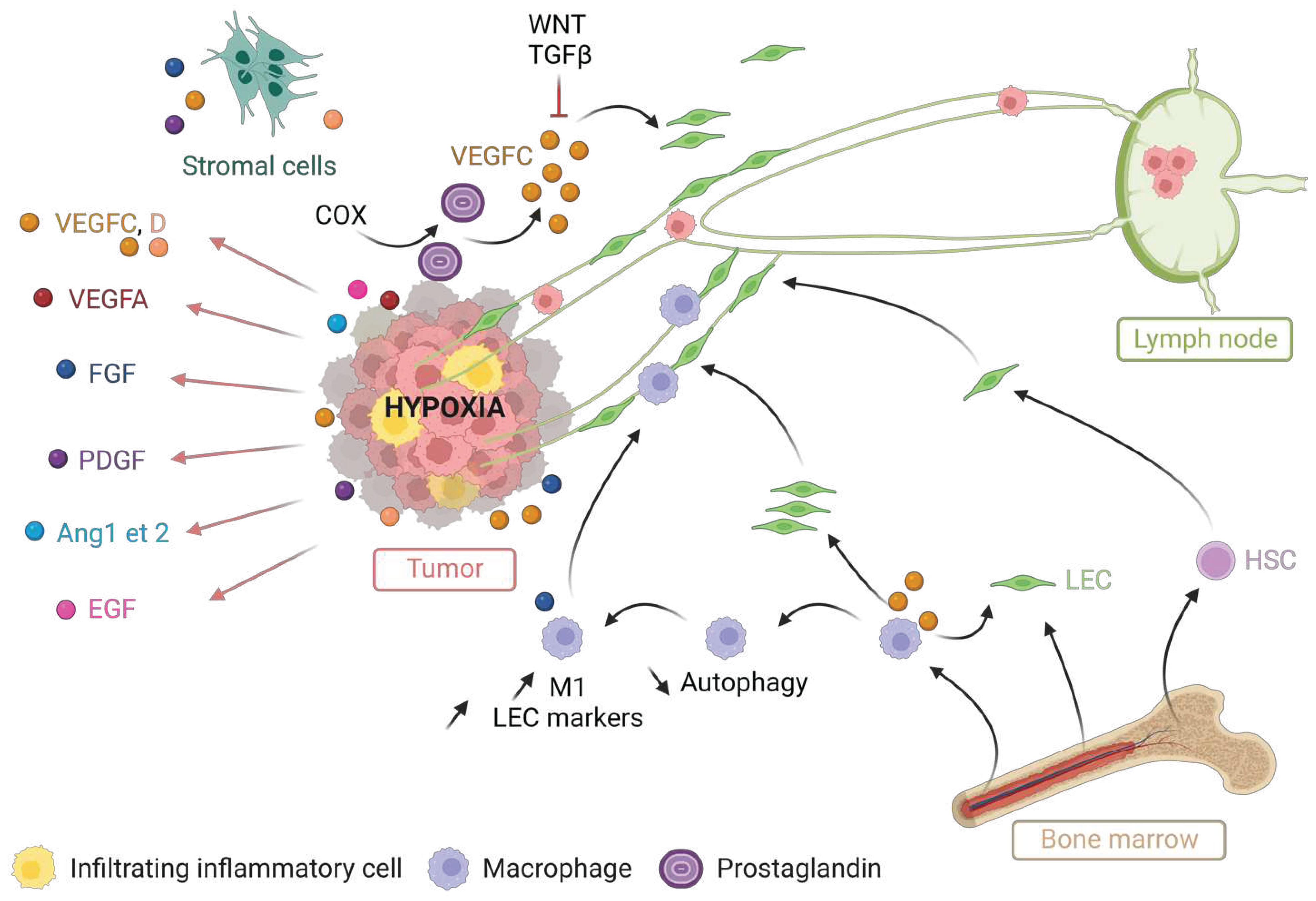
4.2. Molecular mechanisms of tumor lymphangiogenesis
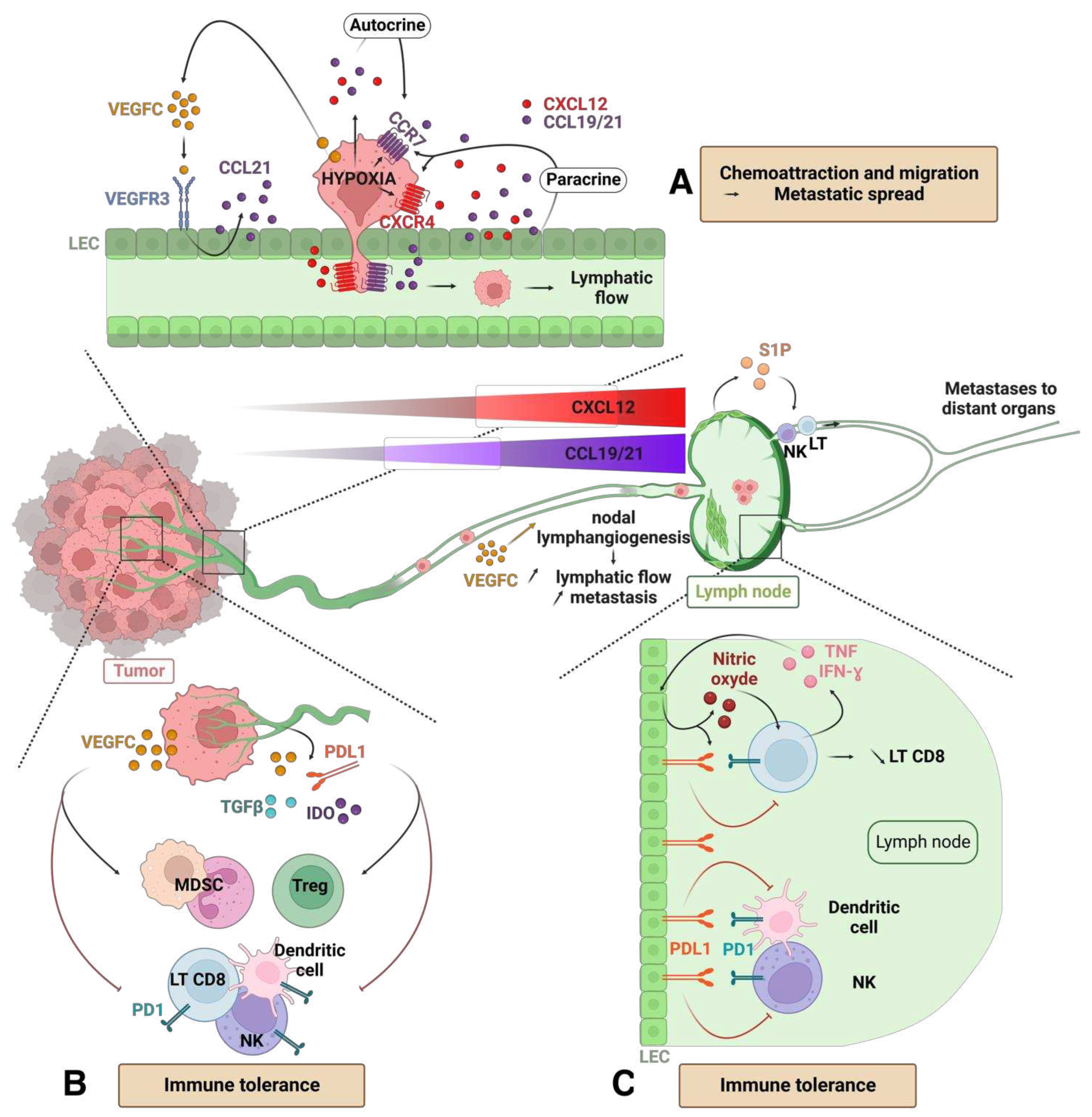
4.3. The dual role of cancer-associated lymphangiogenesis
4.3.1. Harmful role: metastatic dissemination and immune tolerance
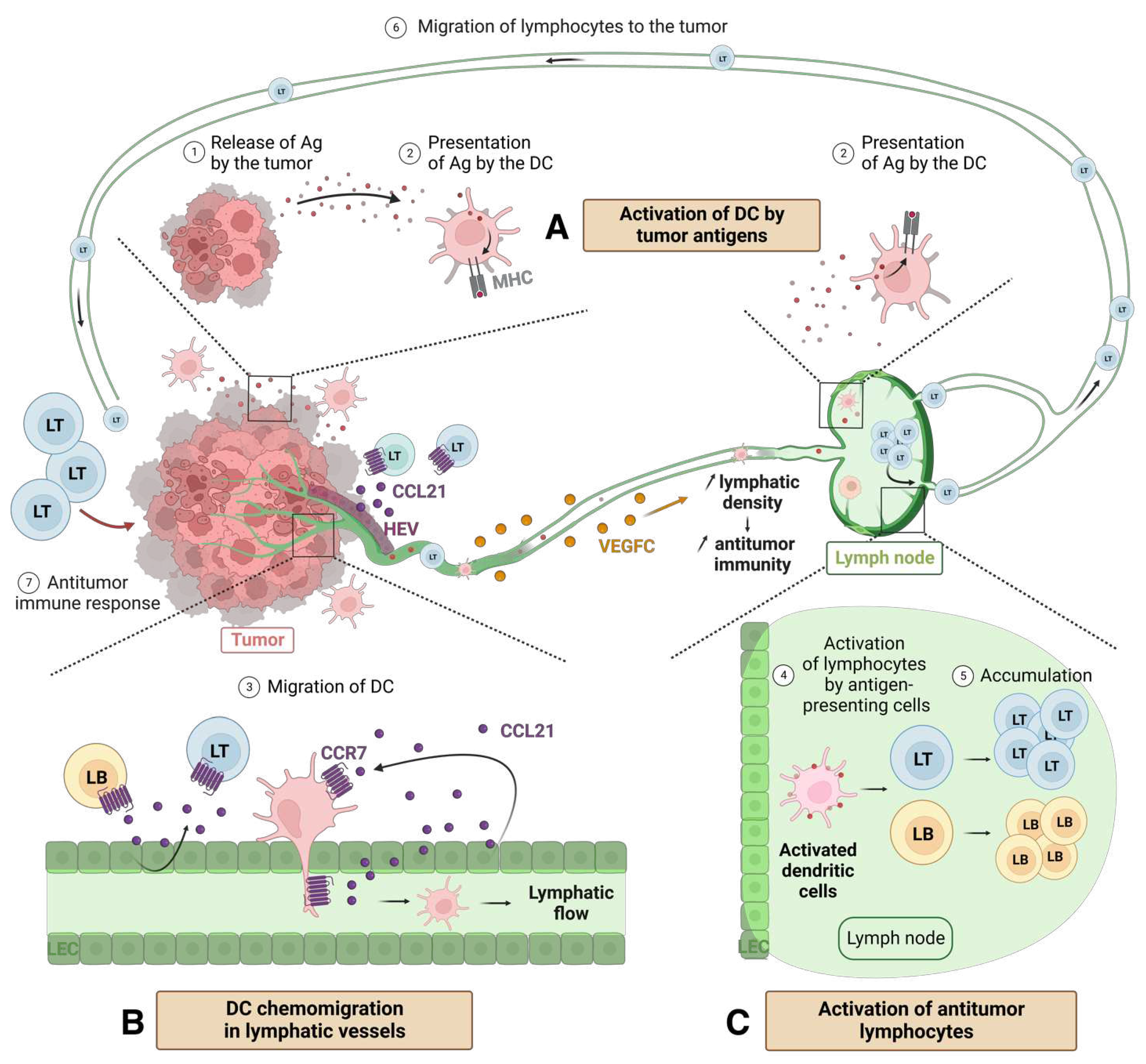
4.3.2. Beneficial role and synergy with the immune system
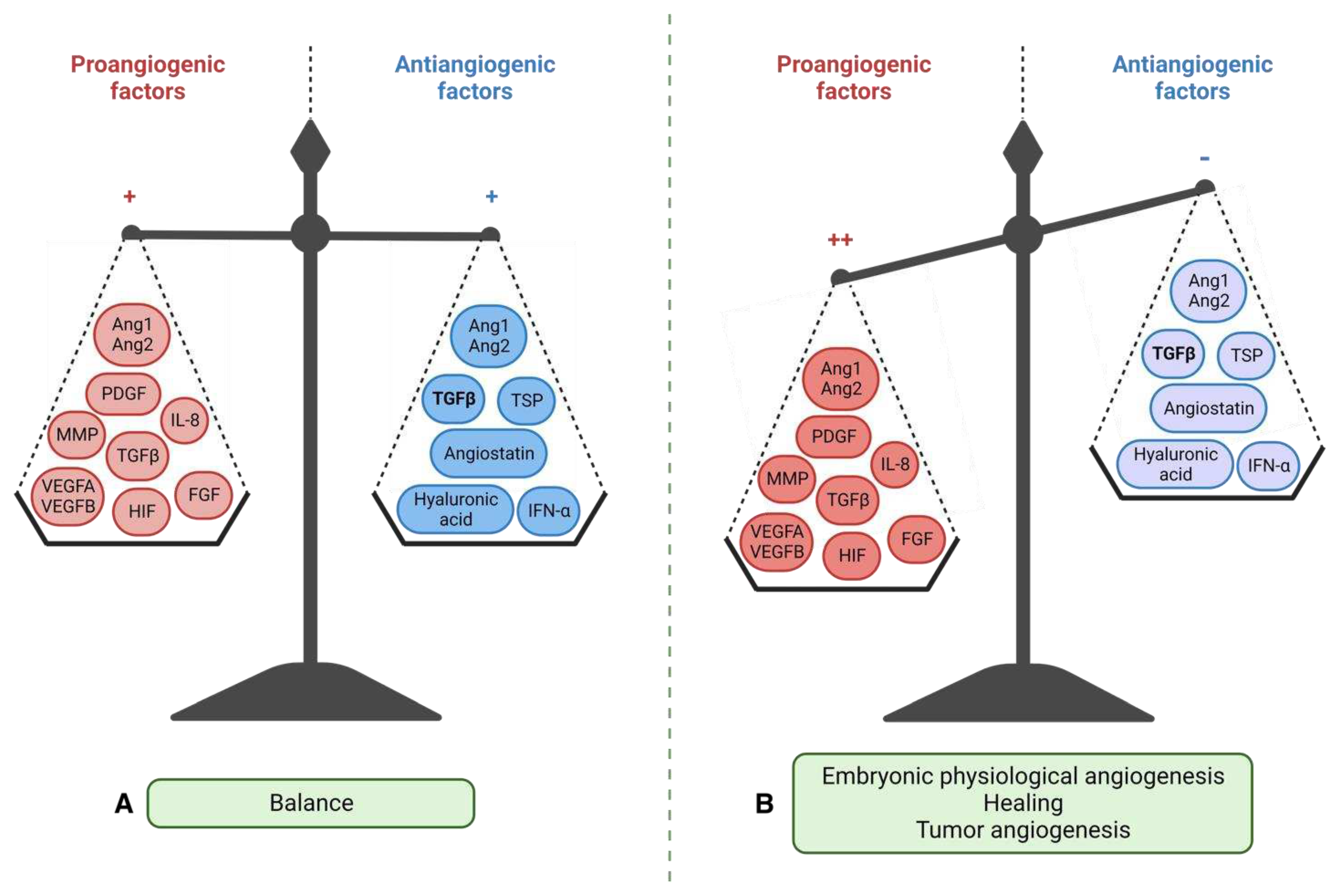
5. Tumor angiogenesis: scientific context and therapeutic failure
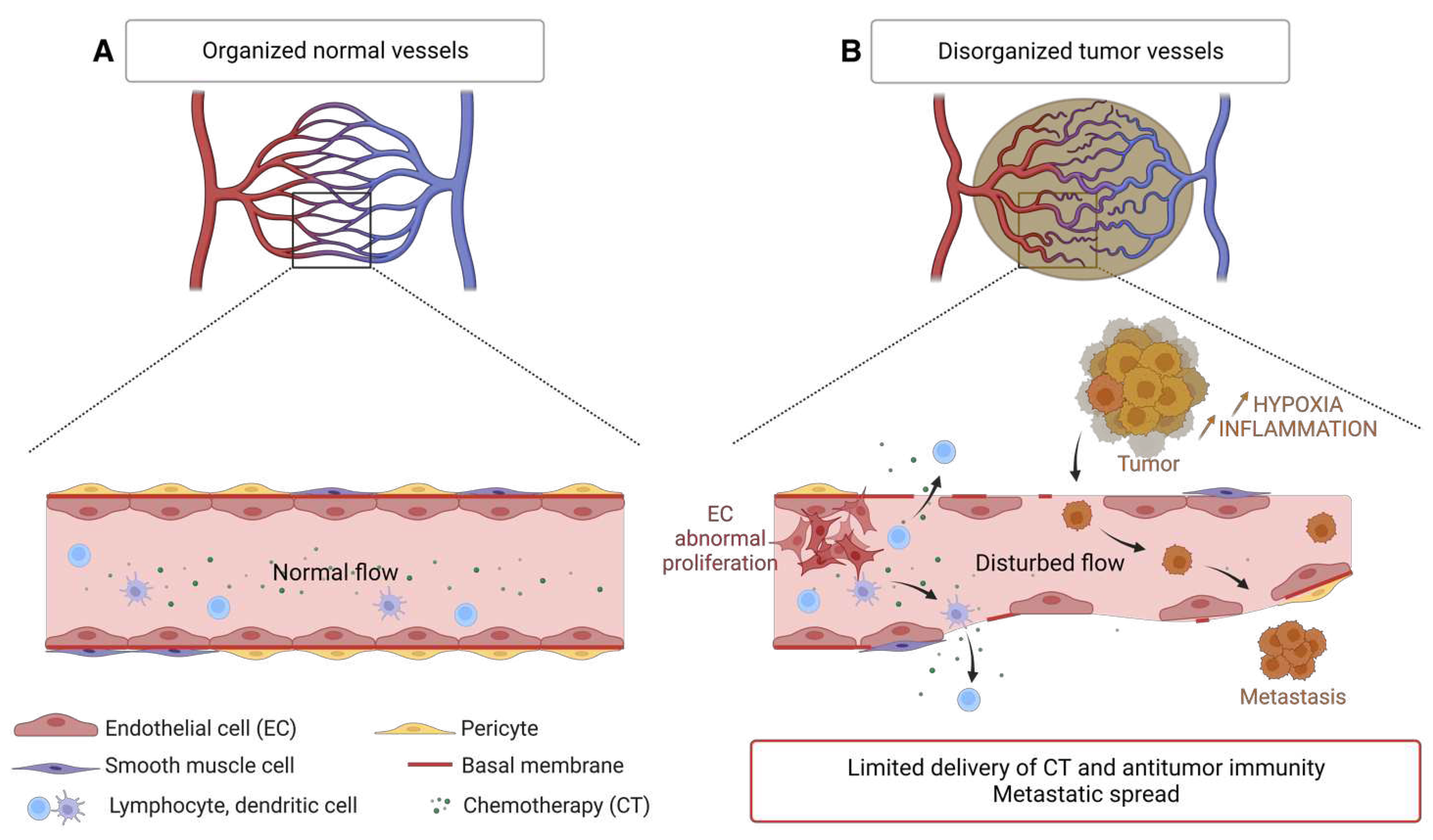
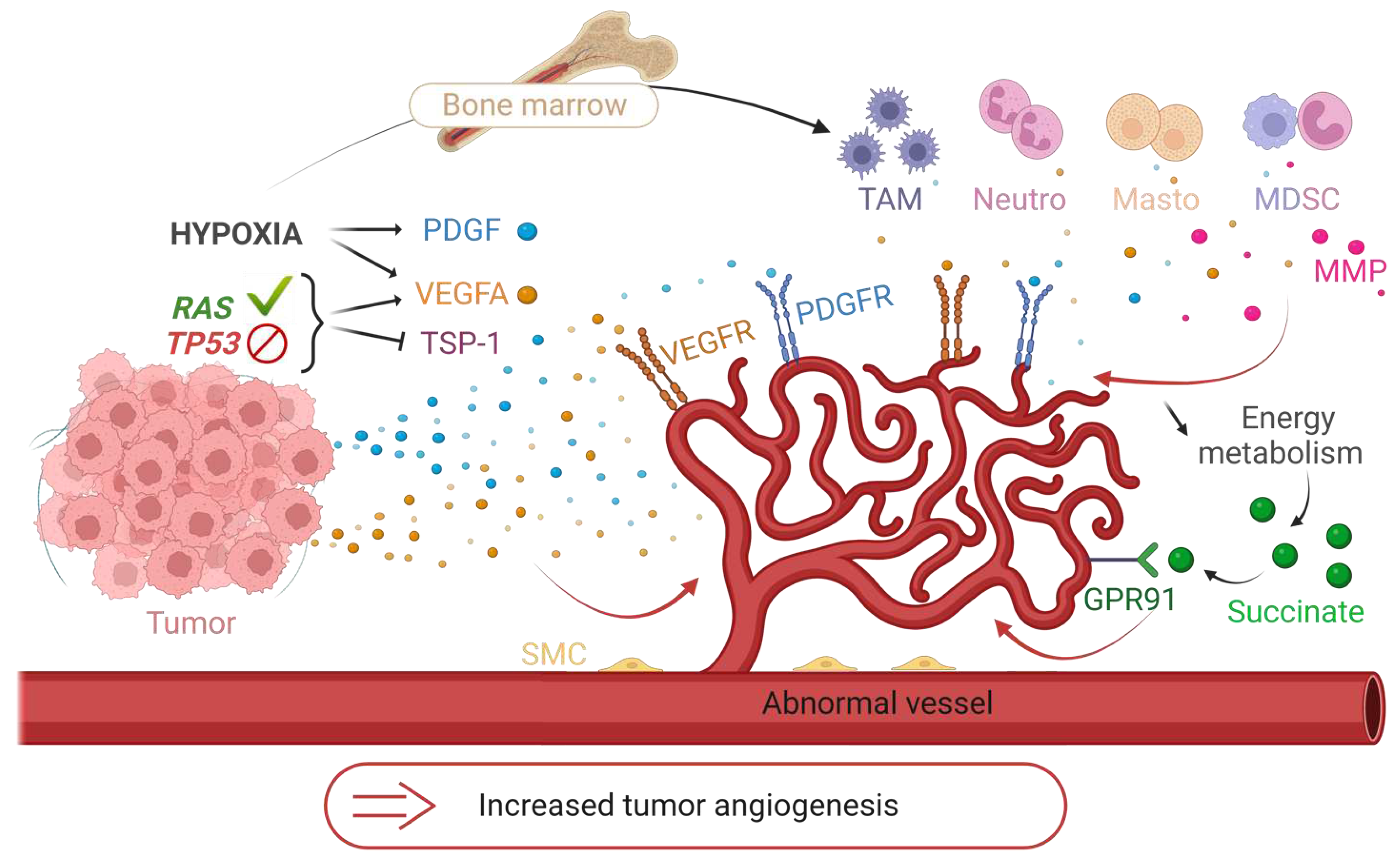
5.1. Tumor neovascularization
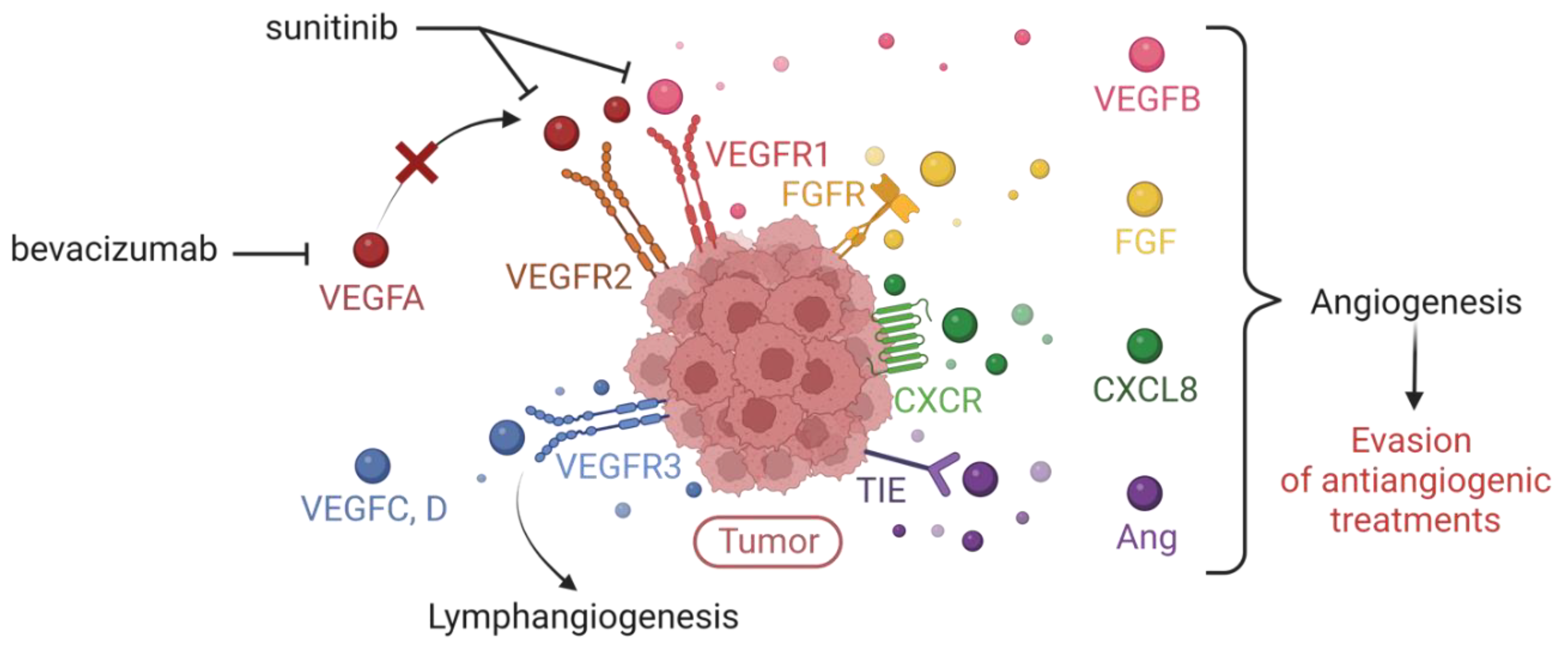
5.2. Mechanisms of angiogenic hijacking
5.3. Anti-angiogenic therapies and their limits
6. From molecular pathology to targeted therapies
6.1. WNT subgroup medulloblastomas
6.2. SHH group medulloblastomas
6.3. Group 3/4 medulloblastomas
7. What about immunotherapies?
7.1. WNT subgroup medulloblastomas
7.2. Natural Killer NK cells
7.3. CAR-T cells
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smoll, N.R. and K.J. Drummond, The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci, 2012. 19(11): p. 1541-4. [CrossRef]
- Bihannic, L. and O. Ayrault, Insights into cerebellar development and medulloblastoma. Bull Cancer, 2016. 103(1): p. 30-40. [CrossRef]
- Louis, D.N., et al., The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol, 2016. 131(6): p. 803-20. [CrossRef]
- Kumar, R., A.P.Y. Liu, and P.A. Northcott, Medulloblastoma genomics in the modern molecular era. Brain Pathol, 2020. 30(3): p. 679-690. [CrossRef]
- Northcott, P.A., et al., Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature, 2012. 488(7409): p. 49-56. [CrossRef]
- Kool, M., et al., Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol, 2012. 123(4): p. 473-84. [CrossRef]
- Northcott, P.A., et al., The whole-genome landscape of medulloblastoma subtypes. Nature, 2017. 547(7663): p. 311-317. [CrossRef]
- Cho, Y.J., et al., Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol, 2011. 29(11): p. 1424-30. [CrossRef]
- Cavalli, F.M.G., et al., Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell, 2017. 31(6): p. 737-754.e6. [CrossRef]
- Schwalbe, E.C., et al., Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. Lancet Oncol, 2017. 18(7): p. 958-971. [CrossRef]
- Louis, D.N., et al., The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol, 2021. 23(8): p. 1231-1251. [CrossRef]
- Hovestadt, V., et al., Medulloblastomics revisited: biological and clinical insights from thousands of patients. Nat Rev Cancer, 2020. 20(1): p. 42-56. [CrossRef]
- Shih, D.J., et al., Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol, 2014. 32(9): p. 886-96. [CrossRef]
- Pui, C.H., et al., Challenging issues in pediatric oncology. Nat Rev Clin Oncol, 2011. 8(9): p. 540-9. [CrossRef]
- Phoenix, T.N., et al., Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell, 2016. 29(4): p. 508-522. [CrossRef]
- Clifford, S.C., et al., Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle, 2006. 5(22): p. 2666-70. [CrossRef]
- Ellison, D.W., et al., beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol, 2005. 23(31): p. 7951-7. [CrossRef]
- Fattet, S., et al., Beta-catenin status in paediatric medulloblastomas: correlation of immunohistochemical expression with mutational status, genetic profiles, and clinical characteristics. J Pathol, 2009. 218(1): p. 86-94. [CrossRef]
- Juraschka, K. and M.D. Taylor, Medulloblastoma in the age of molecular subgroups: a review. J Neurosurg Pediatr, 2019. 24(4): p. 353-363. [CrossRef]
- Ray, S., et al., Subgroup-Specific Diagnostic, Prognostic, and Predictive Markers Influencing Pediatric Medulloblastoma Treatment. Diagnostics (Basel), 2021. 12(1). [CrossRef]
- Zhou, Z., et al., Research progress in molecular pathology markers in medulloblastoma. Explor Target Antitumor Ther, 2023. 4(1): p. 139-156. [CrossRef]
- Sharma, T., et al., Second-generation molecular subgrouping of medulloblastoma: an international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol, 2019. 138(2): p. 309-326. [CrossRef]
- Fults, D.W., M.D. Taylor, and L. Garzia, Leptomeningeal dissemination: a sinister pattern of medulloblastoma growth. J Neurosurg Pediatr, 2019: p. 1-9. [CrossRef]
- Li, M., Y. Deng, and W. Zhang, Molecular Determinants of Medulloblastoma Metastasis and Leptomeningeal Dissemination. Mol Cancer Res, 2021. 19(5): p. 743-752. [CrossRef]
- Aspelund, A., et al., A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med, 2015. 212(7): p. 991-9. [CrossRef]
- Absinta, M., et al., Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife, 2017. 6. [CrossRef]
- Louveau, A., et al., Structural and functional features of central nervous system lymphatic vessels. Nature, 2015. 523(7560): p. 337-41. [CrossRef]
- Mobark, N.A., et al., A case of molecularly profiled extraneural medulloblastoma metastases in a child. BMC Med Genet, 2018. 19(1): p. 10. [CrossRef]
- Campbell, A.N., et al., Extracranial metastases in childhood primary intracranial tumors. A report of 21 cases and review of the literature. Cancer, 1984. 53(4): p. 974-81.
- Rochkind, S., et al., Extracranial metastases of medulloblastoma in adults: literature review. J Neurol Neurosurg Psychiatry, 1991. 54(1): p. 80-6. [CrossRef]
- Goyal, A., et al., Surgical Treatment of Intramedullary Spinal Metastasis in Medulloblastoma: Case Report and Review of the Literature. World Neurosurg, 2018. 118: p. 42-46. [CrossRef]
- Jiang, H., et al., Intramedullary metastasis in medulloblastoma: a case report and literature review. Childs Nerv Syst, 2021. 37(6): p. 2091-2095. [CrossRef]
- Kerjaschki, D., et al., Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol, 2004. 15(3): p. 603-12. [CrossRef]
- Petrova, T.V., et al., Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med, 2004. 10(9): p. 974-81. [CrossRef]
- Skobe, M., et al., Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med, 2001. 7(2): p. 192-8. [CrossRef]
- Vollmar, B., et al., Lymph vessel expansion and function in the development of hepatic fibrosis and cirrhosis. Am J Pathol, 1997. 151(1): p. 169-75.
- Tammela, T. and K. Alitalo, Lymphangiogenesis: Molecular mechanisms and future promise. Cell, 2010. 140(4): p. 460-76. [CrossRef]
- Mohammed, R.A., et al., Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br J Cancer, 2007. 96(7): p. 1092-100. [CrossRef]
- Neuchrist, C., et al., Vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck, 2003. 25(6): p. 464-74. [CrossRef]
- Stacker, S.A., M.E. Baldwin, and M.G. Achen, The role of tumor lymphangiogenesis in metastatic spread. FASEB J, 2002. 16(9): p. 922-34. [CrossRef]
- Wartiovaara, U., et al., Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost, 1998. 80(1): p. 171-5. [CrossRef]
- Schoppmann, S.F., et al., VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery, 2006. 139(6): p. 839-46. [CrossRef]
- Mandriota, S.J., et al., Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J, 2001. 20(4): p. 672-82. [CrossRef]
- He, Y., et al., Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst, 2002. 94(11): p. 819-25. [CrossRef]
- Karpanen, T., et al., Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res, 2001. 61(5): p. 1786-90.
- Shimizu, K., et al., Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci, 2004. 95(4): p. 328-33. [CrossRef]
- Roberts, N., et al., Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res, 2006. 66(5): p. 2650-7. [CrossRef]
- Hirakawa, S., et al., VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med, 2005. 201(7): p. 1089-99. [CrossRef]
- Caunt, M., et al., Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell, 2008. 13(4): p. 331-42. [CrossRef]
- Dadras, S.S., et al., Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol, 2003. 162(6): p. 1951-60. [CrossRef]
- Liang, P., et al., Increased density and diameter of lymphatic microvessels correlate with lymph node metastasis in early stage invasive colorectal carcinoma. Virchows Arch, 2006. 448(5): p. 570-5. [CrossRef]
- Wirzenius, M., et al., Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J Exp Med, 2007. 204(6): p. 1431-40. [CrossRef]
- Hoshida, T., et al., Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res, 2006. 66(16): p. 8065-75. [CrossRef]
- Padera, T.P., et al., Lymphatic metastasis in the absence of functional intratumor lymphatics. Science, 2002. 296(5574): p. 1883-6. [CrossRef]
- Albrecht, I. and G. Christofori, Molecular mechanisms of lymphangiogenesis in development and cancer. Int J Dev Biol, 2011. 55(4-5): p. 483-94. [CrossRef]
- Bracher, A., et al., Crystal structures of the free and ligand-bound FK1-FK2 domain segment of FKBP52 reveal a flexible inter-domain hinge. J Mol Biol, 2013. 425(22): p. 4134-44. [CrossRef]
- Dadras, S.S., An unexpected role for EGF in lymphangiogenesis-mediated melanoma metastasis to sentinel lymph nodes. J Invest Dermatol, 2013. 133(1): p. 14-6. [CrossRef]
- Zhang, X.H., et al., Coexpression of VEGF-C and COX-2 and its association with lymphangiogenesis in human breast cancer. BMC Cancer, 2008. 8: p. 4. [CrossRef]
- Su, J.L., et al., Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res, 2004. 64(2): p. 554-64.
- von Rahden, B.H., et al., Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res, 2005. 65(12): p. 5038-44. [CrossRef]
- Kyzas, P.A., D. Stefanou, and N.J. Agnantis, COX-2 expression correlates with VEGF-C and lymph node metastases in patients with head and neck squamous cell carcinoma. Mod Pathol, 2005. 18(1): p. 153-60. [CrossRef]
- Jiang, S., et al., Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One, 2008. 3(11): p. e3812. [CrossRef]
- Religa, P., et al., Allogenic immune response promotes the accumulation of host-derived smooth muscle cells in transplant arteriosclerosis. Cardiovasc Res, 2005. 65(2): p. 535-45. [CrossRef]
- Zumsteg, A., et al., Myeloid cells contribute to tumor lymphangiogenesis. PLoS One, 2009. 4(9): p. e7067. [CrossRef]
- Kerjaschki, D., The crucial role of macrophages in lymphangiogenesis. J Clin Invest, 2005. 115(9): p. 2316-9. [CrossRef]
- Zhang, Y., et al., Lymphangiogenesis in renal fibrosis arises from macrophages via VEGF-C/VEGFR3-dependent autophagy and polarization. Cell Death Dis, 2021. 12(1): p. 109. [CrossRef]
- Patel, V., et al., Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res, 2011. 71(22): p. 7103-12. [CrossRef]
- Niederleithner, H., et al., Wnt1 is anti-lymphangiogenic in a melanoma mouse model. J Invest Dermatol, 2012. 132(9): p. 2235-44. [CrossRef]
- Oka, M., et al., Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood, 2008. 111(9): p. 4571-9. [CrossRef]
- Ndiaye, P.D., et al., VEGFC acts as a double-edged sword in renal cell carcinoma aggressiveness. Theranostics, 2019. 9(3): p. 661-675. [CrossRef]
- Alitalo, A. and M. Detmar, Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene, 2012. 31(42): p. 4499-508. [CrossRef]
- Angeli, V., et al., B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity, 2006. 24(2): p. 203-15. [CrossRef]
- Kataru, R.P., et al., T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity, 2011. 34(1): p. 96-107. [CrossRef]
- Kataru, R.P., et al., Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood, 2009. 113(22): p. 5650-9. [CrossRef]
- Halin, C., et al., VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood, 2007. 110(9): p. 3158-67. [CrossRef]
- Nemoto, K., et al., Mesenteric lymph flow in endotoxemic guinea pigs. Lymphat Res Biol, 2011. 9(3): p. 129-34. [CrossRef]
- Cromer, W.E., et al., The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis, 2014. 17(2): p. 395-406. [CrossRef]
- Liao, S. and P.Y. von der Weid, Inflammation-induced lymphangiogenesis and lymphatic dysfunction. Angiogenesis, 2014. 17(2): p. 325-34. [CrossRef]
- Dufies, M., et al., Sunitinib stimulates expression of VEGFC by tumor cells and promotes lymphangiogenesis in clear cell renal cell carcinomas. Cancer Res, 2017. 77(5): p. 1212-1226. [CrossRef]
- Bieniasz-Krzywiec, P., et al., Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab, 2019. 30(5): p. 917-936.e10. [CrossRef]
- Ma, Q., et al., Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread. Sci Adv, 2018. 4(8): p. eaat4758. [CrossRef]
- Vaahtomeri, K. and K. Alitalo, Lymphatic Vessels in Tumor Dissemination versus Immunotherapy. Cancer Res, 2020. 80(17): p. 3463-3465. [CrossRef]
- Decio, A., et al., Vascular endothelial growth factor c promotes ovarian carcinoma progression through paracrine and autocrine mechanisms. Am J Pathol, 2014. 184(4): p. 1050-1061. [CrossRef]
- Brown, M., et al., Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science, 2018. 359(6382): p. 1408-1411. [CrossRef]
- Pereira, E.R., et al., Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science, 2018. 359(6382): p. 1403-1407. [CrossRef]
- Jones, D., E.R. Pereira, and T.P. Padera, Growth and Immune Evasion of Lymph Node Metastasis. Front Oncol, 2018. 8: p. 36. [CrossRef]
- Stacker, S.A., et al., Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer, 2014. 14(3): p. 159-72. [CrossRef]
- Kabashima, K., et al., CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol, 2007. 171(4): p. 1249-57. [CrossRef]
- Staller, P., et al., Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature, 2003. 425(6955): p. 307-11. [CrossRef]
- Balkwill, F., Cancer and the chemokine network. Nat Rev Cancer, 2004. 4(7): p. 540-50. [CrossRef]
- Günther, K., et al., Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int J Cancer, 2005. 116(5): p. 726-33. [CrossRef]
- Koizumi, K., et al., CCL21 promotes the migration and adhesion of highly lymph node metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol Rep, 2007. 17(6): p. 1511-6. [CrossRef]
- Müller, A., et al., Involvement of chemokine receptors in breast cancer metastasis. Nature, 2001. 410(6824): p. 50-6. [CrossRef]
- Munson, J.M., R.V. Bellamkonda, and M.A. Swartz, Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res, 2013. 73(5): p. 1536-46. [CrossRef]
- Issa, A., et al., Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res, 2009. 69(1): p. 349-57. [CrossRef]
- Lanati, S., et al., Chemotrap-1: an engineered soluble receptor that blocks chemokine-induced migration of metastatic cancer cells in vivo. Cancer Res, 2010. 70(20): p. 8138-48. [CrossRef]
- Harrell, M.I., B.M. Iritani, and A. Ruddell, Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol, 2007. 170(2): p. 774-86. [CrossRef]
- Hirakawa, S., et al., VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood, 2007. 109(3): p. 1010-7. [CrossRef]
- Garnier, L., A.O. Gkountidi, and S. Hugues, Tumor-Associated Lymphatic Vessel Features and Immunomodulatory Functions. Front Immunol, 2019. 10: p. 720. [CrossRef]
- Dieterich, L.C., et al., Tumor-Associated Lymphatic Vessels Upregulate PDL1 to Inhibit T-Cell Activation. Front Immunol, 2017. 8: p. 66. [CrossRef]
- Lane, R.S., et al., IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J Exp Med, 2018. 215(12): p. 3057-3074. [CrossRef]
- Lund, A.W., et al., VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep, 2012. 1(3): p. 191-9. [CrossRef]
- Baitsch, L., et al., Exhaustion of tumor-specific CD8⁺ T cells in metastases from melanoma patients. J Clin Invest, 2011. 121(6): p. 2350-60. [CrossRef]
- Grigorova, I.L., et al., Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat Immunol, 2009. 10(1): p. 58-65. [CrossRef]
- Pham, T.H., et al., Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med, 2010. 207(1): p. 17-27. [CrossRef]
- Lukacs-Kornek, V., et al., Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol, 2011. 12(11): p. 1096-104. [CrossRef]
- Liu, J., et al., Dendritic cell migration in inflammation and immunity. Cell Mol Immunol, 2021. 18(11): p. 2461-2471. [CrossRef]
- Salmi, M. and S. Jalkanen, Cell-surface enzymes in control of leukocyte trafficking. Nat Rev Immunol, 2005. 5(10): p. 760-71. [CrossRef]
- Roberts, E.W., et al., Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell, 2016. 30(2): p. 324-336. [CrossRef]
- Fridman, W.H., et al., The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer, 2012. 12(4): p. 298-306. [CrossRef]
- Fankhauser, M., et al., Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci Transl Med, 2017. 9(407). [CrossRef]
- Lund, A.W., et al., Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest, 2016. 126(9): p. 3389-402. [CrossRef]
- Bordry, N., et al., Lymphatic vessel density is associated with CD8. Oncoimmunology, 2018. 7(8): p. e1462878. [CrossRef]
- Mlecnik, B., et al., The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med, 2016. 8(327): p. 327ra26. [CrossRef]
- Mempel, T.R., S.E. Henrickson, and U.H. Von Andrian, T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature, 2004. 427(6970): p. 154-9. [CrossRef]
- Qi, H., et al., Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science, 2006. 312(5780): p. 1672-6. [CrossRef]
- Alvarez, D., E.H. Vollmann, and U.H. von Andrian, Mechanisms and consequences of dendritic cell migration. Immunity, 2008. 29(3): p. 325-42. [CrossRef]
- Roozendaal, R., R.E. Mebius, and G. Kraal, The conduit system of the lymph node. Int Immunol, 2008. 20(12): p. 1483-7. [CrossRef]
- Peske, J.D., et al., Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun, 2015. 6: p. 7114. [CrossRef]
- Thompson, E.D., et al., Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med, 2010. 207(8): p. 1791-804. [CrossRef]
- Kimura, T., et al., Lymphatic dysfunction attenuates tumor immunity through impaired antigen presentation. Oncotarget, 2015. 6(20): p. 18081-93. [CrossRef]
- Girard, J.P., C. Moussion, and R. Förster, HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol, 2012. 12(11): p. 762-73. [CrossRef]
- Aloisi, F. and R. Pujol-Borrell, Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol, 2006. 6(3): p. 205-17. [CrossRef]
- Coppola, D., et al., Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol, 2011. 179(1): p. 37-45. [CrossRef]
- Martinet, L., et al., Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res, 2011. 71(17): p. 5678-87. [CrossRef]
- Martinet, L., et al., High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology, 2012. 1(6): p. 829-839.
- Messina, J.L., et al., 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep, 2012. 2: p. 765. [CrossRef]
- Song, E., et al., VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature, 2020. 577(7792): p. 689-694. [CrossRef]
- Varney, M.L. and R.K. Singh, VEGF-C-VEGFR3/Flt4 axis regulates mammary tumor growth and metastasis in an autocrine manner. Am J Cancer Res, 2015. 5(2): p. 616-28.
- Tokuyama, W., et al., Autocrine and paracrine roles of VEGF/VEGFR-2 and VEGF-C/VEGFR-3 signaling in angiosarcomas of the scalp and face. Hum Pathol, 2010. 41(3): p. 407-14. [CrossRef]
- Matsuura, M., et al., Autocrine loop between vascular endothelial growth factor (VEGF)-C and VEGF receptor-3 positively regulates tumor-associated lymphangiogenesis in oral squamoid cancer cells. Am J Pathol, 2009. 175(4): p. 1709-21. [CrossRef]
- Dumond, A., et al., Anti-Vascular Endothelial Growth Factor C Antibodies Efficiently Inhibit the Growth of Experimental Clear Cell Renal Cell Carcinomas. Cells, 2021. 10(5). [CrossRef]
- Ferrara, N. and R.S. Kerbel, Angiogenesis as a therapeutic target. Nature, 2005. 438(7070): p. 967-74. [CrossRef]
- Folkman, J., Tumor angiogenesis: therapeutic implications. N Engl J Med, 1971. 285(21): p. 1182-6. [CrossRef]
- Folkman, J., et al., Isolation of a tumor factor responsible for angiogenesis. J Exp Med, 1971. 133(2): p. 275-88. [CrossRef]
- Aird, W.C., Endothelial cell heterogeneity. Cold Spring Harb Perspect Med, 2012. 2(1): p. a006429. [CrossRef]
- Jain, R.K., Molecular regulation of vessel maturation. Nat Med, 2003. 9(6): p. 685-93. [CrossRef]
- Nagy, J.A., et al., Heterogeneity of the tumor vasculature. Semin Thromb Hemost, 2010. 36(3): p. 321-31. [CrossRef]
- Azzi, S., J.K. Hebda, and J. Gavard, Vascular permeability and drug delivery in cancers. Front Oncol, 2013. 3: p. 211. [CrossRef]
- Stafford, J.H. and P.E. Thorpe, Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia, 2011. 13(4): p. 299-308. [CrossRef]
- Denekamp, J. and B. Hobson, Endothelial-cell proliferation in experimental tumours. Br J Cancer, 1982. 46(5): p. 711-20. [CrossRef]
- Morikawa, S., et al., Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol, 2002. 160(3): p. 985-1000. [CrossRef]
- Inai, T., et al., Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol, 2004. 165(1): p. 35-52. [CrossRef]
- Dwyer, J., et al., Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2. PLoS One, 2012. 7(9): p. e45562. [CrossRef]
- Treps, L. and J. Gavard, [Tumor angiogenesis: when the Tree of Life turns bad]. Med Sci (Paris), 2015. 31(11): p. 989-95. [CrossRef]
- Goel, S., et al., Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev, 2011. 91(3): p. 1071-121. [CrossRef]
- Sapieha, P., et al., [Supply and demand: the influence of energy metabolism on angiogenesis]. Med Sci (Paris), 2009. 25(4): p. 346-8. [CrossRef]
- Ferrara, N., Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev, 2010. 21(1): p. 21-6. [CrossRef]
- Fukumura, D., et al., Role of nitric oxide in tumor microcirculation. Blood flow, vascular permeability, and leukocyte-endothelial interactions. Am J Pathol, 1997. 150(2): p. 713-25.
- Roberts, W.G., et al., Host microvasculature influence on tumor vascular morphology and endothelial gene expression. Am J Pathol, 1998. 153(4): p. 1239-48. [CrossRef]
- Hellebrekers, D.M., et al., Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule-1 by histone modifications. Cancer Res, 2006. 66(22): p. 10770-7. [CrossRef]
- Hellebrekers, D.M., et al., Identification of epigenetically silenced genes in tumor endothelial cells. Cancer Res, 2007. 67(9): p. 4138-48. [CrossRef]
- Bussolati, B., et al., Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J, 2003. 17(9): p. 1159-61. [CrossRef]
- Unger, R.E., et al., Isolation and molecular characterization of brain microvascular endothelial cells from human brain tumors. In Vitro Cell Dev Biol Anim, 2002. 38(5): p. 273-81.
- Hida, K., et al., Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res, 2004. 64(22): p. 8249-55. [CrossRef]
- Holash, J., et al., Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science, 1999. 284(5422): p. 1994-8. [CrossRef]
- Ricci-Vitiani, L., et al., Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature, 2010. 468(7325): p. 824-8. [CrossRef]
- Naito, H., T. Iba, and N. Takakura, Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int Immunol, 2020. 32(5): p. 295-305. [CrossRef]
- Hurwitz, H., Integrating the anti-VEGF-A humanized monoclonal antibody bevacizumab with chemotherapy in advanced colorectal cancer. Clin Colorectal Cancer, 2004. 4 Suppl 2: p. S62-8. [CrossRef]
- Burger, R.A., et al., Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med, 2011. 365(26): p. 2473-83. [CrossRef]
- Escudier, B., et al., Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet, 2007. 370(9605): p. 2103-11. [CrossRef]
- Escudier, B., et al., Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol, 2010. 28(13): p. 2144-50. [CrossRef]
- Rini, B.I., et al., Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet, 2019. 393(10189): p. 2404-2415. [CrossRef]
- Casanovas, O., et al., Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell, 2005. 8(4): p. 299-309. [CrossRef]
- Grepin, R., et al., Acceleration of clear cell renal cell carcinoma growth in mice following bevacizumab/Avastin treatment: the role of CXCL cytokines. Oncogene, 2012. 31(13): p. 1683-94. [CrossRef]
- Huber, H., et al., Angiogenic profile of childhood primitive neuroectodermal brain tumours/medulloblastomas. Eur J Cancer, 2001. 37(16): p. 2064-72. [CrossRef]
- Slavc, I., et al., Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a "MEMMAT-like" Metronomic Antiangiogenic Approach. Cancers (Basel), 2022. 14(20). [CrossRef]
- Pagnuzzi-Boncompagni, M., et al., Antiangiogenic Compound Axitinib Demonstrates Low Toxicity and Antitumoral Effects against Medulloblastoma. Cancers (Basel), 2021. 14(1). [CrossRef]
- Schwinn, S., et al., Cytotoxic effects and tolerability of gemcitabine and axitinib in a xenograft model for c-myc amplified medulloblastoma. Sci Rep, 2021. 11(1): p. 14062. [CrossRef]
- Houschyar, K.S., et al., Wnt Pathway in Bone Repair and Regeneration - What Do We Know So Far. Front Cell Dev Biol, 2018. 6: p. 170. [CrossRef]
- Quaglio, D., et al., Hedgehog signaling pathway inhibitors: an updated patent review (2015-present). Expert Opin Ther Pat, 2020. 30(4): p. 235-250. [CrossRef]
- Robinson, G.W., et al., Vismodegib Exerts Targeted Efficacy Against Recurrent Sonic Hedgehog-Subgroup Medulloblastoma: Results From Phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J Clin Oncol, 2015. 33(24): p. 2646-54. [CrossRef]
- Robinson, G.W., et al., Irreversible growth plate fusions in children with medulloblastoma treated with a targeted hedgehog pathway inhibitor. Oncotarget, 2017. 8(41): p. 69295-69302. [CrossRef]
- Kim, J., et al., Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell, 2013. 23(1): p. 23-34. [CrossRef]
- Menyhárt, O. and B. Győrffy, Molecular stratifications, biomarker candidates and new therapeutic options in current medulloblastoma treatment approaches. Cancer Metastasis Rev, 2020. 39(1): p. 211-233. [CrossRef]
- Bandopadhayay, P., et al., BET bromodomain inhibition of MYC-amplified medulloblastoma. Clin Cancer Res, 2014. 20(4): p. 912-25. [CrossRef]
- Hanaford, A.R., et al., DiSCoVERing Innovative Therapies for Rare Tumors: Combining Genetically Accurate Disease Models with In Silico Analysis to Identify Novel Therapeutic Targets. Clin Cancer Res, 2016. 22(15): p. 3903-14. [CrossRef]
- Archer, T.C., et al., Proteomics, Post-translational Modifications, and Integrative Analyses Reveal Molecular Heterogeneity within Medulloblastoma Subgroups. Cancer Cell, 2018. 34(3): p. 396-410.e8. [CrossRef]
- Esfahani, K., et al., A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol, 2020. 27(Suppl 2): p. S87-S97. [CrossRef]
- Keir, M.E., et al., PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol, 2008. 26: p. 677-704. [CrossRef]
- Murata, D., et al., High programmed cell death 1 ligand-1 expression: association with CD8+ T-cell infiltration and poor prognosis in human medulloblastoma. J Neurosurg, 2018. 128(3): p. 710-716. [CrossRef]
- Martin, A.M., et al., PD-L1 expression in medulloblastoma: an evaluation by subgroup. Oncotarget, 2018. 9(27): p. 19177-19191. [CrossRef]
- Pham, C.D., et al., Differential Immune Microenvironments and Response to Immune Checkpoint Blockade among Molecular Subtypes of Murine Medulloblastoma. Clin Cancer Res, 2016. 22(3): p. 582-95. [CrossRef]
- Gabrilovich, D.I. and S. Nagaraj, Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol, 2009. 9(3): p. 162-74. [CrossRef]
- Gregorio, A., et al., Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology, 2008. 53(1): p. 73-80. [CrossRef]
- Voskamp, M.J., et al., Immunotherapy in Medulloblastoma: Current State of Research, Challenges, and Future Perspectives. Cancers (Basel), 2021. 13(21). [CrossRef]
- Zhang, Y. and B. Huang, The Development and Diversity of ILCs, NK Cells and Their Relevance in Health and Diseases. Adv Exp Med Biol, 2017. 1024: p. 225-244. [CrossRef]
- Shimasaki, N., A. Jain, and D. Campana, NK cells for cancer immunotherapy. Nat Rev Drug Discov, 2020. 19(3): p. 200-218. [CrossRef]
- Castriconi, R., et al., Both CD133+ and CD133- medulloblastoma cell lines express ligands for triggering NK receptors and are susceptible to NK-mediated cytotoxicity. Eur J Immunol, 2007. 37(11): p. 3190-6. [CrossRef]
- Kennis, B.A., et al., Monitoring of intracerebellarly-administered natural killer cells with fluorine-19 MRI. J Neurooncol, 2019. 142(3): p. 395-407. [CrossRef]
- Powell, A.B., et al., Medulloblastoma rendered susceptible to NK-cell attack by TGFβ neutralization. J Transl Med, 2019. 17(1): p. 321. [CrossRef]
- Gauthier, J. and I. Yakoub-Agha, Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: Clinical data to date, current limitations and perspectives. Curr Res Transl Med, 2017. 65(3): p. 93-102. [CrossRef]
- Mohanty, R., et al., CAR T cell therapy: A new era for cancer treatment (Review). Oncol Rep, 2019. 42(6): p. 2183-2195. [CrossRef]
- Gilbertson, R.J., ERBB2 in pediatric cancer: innocent until proven guilty. Oncologist, 2005. 10(7): p. 508-17. [CrossRef]
- Orentas, R.J., et al., Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Front Oncol, 2012. 2: p. 194. [CrossRef]
- Press, M.F., C. Cordon-Cardo, and D.J. Slamon, Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene, 1990. 5(7): p. 953-62.
- Nellan, A., et al., Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J Immunother Cancer, 2018. 6(1): p. 30. [CrossRef]
- Donovan, L.K., et al., Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med, 2020. 26(5): p. 720-731. [CrossRef]
- Tasian, S.K. and R.A. Gardner, CD19-redirected chimeric antigen receptor-modified T cells: a promising immunotherapy for children and adults with B-cell acute lymphoblastic leukemia (ALL). Ther Adv Hematol, 2015. 6(5): p. 228-41. [CrossRef]
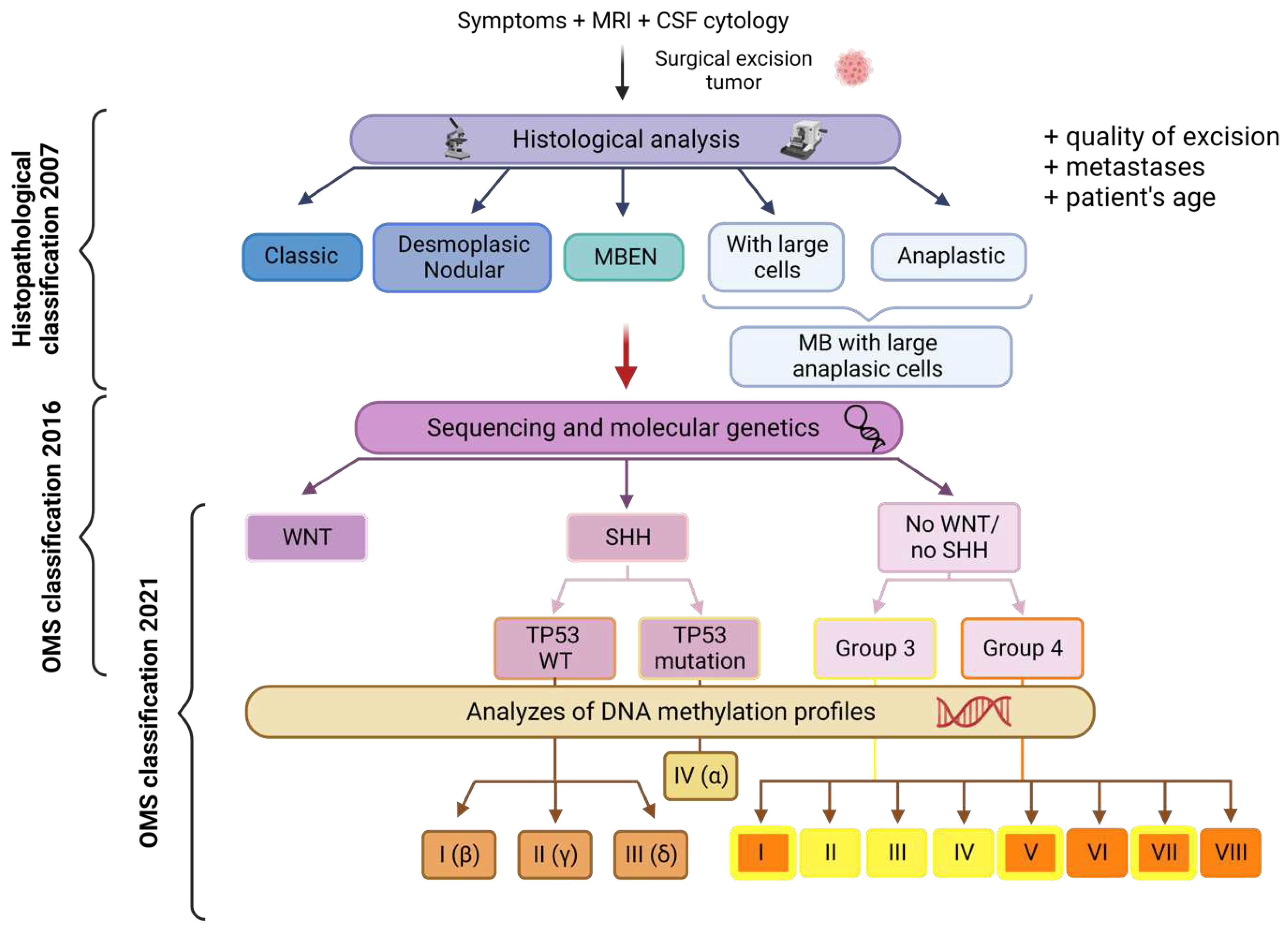
| Subgroup | Subtype | Frequency | Demography | Main genetic events | Metastasis rate | 5-year overall survival |
|---|---|---|---|---|---|---|
| WNT | WNTa | 70% | Infants - adolescents |
CTNNB1, TP53, DDX3X, MLL2/3 mutation Monosomy chromosome 6 |
8.6% | 97% |
| WNTb | 30% | Children – young adults | 21.4% | 100% | ||
| SHH | SHHa | 29% | Children - adolescents | Loss of 9q, 10q, 17p MYCN, GLI2, YAP1 amp; TP53 mutation |
20% | 69.8% |
| SHHb | 16% | Infants | PTEN loss | 33% | 67.3% | |
| SHHg | 31% | Infants | Low copy number alterations | 8.9% | 88% | |
| SHHd | 24% | Young adults | TERT promoter mutation | 9.4% | 88.5% | |
| Group 3 | Group 3a | 47% | Infants - children | i17q; loss of 8q and 17p | 43.4% | 66.2% |
| Group 3b | 26% | Children - adolescents | OTX2 gain and DDX31 loss; activation of GFI1 and GFI1B oncogenes | 20% | 55.8% | |
| Group 3g | 28% | Infants - children | i17q; 8q gain and MYC amplification | 39.4% | 41.9% | |
| Group 4 | Group 4a | 30% | Children - adolescents | i17q; loss of 8p; 7q gain; MYCN and CDK6 amplification | 40% | 66.8% |
| Group 4b | 33% | Children - adolescents | i17q; 17p loss; SNCAIP duplication | 40.7% | 75.4% | |
| Group 4d | 37% | Children - adolescents | i17q; loss of 8p; 7q gain; CDK6 amplification | 38.7% | 82.5% |
| Reference | Title | Phase | Enrollment | Intervention/treatment type |
|---|---|---|---|---|
| NCT00602667 | Risk-Adapted Therapy for Young Children with Embryonal Brain Tumors, Choroid Plexus Carcinoma, High Grade Glioma or Ependymoma | 2 | 293 | Drug: Induction Chemotherapy Drug: Low-Risk Therapy Drug: High-Risk Therapy Drug: Intermediate-Risk Therapy |
| NCT01878617 | A Clinical and Molecular Risk-Directed Therapy for Newly Diagnosed Medulloblastoma | 2 | 660 | Radiation: Craniospinal Irradiation with boost to the primary tumor site Drug: Cyclophosphamide Drug: Cisplatin Drug: Vincristine Drug: Vismodegib Drug: Pemetrexed Drug: Gemcitabine Other: Aerobic Training Other: Neurocognitive Remediation |
| NCT02017964 | Combination Chemotherapy in Treating Younger Patients With Newly Diagnosed, Non-metastatic Desmoplastic Medulloblastoma |
2 | 26 | Drug: Carboplatin Other: Cognitive Assessment Drug: Cyclophosphamide Drug: Etoposide Other: Laboratory Biomarker Analysis Drug: Methotrexate Drug: Vincristine Sulfate |
| NCT02066220 | International Society of Paediatric Oncology (SIOP) PNET 5 Medulloblastoma |
2; 3 | 360 | Radiation: Radiotherapy without Carboplatin Drug: Reduced-intensity maintenance chemotherapy Radiation: Radiotherapy with Carboplatin Drug: Maintenance chemotherapy Radiation: WNT-HR < 16 years Radiation: WNT-HR >= 16 years Drug: Induction Chemotherapy Radiation: SHH-TP53 M0 Radiation: SHH-TP53 M+ (germline) Radiation: SHH-TP53 (somatic) Drug: Vinblastin Maintenance |
| NCT02238899 | Multicenter Register for Children and Young Adults With Intracranial Localized Medulloblastoma, CNS-PNET or Ependymoma | 354 | ||
| NCT02255461 | Palbociclib Isethionate in Treating Younger Patients With Recurrent, Progressive, or Refractory Central Nervous System Tumors | 1 | 35 | Drug: palbociclib isethionate Other: pharmacological study Other: laboratory biomarker analysis |
| NCT02271711 | Expanded Natural Killer Cell Infusion in Treating Younger Patients With Recurrent/Refractory Brain Tumors | 1 | 12 | Other: Laboratory Biomarker Analysis Biological: Natural Killer Cell Therapy |
| NCT02359565 | Pembrolizumab in Treating Younger Patients With Recurrent, Progressive, or Refractory High-Grade Gliomas, Diffuse Intrinsic Pontine Gliomas, Hypermutated Brain Tumors, Ependymoma or Medulloblastoma |
1 | 110 | Procedure: Diffusion Tensor Imaging Procedure: Diffusion Weighted Imaging Procedure: Dynamic Contrast-Enhanced Magnetic Resonance Imaging Procedure: Dynamic Susceptibility Contrast-Enhanced Magnetic Resonance Imaging Other: Laboratory Biomarker Analysis Procedure: Magnetic Resonance Spectroscopic Imaging Biological: Pembrolizumab Procedure: Perfusion Magnetic Resonance Imaging |
| NCT02724579 | Reduced Craniospinal Radiation Therapy and Chemotherapy in Treating Younger Patients With Newly Diagnosed WNT-Driven Medulloblastoma | 2 | 45 | Drug: Cisplatin Drug: Cyclophosphamide Other: Laboratory Biomarker Analysis Drug: Lomustine Radiation: Radiation Therapy Drug: Vincristine Drug: Vincristine Sulfate |
| NCT03130959 | A Study to Evaluate the Safety and Efficacy of Nivolumab Monotherapy and Nivolumab in Combination With Ipilimumab in Pediatric Participants With High Grade Primary Central Nervous System (CNS) Malignancies (CheckMate 908) | 2 | 166 | Biological: Nivolumab Biological: Ipilimumab |
| NCT03500991 | HER2-specific CAR T Cell Locoregional Immunotherapy for HER2-positive Recurrent/Refractory Pediatric CNS Tumors | 1 | 48 | Biological: HER2-specific chimeric antigen receptor (CAR) T cell |
| NCT04023669 | Evaluation of LY2606368 Therapy in Combination With Cyclophosphamide or Gemcitabine for Children and Adolescents With Refractory or Recurrent Group 3/Group 4 or SHH Medulloblastoma Brain Tumors | 1 | 21 | Drug: Prexasertib Drug: Cyclophosphamide Drug: Gemcitabine Biological: filgrastim Biological: peg-filgrastim |
| NCT04743661 | 131I-Omburtamab, in Recurrent Medulloblastoma and Ependymoma | 2 | 62 | Drug: Irinotecan Drug: Temozolomide Drug: Bevacizumab Drug: Omburtamab I-131 Drug: Liothyronine Drug: SSKI Drug: Dexamethasone Drug: Antipyretic Drug: Antihistamine Drug: anti-emetics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





