Submitted:
22 June 2023
Posted:
23 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and discussion
3.1. PLE photoprotective activity
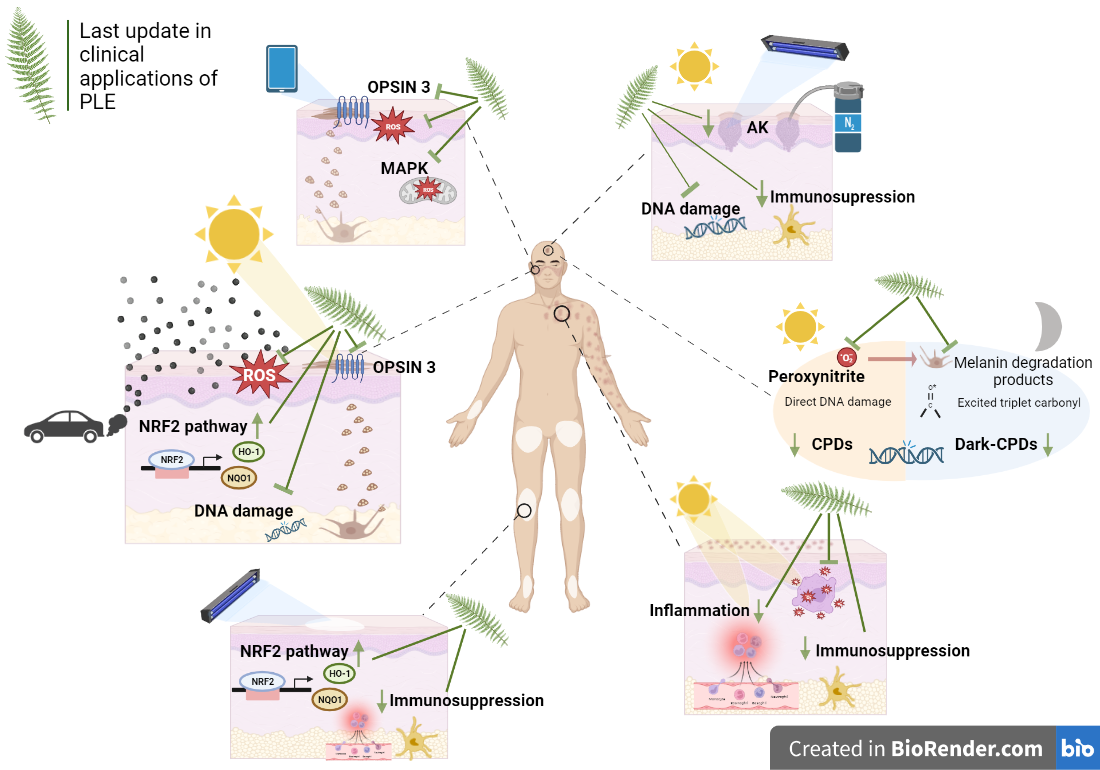
3.2. Clinical applications
3.2.1. Oncodermatology
| Oncodermatology | |||
| Design | Pathology/Focus | Summary / Outcome |
Study Reference |
| Review | General oncodermatology | This review reports the mechanisms through which Polypodium leucotomos acts to evaluate its uses in oncodermatology with references to in vitro and in vivo studies | [13] |
| Review & Book Chapter |
Continuing medical education about skin cancer and sunscreen use | These reviews provide evidence-based recommendations for the use of sunscreen as a preventive strategy against skin cancer, while also considering potential risks and environmental impacts associated with the use of some chemical sunscreen filters. PLE is included as a reference oral sunscreen technology for prevention of photodamage. | [33,34,35,36,37] |
| Reviews & Book chapter | Botanical interventions for photoprotection and skin cancer | These works review the main actives derived from plants with scientific evidence as treatment in photoprotection and offer an overview of cancer and phytotherapy. Specifically, they review the existing literature on the properties of PLE and its potential therapeutic effects in preventing skin damage. These reviews include studies conducted in vitro, in vivo, and clinical trials. | [38,39,40,41,42] |
| Review | Preventive interventions for keratinocyte carcinoma | This manuscript examines the potential of pharmaceuticals, plant-derived phytochemicals, and vitamins for preventing keratinocyte carcinoma. One such reference photoprotectant is PLE, which has been shown to inhibit the development of tumors and acute UV-induced damage in humans. | [43] |
| Clinical study | Field cancerization | This clinical study suggests that a new medical device treatment containing Fernblock® (NMD) is a useful treatment method for improving the precancerous field and preventing the development of new AKs. | [21] |
| Clinical study | Oral cancer | The findings indicate that PLE has the ability to suppress oral cancer cell growth in vitro and prevent tumor development in vivo. Thus, PLE could be a promising natural therapeutic approach for preventing and treating oral cancer. | [32] |
| Preclinical study | Skin cancer markers | This in vitro study suggests that FB could be a promising candidate to complement traditional sunscreens in providing long-lasting skin protection against dark-CPDs formation after irradiation. | [26] |
| Preclinical study | Melanoma | This in vitro research suggests that supplements containing sulforaphane/FB could be used to prevent skin aging and help treat advanced melanoma. | [25] |
| Preclinical study | Skin cancer induced by photopollution | This preclinical study demonstrates the efficacy of PLE in preventing changes in cellular structure, viability, oxidative stress and activation of the melanogenic signaling pathway caused by exposure to both BaP and UVA light. | [28] |
| Actinic keratosis | |||
| Review | Actinic keratosis | This review article examines in vitro experiments and clinical trials that utilize evidence-based therapeutic methods before or after photodynamic therapy (PDT). Specifically, the effectiveness of topical treatments and oral supplementation, such as diclofenac, imiquimod, PLE, among others, as well as mechanical-physical treatments, are evaluated. | [44] |
| Review | Actinic keratosis | In this article, the authors offer expert opinions and practical insights into the treatment of actinic keratosis and field cancerization using monotherapy or a combination of therapies among which PLE is cited. The primary objective is to achieve improved, quicker, and more tolerable clinical outcomes. | [45] |
| Review | Actinic keratosis | This review discusses various physical ablative techniques and drug preparations available for treatment. It emphasizes the need for careful evaluation of efficacy, toxicity, and tolerability data, as well as practical considerations such as treatment protocols and patient preferences, to achieve maximal adherence and prevent treatment failure. It includes PLE as a chemopreventive treatment tool against the development of AK. | [46] |
| Xeroderma pigmentosum | |||
| Review | Xeroderma pigmentosum | The purpose of this review is to present the symptoms, diagnosis, and treatment of XP. It also includes oral PLE as a treatment adjuvant due to its chemoprotective, antioxidative, anti-inflammatory and immunomodulatory properties. All these effects have the potential to lessen the phototoxic effects of UVR and thus reduce UVR-induced skin damage and cancer. | [47] |
3.2.2. Photodermatoses and photoaggravated skin diseases
3.2.2.1. Polymorphous light eruption and actinic prurigo.
3.2.2.2. Solar Urticaria and Photosensitive Lupus
| Photodermatoses and photoaggravated skin diseases | |||
| Design | Pathology/Focus | Summary / Outcome |
Study Reference |
| Review | UVB phototoxicity | The objective of the text is to explore and discuss the potential of various nutraceuticals in preventing or mitigating the effects of phototoxicity caused by UVB radiation. The text provides the mechanisms by which these nutraceuticals, including spirulina, soy isoflavones, and PLE, among others, may offer protection against UVB-induced sunburn, photoaging, and NMSC. | [9] |
| Review | Photodermatoses | This chapter outlines various topical and systemic agents that can trigger phototoxic and photoallergic reactions. In terms of treatment, the chapter mentions that PLE can be used as a systemic antioxidant in conjunction with PUVA to manage cases of PMLE. | [64] |
| Review | Idiopathic photodermatoses | This chapter provides a clinical approach to managing idiopathic photodermatoses, including conditions such as PMLE, actinic prurigo, and idiopathic solar urticaria, among others. Preventing and managing these conditions involves implementing photoprotective measures and increasing the skin's tolerance to sunlight through the use of narrow-band UVB therapy and other forms of phototherapy or photochemotherapy when necessary. In addition, topical or systemic antioxidants like PLE may be helpful in certain cases. | [65] |
| Review | Diet & Photodermatoses | Prior studies have explored the connection between diet and several skin conditions, including rosacea, hidradenitis suppurativa, herpes labialis, and vitiligo. The authors consolidate the findings from existing literature to create clear and concise guidance regarding dietary supplements that could be beneficial or harmful. By doing so, they provide healthcare professionals with evidence-based recommendations to assist their patients, including PLE as a recommended supplement in the treatment of vitiligo. | [66] |
| Case report | Photodermatoses | The case study involves a 55-year-old man who experienced a severe and painful skin eruption with erythema and blisters in sun-exposed areas one month after starting vandetanib treatment. Despite treatment with steroids and avoiding sun exposure, the condition did not improve until the patient began taking oral supplements of PLE. This case highlights the potential of PLE as a safe and effective photoprotective agent for treating refractory phototoxic reactions. | [67] |
| Polymorphous light eruption and actinic prurigo | |||
| Review | PMLE | The purpose of the review is to provide a better understanding of the molecular pathogenesis of PMLE by examining the immunological disturbances associated with the disease. The authors emphasize the potential of PLE as an immunomodulatory and antioxidant agent and suggest it could be used as a preventive therapeutic approach for PMLE treatment. | [53] |
| Review | PMLE | The goal of this article is to provide readers with the latest information on PMLE with regards to its epidemiology, clinical presentation, underlying pathophysiology, available treatments, and prognosis. PLE is presented as a potential treatment for PMLE, and the review cites open-label studies showing that this supplement can reduce the severity, frequency, and rapidity of onset of PMLE reactions. | [68] |
| Review | Actinic prurigo | The aim of this study is to provide a summary of current knowledge related to two types of photodermatoses - actinic prurigo (AP) and hydroa vacciniforme (HV), both of which typically develop during childhood. Among suggested treatment, botanical agents such as PLE may be beneficial in reducing photosensitivity in certain skin conditions like PMLE and solar urticaria. However, further studies are needed to suggest their usefulness in treating AP. | [57] |
| Case report | Actinic prurigo | In this report, the authors describe the successful use of PLE in an 11-year-old girl with AP. PLE treatment led to a significant reduction in her symptoms and no negative side effects were observed. PLE has a wide-ranging impact on the immune system and acts as an antioxidant by promoting an anti-inflammatory environment. | [56] |
| Solar Urticaria and Photosensitive Lupus | |||
| Restrospective analysis | Solar urticaria (SU) | The authors conducted a retrospective analysis in 83 patients with SU. Among the 60 patients who underwent monochromator testing, 35 were confirmed to have SU, with most reacting to VIS and UVA, or UVA alone. The mainstay of treatment for SU is antihistamines and sun avoidance. However, for patients who do not respond to these treatments, other options such as omalizumab may be of potential interest. Also, PLE is sugested as a treatment option for SU without side effects. | [58] |
| Review | Lupus | This review analyses natural actives traditionally used to treat rheumatological conditions, including antimalarials, which could also be beneficially indicated for cutaneous lupus eryhtematosus (CLE). It also suggests their combination with PLE as a photoprotective supplement to control photosensitivity. | [69] |
| Review | Lupus | This text reviews the available evidence regarding local and systemic therapies for CLE and provides healthcare professionals with alternative treatment options for patients who were previously treated with Quinacrine, which is currently unavailable in the USA. Among these options, PLE is proposed with a level of evidence of 5 in accordance to the levels adapted from the Oxford Centre for Evidence Based Medicine. | [70] |
| Clinical cases | Lupus | This article examines the use of thalidomide in the treatment of discoid lupus erythematosus (DLE) and discusses four case studies that demonstrate its success. Two of the case studies included the addition of PLE. Patients who received PLE experienced a longer duration for complete clearance of symptoms; moreover, incorporating PLE helped to lower the thalidomide dosage and thus reduce its side effects. | [61] |
| Clinical study | PMLE | In this prospective study, a standardized extract of P. leucotomos, along with nicotinamide, vitamin D, and zinc, was orally administered to 15 patients suffering PMLE. These patients had not achieved symptom control through the use of only topical photoprotection. Administering a standardized extract of P. leucotomos, nicotinamide, vitamin D, and zinc orally, in conjunction with appropriate topical photoprotection, offers a safe and effective alternative for preventing and minimizing the frequency and severity of outbreaks in individuals with PMLE. | [55] |
| Other photodermatoses: Chronic Actinic Dermatitis | |||
| Case report | Chronic Actinic Dermatitis | In this study a case of a patient with Chronic Actinic Dermatitis (CAD) who showed only partial improvement with dupilumab is described. Initial management included sun avoidance and photoprotective therapy, which included topical Fernblock®, among others. The CAD did not improve, and the treatment continued with topical corticosteroids, immunomodulators, and systemic immunosuppressive agents. The continued implementation of photoprotection measures such as oral supplements, including oral and topical PLE, is recommended due to their proven efficacy as adjuvants to the above-mentioned pharmacological treatments. | [71] |
| Other studies with PL extracts in photodermatoses: non- Fernblock® PL extracts | |||
| Review | Rosacea | The purpose of this study is to explain the origin of rosacea, with a particular focus on the influence of UV radiation and exposome on the development of this skin condition. Additionally, this review highlights the importance of non-pharmacological approaches, with specific emphasis on photoprotection strategies in managing rosacea, using for example an extract of P. leucotomos. | [72] |
3.2.3. Pigmentary disorders
3.2.3.1. Vitiligo
3.2.3.2. Hyperpigmentation disorders
| Pigmentary disorders | |||
| Design | Pathology/Focus | Summary / Outcome |
Study Reference |
| Reviews & Book chapters |
Pigmentary disorders | Pigmentary disorders (melasma, vitiligo, periocular hyperpigmentation, pigmented contact dermatitis and lichen planus pigmentosus), are over-represented in women in most societies. Their mechanisms and future therapies, including PLE are reviewed. | [94,95] |
| Review | Pathways & ingredients involved in pigmentary disorders | The text provides an overview of the role oxidative stress plays in melanogenesis, particularly in response to skin exposure to UVR and VIS. It also discusses various pathways involved in pigmentary disorders. Additionally, the text offers guidance on effective approaches to modulate melanogenesis, including the use of vitamins, PLE, niacinamide, and other options, such as lightening agents, that can aid in the better management of pigmentary disorders. | [80,96,97,98,99,100] |
| Review | Visible light and hyperpigmentation | These reviews focus on the role of VIS in hyperpigmentation disorders (melasma and PIH) and analyze the direct and indirect effects of blue light emitted by digital devices reported on in vitro and in vivo studies. Recent advances in understanding the protective role of PLE against UVA and VIS have led to it being cited as a reference agent in preclinical and clinical studies. | [95,101] |
| Review | Management of hyperpigmentation: Dermatological procedures | This review focuses on the medical and dermoaesthetic procedures for hyperpigmentation; and on challenges (resistance, recurrence, adverse effect) in the management of pigmentary disorders such as melasma and PIH. PLE is included as a reference compound due to studies that suggest its beneficial activity in treating dyschromias. | [102,103,104] |
| Review | Management of hyperpigmentation: Topical | The review presents alternative ways to manage hyperpigmentation, including PLE, glutathione, and thiamidol. It also provides a table summarizing the scientific evidence supporting their effectiveness. | [105] |
| Review | Management of hyperpigmentation: Oral | The texts provide a review of literature on oral treatments for hyperpigmentation, with a specific focus on examining the clinical evidence that supports the use of several oral treatments, including PLE and others. | [106,107] |
| Review | Management of PIH | These reviews describe the first-line treatments for epidermal PIH (based on topical or oral skin lightening agents) and the available adjunctive therapies for patients refractory to first-line treatment or for dermal PIH (peels, laser, etc.). They also analyze the use of sunscreens for the treatment of melasma and PIH. PLE is included as a systemic skin-lightening agent, among others. It is noted that PLE is the only ingredient listed that does not have any reported adverse effects. | [108,109,110] |
| Clinical study | Skin pigmentation induced by VIS+UVA light | This study evaluates the role of topical antioxidants in protecting against VIS+UVA-induced effects in skin phototypes I-VI. Topical antioxidants inhibit erythema in phototypes I-III and reduce pigmentation in phototypes IV-VI. PLE is used as a reference compound to compare its antioxidant properties with those of other compounds. | [111] |
| Melasma | |||
| Reviews & Book chapters | Management of Melasma: Focus on topical and oral treatments |
These articles discuss various techniques for treating melasma and evaluate the effectiveness and safety of ingredients like hydroquinone and tranexamic acid, as well as delivery systems that improve the depigmentation activity of certain agents. Additionally, all these reviews place PLE among the most effective oral agents recommended for melasma. | [79,112,113,114,115,116,117,118,119] Sistematic review and metanalysis:[120,121,122] Book chapters: [123,124] |
| Review | Management of Melasma: Focus on dermatological procedures |
The main objective of these reviews was to analyze the available evidence on the efficacy and safety of microneedling alone or in combination with topical agents in reducing pigmentation and improving the quality of life for adult patients with melasma. Oral PLE treatment was included as a therapeutic option for melasma suggesting that combined therapies tend to produce better results compared to monotherapy. | [125,126,127] |
| Review | Melasma | The review aims to provide a comprehensive understanding of the role of oxidative stress in melasma and the potential therapeutic benefits of various antioxidants for individuals with this condition. Here, PLE is considered a principal antioxidant for treating melasma, and a summary of clinical studies is included in these documents. | [128,129] |
| Review | Melasma pathways | The manuscript provides a review of the processes and pathways responsible for skin pigmentation, specifically the changes in melanogenesis that lead to melasma and resulting hyperpigmentation. The paper also discusses current treatments and therapies, including those administered topically, orally, and through phototherapy, with a particular focus on the effects of cosmetics. PLE is included as a plant-based oral treatment. | [130] |
| Clinical study | Pigmentation disorders | This study aims to assess the effectiveness of PLE in preventing VIS-induced effects in human skin. PLE treatment induces a significant decrease in persistent pigment darkening and delayed tanning and reduces expression of several damage markers. The study provides scientific evidence to position PLE as a treatment for pigmentation disorders. | [131] |
| Vitiligo | |||
| Review | Vitiligo treatments | These reviews discuss the pathogenesis of vitiligo and focus on treatment options for the disease including standard drug treatments, phototherapy (NB-UVB and PUVA) and the effectiveness of antioxidant therapies. Moreover, in the majority of cases, antioxidant therapies on their own are not capable of producing significant clinical improvements, except perhaps in mild cases, and they must be used alongside standard drug treatments in order to achieve noticeable outcomes, such as PLE in concomitance with NB-UVB or PUVA. | [76,132,133,134,135,136,137,138,139,140] Focused on PUVA: [141,142,143] |
| Review | Safety in vitiligo treatments | These reviews concentrate on the potential harm related to the use of medicinal plants, including PLE, and offer a summary of adverse drug reactions (ADRs) that have been reported in national and global individual case safety report databases. | [144,145] |
| Review | The role of Nrf2-ARE in vitiligo | This paper examines the role of NRF2 in vitiligo and reviews several agents known as NRF2 activators, including PLE. It suggests that PLE's efficacy in the treatment of vitiligo is the results of its activation of the NRF2 pathway. | [146] |
| Clinical study | PLE in vitiligo | This study involved 44 patients with generalized vitiligo who received either combined treatment of NB-UVB phototherapy and oral PLE or NB-UVB phototherapy and placebo. The results showed that oral PLE combined with NB-UVB improved repigmentation and increased response rate as compared to NB-UVB alone. | [75] |
| Other studies with PL extracts in melasma: non- Fernblock® PL extracts | |||
| Case report | Melasma | The aim of this research was to examine the practical outcomes of a treatment plan that combined multiple interventions: one conducted at home and the other performed in a clinical setting. The protocol consisted of using a peel containing trichloroacetic acid, phytic acid, and ascorbic acid, in combination with oral antioxidant supplements containing an extract from PL. Additionally, topical products were provided to individuals with persistent melasma. The findings of the study suggest that this treatment protocol could represent an effective approach for managing melasma. | [92] |
| Chemical assay with different PL extracts | Photoaging, hyperpigmentation | This study evaluates the antioxidant capacity of different hydrophilic and lipophilic fern extracts. All ferns present antioxidant activity and potential to inhibit hyperpigmentation (anti-tyrosinase activity). This report concluded that hydrophilic extracts are more potent and effective. | [93] |
3.2.4. Extrinsic aging
| Extrinsic aging | |||
| Design | Pathology/Focus | Summary / Outcome |
Study Reference |
| Review | Skin aging | The present study reviews the literature on the underlying causes and pathophysiological processes of skin aging, healthy skin aging, and basic protective antiaging approaches. PLE is regarded as a reference antioxidant due to its phenolic content and it can be used orally or topically to counteract skin aging due to its capacity to reduce the harmful effects of UVR and its photoprotective, antioxidant, anti-inflammatory and antiaging properties. | [152] |
| Review | Photoaging | These reviews evaluate the ability of sunscreens to protect against photoaging and analyze the ideal characteristics of sunscreen, taking into account the impact of VIS and IR on skin aging (apart from UVR). This document includes PLE as a reference compound for oral photoprotection and its role in the prevention of photoaging. | [7,153,154] |
| Review | Photoaging pathway | This article discusses the mechanisms of photoaging, specifically in human dermal fibroblasts. PLE is included for its role in preventing photoaging caused by VIS and IR radiation by decreasing MMP-1 and Cat K levels and preventing changes in the expression of fibrillin 1, fibrillin 2, and elastin. | [155] |
| Review | Ingredients & Photoaging | These reviews provide a summary of the pathways involved in skin aging and explore various therapeutic approaches that utilize natural actives. The reviews cite PLE as one of the main natural actives, extensively researched and with robust scientific evidence supporting its use and its role as a reference product. | [156,157,158,159,160,161,162,163,164,165,166] |
| Clinical study | Sunburn-Photoaging-Skin cancer | This study compares the efficacy of two identical sunscreens, one containing PLE and the other not. The presence of PLE in the formulation provided a significantly greater reduction in skin damage triggered by solar radiation (reduction of erythema, pigmentation, DNA damage, collagen breakdown and immunosuppression). | [29] |
| Clinical study | Photoaging induced by IR and VIS | This pilot study evaluates the effect of oral PLE against the IR and VIS-induced photodamage. PLE attenuates IR/VIS-induced MMP1 overexpression. This study reinforces the anti-photoaging potential of PLE. | [150] |
| Clinical study | Photoaging induced by VIS | Twenty-two participants were exposed to VIS before taking PLE and then observed for 7 days to establish a baseline response. After 28 days of taking PLE, VIS was administered to the opposite side of the participant's back. Instrumental assessments showed a statistically significant decrease in persistent pigment darkening and delayed tanning in patients after PLE administration. | [131] |
| In vitro study | Photoaging induced by blue light | This study assesses the capacity of PLE to reduce pigmentation induced by blue light from digital devices. PLE prevents cell death, alteration of mitochondrial morphology, and phosphorylation of p38 triggered by blue light. PLE also prevents melanin photodegradation through regulation of Opsin-3 in melanocytes. | [82] |
| In vitro study | Pollution and aging | This study evaluates the potential of PLE to protect against xenotoxic stress related to exposure to fine particulate pollutants. PLE can reduce pollution-induced stress through modulation of NRF2 pathway. | [10] |
| Other studies with PL extracts in photoaging: non- Fernblock® PL extracts | |||
| Clinical study | Photoaging | This study evaluates the photoprotective properties of an oral food supplement containing: vitamins A, C, D3, E, selenium, lycopene, lutein, green tea, P. leucotomos and grape extracts. Oral intake of this supplement increases MED and FRAP. In general, it improves antioxidant status of skin and exerts photoprotective effects. | [151] |
4. Conclusions and future perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Yeager, D.G.; Lim, H.W. What’s New in Photoprotection: A Review of New Concepts and Controversies. Dermatol Clin 2019, 37, 149–157. [Google Scholar] [CrossRef]
- Parrado, C.; Philips, N.; Gilaberte, Y.; Juarranz, A.; González, S. Oral Photoprotection: Effective Agents and Potential Candidates. Front Med (Lausanne) 2018, 5. [Google Scholar] [CrossRef]
- García, F.; Pivel, J.P.; Guerrero, A.; Brieva, A.; Martínez-Alcázar, M.P.; Caamaño-Somoza, M.; González, S. Phenolic Components and Antioxidant Activity of Fernblock, an Aqueous Extract of the Aerial Parts of the Fern Polypodium Leucotomos. Methods Find Exp Clin Pharmacol 2006, 28, 157–160. [Google Scholar] [CrossRef]
- González, S.; Lucena, S.R.; Delgado, P.; Juarranz, A. Comparison of Several Hydrophilic Extracts of Polypodium Leucotomos Reveals Different Antioxidant Moieties and Photoprotective Effects in Vitro. Journal of Medicinal Plants Research 2018, 13. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Polypodium Leucotomos Extract (PLE): New Study Gives Evidence-Based Insight into “Ain’t Nothing Like the Real Thing. ” J Clin Aesthet Dermatol 2019, 12, 45. [Google Scholar] [PubMed]
- Parrado, C.; Nicolas, J.; Juarranz, A.; Gonzalez, S. The Role of the Aqueous Extract Polypodium Leucotomos in Photoprotection. Photochem Photobiol Sci 2020, 19, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Pourang, A.; Dourra, M.; Ezekwe, N.; Kohli, I.; Hamzavi, I.; Lim, H.W. The Potential Effect of Polypodium Leucotomos Extract on Ultraviolet- and Visible Light-Induced Photoaging. Photochemical and Photobiological Sciences 2021, 20, 1229–1238. [Google Scholar] [CrossRef]
- Barry, E.S.; Merkebu, J.; Varpio, L. State-of-the-Art Literature Review Methodology: A Six-Step Approach for Knowledge Synthesis. Perspect Med Educ 2022, 11, 281–288. [Google Scholar] [CrossRef]
- McCarty, M.F.; Benzvi, C.; Vojdani, A.; Lerner, A. Nutraceutical Strategies for Alleviation of UVB Phototoxicity. Exp Dermatol 2023. [Google Scholar] [CrossRef]
- Delgado-Wicke, P.; Rodríguez-Luna, A.; Ikeyama, Y.; Honma, Y.; Kume, T.; Gutierrez, M.; Lorrio, S.; Juarranz, Á.; González, S. Fernblock® Upregulates NRF2 Antioxidant Pathway and Protects Keratinocytes from PM2.5-Induced Xenotoxic Stress. Oxid Med Cell Longev 2020, 2020. [Google Scholar] [CrossRef]
- Jańczyk, A.; Garcia-Lopez, M.A.; Fernandez-Peñas, P.; Alonso-Lebrero, J.L.; Benedicto, I.; López-Cabrera, M.; Gonzalez, S. A Polypodium Leucotomos Extract Inhibits Solar-Simulated Radiation-Induced TNF-α and INOS Expression, Transcriptional Activation and Apoptosis. Exp Dermatol 2007, 16, 823–829. [Google Scholar] [CrossRef]
- Zattra, E.; Coleman, C.; Arad, S.; Helms, E.; Levine, D.; Bord, E.; Guillaume, A.; El-Hajahmad, M.; Zwart, E.; Van Steeg, H.; et al. Polypodium Leucotomos Extract Decreases UV-Induced Cox-2 Expression and Inflammation, Enhances DNA Repair, and Decreases Mutagenesis in Hairless Mice. Am J Pathol 2009, 175, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Calzari, P.; Vaienti, S.; Nazzaro, G. Uses of Polypodium Leucotomos Extract in Oncodermatology. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Viera, M.H.; Amini, S.; Huo, R.; Perez, O.; Ruiz, P.; Amador, A.; Elgart, G.; Berman, B. Decrease of Ultraviolet A Light-Induced “Common Deletion” in Healthy Volunteers after Oral Polypodium Leucotomos Extract Supplement in a Randomized Clinical Trial. J Am Acad Dermatol 2010, 62, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Yanes, E.; Juarranz, Á.; Cuevas, J.; Gonzalez, S.; Mallol, J. Polypodium Leucotomos Decreases UV-Induced Epidermal Cell Proliferation and Enhances P53 Expression and Plasma Antioxidant Capacity in Hairless Mice. Exp Dermatol 2012, 21, 638–640. [Google Scholar] [CrossRef]
- Philips, N.; Smith, J.; Keller, T.; Gonzalez, S. Predominant Effects of Polypodium Leucotomos on Membrane Integrity, Lipid Peroxidation, and Expression of Elastin and Matrixmetalloproteinase-1 in Ultraviolet Radiation Exposed Fibroblasts, and Keratinocytes. J Dermatol Sci 2003, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Conte, J.; Chen, Y.J.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial Regulation of Matrixmetalloproteinases and Their Inhibitors, Fibrillar Collagens and Transforming Growth Factor-β by Polypodium Leucotomos, Directly or in Dermal Fibroblasts, Ultraviolet Radiated Fibroblasts, and Melanoma Cells. Arch Dermatol Res 2009, 301, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Kohli, I.; Shafi, R.; Isedeh, P.; Griffith, J.L.; Al-Jamal, M.S.; Silpa-archa, N.; Jackson, B.; Athar, M.; Kollias, N.; Elmets, C.A.; et al. The Impact of Oral Polypodium Leucotomos Extract on Ultraviolet B Response: A Human Clinical Study. J Am Acad Dermatol 2017, 77, 33–41. [Google Scholar] [CrossRef]
- Middelkamp-Hup, M.A.; Pathak, M.A.; Parrado, C.; Goukassian, D.; Rius-Díaz, F.; Mihm, M.C.; Fitzpatrick, T.B.; González, S. Oral Polypodium Leucotomos Extract Decreases Ultraviolet-Induced Damage of Human Skin. J Am Acad Dermatol 2004, 51, 910–918. [Google Scholar] [CrossRef]
- Auriemma, M.; Di Nicola, M.; Gonzalez, S.; Piaserico, S.; Capo, A.; Amerio, P. Polypodium Leucotomos Supplementation in the Treatment of Scalp Actinic Keratosis. Dermatologic Surgery 2015, 41, 898–902. [Google Scholar] [CrossRef]
- De Unamuno Bustos, B.; Aguilera, N.C.; Azorín García, I.; Andrino, A.C.; Ros, M.L.; Rodrigo, R.; Vitale, M.; González, S.; Botella Estrada, R. Long-Term Efficacy of a New Medical Device Containing Fernblock ® and DNA Repair Enzyme Complex in the Treatment and Prevention of Cancerization Field in Patients with Actinic Keratosis. J Clin Exp Dermatol Res 2019, 10, 499. [Google Scholar] [CrossRef]
- Lamberti, A.; Cartocci, A.; Donelli, C.; Cortonesi, G.; Trovato, E.; Milani, M.; Rubegni, P.; Cinotti, E. Prevention Strategies in Patients Affected by Actinic Keratosis of the Head: A 12-Month, Prospective, Assessor-Blinded, Controlled Study with Lesion-Directed Treatment Associated with Medicalized Photoprotection. 2022. [Google Scholar] [CrossRef]
- Pellacani, G.; Peris, K.; Ciardo, S.; Pezzini, C.; Tambone, S.; Farnetani, F.; Longo, C.; Chello, C.; Gonzalez, S. The Combination of Oral and Topical Photoprotection with a Standardized Polypodium Leucotomos Extract Is Beneficial against Actinic Keratosis. Photodermatol Photoimmunol Photomed 2023. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, P.; Carrera, C.; Puig-Butille, J.A.; Badenas, C.; Lecha, M.; González, S.; Malvehy, J.; Puig, S. Benefits of Oral Polypodium Leucotomos Extract in MM High-Risk Patients. J Eur Acad Dermatol Venereol 2013, 27, 1095–1100. [Google Scholar] [CrossRef]
- Serini, S.; Guarino, R.; Vasconcelos, R.O.; Celleno, L.; Calviello, G. The Combination of Sulforaphane and Fernblock® XP Improves Individual Beneficial Effects in Normal and Neoplastic Human Skin Cell Lines. Nutrients 2020, Vol. 12, Page 1608 2020, 12, 1608. [Google Scholar] [CrossRef]
- Portillo-Esnaola, M.; Rodríguez-Luna, A.; Nicolás-Morala, J.; Gallego-Rentero, M.; Villalba, M.; Juarranz, Á.; González, S. Formation of Cyclobutane Pyrimidine Dimers after UVA Exposure (Dark-CPDs) Is Inhibited by an Hydrophilic Extract of Polypodium Leucotomos. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Brash, D.E. Chemiexcitation of Melanin Derivatives Induces DNA Photoproducts Long after UV Exposure. Science (1979) 2015, 347, 842–847. [Google Scholar] [CrossRef]
- Gallego-Rentero, M.; Nicolás-Morala, J.; Alonso-Juarranz, M.; Carrasco, E.; Portillo-Esnaola, M.; Rodríguez-Luna, A.; González, S. Protective Effect of the Hydrophilic Extract of Polypodium Leucotomos, Fernblock®, against the Synergistic Action of UVA Radiation and Benzo[a]Pyrene Pollutant. Antioxidants 2022, 11. [Google Scholar] [CrossRef]
- Schalka, S.; Donato, L.C. Evaluation of Effectiveness of a Sunscreen Containing Polypodium Leucatomos Extract in Reducing the Sun Damage to the Skin. Surgical & Cosmetic Dermatology 2019, 11, 310–318. [Google Scholar] [CrossRef]
- Aguilera, J.; Vicente-Manzanares, M.; de Gálvez, M.V.; Herrera-Ceballos, E.; Rodríguez-Luna, A.; González, S. Booster Effect of a Natural Extract of Polypodium Leucotomos (Fernblock®) That Improves the UV Barrier Function and Immune Protection Capability of Sunscreen Formulations. Front Med (Lausanne) 2021, 8. [Google Scholar] [CrossRef]
- González-Morán, A.; Piquero-Casals, J. Use of a Topical Film-Forming Medical Device Containing Repairsomes® in a Patient with Xeroderma Pigmentosum to Avoid Progression to Skin Cancerization. Clin Cosmet Investig Dermatol 2020, 13, 677–681. [Google Scholar] [CrossRef]
- Lacerda, P.A.; Oenning, L.C.; Bellato, G.C.; Lopes-Santos, L.; de Antunes, N.J.; Mariz, B.A.L.A.; Teixeira, G.; Vasconcelos, R.; Simões, G.F.; de Souza, I.A.; et al. Polypodium Leucotomos Targets Multiple Aspects of Oral Carcinogenesis and It Is a Potential Antitumor Phytotherapy against Tongue Cancer Growth. Front Pharmacol 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- González, S.; De Gálvez, M.V.; De Troya, M.; Rodríguez-Luna, A.; Calzavara-Pinton, P. Personalized Medical Photoprotection: Determining Optimal Measures for Susceptible Patient Groups. Open Dermatol J 2023, 17. [Google Scholar] [CrossRef]
- González, S.; Aguilera, J.; Berman, B.; Calzavara-Pinton, P.; Gilaberte, Y.; Goh, C.L.; Lim, H.W.; Schalka, S.; Stengel, F.; Wolf, P.; et al. Expert Recommendations on the Evaluation of Sunscreen Efficacy and the Beneficial Role of Non-Filtering Ingredients. Front Med (Lausanne) 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Abisaad, J.A.; Rojas, K.D.; Marchetti, M.A.; Jaimes, N. Skin Cancer: Primary, Secondary, and Tertiary Prevention. Part I. J Am Acad Dermatol 2022, 87, 255–268. [Google Scholar] [CrossRef]
- Passeron, T.; Lim, H.W.; Goh, C.L.; Kang, H.Y.; Ly, F.; Morita, A.; Ocampo Candiani, J.; Puig, S.; Schalka, S.; Wei, L.; et al. Photoprotection According to Skin Phototype and Dermatoses: Practical Recommendations from an Expert Panel. Journal of the European Academy of Dermatology and Venereology 2021, 35, 1460–1469. [Google Scholar] [CrossRef]
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The Efficacy and Safety of Sunscreen Use for the Prevention of Skin Cancer. CMAJ 2020, 192, E1802–E1808. [Google Scholar] [CrossRef]
- Philips, N.; Richardson, R.; Siomyk, H.; Bynum, D.; Gonzalez, S. “Skin Cancer, Polyphenols, and Oxidative Stress” or Counteraction of Oxidative Stress, Inflammation, Signal Transduction Pathways, and Extracellular Matrix Remodeling That Mediate Skin Carcinogenesis by Polyphenols. Cancer: Oxidative Stress and Dietary Antioxidants 2021, 439–450. [Google Scholar] [CrossRef]
- Araújo Lacerda, P.; Marinho Ottoni Costa, L.; Cuoghi Bellato, G.; Ayaka Yamashita, M.; Lopes-Santos, L.; Augusto, T.M.; Karla Cervigne, N. Perspectives on Cancer and Phytotherapy: An Overview Focusing on Polypodium Leucotomos Therapeutic Properties. J Cancer Prev Curr Res 2021, Volume 12, 9–18. [Google Scholar] [CrossRef]
- Subhadarshani, S.; Athar, M.; Elmets, C.A. Photocarcinogenesis. Curr Dermatol Rep 2020, 9, 189–199. [Google Scholar] [CrossRef]
- Bhatia, B.K.; Lim, H.W.; Hamzavi, I.H. Comprehensive Dermatologic Drug Therapy. In Comprehensive Dermatologic Drug Therapy; Wolverton, D.S.E., Wu, J.J., Eds.; 2020; Vol. 23, pp. 263–270.
- Zimmerman, C. Herbs for Low-Risk Skin Cancers and Precancers. https://home.liebertpub.com/act 2019, 25, 163–166. [Google Scholar] [CrossRef]
- Pihl, C.; Togsverd-Bo, K.; Andersen, F.; Haedersdal, M.; Bjerring, P.; Lerche, C.M. Keratinocyte Carcinoma and Photoprevention: The Protective Actions of Repurposed Pharmaceuticals, Phytochemicals and Vitamins. Cancers 2021, Vol. 13, Page 3684 2021, 13, 3684. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Mazzetto, R.; Sartor, E.; Bortoletti, C. Combination-Based Strategies for the Treatment of Actinic Keratoses with Photodynamic Therapy: An Evidence-Based Review. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Gilaberte, Y.; Del Rio, R.; Macaya-Pascual, A.; Granger, C.; López-Estebaranz, J.L. Management Pearls on the Treatment of Actinic Keratoses and Field Cancerization. Dermatol Ther (Heidelb) 2020, 10, 903–915. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Calzavara-Pinton, I.; Rovati, C.; Rossi, M. Topical Pharmacotherapy for Actinic Keratoses in Older Adults. Drugs Aging 2022, 39, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Xeroderma Pigmentosum: An Updated Review. Drugs Context 2022, 11. [Google Scholar] [CrossRef]
- Oakley, A.M.; Badri, T.; Harris, B.W. Photosensitivity. StatPearls 2022. [Google Scholar]
- Ali, N.; Rosenblum, M.D. Regulatory T Cells in Skin. Immunology 2017, 152, 372. [Google Scholar] [CrossRef]
- Capote, R.; Alonso-Lebrero, J.L.; García, F.; Brieva, A.; Pivel, J.P.; González, S. Polypodium Leucotomos Extract Inhibits Trans-Urocanic Acid Photoisomerization and Photodecomposition. J Photochem Photobiol B 2006, 82, 173–179. [Google Scholar] [CrossRef]
- Mulero, M.; Rodríguez-yanes, E.; Nogués, M.R.; Giralt, M.; Romeu, M.; González, S.; Mallol, J. Polypodium Leucotomos Extract Inhibits Glutathione Oxidation and Prevents Langerhans Cell Depletion Induced by UVB/UVA Radiation in a Hairless Rat Model. Exp Dermatol 2008, 17, 653–658. [Google Scholar] [CrossRef]
- Rodríguez-Yanes, E.; Cuevas, J.; González, S.; Mallol, J. Oral Administration of Polypodium Leucotomos Delays Skin Tumor Development and Increases Epidermal P53 Expression and the Anti-Oxidant Status of UV-Irradiated Hairless Mice. Exp Dermatol 2014, 23, 526–528. [Google Scholar] [CrossRef]
- Kadurina, M.; Kazandjieva, J.; Bocheva, G. Immunopathogenesis and Management of Polymorphic Light Eruption. Dermatol Ther 2021, 34, e15167. [Google Scholar] [CrossRef] [PubMed]
- George, E.A.; Baranwal, N.; Kang, J.H.; Qureshi, A.A.; Drucker, A.M.; Cho, E. Photosensitizing Medications and Skin Cancer: A Comprehensive Review. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Valladares Narganes, L.M. Actividad Fotoprotectora Del Extracto Estandarizado de Polypodium Leucotomos, Nicotinamida, Vitamina D y Cinc En La Prevención y La Reducción de Brotes En Los Pacientes Con Erupción Polimorfa Lumínica. Piel: Formación continua en dermatología, ISSN 0213-9251, Vol. 37, No. 1 (Enero), 2022, págs. 7-12 2022, 37, 7–12. [Google Scholar] [CrossRef]
- Stump, M.; Dhinsa, H.; Powers, J.; Stone, M. Attenuation of Actinic Prurigo Eruptions with Polypodium Leucotomos Supplementation. Pediatr Dermatol 2022, 39, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.L.; DeLeo, V.A. Actinic Prurigo and Hydroa Vacciniforme. Curr Dermatol Rep 2019, 8, 63–72. [Google Scholar] [CrossRef]
- Photiou, L.; Foley, P.; Ross, G. Solar Urticaria – An Australian Case Series of 83 Patients. Australasian Journal of Dermatology 2019, 60, 110–117. [Google Scholar] [CrossRef]
- Caccialanza, M.; Percivalle, S.; Piccinno, R.; Brambilla, R. Photoprotective Activity of Oral Polypodium Leucotomos Extract in 25 Patients with Idiopathic Photodermatoses. Photodermatol Photoimmunol Photomed 2007, 23, 46–47. [Google Scholar] [CrossRef]
- Caccialanza, M.; Recalcati, S.; Piccinno, R. Oral Polypodium Leucotomos Extract Photoprotective Activity in 57 Patients with Idiopathic Photodermatoses. G Ital Dermatol Venereol 2011, 146, 85–87. [Google Scholar]
- Malara, G.; Verduci, C.; Altomonte, M.; Cuzzola, M.; Trifiro, C.; Politi, C.; Tripepi, G. Thalidomide and Discoid Lupus Erythematosus: Case Series and Review of Literature. Drugs Context 2022, 11. [Google Scholar] [CrossRef]
- Segars, K.; McCarver, V.; Miller, R.A. Dermatologic Applications of Polypodium Leucotomos: A Literature Review. J Clin Aesthet Dermatol 2021, 14, 50. [Google Scholar]
- Breithaupt, A.D.; Jacob, S.E. Subacute Cutaneous Lupus Erythematosus: A Case Report of Polypodium Leucotomos as an Adjuvant Therapy - PubMed. Cutis 2012, 89, 183–184. [Google Scholar] [PubMed]
- Whitney, A.; Prok, L.D. Dermatology Secrets 2020, A. Whitney & L. D. Prok, Eds.; 6a ed.). Elsevier. https://books.google.at/books?id=CJ8FEAAAQBAJ.
- Hölzle, E.; Dawe, R. The Idiopathic Photodermatoses and Skin Testing. Harper’s Textbook of Pediatric Dermatology 2019, 943–956. [Google Scholar] [CrossRef]
- Jamgochian, M.; Alamgir, M.; Rao, B. Diet in Dermatology: Review of Diet’s Influence on the Conditions of Rosacea, Hidradenitis Suppurativa, Herpes Labialis, and Vitiligo. Am J Lifestyle Med 2021, 17, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Korman, A.M.; Reynolds, K.A.; Nabhan, F.; Konda, B.; Shah, M.H.; Kaffenberger, B.H. Vandetanib-Induced Phototoxic Drug Eruption Treated with Polypodium Leucotomos Extract: A Case Report and Review of the Literature. J Clin Aesthet Dermatol 2019, 12, 35. [Google Scholar]
- Artz, C.E.; Farmer, C.M.; Lim, H.W. Polymorphous Light Eruption: A Review. Curr Dermatol Rep 2019, 8, 110–116. [Google Scholar] [CrossRef]
- Lubov, J.E.; Jamison, A.S.; Baltich Nelson, B.; Amudzi, A.A.; Haas, K.N.; Richmond, J.M. Medicinal Plant Extracts and Natural Compounds for the Treatment of Cutaneous Lupus Erythematosus: A Systematic Review. Front Pharmacol 2022, 13, 188. [Google Scholar] [CrossRef]
- Yan, D.; Borucki, R.; Sontheimer, R.D.; Werth, V.P. Candidate Drug Replacements for Quinacrine in Cutaneous Lupus Erythematosus. Lupus Sci Med 2020, 7, e000430. [Google Scholar] [CrossRef]
- Verma, L.; Pratt, M. A Case Report of Therapeutically Challenging Chronic Actinic Dermatitis. SAGE Open Med Case Rep 2019, 7, 2050313X1984523. [Google Scholar] [CrossRef]
- Morgado-Carrasco, D.; Granger, C.; Trullas, C.; Piquero-Casals, J. Impact of Ultraviolet Radiation and Exposome on Rosacea: Key Role of Photoprotection in Optimizing Treatment. J Cosmet Dermatol 2021, 20, 3415–3421. [Google Scholar] [CrossRef]
- Shahbazi, A.; Zargar, S.J.; Aghdami, N.; Habibi, M. The Story of Melanocyte: A Long Way from Bench to Bedside. Cell Tissue Bank 2023. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, X.; Ying, J.; Cai, Y.; Qiu, Y.; Xiang, W. Evaluating the Quality of Life among Melasma Patients Using the MELASQoL Scale: A Systematic Review and Meta-Analysis. PLoS One 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, A.; Damiani, G.; Iacovelli, P.; Conic, R.R.Z.; Gonzalez, S.; Morrone, A. NB-UVB plus Oral Polypodium Leucotomos Extract Display Higher Efficacy than NB-UVB Alone in Patients with Vitiligo. Dermatol Ther 2021, 34. [Google Scholar] [CrossRef] [PubMed]
- Qadir, A.; Ullah, S.N.M.N.; Jahan, S.; Ali, A.; Khan, N. Drug Delivery of Natural Products through Nano-Carriers for Effective Vitiligo Therapy: A Compendia Review. J Cosmet Dermatol 2022, 21, 5386–5404. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Meng, X.; Song, Z.; Lin, J. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) as a Potential Therapeutic Target for Vitiligo. Arch Biochem Biophys 2020, 696, 108670. [Google Scholar] [CrossRef] [PubMed]
- Kahremany, S.; Hofmann, L.; Gruzman, A.; Dinkova-Kostova, A.T.; Cohen, G. NRF2 in Dermatological Disorders: Pharmacological Activation for Protection against Cutaneous Photodamage and Photodermatosis. Free Radic Biol Med 2022, 188, 262–276. [Google Scholar] [CrossRef]
- Hatem, S.; El Hoffy, N.M.; Elezaby, R.S.; Nasr, M.; Kamel, A.O.; Elkheshen, S.A. Background and Different Treatment Modalities for Melasma: Conventional and Nanotechnology-Based Approaches. J Drug Deliv Sci Technol 2020, 60, 101984. [Google Scholar] [CrossRef]
- Nautiyal, A.; Wairkar, S. Management of Hyperpigmentation: Current Treatments and Emerging Therapies. Pigment Cell Melanoma Res 2021, 34, 1000–1014. [Google Scholar] [CrossRef]
- Mohammad, T.F.; Kohli, I.; Nicholson, C.L.; Do, G.T.; Chaowattanapanit, S.; Nahhas, A.F.; Braunberger, T.L.; Lim, H.W.; Mda, I.H.H. Oral Polypodium Leucotomos Extract and Its Impact on Visible Light-Induced Pigmentation in Human Subjects, 2019; Vol. 18.
- Portillo, M.; Mataix, M.; Alonso-Juarranz, M.; Lorrio, S.; Villalba, M.; Rodríguez-Luna, A.; González, S. The Aqueous Extract of Polypodium Leucotomos (Fernblock®) Regulates Opsin 3 and Prevents Photooxidation of Melanin Precursors on Skin Cells Exposed to Blue Light Emitted from Digital Devices. Antioxidants (Basel) 2021, 10, 1–16. [Google Scholar] [CrossRef]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J Invest Dermatol 2018, 138, 171–178. [Google Scholar] [CrossRef]
- Zhou, S.; Yamada, R.; Sakamoto, K. Low Energy Multiple Blue Light-Emitting Diode Light Irradiation Promotes Melanin Synthesis and Induces DNA Damage in B16F10 Melanoma Cells. PLoS One 2023, 18. [Google Scholar] [CrossRef]
- Ceresnie, M.S.; Patel, J.; Lim, H.W.; Kohli, I. The Cutaneous Effects of Blue Light from Electronic Devices: A Systematic Review with Health Hazard Identification. Photochem Photobiol Sci 2023, 22, 457–464. [Google Scholar] [CrossRef] [PubMed]
- de Gálvez, E.N.; Aguilera, J.; Solis, A.; de Gálvez, M.V.; de Andrés, J.R.; Herrera-Ceballos, E.; Gago-Calderon, A. The Potential Role of UV and Blue Light from the Sun, Artificial Lighting, and Electronic Devices in Melanogenesis and Oxidative Stress. J Photochem Photobiol B 2022, 228. [Google Scholar] [CrossRef] [PubMed]
- Ramser, A.; Casey, A. Blue Light and Skin Health. J Drugs Dermatol 2022, 21, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Suitthimeathegorn, O.; Yang, C.; Ma, Y.; Liu, W. Direct and Indirect Effects of Blue Light Exposure on Skin: A Review of Published Literature. Skin Pharmacol Physiol 2022, 35, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Felsner, I.; Brenden, H.; Marini, A.; Jaenicke, T.; Aue, N.; Welss, T.; Uthe, I.; Krutmann, J. Air Pollution-Induced Tanning of Human Skin. Br J Dermatol 2021, 185, 1026–1034. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, E.J.; Luo, E.; Choi, J.; Kim, J.Y.; Kim, S.; Kim, S.H.; Bae, Y.J.; Park, S.; Lee, J.; et al. Particulate Matter Promotes Melanin Production through Endoplasmic Reticulum Stress‒Mediated IRE1α Signaling. J Invest Dermatol 2022, 142, 1425–1434. [Google Scholar] [CrossRef]
- Goh, C.-L.; Chuan, S.Y.; Tien, S.; Thng, G.; Vitale, M.A.; Delgado-Rubin, A. Double-Blind, Placebo-Controlled Trial to Evaluate the Effectiveness of Polypodium Leucotomos Extract in the Treatment of Melasma in Asian Skin: A Pilot Study - PubMed. J Clin Aesthet Dermatol 2018, 11, 14–19. [Google Scholar]
- Piquero-Casals, J.; Granger, C.; Piquero-Casals, V.; Garre, A.; Mir-Bonafé, J.F. A Treatment Combination of Peels, Oral Antioxidants, and Topical Therapy for Refractory Melasma: A Report of 4 Cases. Clin Cosmet Investig Dermatol 2020, 13, 209–213. [Google Scholar] [CrossRef]
- Farràs, A.; Cásedas, G.; Les, F.; Terrado, E.M.; Mitjans, M.; López, V. Evaluation of Anti-Tyrosinase and Antioxidant Properties of Four Fern Species for Potential Cosmetic Applications. Forests 2019, Vol. 10, Page 179 2019, 10, 179. [Google Scholar] [CrossRef]
- Sinha, S.; Sarkar, R.; Upadhyaya, A. Pigmentary Disorders in Women. Skin Diseases in Females 2022, 181–223. [Google Scholar] [CrossRef]
- Bhatia, B.K.; Huggins, R.H.; Tisack, A. Pigmentary Disorders. Practical Guide to Dermatology 2020, 213–222. [Google Scholar] [CrossRef]
- Xing, X.; Dan, Y.; Xu, Z.; Xiang, L. Implications of Oxidative Stress in the Pathogenesis and Treatment of Hyperpigmentation Disorders. Oxid Med Cell Longev 2022, 2022. [Google Scholar] [CrossRef]
- Kasraee, B. Skin Depigmenting Agents: Where Do We Stand? Pigmentation Disorders - Etiology and Recent Advances in Treatments, 2022. [Google Scholar] [CrossRef]
- Shah, S.; Shah, R.M.; Patel, S.; Patel, S.; Doshi, S.; Lio, P. Integrative Approaches to Hyperpigmentation Therapy, 2022.
- Nahhas, A.F.; Abdel-Malek, Z.A.; Kohli, I.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. The Potential Role of Antioxidants in Mitigating Skin Hyperpigmentation Resulting from Ultraviolet and Visible Light-Induced Oxidative Stress. Photodermatol Photoimmunol Photomed 2019, 35, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.K. Skin Lightening & Management of Hyperpigmentation. Pharmaceutical Sciences & Analytical Research Journal 2019, 2019, 180020. [Google Scholar]
- Cohen, L.; Brodsky, M.A.; Zubair, R.; Kohli, I.; Hamzavi, I.H.; Sadeghpour, M. Cutaneous Interaction with Visible Light: What Do We Know. J Am Acad Dermatol 2020. [Google Scholar] [CrossRef]
- Ko, D.; Wang, R.F.; Ozog, D.; Lim, H.W.; Mohammad, T.F. Disorders of Hyperpigmentation. Part II. Review of Management and Treatment Options for Hyperpigmentation. J Am Acad Dermatol 2023, 88, 291–320. [Google Scholar] [CrossRef]
- Liu, W.; Krutmann, J.; Tian, Y.; Granger, C.; Piquero-Casals, J.; Trullàs, C.; Passeron, T.; Lim, H.W.; Lai, W. Commentary: Facial Aesthetic Dermatological Procedures and Photoprotection in Chinese Populations. Dermatol Ther (Heidelb) 2023, 13, 13–27. [Google Scholar] [CrossRef]
- Sowash, M.; Alster, T. Review of Laser Treatments for Post-Inflammatory Hyperpigmentation in Skin of Color. Am J Clin Dermatol 2023, 1–16. [Google Scholar] [CrossRef]
- Charoo, N.A. Hyperpigmentation: Looking beyond Hydroquinone. J Cosmet Dermatol 2022, 21, 4133–4145. [Google Scholar] [CrossRef]
- Shimshak, S.J.E.; Tolaymat, L.M.; Haga, C.B.; Dawson, N.L.; Gillis, M.S.; Yin, M.; Kirsch, B.; Cooper, M.; Sluzevich, J.C. A Review of Oral Therapies for the Treatment of Skin Hyperpigmentation. Journal of Cutaneous Medicine and Surgery 2021, 26, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; An, X.; Qu, L.; Wang, F. Review on Oral Plant Extracts in Skin Whitening. Food Science and Technology 2022, 42, e83922. [Google Scholar] [CrossRef]
- Garg, S.; Tuknayat, A.; Garg, S.; Tuknayat, A. Tips for Managing Post-Inflammatory Hyperpigmentation of Acne. Cosmoderma 2021, 1, 28. [Google Scholar] [CrossRef]
- Fatima, S.; Braunberger, T.; Mohammad, T.; Kohli, I.; Hamzavi, I. The Role of Sunscreen in Melasma and Postinflammatory Hyperpigmentation. Indian J Dermatol 2020, 65, 5. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Das, A. Postinflammatory Hyperpigmentation: What We Should Know. Pigment International 2019, 6, 57. [Google Scholar]
- Lyons, A.B.; Zubair, R.; Kohli, I.; Nahhas, A.F.; Braunberger, T.L.; Mokhtari, M.; Ruvolo, E.; Lim, H.W.; Hamzavi, I.H. Mitigating Visible Light and Long Wavelength UVA1-Induced Effects with Topical Antioxidants. Photochem Photobiol 2022, 98, 455–460. [Google Scholar] [CrossRef]
- Tan, S.; Aw, D. A Review of Oral Treatments for Melasm. Hong Kong J. Dermatol. Venereol. 2021, 29, 62–70. [Google Scholar]
- Yuniandari, A.A.A.A.N.; Wijayanti, D. Evaluating The Efficacy And Safety Of Tranexamic Acid To Hydroquinone And Triple Combination Cream In The Treatment Of Melasma. Jurnal Health Sains 2022, 3, 1643–1657. [Google Scholar] [CrossRef]
- Jung Thapa, R.; Chandra Karki, B.; Chandra Yadav, S. Management of Melasma: Emerging Facts. Journal of Tumor Science Research 2022, 1, 1–6. [Google Scholar] [CrossRef]
- McKesey, J.; Tovar-Garza, A.; Pandya, A.G. Melasma Treatment: An Evidence-Based Review. Am J Clin Dermatol 2020, 21, 173–225. [Google Scholar] [CrossRef]
- Grimes, P.E.; Ijaz, S.; Nashawati, R.; Kwak, D. New Oral and Topical Approaches for the Treatment of Melasma. Int J Womens Dermatol 2019, 5, 30–36. [Google Scholar] [CrossRef]
- Babbush, K.M.; Babbush, R.A.; Khachemoune, A. Treatment of Melasma: A Review of Less Commonly Used Antioxidants. Int J Dermatol 2021, 60, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Cassiano, D.P.; Espósito, A.C.C.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Miot, L.D.B.; Miot, H.A.; Bagatin, E. Update on Melasma—Part II: Treatment. Dermatology and Therapy 2022 12:9 2022, 12, 1989–2012. [Google Scholar] [CrossRef]
- Katoch, S.; Sarkar, R. Approach to a Case of Melasma. Pigment International 2021, 8, 195. [Google Scholar]
- Liu, Y.; Wu, S.; Wu, H.; Liang, X.; Guo, D.; Zhuo, F. Comparison of the Efficacy of Melasma Treatments: A Network Meta-Analysis of Randomized Controlled Trials. Front Med (Lausanne) 2021, 8, 1587. [Google Scholar] [CrossRef]
- Neagu, N.; Conforti, C.; Agozzino, M.; Marangi, G.F.; Morariu, S.H.; Pellacani, G.; Persichetti, P.; Piccolo, D.; Segreto, F.; Zalaudek, I.; et al. Melasma Treatment: A Systematic Review. Journal of Dermatological Treatment 2022, 33, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Ayu, S.; Suryantari, A.; Putu, N.; Sweta, T.B.; Veronica, E.; Ngurah, G.; Rai, B.; Hartawan, M.; Luh, N.; Ratih, P.; et al. Systematic Review of Melasma Treatments: Advantages and Disadvantages. Bali Dermatology and Venereology 2020, 3, 37–51. [Google Scholar] [CrossRef]
- da Cunha, M.G.; da Urzedo, A.P.S.; da Cunha, M.G.; da Urzedo, A.P.S. Melasma: A Review about Pathophysiology and Treatment. Pigmentation Disorders - Etiology and Recent Advances in Treatments 2022. [Google Scholar] [CrossRef]
- Handog, E.B.; Datuin, M.S. Evangeline B Handog Melasma: A Monograph - Oral Agents in the Treatment of Melasma, 2020, Edited by R. Sarkar.
- Brasil dos Santos, J.; Nagem Lopes, L.P.; de Lima, G.G.; Teixeira da Silva, R.; da Silva e Souza Lorca, B.; Miranda Pinheiro, G.; Faria de Freitas, Z.M. Microneedling with Cutaneous Delivery of Topical Agents for the Treatment of Melasma: A Systematic Review. J Cosmet Dermatol 2022, 21, 5680–5695. [Google Scholar] [CrossRef]
- Kamal, K.; Heitmiller, K.; Christman, M. Lasers, Lights, and Compounds for Melasma in Aesthetics. Clin Dermatol 2022, 40, 249–255. [Google Scholar] [CrossRef]
- Mahajan, V.K.; Patil, A.; Blicharz, L.; Kassir, M.; Konnikov, N.; Gold, M.H.; Goldman, M.P.; Galadari, H.; Goldust, M. Medical Therapies for Melasma. J Cosmet Dermatol 2022, 21, 3707–3728. [Google Scholar] [CrossRef]
- Babbush, K.M.; Khachemoune, A.; Babbush Bs, K.M.; Babbush, R.A.; Facms, F. The Therapeutic Use of Antioxidants for Melasma. J Drugs Dermatol 2020, 19, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Babbush, K.M.; Babbush, R.A.; Khachemoune, A. Treatment of Melasma: A Review of Less Commonly Used Antioxidants. Int J Dermatol 2021, 60, 166–173. [Google Scholar] [CrossRef]
- Maddaleno, A.S.; Camargo, J.; Mitjans, M.; Vinardell, M.P. Melanogenesis and Melasma Treatment. Cosmetics 2021, Vol. 8, Page 82 2021, 8, 82. [Google Scholar] [CrossRef]
- Mohammad, T.F.; Kohli, I.; Nicholson, C.L.; Treyger, G.; Chaowattanapanit, S.; Nahhas, A.F.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. Oral Polypodium Leucotomos Extract and Its Impact on Visible Light-Induced Pigmentation in Human Subjects. J Drugs Dermatol. 2019, 18, 1198–1203. [Google Scholar] [PubMed]
- Guarneri, F.; Bertino, L.; Pioggia, G.; Casciaro, M.; Gangemi, S. Therapies with Antioxidant Potential in Psoriasis, Vitiligo, and Lichen Planus. Antioxidants 2021, Vol. 10, Page 1087 2021, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Bouceiro Mendes, R.; Alpalhão, M.; Filipe, P. UVB Phototherapy in the Treatment of Vitiligo: State of the Art and Clinical Perspectives. Photodermatol Photoimmunol Photomed 2022, 38, 215–223. [Google Scholar] [CrossRef]
- Wang, E.; Rodrigues, M.; Dermatology, C. An Update and Review of Narrowband Ultraviolet B Phototherapy for Vitiligo. Dermatological Reviews 2022, 3, 326–335. [Google Scholar] [CrossRef]
- Sun, M.C.; Xu, X.L.; Lou, X.F.; Du, Y.Z. Recent Progress and Future Directions: The Nano-Drug Delivery System for the Treatment of Vitiligo. Int J Nanomedicine 2020, 15, 3267–3279. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Lam, J.M.; Leong, K.F.; Hon, K.L. Vitiligo: An Updated Narrative Review. Curr Pediatr Rev 2020, 17, 76–91. [Google Scholar] [CrossRef]
- Ali, S.A.; Parveen, N.; Ali, A.S. Promoting Melanocyte Regeneration Using Different Plants and Their Constituents. In Herbal Medicine Back to the Future: Cancer Therapy; 2019; pp. 247–276. [Google Scholar] [CrossRef]
- Taïeb, A.; Picardo, M. Management Overview. In Vitiligo; Springer: Cham, 2019; pp. 345–351. [Google Scholar]
- Picardo, M.; Taïeb, A.V.; Picardo, M. , Taïeb, A., Eds.; Springer International Publishing: Cham, 2019; ISBN 978-3-319-62958-2. [Google Scholar]
- Böhm, M.; Schunter, J.A.; Fritz, K.; Salavastru, C.; Dargatz, S.; Augustin, M.; Tanew, A. S1 Guideline: Diagnosis and Therapy of Vitiligo. J Dtsch Dermatol Ges 2022, 20, 365–378. [Google Scholar] [CrossRef]
- Maghfour, J.; Hamzavi, I.H.; Tasneem, I.; Mohammad, F. An Updated Review on Systemic and Targeted Therapies for Vitiligo. Dermatological Reviews 2022, 3, 313–325. [Google Scholar] [CrossRef]
- Dutta, R.; Kumar, T.; Ingole, N. Diet and Vitiligo: The Story So Far. Cureus 2022, 14. [Google Scholar] [CrossRef]
- Arora, P. Nutraceuticals in Vitiligo: Not Just “Designer” Foods. Pigment International 2022, 9, 147. [Google Scholar] [CrossRef]
- Lotti, T.; Agarwal, K.; Podder, I.; Satolli, F.; Kassir, M.; Schwartz, R.A.; Wollina, U.; Grabbe, S.; Navarini, A.A.; Mueller, S.M.; et al. Safety of the Current Drug Treatments for Vitiligo. Expert Opin Drug Saf 2020, 19, 499–511. [Google Scholar] [CrossRef]
- Hussain, I. The Safety of Medicinal Plants Used in the Treatment of Vitiligo and Hypermelanosis: A Systematic Review of Use and Reports of Harm. Clin Cosmet Investig Dermatol 2021, 14, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Meng, X.; Song, Z.; Lin, J. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) as a Potential Therapeutic Target for Vitiligo. Arch Biochem Biophys 2020, 696, 108670. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and Biological Impact of the Exposome on the Skin. J Eur Acad Dermatol Venereol 2020, 34, 4–25. [Google Scholar] [CrossRef]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr Med Chem 2018, 25, 5469–5486. [Google Scholar] [CrossRef] [PubMed]
- Zamarrón, A.; Lorrio, S.; González, S.; Juarranz, Á. Fernblock Prevents Dermal Cell Damage Induced by Visible and Infrared A Radiation. Int J Mol Sci 2018, 19, 2250. [Google Scholar] [CrossRef]
- Truchuelo, M.T.; Jiménez, N.; Dí as, I.J.; Gallego-Rentero, M.; Alonso-Juarranz, M.; Gonzalez, S. A Pilot Study to Assess the Effects of an Oral Photo Protector of Botanical Origin against Visible and Infrared Radiations in Human Volunteers. J Dermatol Dis 2019, 6, 1–3. [Google Scholar]
- Granger, C.; Aladren, S.; Delgado, J.; Garre, A.; Trullas, C.; Gilaberte, Y. Prospective Evaluation of the Efficacy of a Food Supplement in Increasing Photoprotection and Improving Selective Markers Related to Skin Photo-Ageing. Dermatol Ther (Heidelb) 2020, 10, 163–178. [Google Scholar] [CrossRef]
- Bay, E.Y.; Topal, I.O. Aging Skin and Anti-Aging Strategies. Explor Res Hypothesis Med 2022. [Google Scholar] [CrossRef]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am J Clin Dermatol 2021, 22, 819. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, J.Y.; Martinez, R.M.; Morocho-Jácome, A.L.; Castillo-Gómez, T.S.; Pereda-Contreras, V.J.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Skin Impacts from Exposure to Ultraviolet, Visible, Infrared, and Artificial Lights – a Review. J Cosmet Laser Ther 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Lee, L.Y.; Liu, S.X. Pathogenesis of Photoaging in Human Dermal Fibroblasts. Int J Dermatol Venereol 2021, 37–42. [Google Scholar] [CrossRef]
- Tanveer, M.A.; Rashid, H.; Tasduq, S.A. Molecular Basis of Skin Photoaging and Therapeutic Interventions by Plant-Derived Natural Product Ingredients: A Comprehensive Review. Heliyon 2023, 9, e13580. [Google Scholar] [CrossRef] [PubMed]
- Bharadvaja, N.; Gautam, S.; Singh, H. Natural Polyphenols: A Promising Bioactive Compounds for Skin Care and Cosmetics. Mol Biol Rep 2023, 50, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Valencia, S.; Mejía-Giraldo, J.C.; Puertas-Mejía, M.Á. Algae Metabolites as an Alternative in Prevention and Treatment of Skin Problems Associated with Solar Radiation and Conventional Photo-Protection. Braz J Pharm Sci 2023, 58, e201046. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Garcia-Baeza, A.; Vidal-Limon, H.R.; Balderas-Renteria, I.; Ramírez-Cabrera, M.A.; Ramirez-Estrada, K. Plant Secondary Metabolites against Skin Photodamage: Mexican Plants, a Potential Source of UV-Radiation Protectant Molecules. Plants 2022, Vol. 11, Page 220 2022, 11, 220. [Google Scholar] [CrossRef]
- González, S.; Parrado, C.; Juarranz, Á. Introduction to Special Issue “Recent Advances in Skin Anti-Aging Agents. ” Plast Aesthet Res 2022, 9, 32. [Google Scholar] [CrossRef]
- Cândido, T.M.; Ariede, M.B.; Lima, F.V.; de Guedes, L.S.; Velasco, M.V.R.; Baby, A.R.; Rosado, C. Dietary Supplements and the Skin: Focus on Photoprotection and Antioxidant Activity—A Review. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural Products and Extracts from Plants as Natural UV Filters for Sunscreens: A Review. Animal Model Exp Med 2022. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Lorz, L.R.; Lee, J.; Cho, J.Y. In Vitro Photoprotective, Anti-Inflammatory, Moisturizing, and Antimelanogenic Effects of a Methanolic Extract of Chrysophyllum Lucentifolium Cronquist. Plants 2022, Vol. 11, Page 94 2021, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.G.; Kim, N. Dietary Supplements in Dermatology: A Review of the Evidence for Zinc, Biotin, Vitamin D, Nicotinamide, and Polypodium. J Am Acad Dermatol 2021, 84, 1042–1050. [Google Scholar] [CrossRef]
- Torres, A.E.; Luk, K.M.; Lim, H.W. Botanicals for Photoprotection. Plast Aesthet Res 2020, 7, 57. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Calzavara-Pinton, I.; Arisi, M.; Rossi, M.T.; Scapagnini, G.; Davinelli, S.; Venturini, M. Cutaneous Photoprotective Activity of a Short-Term Ingestion of High-Flavanol Cocoa: A Nutritional Intervention Study. Photochem Photobiol 2019, 95, 1029–1034. [Google Scholar] [CrossRef]
- Tsuruta, Y.; Katsuyama, Y.; Okano, Y.; Ozawa, T.; Yoshimoto, S.; Ando, H.; Masaki, H.; Ichihashi, M. Possible Involvement of Dermal Fibroblasts in Modulating Nrf2 Signaling in Epidermal Keratinocytes. Biol Pharm Bull 2023, 46, 725–729. [Google Scholar] [CrossRef]
- Tabolacci, E.; Tringali, G.; Nobile, V.; Duca, S.; Pizzoferrato, M.; Bottoni, P.; Maria Elisabetta, C. Rutin Protects Fibroblasts from UVA Radiation through Stimulation of Nrf2 Pathway. Antioxidants 2023, 12. [Google Scholar] [CrossRef]
- Gonzalez, S.; Gilaberte, Y.; Philips, N.; Juarranz, A. Fernblock, a Nutriceutical with Photoprotective Properties and Potential Preventive Agent for Skin Photoaging and Photoinduced Skin Cancers. Int J Mol Sci 2011, 12, 8466–8475. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim Biophys Acta Mol Basis Dis 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Jeayeng, S.; Wongkajornsilp, A.; Slominski, A.T.; Jirawatnotai, S.; Sampattavanich, S.; Panich, U. Nrf2 in Keratinocytes Modulates UVB-Induced DNA Damage and Apoptosis in Melanocytes through MAPK Signaling. Free Radic Biol Med 2017, 108, 918. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.A.; Rashid, H.; Nazir, L.A.; Archoo, S.; Shahid, N.H.; Ragni, G.; Umar, S.A.; Tasduq, S.A. Trigonelline, a Plant Derived Alkaloid Prevents Ultraviolet-B-Induced Oxidative DNA Damage in Primary Human Dermal Fibroblasts and BALB/c Mice via Modulation of Phosphoinositide 3-Kinase-Akt-Nrf2 Signalling Axis. Exp Gerontol 2023, 171, 112028. [Google Scholar] [CrossRef] [PubMed]
- Kobaisi, F.; Fayyad, N.; Rezvani, H.R.; Fayyad-Kazan, M.; Sulpice, E.; Badran, B.; Fayyad-Kazan, H.; Gidrol, X.; Rachidi, W. Signaling Pathways, Chemical and Biological Modulators of Nucleotide Excision Repair: The Faithful Shield against UV Genotoxicity. Oxid Med Cell Longev 2019, 2019. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.; Gong, Q.; Cheng, Z.; Ran, C.; Liu, K.; Shi, C. Cold Atmospheric Plasma Alleviates Radiation-Induced Skin Injury by Suppressing Inflammation and Promoting Repair. Free Radic Biol Med 2023, 204, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Rojo De La Vega, M.; Krajisnik, A.; Zhang, D.D.; Wondrak, G.T. Nutrients Targeting NRF2 for Improved Skin Barrier Function and Photoprotection: Focus on the Achiote-Derived Apocarotenoid Bixin. 2017. [Google Scholar] [CrossRef]
- Yokoi, K.; Yasumizu, Y.; Ohkura, N.; Shinzawa, K.; Okuzaki, D.; Shimoda, N.; Ando, H.; Yamada, N.; Fujimoto, M.; Tanemura, A. Increased Anti-Oxidative Action Compensates for Collagen Tissue Degeneration in Vitiligo Dermis. Pigment Cell Melanoma Res 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





