Submitted:
16 June 2023
Posted:
19 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Manifestations of Our 80 Participants
3.2. Our 22 Candidate Genes
3.3. Case Reports
3.3.1. Case 1: Ryan
3.3.2. Case 2: Spencer
3.3.3. Case 3: Lily
3.3.4. Case 4: Liliana
3.3.5. Case 5: Sydney
3.3.6. Case 6: Madeline
3.3.7. Case 7: Kate
3.3.8. Case 8: Mollie
4. Discussion
4.1. Clinical Pearls from the Case Reports - Phenotypic Heterogeneity
4.2. Clinical Pearls from the Case Reports - Genotypic Heterogeneity
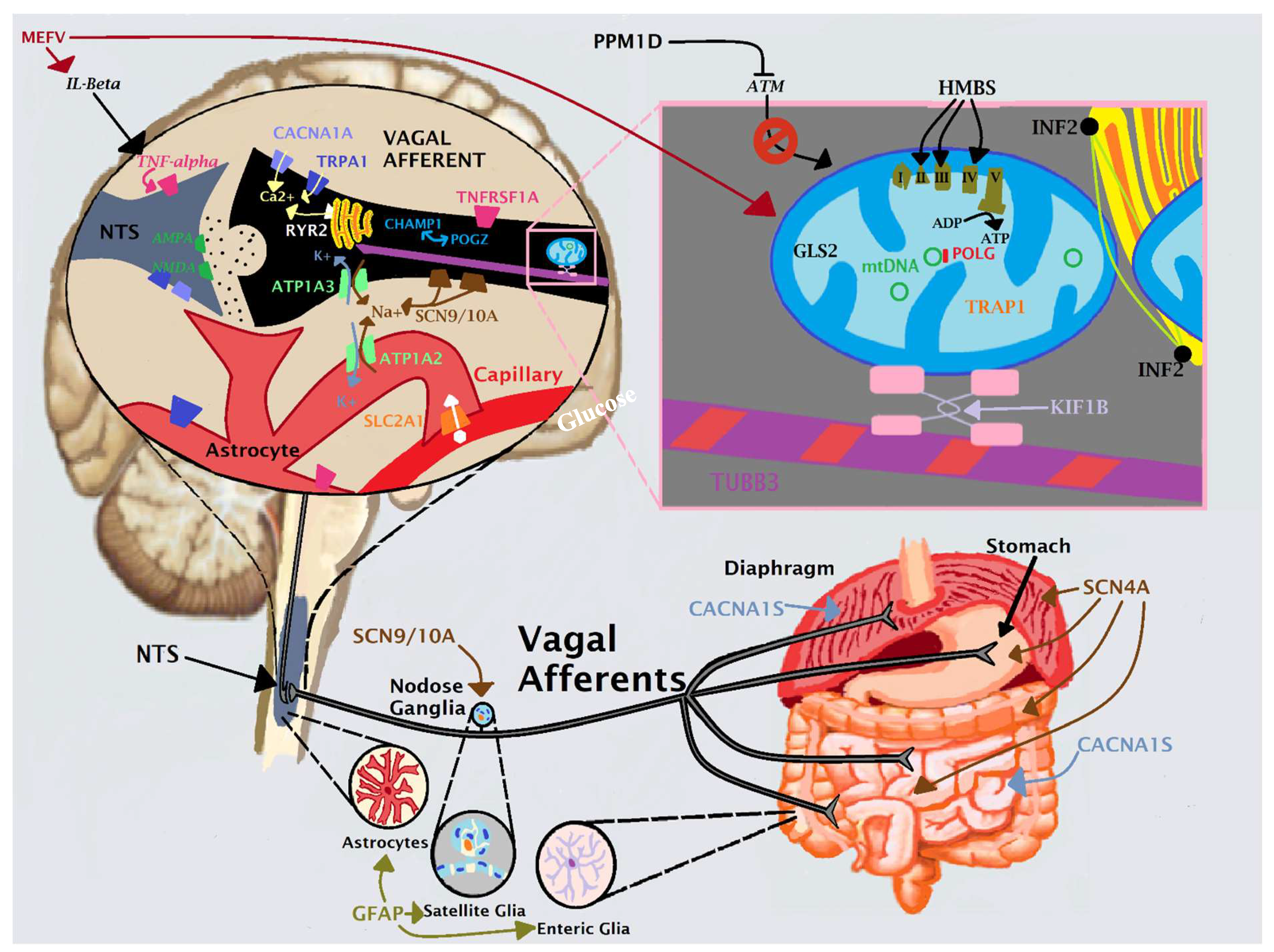
4.3. An Anatomical Model for CVS: Vagal Afferent Unit
4.4. Treatment of CVS as Informed by Our Models and Case Reports
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bar, O. , Ebenau, L., Weiner, K., Mintz, M. and Boles, R. (2023). Whole exome/genome sequencing in cyclic vomiting syndrome reveals multiple candidate genes, suggesting a model of elevated intracellular cations and mitochondrial dysfunction. Frontiers in Neurology, 14(1664-2295). [CrossRef]
- Rome IV Criteria - Rome Foundation. Available online: https://theromefoundation.org/rome-iv /rome-iv-criteria/ (Accessed 21 October 2020).
- Abu-Arafeh, I. and Russell, G., 1995. Cyclical vomiting syndrome in children: a population-based study. Journal of pediatric gastroenterology and nutrition, 21(4), pp.454-458. [CrossRef]
- Li, B.U., Lefevre, F., Chelimsky, G.G., Boles, R.G., Nelson, S.P., Lewis, D.W., Linder, S.L., Issenman, R.M. and Rudolph, C.D., 2008. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition consensus statement on the diagnosis and management of cyclic vomiting syndrome. Journal of pediatric gastroenterology and nutrition, 47(3), pp.379-393. [CrossRef]
- Venkatesan, T., Levinthal, D.J., Tarbell, S.E., Jaradeh, S.S., Hasler, W.L., Issenman, R.M., Adams, K.A., Sarosiek, I., Stave, C.D., Sharaf, R.N. and Sultan, S., 2019. Guidelines on management of cyclic vomiting syndrome in adults by the American Neurogastroenterology and Motility Society and the Cyclic Vomiting Syndrome Association. Neurogastroenterology & Motility, 31, p.e13604. [CrossRef]
- Li, B.U.K., Murray, R.D., Heitlinger, L.A., Robbins, J.L. and Hayes, J.R., 1999. Is cyclic vomiting syndrome related to migraine?. The Journal of pediatrics, 134(5), pp.567-572. [CrossRef]
- Enokizono, T., Nemoto, K., Fujiwara, J., Tanaka, R. and Ohto, T., 2017. Cyclic vomiting syndrome after acute autonomic and sensory neuropathy. Pediatrics International: Official Journal of the Japan Pediatric Society, 59(4), pp.503-505. [CrossRef]
- Sato, T., Igarashi, N., Minami, S., Okabe, T., Hashimoto, H., Hasui, M. and Kato, E., 1988. Recurrent attacks of vomiting, hypertension and psychotic depression: a syndrome of periodic catecholamine and prostaglandin discharge. European Journal of Endocrinology, 117(2), pp.189-197. [CrossRef]
- Boles, R. G., Adams, K. and Li, B. U. K., 2005. Maternal inheritance in cyclic vomiting syndrome. American Journal of Medical Genetics Part A, 133(1), pp.71-77. [CrossRef]
- Lee, J., Wong, S. A., Li, B. U. K. and Boles, R. G., 2015. NextGen nuclear DNA sequencing in cyclic vomiting syndrome reveals a significant association with the stress-induced calcium channel (RYR2). Neurogastroenterology & Motility, 27(7), pp.990-996. [CrossRef]
- Wasilewski, A., Lewandowska, U., Mosinska, P., Watala, C., Storr, M., Fichna, J. and Venkatesan, T., 2017. Cannabinoid receptor type 1 and mu-opioid receptor polymorphisms are associated with cyclic vomiting syndrome. Official journal of the American College of Gastroenterology| ACG, 112(6), pp.933-939. [CrossRef]
- Fleisher, D. R., Gornowicz, B., Adams, K., Burch, R. and Feldman, E. J., 2005. Cyclic vomiting syndrome in 41 adults: the illness, the patients, and problems of management. BMC medicine, 3(1), pp.1-12. [CrossRef]
- Abell, T.L., Adams, K.A., Boles, R.G., Bousvaros, A., Chong, S.K.F., Fleisher, D.R., Hasler, W.L., Hyman, P.E., Issenman, R.M., Li, B.U.K. and Linder, S.L., 2008. Cyclic vomiting syndrome in adults. Neurogastroenterology & Motility, 20(4), pp.269-284. [CrossRef]
- Boles, R. G., Powers, A. L. and Adams, K., 2006. Cyclic vomiting syndrome plus. Journal of child neurology, 21(3), pp.182-189. [CrossRef]
- Wang, Q., Ito, M., Adams, K., Li, B.U., Klopstock, T., Maslim, A., Higashimoto, T., Herzog, J. and Boles, R.G., 2004. Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. American Journal of Medical Genetics Part A, 131(1), pp.50-58. [CrossRef]
- Zaki, E.A., Freilinger, T., Klopstock, T., Baldwin, E.E., Heisner, K.R.U., Adams, K., Dichgans, M., Wagler, S. and Boles, R.G., 2009. Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia, 29(7), pp.719-728. [CrossRef]
- Landrum, M.J., Lee, J.M., Benson, M., Brown, G., Chao, C., Chitipiralla, S., Gu, B., Hart, J., Hoffman, D., Hoover, J. and Jang, W., 2016. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic acids research, 44(D1), pp.D862-D868. [CrossRef]
- Boles, R.G., 2011. High degree of efficacy in the treatment of cyclic vomiting syndrome with combined co-enzyme Q10, L-carnitine and amitriptyline, a case series. BMC neurology, 11(1), pp.1-5. [CrossRef]
- Fan, C., Mao, N., Lehmann-Horn, F., Bürmann, J. and Jurkat-Rott, K., 2017. Effects of S906T polymorphism on the severity of a novel borderline mutation I692M in Na v 1.4 cause periodic paralysis. Clinical Genetics, 91(6), pp.859-867. [CrossRef]
- Kuzmenkin, A., Jurkat-Rott, K., Lehmann-Horn, F. and Mitrovic, N., 2003. Impaired slow inactivation due to a polymorphism and substitutions of Ser-906 in the II-III loop of the human Na v 1.4 channel. Pflügers Archiv, 447, pp.71-77. [CrossRef]
- Binda, A., Renna, L.V., Bosè, F., Brigonzi, E., Botta, A., Valaperta, R., Fossati, B., Rivolta, I., Meola, G. and Cardani, R., 2018. SCN4A as modifier gene in patients with myotonic dystrophy type 2. Scientific reports, 8(1), pp.1-10. [CrossRef]
- Karczewski, K.J., Francioli, L.C., Tiao, G., Cummings, B.B., Alföldi, J., Wang, Q., Collins, R.L., Laricchia, K.M., Ganna, A., Birnbaum, D.P. and Gauthier, L.D., 2020. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature, 581(7809), pp.434-443. [CrossRef]
- Pirone, A., Schredelseker, J., Tuluc, P., Gravino, E., Fortunato, G., Flucher, B.E., Carsana, A., Salvatore, F. and Grabner, M., 2010. Identification and functional characterization of malignant hyperthermia mutation T1354S in the outer pore of the Cavα1S-subunit. American Journal of Physiology-Cell Physiology, 299(6), pp.C1345-C1354. [CrossRef]
- Zhou, Y., Fang, L., Jiang, L., Wen, P., Cao, H., He, W., Dai, C. and Yang, J., 2012. Uric acid induces renal inflammation via activating tubular NF-κB signaling pathway. PloS one, 7(6), p.e39738. [CrossRef]
- Fejzo, M.S., Myhre, R., Colodro-Conde, L., MacGibbon, K.W., Sinsheimer, J.S., Reddy, M.P.L., Pajukanta, P., Nyholt, D.R., Wright, M.J., Martin, N.G. and Engel, S.M., 2017. Genetic analysis of hyperemesis gravidarum reveals association with intracellular calcium release channel (RYR2). Molecular and cellular endocrinology, 439, pp.308-316. [CrossRef]
- Nozawa, R.S., Nagao, K., Masuda, H.T., Iwasaki, O., Hirota, T., Nozaki, N., Kimura, H. and Obuse, C., 2010. Human POGZ modulates dissociation of HP1α from mitotic chromosome arms through Aurora B activation. Nature cell biology, 12(7), pp.719-727. [CrossRef]
- Assia Batzir, N., Posey, J.E., Song, X., Akdemir, Z.C., Rosenfeld, J.A., Brown, C.W., Chen, E., Holtrop, S.G., Mizerik, E., Nieto Moreno, M. and Payne, K., 2020. Phenotypic expansion of POGZ-related intellectual disability syndrome (White-Sutton syndrome). American Journal of Medical Genetics Part A, 182(1), pp.38-52. [CrossRef]
- Human DNA Polymerase Gamma Mutation Database. Available online: https://tools.niehs.nih.gov/polg/index.cfm/main/reference (Accessed 01 November 2022).
- Jansen, S., Geuer, S., Pfundt, R., Brough, R., Ghongane, P., Herkert, J.C., Marco, E.J., Willemsen, M.H., Kleefstra, T., Hannibal, M. and Shieh, J.T., 2017. De novo truncating mutations in the last and penultimate exons of PPM1D cause an intellectual disability syndrome. The American Journal of Human Genetics, 100(4), pp.650-658. [CrossRef]
- Breitbart, R.E., Liang, C.S., Smoot, L.B., Laheru, D.A., Mahdavi, V. and Nadal-Ginard, B., 1993. A fourth human MEF2 transcription factor, hMEF2D, is an early marker of the myogenic lineage. Development, 118(4), pp.1095-1106. [CrossRef]
- Rashid, A.J., Cole, C.J. and Josselyn, S.A., 2014. Emerging roles for MEF2 transcription factors in memory. Genes, Brain and Behavior, 13(1), pp.118-125. [CrossRef]
- Clauw, D.J. and Chrousos, G.P., 1997. Chronic pain and fatigue syndromes: overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmunomodulation, 4(3), pp.134-153. [CrossRef]
- Eccles, J.A. and Davies, K.A., 2021. The challenges of chronic pain and fatigue. Clinical Medicine, 21(1), p.19. [CrossRef]
- Steinsvik, E.K., Hausken, T., Fluge, Ø., Mella, O. and Gilja, O.H.., 2023. Gastric dysmotility and gastrointestinal symptoms in myalgic encephalomyelitis/chronic fatigue syndrome. Scandinavian Journal of Gastroenterology, pp.1-8. [CrossRef]
- Andrews, P., Hoyle, C. H. V., Ngoka, T. and Smith, G. E., 2003. Differences in the vagal innervation of the oesophagus may explain the lack of emesis in rodents. Neurogastroenterol Motil, 15, pp.28.
- Andrews, P. L. and Horn, C. C., 2006. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Autonomic Neuroscience, 125(1-2), pp.100-115. [CrossRef]
- Ricardo, J. A. and Koh, E. T., 1978. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain research, 153(1), pp.1-26. [CrossRef]
- Hejazi, R. A. and McCallum, R. W., 2011. Review article: cyclic vomiting syndrome in adults–rediscovering and redefining an old entity. Alimentary pharmacology & therapeutics, 34(3), pp.263-273. [CrossRef]
- Ellingsen, D.M., Garcia, R.G., Lee, J., Lin, R.L., Kim, J., Thurler, A.H., Castel, S., Dimisko, L., Rosen, B.R., Hadjikhani, N. and Kuo, B., 2017. Cyclic Vomiting Syndrome is characterized by altered functional brain connectivity of the insular cortex: a cross-comparison with migraine and healthy adults. Neurogastroenterology & Motility, 29(6), p.e13004. [CrossRef]
- Kawai, Y., 2018. Differential ascending projections from the male rat caudal nucleus of the tractus solitarius: an interface between local microcircuits and global macrocircuits. Frontiers in Neuroanatomy, 12, 63. [CrossRef]
- Fleisher, D.R., 1997. Cyclic vomiting syndrome: a paroxismal disorder of brain-gut interaction. Journal of pediatric gastroenterology and nutrition, 25, pp.13-15. [CrossRef]
- Taché, Y., 1999. Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Digestive diseases and sciences, 44(8 Suppl), pp.79S-86S.
- Taché, Y., Martinez, V., Million, M. and Wang, L., 2001. III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. American Journal of Physiology-Gastrointestinal and Liver Physiology, 280(2), pp.G173-G177. [CrossRef]
- Ammar, T., & Renaud, J. M. (2015). Diaphragm of hyperkalemic periodic paralysis mouse has no contractility abnormality compared to the robust abnormalities in EDL and soleus. The FASEB Journal, 29, 947-8. [CrossRef]
- Schuster, F., Johannsen, S., Isbary, S., Türkmeneli, I., & Roewer, N. (2018). In vitro effects of levosimendan on muscle of malignant hyperthermia susceptible and non-susceptible swine. BMC anesthesiology, 18(1), 1-5. [CrossRef]
- Fung, C., & Vanden Berghe, P. (2020). Functional circuits and signal processing in the enteric nervous system. Cellular and Molecular Life Sciences, 77(22), 4505-4522. 22. [CrossRef]
- Spear, E. T., & Mawe, G. M. (2019). Enteric neuroplasticity and dysmotility in inflammatory disease: key players and possible therapeutic targets. American Journal of Physiology-Gastrointestinal and Liver Physiology, 317(6), G853-G861. [CrossRef]
- Morales-Soto, W. and Gulbransen, B.D., 2019. Enteric glia: a new player in abdominal pain. Cellular and molecular gastroenterology and hepatology, 7(2), pp.433-445. [CrossRef]
- Grundmann, D., Loris, E., Maas-Omlor, S., Huang, W., Scheller, A., Kirchhoff, F., & Schäfer, K. H. (2019). Enteric glia: S100, GFAP, and beyond. The Anatomical Record, 302(8), 1333-1344. [CrossRef]
- Mayer, E. A., Aziz, Q., Coen, S., Kern, M., Labus, J. S., Lane, R., ... & Tracey, I. (2009). Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterology & Motility, 21(6), 579-596. [CrossRef]
- Van Calcar, Sandra C., Cary O. Harding, and Jon A. Wolff. L-carnitine administration reduces number of episodes in cyclic vomiting syndrome. Clinical pediatrics 41.3 (2002): 171-174. [CrossRef]
- Boles, R. G., Lovett-Barr, M. R., Preston, A., Li, B. U., & Adams, K. (2010). Treatment of cyclic vomiting syndrome with co-enzyme Q10 and amitriptyline, a retrospective study. BMC neurology, 10(1), 1-5. [CrossRef]
- Tillman, E. M., & Harvath, E. M. (2022). Cyclic Vomiting Syndrome in Pediatric Patients: A Review of Therapeutics. The Journal of Pediatric Pharmacology and Therapeutics, 27(1), 12-18. [CrossRef]
- Li, B. U. (2018). Managing cyclic vomiting syndrome in children: beyond the guidelines. European journal of pediatrics, 177(10), 1435-1442. [CrossRef]
- Ala, M., Jafari, R.M., Ala, M., Agbele, A.T., Hejazi, S.M., Tavangar, S.M., Mahdavi, S.R.M. and Dehpour, A.R., 2020. Sumatriptan alleviates radiation-induced oral mucositis in rats by inhibition of NF-kB and ERK activation, prevention of TNF-α and ROS release. Archives of oral biology, 119, p.104919. [CrossRef]
- Gharishvandi, F., Abdollahi, A., Shafaroodi, H., Jafari, R.M., Pasalar, P. and Dehpour, A.R., 2020. Involvement of 5-HT1B/1D receptors in the inflammatory response and oxidative stress in intestinal ischemia/reperfusion in rats. European journal of pharmacology, 882, p.173265. [CrossRef]
- Leenhardt, A., Denjoy, I. and Guicheney, P., 2012. Catecholaminergic polymorphic ventricular tachycardia. Circulation: Arrhythmia and Electrophysiology, 5(5), pp.1044-1052. [CrossRef]
- Taverna, S., Sancini, G., Mantegazza, M., Franceschetti, S. and Avanzini, G., 1999. Inhibition of transient and persistent Na+ current fractions by the new anticonvulsant topiramate. Journal of Pharmacology and Experimental Therapeutics, 288(3), pp.960-968.
- Zhang, X.L., Velumian, A.A., Jones, O.T. and Carlen, P.L., 2000. Modulation of high-voltage–activated calcium channels in dentate granule cells by topiramate. Epilepsia, 41, pp.52-60. [CrossRef]
- Biton, V., 2007. Clinical pharmacology and mechanism of action of zonisamide. Clinical neuropharmacology, 30(4), pp.230-240. [CrossRef]
- Brodie, M.J., Ben-Menachem, E., Chouette, I. and Giorgi, L., 2012. Zonisamide: its pharmacology, efficacy and safety in clinical trials. Acta neurologica scandinavica, 126, pp.19-28. [CrossRef]
- Deshpande, L.S. and DeLorenzo, R.J., 2014. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Frontiers in neurology, 5, p.11. [CrossRef]
- Wang, S.J., Sihra, T.S. and Gean, P.W., 2001. Lamotrigine inhibition of glutamate release from isolated cerebrocortical nerve terminals (synaptosomes) by suppression of voltage-activated calcium channel activity. Neuroreport, 12(10), pp.2255-2258. [CrossRef]
- Gerner, P., Haderer, A.E., Mujtaba, M., Sudoh, Y., Narang, S., Abdi, S., Srinivasa, V., Pertl, C. and Kuo Wang, G., 2003. Assessment of differential blockade by amitriptyline and its N-methyl derivative in different species by different routes. The Journal of the American Society of Anesthesiologists, 98(6), pp.1484-1490. [CrossRef]
- Schiavon, C.R., Shadel, G.S. and Manor, U., 2021. Impaired mitochondrial mobility in Charcot-Marie-Tooth disease. Frontiers in Cell and Developmental Biology, 9, p.624823. [CrossRef]
- Massaro, A.M. and Lenz, K.L., 2005. Aprepitant: a novel antiemetic for chemotherapy-induced nausea and vomiting. Annals of Pharmacotherapy, 39(1), pp.77-85. [CrossRef]
- Currow, D.C., Stuart-Harris, R.C. and Noble, P.D., 1995. The clinical use of ondansetron. Medical journal of Australia, 162(3), pp.145-149.
- Chelimsky, T. C. and Chelimsky, G. G., 2007. Autonomic abnormalities in cyclic vomiting syndrome. Journal of pediatric gastroenterology and nutrition, 44(3), pp.326-330. [CrossRef]
| Sex | Female: 46, Male: 34 |
|---|---|
| Race/Ethnicity | Western Eurasian: 61 (European (EU, 1 patient had distant Finnish ancestry): 51, Ashkenazi (AS): 3, Middle Eastern: (ME) 1, EU/AS: 5, EU/ME: 1), South Asian (SA): 3, African American (AA): 2, East Asian (EA): 2, Latin American (LA): 1, EU/LA: 6, EU/SA: 2, EU/EA: 2, AA/LA: 1 |
| Age of Onset | < 1 year: 14, 1-3 years: 11, 3-6: 7, 6-12: 12, 12-18: 21, > 18: 7, NR: 8 |
| Episode Triggers | None: 30, Stress/anxiety: 26, Fatigue/exhaustion: 26, Illness: 22, Temperature: 9, Food: 7, Menses: 6, Overexcitement: 5, Other: 141 |
| Prodrome2 | None: 50, Pain: 15, Sensory: 6, Behavioral: 5, Other: 181 |
| Manifestations During Episodes | Nausea and Vomiting 803, None: 7, Fatigue/Lethargy: 62, Pain: 48, Sensory: 36, Dysautonomia: 28, GI: 22, Muscular: 22, Neuro: 12, Behavioral: 4, Other: 231 |
| Episode Frequency | < 2 weeks: 16, 2-5 weeks: 28, 5-9: 9, 9-13: 8, 13-26: 8, > 26: 2, Varies substantially: 6, NR: 3 |
| Episode Duration | < 4 hours: 10, 4-12 hours: 10, 12-24: 5, 24-48: 11, 48-96: 11, 96-168: 13, > 168: 5, Varies: 11, NR: 4 |
| Pain (60/80, 75%) | Headache: 51 (migraine 39/51), Joints: 22, Abdomen: 20, Extremities: 20, Neck/back: 18, Muscles: 10, Throat/lymph nodes: 7, Chest: 7, Pelvic: 32 |
|---|---|
| Gastrointestinal (58/80, 72.5%) | Bowel3: 42, Nausea: 30, GERD: 10, Other: 202 |
| Dysautonomia (57/80, 71.25%) | Temperature intolerance4: 35, POTS-like manifestations5: 32, Unexplained fevers: 5, Other: 82 |
| Allergy & Immunity (33/80, 41.25%) | Immunodeficiency6: 16, Pruritis: 15, Rash: 12 (urticaria 5/12), Asthma: 9, MCAS: 4, Other: 32 |
| Sleep (45/80, 56%) | Parasomnia: 28, Insomnia: 21, Hypersomnia: 3, Other: 12 |
| Psychological (54/80, 68%) | Anxiety: 51, Depression: 22 (suicide attempt 2/22), Mood instability: 3, Other: 52 |
| Neurological7 (47/80, 68%) | Muscular: 18 (hypotonia 8, muscle weakness 5, other 53), Tinnitus: 13, Abnormal movements: 14 (tics 7, tremors 5, other 43), Sensory 13 (photophobia 8, aversions 1, other 2), Ophthalmological: 8, Paresthesia: 7, Seizures: 7, Ataxia: 7, Neuropathy: 5, Periodic paralysis: 5, Other: 172 |
| Neurodevelopmental (31/80, 40%) | ADHD: 14, ASD: 10, Learning disability: 8, Intellectual disability: 7, Other: 22 |
| Fatigue (56/80, 70%) | |
| Urinary (20/80, 25%) | Frequency: 11, Hesitancy: 4, Enuresis: 4, Dysuria: 3, Other: 82 |
| Connective (9/80, 11%) | Hypermobility: 6, Ehlers-Danlos syndrome: 3 |
| Endocrine (10/80, 12.5%) | Thyroid: 5, Other: 52 |
| Other (21/80, 26.25%) | Heme: 8, Syndromic: 3, Cardiac: 3, Other: 72 |
| Gene | Points by Our Study | Points by Literature | Composite | Expression, with Emphasis per Our Model | Protein Function |
| ATP1A2 | 1 | 21 | Muscle, brain, and glia (mainly astrocytes) | Maintains the electrochemical gradients of Na and K ions across the plasma membrane; requires ATP | |
| ATP1A3 | 0 | 26 | Most prominently in GABA interneurons; in vagal afferents | Maintains the electrochemical gradients of Na and K ions across the plasma membrane; requires ATP | |
| CACNA1A | 19 | 14 | In most CNS synapses including the NTS; also in vagal afferents | Transmembrane pore-forming subunit of a voltage-gated calcium channel | |
| CACNA1S | 17 | 0 | Skeletal muscle (e.g., diaphragm) | Transmembrane pore-forming subunit of a voltage-gated calcium channel | |
| CHAMP1 | 1 | 6 | Ubiquitous (essentially uniform in all cell types) | Enables chromosome segregation. Associated with expression of genes involving cations and neurotransmitter transport | |
| GFAP | 3 | 18 | Glial cells only | Intermediate filaments in glia. Related to regulation of voltage-gated Na+, K+ and Ca2+ channels | |
| HMBS | 0 | 6 | Ubiquitous | Intermediate step in heme production, which is necessary for cytochrome production in the mitochondrial respiratory chain | |
| MEFV | 12 | 2 | Ubiquitous, yet higher in blood cells | Modulates innate immunity. Promotes inflammasome formation and maturation of IL-beta, which can induce TNF signaling | |
| OTC | 0 | 54 | Ubiquitous, yet higher in liver | Mitochondrial matrix enzyme involved in the urea cycle to detoxify ammonia into urea for excretion | |
| POGZ | 7 | 10 | Ubiquitous | Regulates mitotic progression. Interacts with CHAMP1 and thus may regulate cation transport | |
| POLG | 9 | 2 | Ubiquitous, highest in muscle & nerve | Replication and proof-reading of mitochondrial DNA | |
| PPM1D | 3 | 8 | Ubiquitous, including nervous tissue | Regulates the DNA damage response through p53 & ATM. ATM affects mitochondrial homeostasis & modulates mitophagy. | |
| RYR2 | 15 | 4 | Highest in heart and nerve, including brain and vagus | Stress-activated calcium channel on the endoplasmic reticulum | |
| SCN4A | 20 | 0 | Skeletal muscle (e.g., diaphragm), also in brain | Transmembrane pore-forming subunit of a voltage-gated sodium channel | |
| SCN9A | 8 | 0 | Ubiquitous, high in sensory neurons (e.g., in viscera, vagus) | Transmembrane pore-forming subunit of a voltage-gated sodium channel | |
| SCN10A | 9 | 0 | Ubiquitous, including in sensory neurons (e.g., vagus) | Transmembrane pore-forming subunit of a voltage-gated sodium channel | |
| SLC2A1 | 0 | 11 | Brain astrocytes, placenta, and erythrocytes | Major glucose transporter across the blood-brain barrier | |
| TNFRSF1A | 10 | 0 | Ubiquitous including brain, vagus, and glia | Binds TNF-alpha, important in inflammation; involved in mitophagy and glial/neuronal excitation; binds TRAP1 | |
| TRAP1 | 14 | 2 | Mitochondria; ubiquitous, including brain, vagus, and glia | Mitochondrial chaperone protein induced in times of oxidative stress; involved in mitophagy | |
| TRPA1 | 7 | 0 | Ubiquitous, highest in gut, also present in nerve (e.g., vagus) | Non-specific cation channel involved in perception of pain, cold, itch, sound, and oxygen concentration | |
| TUBB3 | 0 | 9 | Highest in brain, present in vagus | A beta tubulin protein that forms microtubules in neurons | |
| mtDNA | - | - | Ubiquitous, highest in nerve and muscle | Encodes subunits of the respiratory chain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




