Submitted:
20 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Imaging for diagnosis
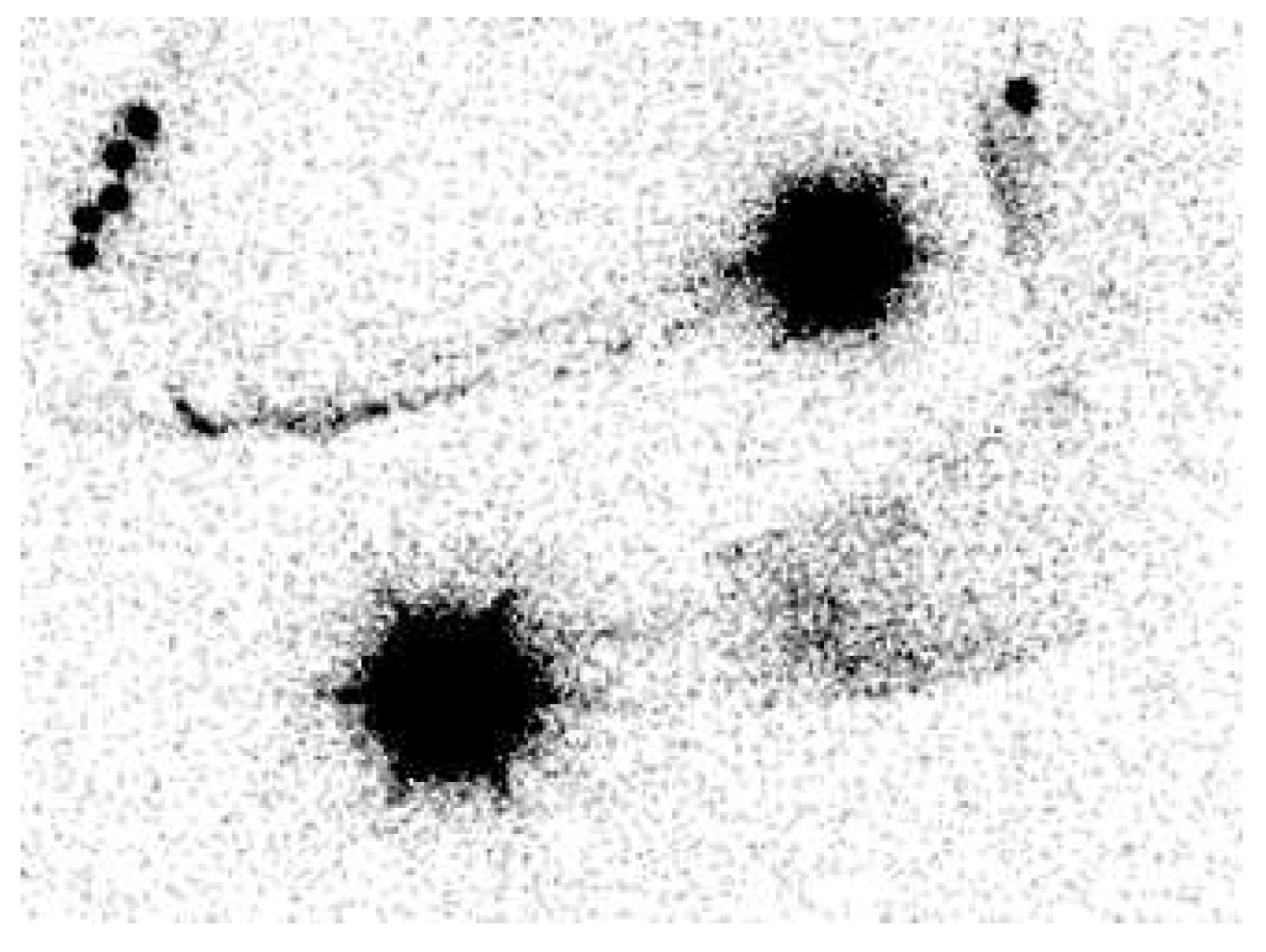
2.1. Lymphoscintigraphy
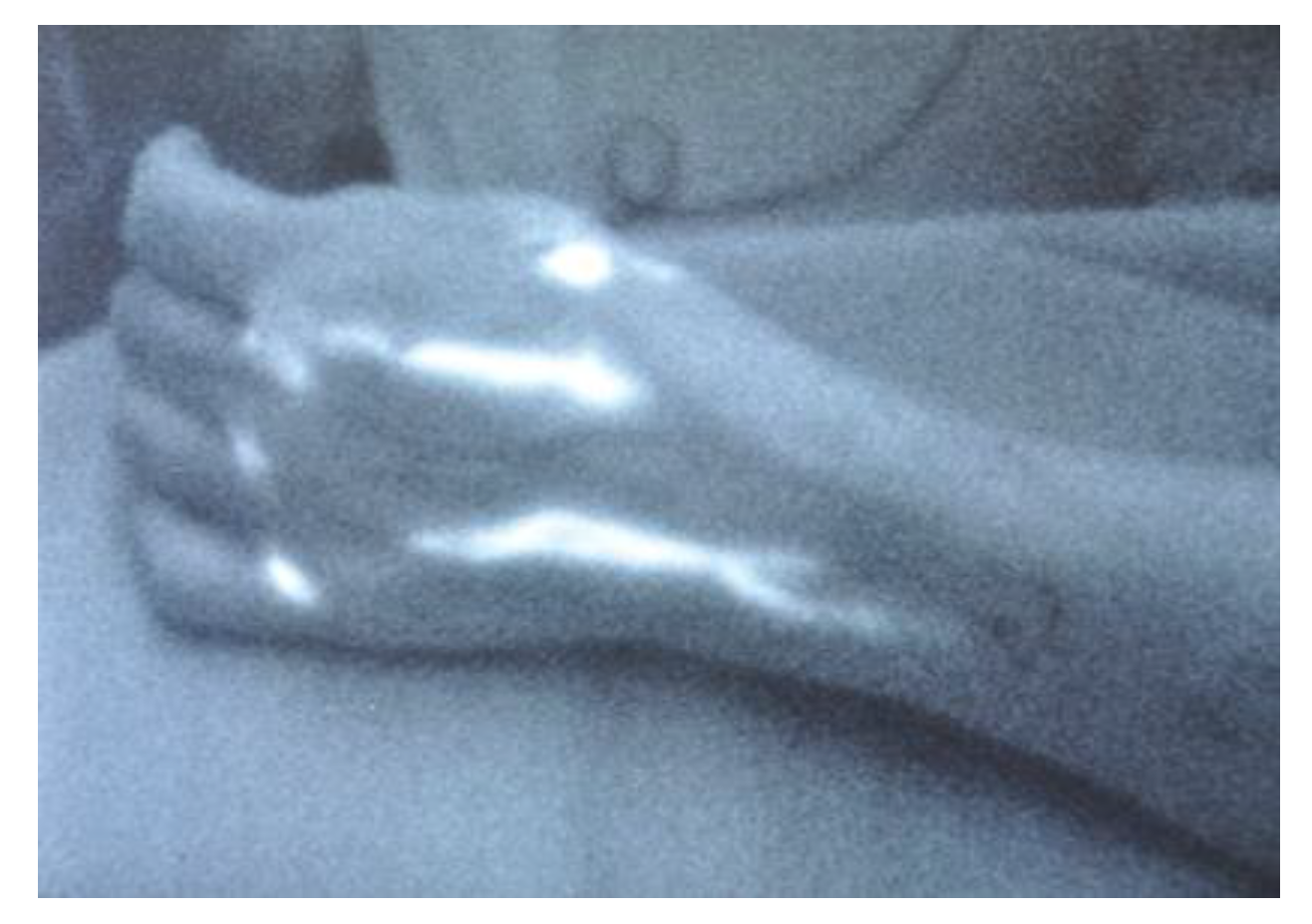
2.2. Indocyanine green lymphography
3. Imaging for treatment
3.1. Indocyanine green lymphography
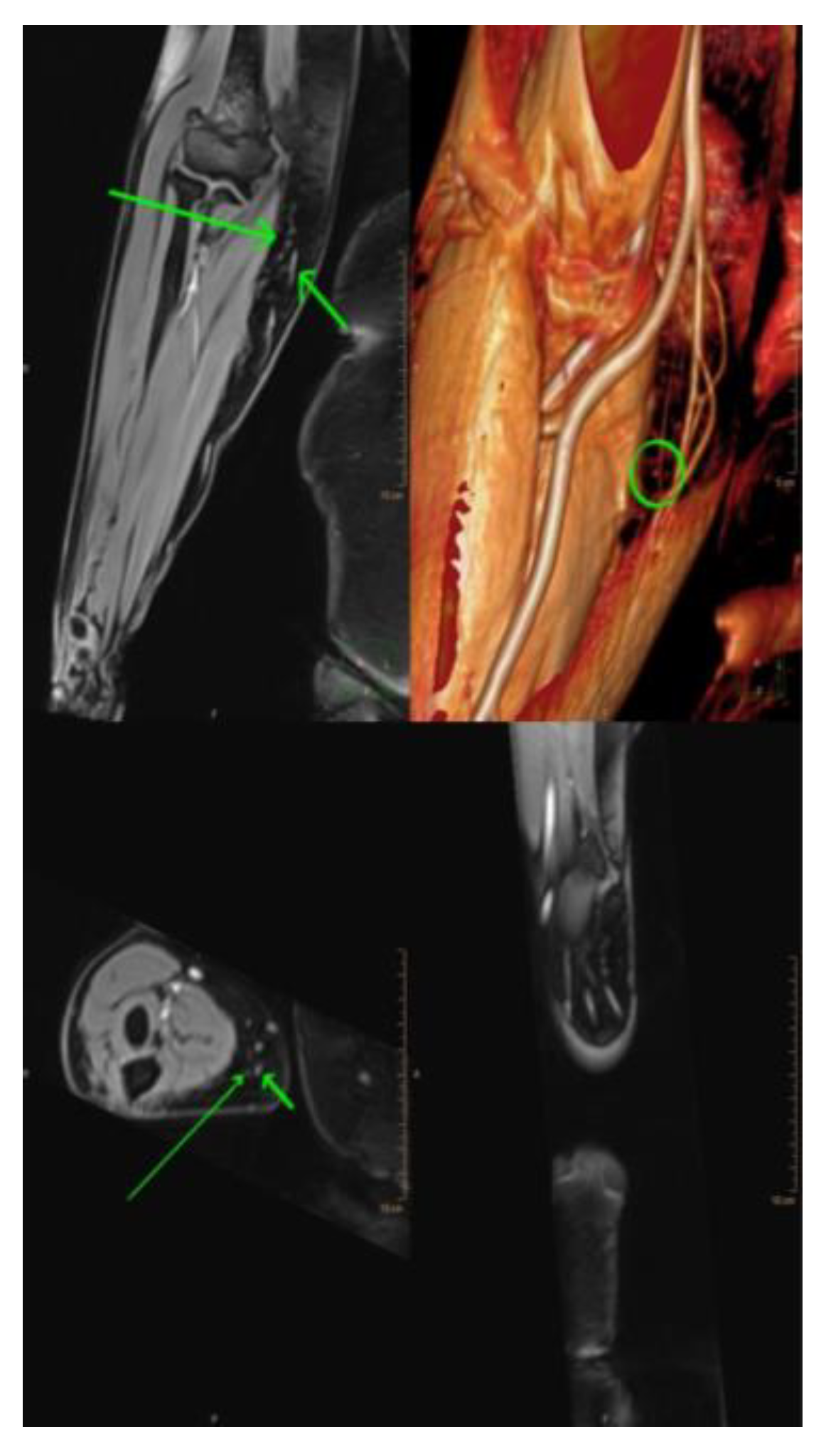
3.2. Magnetic resonance lymphangiography (MRL)
3.3. Single-photon emission computed tomography/computed tomography (SPECT/CT)
3.4. Ultra high-frequency ultrasound (UHF-US)
3.5. Photoacoustic (PA) or optoacoustic imaging
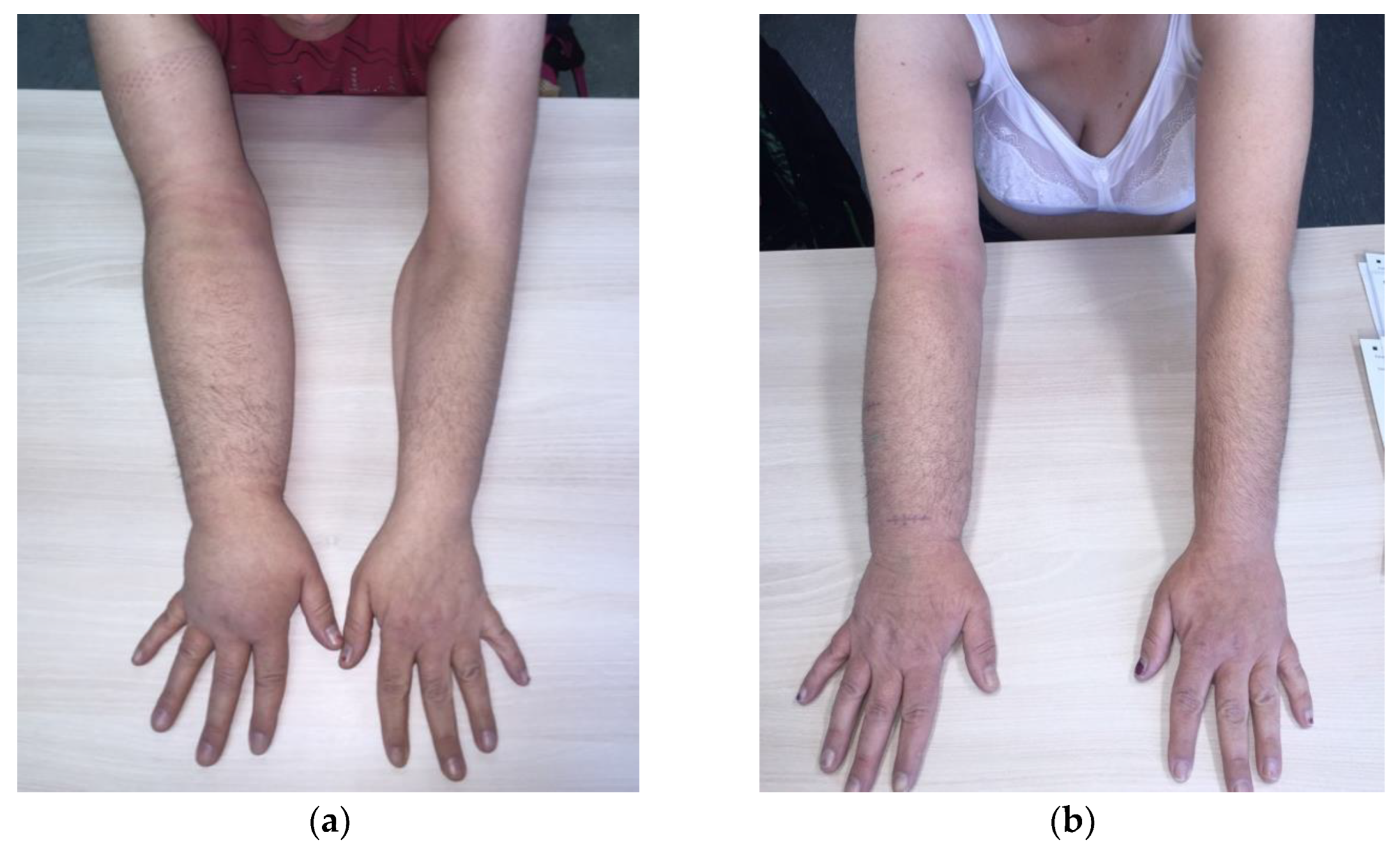
4. Surgical treatment
4.1. Debulking procedures
4.1.1. Suction-assisted lipectomy (SAL)
4.1.2. Direct excision
4.2. Physiologic procedures
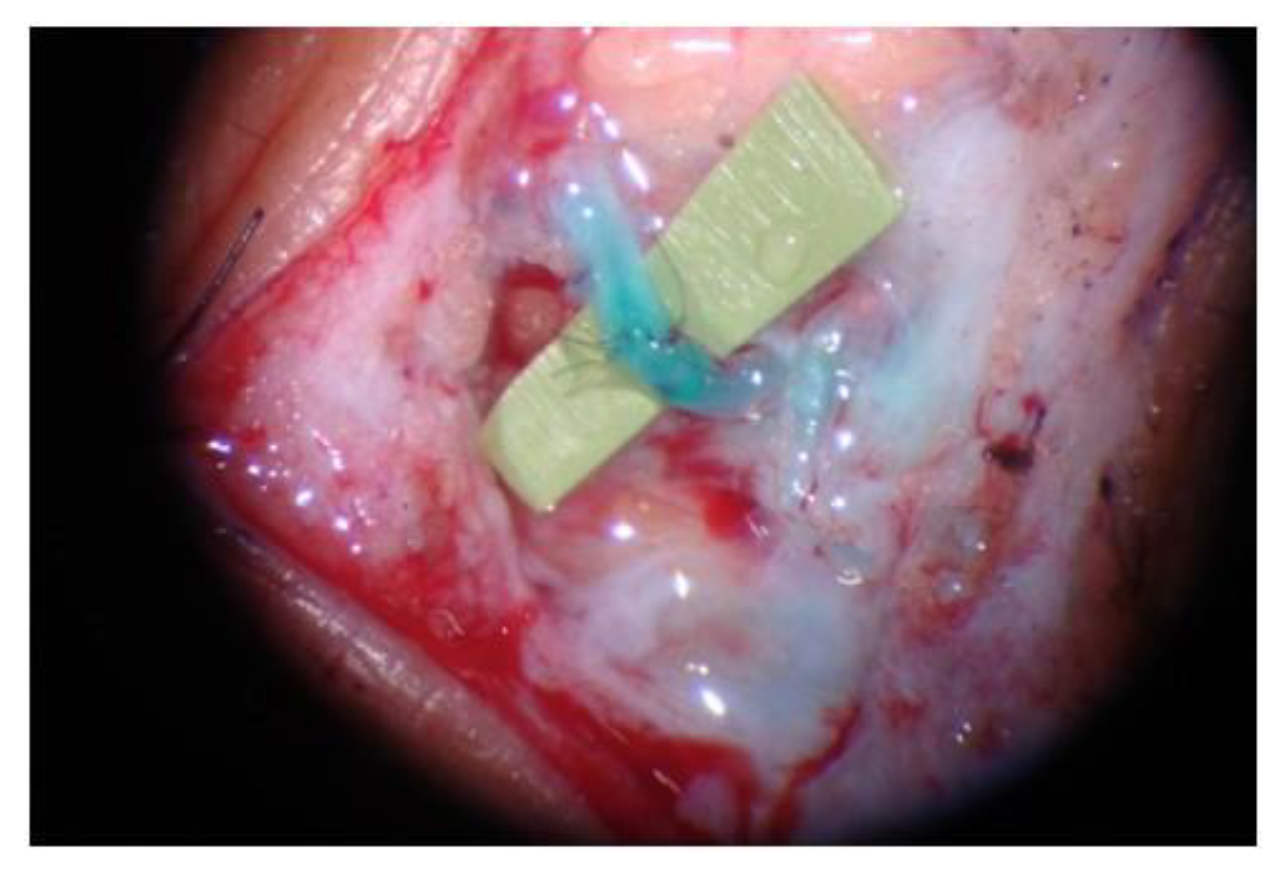
4.2.1. LVA
4.2.2. VLNT
4.2.2.1. Groin lymph node flap
4.2.2.2. Lateral thoracic lymph node flap
4.2.2.3. Supraclavicular lymph node flap
4.2.2.4. Submental lymph node flap
4.2.2.5. Omental lymph node flap
4.2.2.6. Jejunal mesenteric lymph node flaps
4.3. Preventive lymphatic surgery (LYMPHA approach) [63]
5. Discussion
6. Conclusion
Supplementary Materials
Funding
Informed Consent Statement
Conflicts of Interest
References
- Zeltzer AA, Anzarut A, Hamdi M. A Review of Lymphedema for the Hand and Upper-Extremity Surgeon. J Hand Surg Am 2018, 43, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Zeltzer AA, Brussaard C, Koning M, et al. MR lymphography in patients with upper limb lymphedema: The GPS for feasibility and surgical planning for lympho-venous bypass. J Surg Oncol 2018, 118, 407–415. [Google Scholar] [CrossRef]
- Will PA, Wan Z, Seide SE, et al. Supermicrosurgical treatment for lymphedema: a systematic review and network meta-analysis protocol. Syst Rev 2022, 11, 18. [Google Scholar] [CrossRef]
- Winters H, Tielemans HJP, Paulus V, Hummelink S, Slater NJ, Ulrich DJO. A systematic review and meta-analysis of vascularized lymph node transfer for breast cancer-related lymphedema. J Vasc Surg Venous Lymphat Disord 2022, 10, 786–795.e1. [Google Scholar] [CrossRef] [PubMed]
- Beederman M, Garza RM, Agarwal S, Chang DW. Outcomes for Physiologic Microsurgical Treatment of Secondary Lymphedema Involving the Extremity. Ann Surg 2022, 276, e255–e263. [Google Scholar] [CrossRef]
- Verhey EM, Kandi LA, Lee YS, et al. Outcomes of Lymphovenous Anastomosis for Lower Extremity Lymphedema: A Systematic Review. Plast Reconstr Surg Glob Open 2022, 10, e4529. [Google Scholar] [CrossRef]
- Knackstedt R, Chen WF. Current Concepts in Surgical Management of Lymphedema. Phys Med Rehabil Clin N Am 2022, 33, 885–899. [Google Scholar] [CrossRef]
- Beederman M, Chang DW. Advances in surgical treatment of lymphedema. Arch Plast Surg 2021, 48, 670–677. [Google Scholar] [CrossRef]
- Pons G, Clavero JA, Alomar X, Rodríguez-Bauza E, Tom LK, Masia J. Preoperative planning of lymphaticovenous anastomosis: The use of magnetic resonance lymphangiography as a complement to indocyanine green lymphography. J Plast Reconstr Aesthet Surg 2019, 72, 884–891. [Google Scholar] [CrossRef]
- Chang EI, Chu CK, Chang EI. Advancements in imaging technology for microvascular free tissue transfer. J Surg Oncol 2018, 118, 729–735. [Google Scholar] [CrossRef]
- Akita S, Unno N, Maegawa J, et al. A phase III, multicenter, single-arm study to assess the utility of indocyanine green fluorescent lymphography in the treatment of secondary lymphedema. J Vasc Surg Venous Lymphat Disord 2022, 10, 728–737.e3. [Google Scholar] [CrossRef] [PubMed]
- Guerrini S, Gentili F, Mazzei FG, Gennaro P, Volterrani L, Mazzei MA. Magnetic resonance lymphangiography: with or without contrast? Diagn Interv Radiol 2020, 26, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Park KE, Allam O, Chandler L, et al. Surgical management of lymphedema: a review of current literature. Gland Surg 2020, 9, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Dayan JH, Wiser I, Verma R, et al. Regional Patterns of Fluid and Fat Accumulation in Patients with Lower Extremity Lymphedema Using Magnetic Resonance Angiography. Plast Reconstr Surg 2020, 145, 555–563. [Google Scholar] [CrossRef]
- Forte AJ, Boczar D, Huayllani MT, et al. Use of magnetic resonance imaging lymphangiography for preoperative planning in lymphedema surgery: A systematic review. Microsurgery 2021, 41, 384–390. [Google Scholar] [CrossRef]
- Gentileschi S, Albanese R, Pino V, et al. SPECT/CT and fusion ultrasound to target the efferent groin lymph node for lymphatic surgery. Microsurgery 2019, 39, 605–612. [Google Scholar] [CrossRef]
- Du X, Liu C. Application of imaging in lymphedema surgical therapies. Gland Surg 2020, 9, 582–588. [Google Scholar] [CrossRef]
- Iimura T, Fukushima Y, Kumita S, Ogawa R, Hyakusoku H. Estimating Lymphodynamic Conditions and Lymphovenous Anastomosis Efficacy Using (99m)Tc-phytate Lymphoscintigraphy with SPECT-CT in Patients with Lower-limb Lymphedema. Plast Reconstr Surg Glob Open 2015, 3, e404. [Google Scholar] [CrossRef]
- Weiss M, Baumeister RG, Frick A, Wallmichrath J, Bartenstein P, Rominger A. Primary lymphedema of the lower limb: the clinical utility of single photon emission computed tomography/CT. Korean J Radiol 2015, 16, 188–195. [Google Scholar] [CrossRef]
- Hayashi A, Giacalone G, Yamamoto T, et al. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast Reconstr Surg Glob Open 2019, 7, e2086. [Google Scholar] [CrossRef]
- van Heumen S, Riksen JJM, Bramer WM, van Soest G, Vasilic D. Imaging of the Lymphatic Vessels for Surgical Planning: A Systematic Review. Ann Surg Oncol 2023, 30, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Bianchi A, Visconti G, Hayashi A, Santoro A, Longo V, Salgarello M. Ultra-High frequency ultrasound imaging of lymphatic channels correlates with their histological features: A step forward in lymphatic surgery. J Plast Reconstr Aesthet Surg 2020, 73, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Suzuki Y, Kajita H, Watanabe S, et al. Application of Photoacoustic Imaging for Lymphedema Treatment. J Reconstr Microsurg 2022, 38, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Suzuki Y, Kajita H, Watanabe S, et al. Surgical Applications of Lymphatic Vessel Visualization Using Photoacoustic Imaging and Augmented Reality. J Clin Med 2021, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Gallagher K, Marulanda K, Gray S. Surgical Intervention for Lymphedema. Surg Oncol Clin N Am 2018, 27, 195–215. [Google Scholar] [CrossRef]
- Schaverien MV, Coroneos CJ. Surgical Treatment of Lymphedema. Plast Reconstr Surg 2019, 144, 738–758. [Google Scholar] [CrossRef]
- Greene AK, Maclellan RA. Operative Treatment of Lymphedema Using Suction-Assisted Lipectomy. Ann Plast Surg 2016, 77, 337–340. [Google Scholar] [CrossRef]
- Greene AK, Maclellan R. Management of Lymphedema with Suction-Assisted Lipectomy. Plastic and Reconstructive Surgery 2014, 134, 36. [Google Scholar] [CrossRef]
- Van der Walt JC, Perks TJ, Zeeman BJ, Bruce-Chwatt AJ, Graewe FR. Modified Charles procedure using negative pressure dressings for primary lymphedema: a functional assessment. Ann Plast Surg 2009, 62, 669–675. [Google Scholar] [CrossRef]
- Carl HM, Walia G, Bello R, et al. Systematic Review of the Surgical Treatment of Extremity Lymphedema. J Reconstr Microsurg 2017, 33, 412–425. [Google Scholar] [CrossRef]
- Gupta R, Mathijs E, Hart J, Bates J, Powers J, Chaiyasate K. May-Thurner Syndrome and Lymphedema Reconstruction. Plast Reconstr Surg Glob Open 2022, 10, e4377. [Google Scholar] [CrossRef] [PubMed]
- Chang DW, Masia J, Garza R, 3rd, Skoracki R, Neligan PC. Lymphedema: Surgical and Medical Therapy. Plast Reconstr Surg 2016, 138, 209s–218s. [Google Scholar] [CrossRef] [PubMed]
- Hanson SE, Chang EI, Schaverien MV, Chu C, Selber JC, Hanasono MM. Controversies in Surgical Management of Lymphedema. Plast Reconstr Surg Glob Open 2020, 8, e2671. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni MF, Fontein DBY, Arvanitakis M, Giovanoli P. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery 2017, 37, 947–953. [Google Scholar] [CrossRef]
- de Sire A, Losco L, Lippi L, et al. Surgical Treatment and Rehabilitation Strategies for Upper and Lower Extremity Lymphedema: A Comprehensive Review. Medicina (Kaunas) 2022, 58, 954. [Google Scholar] [CrossRef]
- Martins de Carvalho F, Almeida A, Silva Á, Marques M. Treatment of Lymphorrhea Associated with an Amputation Stump with Lymphaticovenular Anastomosis. Acta Med Port 2022, 35, 384–387. [Google Scholar] [CrossRef]
- Yang JC-S, Yen Y-H, Wu S-C, Lin W-C, Chiang M-H, Hsieh C-H. Supermicrosurgical Lymphaticovenous Anastomosis as an Alternative Treatment Option for Patients with Lymphorrhea. Plastic and Reconstructive Surgery 2019, 144, 1214–1224. [Google Scholar] [CrossRef]
- Cheng MH, Huang JJ, Wu CW, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg 2014, 133, 192e–198e. [Google Scholar] [CrossRef]
- De Brucker B, Zeltzer A, Seidenstuecker K, Hendrickx B, Adriaenssens N, Hamdi M. Breast Cancer-Related Lymphedema: Quality of Life after Lymph Node Transfer. Plast Reconstr Surg 2016, 137, 1673–1680. [Google Scholar] [CrossRef]
- Scaglioni MF, Arvanitakis M, Chen YC, Giovanoli P, Chia-Shen Yang J, Chang EI. Comprehensive review of vascularized lymph node transfers for lymphedema: Outcomes and complications. Microsurgery 2018, 38, 222–229. [Google Scholar] [CrossRef]
- Zeltzer AA, Anzarut A, Braeckmans D, et al. The vascularized groin lymph node flap (VGLN): Anatomical study and flap planning using multi-detector CT scanner. The golden triangle for flap harvesting. J Surg Oncol 2017, 116, 378–383. [Google Scholar] [CrossRef]
- Vignes S, Blanchard M, Yannoutsos A, Arrault M. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg 2013, 45, 516–520. [Google Scholar] [CrossRef]
- Sulo E, Hartiala P, Viitanen T, Mäki M, Seppänen M, Saarikko A. Risk of donor-site lymphatic vessel dysfunction after microvascular lymph node transfer. J Plast Reconstr Aesthet Surg 2015, 68, 551–558. [Google Scholar] [CrossRef]
- Viitanen TP, Mäki MT, Seppänen MP, Suominen EA, Saaristo AM. Donor-site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg 2012, 130, 1246–1253. [Google Scholar] [CrossRef]
- Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015, 135, 277–285. [Google Scholar] [CrossRef]
- Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg 2006, 243, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Chang EI, Masià J, Smith ML. Combining Autologous Breast Reconstruction and Vascularized Lymph Node Transfer. Semin Plast Surg 2018, 32, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I. Optimizing Treatment of Breast Cancer Related Lymphedema Using Combined DIEP Flap and Lymphedema Surgery. Arch Plast Surg 2022, 49, 150–157. [Google Scholar] [CrossRef]
- Schaverien MV, Badash I, Patel KM, Selber JC, Cheng MH. Vascularized Lymph Node Transfer for Lymphedema. Semin Plast Surg 2018, 32, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Barreiro GC, Baptista RR, Kasai KE, et al. Lymph fasciocutaneous lateral thoracic artery flap: anatomical study and clinical use. J Reconstr Microsurg 2014, 30, 389–396. [Google Scholar] [CrossRef]
- Tinhofer IE, Meng S, Steinbacher J, et al. The surgical anatomy of the vascularized lateral thoracic artery lymph node flap-A cadaver study. J Surg Oncol 2017, 116, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Coroneos CJ, Asaad M, Wong FC, et al. Outcomes and technical modifications of vascularized lymph node transplantation from the lateral thoracic region for treatment of lymphedema. J Surg Oncol 2022, 125, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Visconti G, Bianchi A, Salgarello M, et al. Lymphatic Tissue Transfer: Ultrasound-Guided Description and Preoperative Planning of Vascularised Lymph Nodes, Lymphatic Units, and Lymphatic Vessels Transfers. J Pers Med 2022, 12. [Google Scholar] [CrossRef]
- Maldonado AA, Chen R, Chang DW. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: A prospective study of 100 consecutive cases. J Surg Oncol 2017, 115, 68–71. [Google Scholar] [CrossRef]
- Mazerolle P, Meresse T, Gangloff D, Kolsi K, Dupret-Bories A. Vascularized lymph node transfer with submental free flap. Eur Ann Otorhinolaryngol Head Neck Dis 2020, 137, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Paulus VAA, Winters H, Hummelink S, Schulten S, Ulrich DJO, Vasilic D. Submental flap for vascularized lymph node transfer; a CTA-based study on lymph node distribution. J Surg Oncol 2020, 122, 1226–1231. [Google Scholar] [CrossRef]
- Tzou CH, Meng S, Ines T, et al. Surgical anatomy of the vascularized submental lymph node flap: Anatomic study of correlation of submental artery perforators and quantity of submental lymph node. J Surg Oncol 2017, 115, 54–59. [Google Scholar] [CrossRef]
- Gustafsson J, Chu SY, Chan WH, Cheng MH. Correlation between Quantity of Transferred Lymph Nodes and Outcome in Vascularized Submental Lymph Node Flap Transfer for Lower Limb Lymphedema. Plast Reconstr Surg 2018, 142, 1056–1063. [Google Scholar] [CrossRef]
- Danforth R, Skoracki R. Intra-abdominal donors for vascularized lymph node transfer: an update and review. Plastic and Aesthetic Research 2021, 8, 48. [Google Scholar] [CrossRef]
- Kenworthy EO, Nelson JA, Verma R, Mbabuike J, Mehrara BJ, Dayan JH. Double vascularized omentum lymphatic transplant (VOLT) for the treatment of lymphedema. J Surg Oncol 2018, 117, 1413–1419. [Google Scholar] [CrossRef]
- Nguyen AT, Suami H, Hanasono MM, Womack VA, Wong FC, Chang EI. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J Surg Oncol 2017, 115, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kraft CT, Eiferman D, Jordan S, Skoracki RJ. Complications after vascularized jejunal mesenteric lymph node transfer: A 3-year experience. Microsurgery 2019, 39, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: a new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol 2009, 16, 703–708. [Google Scholar] [CrossRef]
- Ciudad P, Escandón JM, Bustos VP, Manrique OJ, Kaciulyte J. Primary Prevention of Cancer-Related Lymphedema Using Preventive Lymphatic Surgery: Systematic Review and Meta-analysis. Indian J Plast Surg 2022, 55, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Viviano SL, Neligan PC. Updates on Excisional Procedures for Lymphedema. Adv Wound Care (New Rochelle) 2022, 11, 419–427. [Google Scholar] [CrossRef]
- Chang DW, Dayan J, Greene AK, et al. Surgical Treatment of Lymphedema: A Systematic Review and Meta-Analysis of Controlled Trials. Results of a Consensus Conference. Plast Reconstr Surg 2021, 147, 975–993. [Google Scholar] [CrossRef]
| Imaging modalities | Advantages | Limitations |
|---|---|---|
| LSG |
|
|
| ICG-L |
|
|
| MRL |
|
|
| SPECT/CT |
|
|
| UHF-US |
|
|
| PA imaging |
|
|
| VLNT | Advantages | Limitations |
|---|---|---|
| Groin flap |
|
|
| Lateral thoracic flap |
|
|
| Supraclavicular flap |
|
|
| Submental flap |
|
|
| Omental flap |
|
|
| Jejunal flap |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





