1. Introduction
Ichthyoses are a group of heterogeneous inborn diseases with the main feature of a defective epidermal barrier that causes hyperkeratosis, skin scaling, and inflammation, affecting the skin only (non-syndromic) or associated with internal organ disorders (syndromic). Further division is based on the manifestations at birth (congenital forms) or during the first year of life (vulgar forms). In 2009, the First Ichthyosis Consensus Conference established an international consensus on the nomenclature and classification of inherited ichthyoses [
1]. Further advances in diagnosis and treatment are primarily related to advances in clinical diagnostic tools, molecular testing, and genetic therapy [
2,
3,
4].
Common forms of ichthyosis, ichthyosis vulgaris (IV), and recessive X-linked ichthyosis (RXLI) are characterized by a prevalence of IV (1:250–1000) and RXLI (1:2000–6000), respectively [
1,
4,
5,
6,
7]. Form IV (OMIM 146700), which shows semi-dominant inheritance, is the mildest form, characterized by scaling, xerosis, pruritus, and eczema. Symptoms usually manifest in the first few years of life. IV is characterized by frequent atopic manifestations [
1,
2,
4]. RXLI (OMIM 308100) is the second most frequent type of ichthyosis and represents a benign form of ichthyosis that affects men almost exclusively. It develops because of the accumulation of undegraded cholesterol sulfate, which is responsible for scale formation during steroid sulfatase deficiency [
1,
2,
4]. RXLI is associated with the occurrence of pre-Descemet’s membrane corneal dystrophy (PDCD), which is described in 50% of patients [
1]. Dystrophy is characterized by the accumulation of polymorphic grey hyperreflective opacities located anterior to Descemet’s membrane on slit-lamp examination. In vivo confocal microscopy (IVCM) has revealed enlarged hyperreflective keratocytes with extracellular deposits in the posterior corneal stroma [
8,
9,
10,
11].
Autosomal recessive congenital ichthyosis (ARCI) is a heterogeneous group of recurrent inherited disorders with congenital ichthyosis but no extracutaneous involvement [
1,
3]. Its prevalence is estimated to be 1 in 100,000 individuals. ARCI refers to harlequin ichthyosis (HI), lamellar ichthyosis (LI), and congenital ichthyosiform erythroderma (CIE). Most phenotypes are severe; however, minor variants exist, such as the self-healing collodion baby (SHCB) and bathing suit ichthyosis (BSI). ARCI is caused by mutations in more than a dozen different genes, including TGM1 (Transglutaminase-1), ABCA12 (ATP-binding cassette sub-family A member 12), CYP4F22 (Cytochrome P450 4F22), ALOXE3/ALOX12B (epidermal lipoxygenase-3 /12R-lipoxygenase), NIPAL4 (magnesium transporter NIPA4), CERS3 (ceramide synthase-3), SDR9C7 (short-chain dehydrogenase/reductase family 9C member 7), PNPLA1 (patatin-like phospholipase domain-containing protein 1), SLC27A4 (long-chain fatty acid transport protein 4), and LIPN (epidermal lipase N) [
4].
HI (OMIM 242500) is a severe condition that is associated with increased perinatal mortality. The clinical features of HI include gray or yellowish thick scales with severe collodion membranes, extreme ectropion and eclabium, contractures, a broadened nose, synechiae of the auricles, and sometimes, toes. HI is caused by a loss-of-function mutation in the adenosine triphosphate (ATP)-binding cassette subfamily A member 12 (ABCA12), which leads to lipid transport disruption in keratinizing keratinocytes in the upper epidermis [
1,
4,
12]. The LI phenotype (OMIM 242300) can be milder than that of harlequin and phenotypic heterogeneity may exist. Generally, the phenotype is characterized by generalized large brownish or dark scales, often combined with palmoplantar keratoderma, ectropion, and anhidrosis [
1,
2,
3,
4]. Bathing suit ichthyosis (BSI) is a minor variant of LI that is caused by mutations in TGM1. Patients develop a scaling pattern that only affects the trunk and warmer skin areas such as the axillary region or scalp [
13,
14].
Although each variant of ichthyosis has its own characteristics and intensity of abnormalities, ocular involvement is regarded as a major feature of the disease. There is a broad range of ocular findings described in the literature, mainly involving the ocular surface and anterior eye segment, but there are limited reports on the association of ichthyosis with glaucoma, optic neuropathy, coloboma of the iris, choroid, and retina, and crystalline macular dystrophy [
15,
16,
17].
Ocular surface involvement includes a wide variety of symptoms, such as the scaling of eyelids, cicatricial ectropion, madarosis, entropion, chronic keratoconjunctivitis, meibomian gland dysfunction, epithelial corneal defect, punctate keratitis, corneal ulcer, scarring and perforation, limbal stem cells deficiency and neovascularization, band keratopathy, PDCD, corneal irregularity, thinning and keratoconus [
16,
17,
18,
19,
20].
Ocular findings in ichthyosis are usually based on slit-lamp examinations, relatively small patient sample sizes, or case reports. However, in recent years, multimodal imaging in ophthalmology combining topography, pachymetry, corneal biomechanics, and morphology data has provided significant new insights into the diagnosis of ocular surface, corneal, and anterior eye segment diseases, especially in cases of rare congenital diseases, such as corneal dystrophies [
8,
21,
22].
Anterior eye segment optical coherence tomography (AS OCT) is a precise, contactless technique for obtaining high-resolution ocular tissue images. Swept-source optical coherence tomography (SS-OCT), introduced in 2005, is a next-generation Fourier-domain OCT with a scanning speed of 50,000 A-scans/s and axial resolution of 10 μm. It allows the observation of anterior eye segment morphology, as well as topography, pachymetry, and Fourier indices analysis [
23,
24,
25].
In vivo confocal microscopy (IVCM) enables in vivo corneal imaging with an axial resolution of 1 μm. IVCM is widely used to analyze the microscopic structure of the corneal layers, from the epithelium to the endothelium. This technique is useful for several clinical conditions, including infectious keratitis, dry eye disease (DED), corneal dystrophies, and degenerations [
26,
27].
We present a report on the clinical features of patients with various ichthyoses based on multimodal imaging, including ocular surface assessment, SS-OCT, and IVCM findings. By combining these imaging methods, it was possible to further characterize the appearance of the ocular surface, determine the depth of changes, and provide a profound understanding of the features associated with different variants of ichthyosis.
3. Results
The study group consisted of five patients (P1-P5) aged 13–66 years (three females and two males. BCVA ranged from light perception (LP) to 1,0 (median 0,25). Additionally, genetic analysis results were obtained from six family members of patients P1 and P5 (aged 10–58 years; four females and two males), and slit-lamp examination results were obtained from five family members (aged 10–58 years; three females and two males).
3.1. General demographic information and medical history
A summary of demographic and general medical history data is presented in
Table 1.
The family history was positive in P1, who was diagnosed with LI, and P4, who was diagnosed with HI. The patient’s P1 female sibling was also affected but was not included in the study group (no consent). The patient’s P4 male sibling died shortly after birth because of ichthyotic complications. Otherwise, no diagnosis of ichthyosis was made in any family member in the study group.
ARCI was diagnosed based on dermatological consultation in all patients except P3, who was diagnosed with IV (P1, P2 – LI, P4 – HI, P5 – BSI, LI minor variant).
Two patients had genetic confirmation of the diagnosis (P1 and P5), and genetic analysis results were obtained from six family members. Next-generation sequencing (NGS) was performed using a MiSeq sequencer (Illumina, San Diego, CA, USA) with the SeqCap EZ HyperCap protocol and the NimbleGen SeqCap EZ probe kit (Roche Sequencing Solutions, Inc., Basel, Switzerland). NGS analyses with a mean read coverage of 121,1x. The results were confirmed by Sanger sequencing. The Human Genome Variation Society nomenclature (HGVS v15,11) was used to describe revealed mutations. To analyze TGM1, a reference sequence with accession number NM_000359.3 (HGMD) was used. The known homozygous, pathogenic variant c.[943C>T](p.Arg315Cys) was found following TGM1 analysis in P5 ( proband). TGM1 sequencing of both parents and the proband’s sister revealed the same variant in a heterozygous state. The known heterogeneous mutation c.579G>A (p.Trp193Ter) on one allele and c.1135G>C (p.Val379Leu) in the second allele of TGM1 gene was found in patient P1 (proband). The patient’s P1 sister (18 years old) with the LI phenotype presented with identical genetic results. Moreover, the patient’s P1 mother was identified with the mutation p.Trp193Ter in one allele and the P1 father was identified with p.Val379Leu in one allele of the TGM1 gene.
3.2. Slit lamp findings
Severe involvement of the eyelids with cicatricial ectropion of the upper and lower eyelids was present in two patients (P2 and P4) diagnosed with LI and HI. P1, diagnosed with LI, had ectropion in the lower eyelids, whereas the upper eyelids were spared. The eyelids of P3 diagnosed with IV and those of P5, diagnosed with BSI, had normal anatomy. All patients presented with hyperkeratinization of the lid margin, with scaling and crusting at the base of the lashes at different severity levels (
Figure 1). Patients P1, P2, and P4 were severely affected, with keratinization crossing the lid margin towards the conjunctival fornices. P3 had moderate involvement compared to P1, P2, and P4 while scaling and keratinization were mild in P5.
All patients were diagnosed with moderate-to-severe DED. Abnormal TBUT and fluorescein staining were observed in all eyes. A summary of TBUT and fluorescein staining results are presented in
Table 2. Exposure keratopathy with corneal scarring and peripheral vascularization of different severity due to ectropion was diagnosed in patients P1, P2, and P4. P2, with bilateral ectropion, severe eyelid deformation, and exposure keratopathy, was diagnosed with a diffuse corneal scar after corneal perforation in the temporal paracentral quadrant, anterior iris synechiae, shallow anterior eye chamber, and cataract (
Figure 1b,c). Notably, corneal scarring with peripheral vascularization and limbal stem cell deficiency was present not only in patients diagnosed with ectropion but also in P3 (
Figure 1d,e). In addition, lipid keratopathy with corneal vascularization and calcification was noted in P4 (
Figure 1f).
Representative slit-lamp photographs are shown in
Figure 1. Slit-lamp examination of five family members of the patient did not reveal any abnormalities.
3.3. Optical Coherence Tomography
Corneal topography and pachymetry results
The detailed results of the corneal parameters are presented in
Table S1, and the summarized results are presented in
Table 2. Three patients, P2 (LI), P3 (IV), and P4 (HI), were diagnosed with ectasia patterns based on ESI. The results ranged from 7 to 95% of the probability of a corneal ectasia pattern. The CAT ranged from 480 to 734 µm. The corneas showed a significant pattern of irregularity, with a large difference between CAT and CTT ranging from 5 to 375 µm. The the thinnest point displacement from the inferotemporal region was observed at P2.
The results of corneal shape parameters of the left eye of P2, and the right eye of P3 which were severely affected (
Figure 1c,d), were significantly different in terms of keratometric, posterior, and real keratometry readings, and cylinder results. Both corneas were very steep (kAvgK 66,2 and 55,4 respectively) with a high degree of keratometric astigmatism (kCYL 6,2 and 3,8 respectively).
Figure 2 shows the ESI results of severely affected eyes with ectasia patterns in P2 and P3.
Fourier indices
The detailed FI results for all the parameters are presented in
Table S2, and the summarized results are presented in
Table 3. All patients revealed abnormalities in at least one parameter, namely regular astigmatism for at least one eye at 3 or 6 mm diameter for keratometric, anterior, or posterior analysis.
The FI parameters that showed abnormalities in at least one eye were primarily located on the posterior corneal surface and included 3 mm p Reg, Astigmatism, 3 mm p Asymmetry, 3 mm p Higher Order, 6 mm p Reg, Astigmatism, and 6 mm p Higher Order. Patients P2, P3, and P4 had abnormalities in almost all anterior, keratometric, and posterior FI, which aligned with the ectasia corneal pattern in the topography results (
Figure 2).
Morphology
Most morphological OCT findings are related to severe DED, limbal stem cell deficiency, exposure keratopathy, scarring, and corneal surface irregularity. Scar tissue was visible as an irregular area of hyperreflectivity in the anterior stroma. Scarring was evident in all patients except P5. The most significant corneal changes due to keratopathy exposure were observed in the paracentral lower corneal quadrant. Local irregularities in the anterior and posterior corneal surfaces were also visible in all patients, except for P5. Anterior iris synechiae were present in P2 and P3. Local (P3) or generalized (P2) corneal edema was present in two patients. Representative images of the OCT scans are shown in
Figure 3.
3.4. In Vivo Confocal Microscopy
Representative IVCM images of each patient are shown in
Figure 4. The epithelium showed different degrees of abnormality, including epithelial squamous metaplasia, an irregular shape, and hyperreflectivity pattern of cells with hyperreflective patches. The nerve plexus showed normal anatomy only in both eyes of P5 and the RE of P4. Otherwise, it was completely or partially invisible because of corneal scarring related to exposure keratopathy.
Figure 4c shows a tortuous nerve plexus with decreased nerve density and haze present at a depth of the Bowman layer in P3. Stromal changes in patients P1-P5 ranged in severity and form and mostly included keratocyte pleomorphism, enlarged hyperreflective activated keratocytes, stromal haze, scarring, hyperreflective opacities, vascularization, crystalline lipid keratopathy, and stromal hyperreflective folds. The mildest affected individual, P5, showed only corneal stromal abnormalities in the form of multiple stromal microdot deposits within the stroma. The endothelial layer also showed abnormalities (P1, P3, and P4) or was impossible to visualize because of the massive scarring (P2). Hyperreflective opacities and pleomorphism were also observed.
4. Discussion
Although ocular involvement may be regarded as a significant feature of ichthyoses and includes multiple symptoms, it usually presents based on slit-lamp examination, in relatively small patient sample sizes, or as case reports.
In this study, we aimed to define multimodal characteristic features of the ocular surface in patients with ichthyosis. We evaluated the results of the slit-lamp examination, anterior segment SS-OCT, and IVCM in patients with IV and ARCI, including HI, LI, and BSI. To date, only a few reports have included detailed objective ocular surface assessments based on novel imaging techniques such as meibography, optical coherence tomography, Scheimpflug imaging, and confocal microscopy [
18,
29,
30,
31]. Multimodal imaging is mostly available for specific syndromes, including keratitis-ichthyosis-deafness (KID), Ichthyosis Follicularis, Alopecia, and Photophobia Syndrome (IFAP), Sjögren-Larsson syndrome (SLS) or Pre-Descemet’s membrane corneal dystrophy (PDCD) [
8,
9,
10,
11,
32,
33,
34,
35,
36,
37].
Based on our results, several significant ocular surface abnormalities were identified, consistent with previous research; however, the study also provided new data, especially related to ectasia patterns and Fourier index aberrations based on SS-OCT, and insight into microscopic structural changes revealed by IVCM. The corneal epithelium plays a crucial role in maintaining ocular surface homeostasis and is an important barrier to pathogens and environmental agents. The epithelial abnormalities in our group included epithelial squamous metaplasia, irregular shape, and of cells with hyperreflective patches. These features may be compared to those of patients with severe dry eyes, including epithelial squamous metaplasia with an increase in desquamation, enlarged cells, pyknotic nuclei, and lower cell density compared to normal. In addition, several abnormalities related to the nerve plexus were observed in the study group. The nerve plexus showed normal anatomy only at P5 and at the RE of P4. Otherwise, it is completely or partially invisible because of corneal scarring, which is mostly related to exposure keratopathy. Reductions in sub-basal nerves have also been described in congenital diseases, such as congenital corneal anesthesia (CCA) and corneal dystrophies, and acquired diseases, such as dry eye, diabetes, and infectious keratitis, such as herpes simplex, bacterial, fungal, and Acanthamoeba keratitis [
38,
39,
40,
41]. In ichthyoses, nerve plexus abnormalities may be caused by dry eye, keratopathy, excessive scarring, and limbal stem cell deficiency. Corneal vascularization is secondary to keratopathy and the underlying limbal stem cell deficiency. Notably, corneal scarring with peripheral vascularization and local or diffuse limbal stem cell deficiency was present not only in patients diagnosed with ectropion but also in P3 (IV, normal eyelid anatomy). All patients were characterized by stromal involvement ranging from the mildest stromal microdot deposits (P5) to severe scarring (P2). However, the origin of the microdot deposits remains unclear. One theory is that microdots consist of lipofuscin granules of intracellular origin. They have been reported in healthy subjects and are regarded as early-stage irreversible corneal stromal alterations due to hypoxia [
42]. Utheim et al. reported an accumulation of microdots in the aging cornea, primarily in the subepithelial stromal region [
43]. This conclusion does not align with our findings since P5 is young (13 years old) and presented with microdot accumulation in the anterior and posterior stroma.
To assess the anterior and posterior corneal surface disturbances, we used anterior segment SS-OCT. To date, no published data are available regarding the SS-OCT findings in patients with ichthyosis. However, a study on topographic and biomechanical evaluation of the cornea in patients with ichthyosis vulgaris was conducted by Kara N. et al. [
29]. There was another reported case of marginal pellucid degeneration in a patient with IV disease (44). Moreover, Palamar M. et al., based on Scheimpflug imaging, detected bilateral keratoconus in two out of 12 patients with genetically confirmed LI (19). Kara N. et al. revealed that while corneal topographic findings and corneal hysteresis (CH) in patients with IV were similar to those in healthy subjects, the mean corneal resistance factor (CRF) and central corneal thickness (CCT) were significantly lower in patients with IV [
29]. These results are contrary to our findings because, in the case of patient P3, diagnosed with IV, we revealed a significant corneal ectasia pattern in both eyes (CTT, 356 µm and 459 µm; CAT-CTT, 217 µm and 93 µm; ESI, 95% and 50%, respectively, for RE and LE;
Table S2 and
Table 2). It should be emphasized that ichthyosis vulgaris was strongly associated with atopy, which may have resulted in the diagnosis of ectatic corneal disorders in our patient. Bilateral ectasia patterns were observed in patients P2 (LI) and P4 (HI). Considering the study group. We demonstrated an ectasia profile in five of ten eyes with various ichthyotic forms, including IV, LI, and HI. The high percentage of ectopic patterns in our study group may be explained by the high sensitivity and specificity of SS-OCT in diagnosing and grading keratoconus compared to the slit lamp examination used in other studies [
23,
24,
25]. A recent Scheimpflug imaging study by Palamar M. et al. suggested that the rate of keratoconus is underestimated in patients with ichthyosis and that all patients should undergo topographic screening for ectatic disorders [
18]. Based on this, one can conclude that the rate of keratoconus is significantly higher in patients with ichthyosis than in the general population (approximately 1.38 per 1000 population) [
44].
Our study demonstrated that all patients revealed abnormalities in at least one parameter of the Fourier indices, namely regular astigmatism for at least one eye of 3 or 6 mm diameter for keratometric, anterior, or posterior analysis. The high incidence of FI abnormalities can be explained by the fact that the results may be compromised by subclinical, non-specific abnormalities of the corneal surface, and by the influence of other factors, such as environmental factors, and tear film instability. Abnormalities in the tear film have been previously reported in ichthyosis and were confirmed by our abnormal TBUT and fluorescein staining results [
17,
30]. All patients from our study group were diagnosed with DED. Subsequently, punctate keratitis may be due to tear film instability caused by MGD or secondary cicatricial eyelid margin contraction. Corneal exposure often leads to ulcers, perforations, and severe scarring. Based on a series of 10 patients with LI and cicatricial lagophthalmos published by Cruz A. et al., 30% developed corneal exposure leading to loss of useful vision [
45]. Our results are similar because exposure keratopathy with corneal scarring and peripheral vascularization of different severities due to ectropion was diagnosed in three patients (P1, P2, and P4). The median BCVA was 0,25, ranging from LP to 1,0. The severely affected P2 with LI was diagnosed as a diffuse corneal scar after a corneal perforation in the temporal paracentral quadrant (Figure 1c). Notably, corneal scarring with peripheral vascularization and limbal stem cell deficiency was present not only in patients diagnosed with ectropion, but also in P3 with IV and normal eyelid anatomy (
Figure 1d,e).
This study has certain limitations. The main limitation was the small sample size; five patients were diagnosed with different ichthyosis forms: IV, LI, HI, and BSI. A larger series is needed to confirm our results concerning SS-OCT and IVCM findings. Additionally, this was not a screening study. The study group consisted of patients referred by an ophthalmologist. Therefore, the severity of ophthalmic findings may be overestimated compared with the entire patient population. Moreover, no reliable statistical analyses were possible for this small and diverse study group. The repeatability and reproducibility of SS-OCT have been proven in healthy corneas; however, studies with larger samples of unhealthy eyes are lacking. To the best of our knowledge, this is the first observational study to assess a wide range of corneal parameters based on SS-OCT in ichthyosis.
It is also worth noting that genetic counseling plays a crucial role in the final diagnosis and might enable physicians to predict the possibility of upcoming ocular problems in patients with ichthyosis. Two patients in our study group had genetically confirmed ARCI.
TGM1 gene mutations have been identified. Mutations in this gene are the predominant cause of ARCI, particularly the LI subtype. The known homozygous pathogenic variant c.[943C>T];(p.Arg315Cys) was found following TGM1 gene analysis in P5 (proband), and was strongly linked to BSI [
14,
46]. The suggested effect of p.Arg315Cys on TGase-1 function includes low specific activity, presumably because of protein misfolding or excessively stable proteins that cannot be processed. The other three patients were diagnosed based on dermatological consultation results, which also occurred in most ichthyosis studies [
16,
17,
19,
20,
29].
In summary, our multimodal study revealed several characteristic ocular surface features that may be overlooked when using slit-lamp examination alone. These early changes include DED, punctate keratopathy, and peripheral vascularization, which may occur regardless of anatomical eyelid disturbances. Therefore, patients with ichthyosis should be examined and treated for the early onset of DED. We also revealed that IVCM might aid in assessing ocular surface disease severity and lead to an improved understanding of the pathophysiological mechanisms of this complex disease. Furthermore, owing to the visualization of subclinical findings, IVCM may allow for the detection of ocular complications at much earlier stages. Our study also showed that SS-OCT could detect anterior and posterior corneal surface abnormalities and reveal subclinical ectatic patterns. Screening ichthyosis patients for possible keratoconus is a significant point to acknowledge. Moreover, SS-OCT allows the evaluation of the distribution of corneal opacities and illustrates the depth of scarring and shape abnormalities.
Author Contributions
A.N. and A.M. contributed equally to this study. Conceptualization, A.N., A.M.; methodology, A.N., A.M.; software, S.T.; validation, A.N., E.W. and S.T.; formal analysis, A.N; investigation, A.N., A.M.; resources, S.T., J.L-K..; data curation, A.N., A.M..; writing—original draft preparation, A.M..; writing—review and editing, A.N..; visualization, A.M..; supervision, A.N.; project administration, E.W. ; funding acquisition, A.N. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Representative slit-lamp eye photographs of the study group. The quality of photos is compromised due to the increased light sensitivity among ichthyosis patients. 1A.(mag. 10x, after installation of fluorescein) P1. RE. (LI) Excessive scaling with hyperkeratinization of the lid margin and crust and scales at the base of eyelashes. Severe exposure keratopathy with significant, irregular fluorescein staining located at the lower half of the cornea. 1B. (mag. 10x) P2. RE. (LI) Cicatricial ectropion of the upper and lower eyelid with eyelid margin deformation and hyperkeratinization (X), arcus lipoides, peripheral vascularization at the two third of the lower corneal part (arrowheads), corneal scarring with the epithelium irregularity due to exposure keratopathy (arrow). 1C. (mag. 10x) P2. LE. (LI) Cicatricial ectropion of the upper and lower eyelid with eyelid margin hyperkeratinization and crust at the base of eyelashes (X); arcus lipoides with mild corneal peripheral vascularization, diffuse, dense, central and paracentral corneal scar with temporal focal pigmentation subsequent to corneal perforation (arrowhead). 1D. (mag. 16x) P3. RE. (IV) Localized, central irregular scar involving the lower peripheral cornea. Invading vessels from the lower periphery (arrow). 1E. (mag. 10x) P3. LE. (IV) Hiperkeratinisation of the lower lid exceeding the lid margin, central, diffuse corneal scar with vessels ingrowth from the lower corneal part (arrow). 1F. (mag. 10x) P4. LE. (HI) Upper eyelid deformation. Paracentral area of reticular vascularization, lipid keratopathy, and calcification.
Figure 1.
Representative slit-lamp eye photographs of the study group. The quality of photos is compromised due to the increased light sensitivity among ichthyosis patients. 1A.(mag. 10x, after installation of fluorescein) P1. RE. (LI) Excessive scaling with hyperkeratinization of the lid margin and crust and scales at the base of eyelashes. Severe exposure keratopathy with significant, irregular fluorescein staining located at the lower half of the cornea. 1B. (mag. 10x) P2. RE. (LI) Cicatricial ectropion of the upper and lower eyelid with eyelid margin deformation and hyperkeratinization (X), arcus lipoides, peripheral vascularization at the two third of the lower corneal part (arrowheads), corneal scarring with the epithelium irregularity due to exposure keratopathy (arrow). 1C. (mag. 10x) P2. LE. (LI) Cicatricial ectropion of the upper and lower eyelid with eyelid margin hyperkeratinization and crust at the base of eyelashes (X); arcus lipoides with mild corneal peripheral vascularization, diffuse, dense, central and paracentral corneal scar with temporal focal pigmentation subsequent to corneal perforation (arrowhead). 1D. (mag. 16x) P3. RE. (IV) Localized, central irregular scar involving the lower peripheral cornea. Invading vessels from the lower periphery (arrow). 1E. (mag. 10x) P3. LE. (IV) Hiperkeratinisation of the lower lid exceeding the lid margin, central, diffuse corneal scar with vessels ingrowth from the lower corneal part (arrow). 1F. (mag. 10x) P4. LE. (HI) Upper eyelid deformation. Paracentral area of reticular vascularization, lipid keratopathy, and calcification.
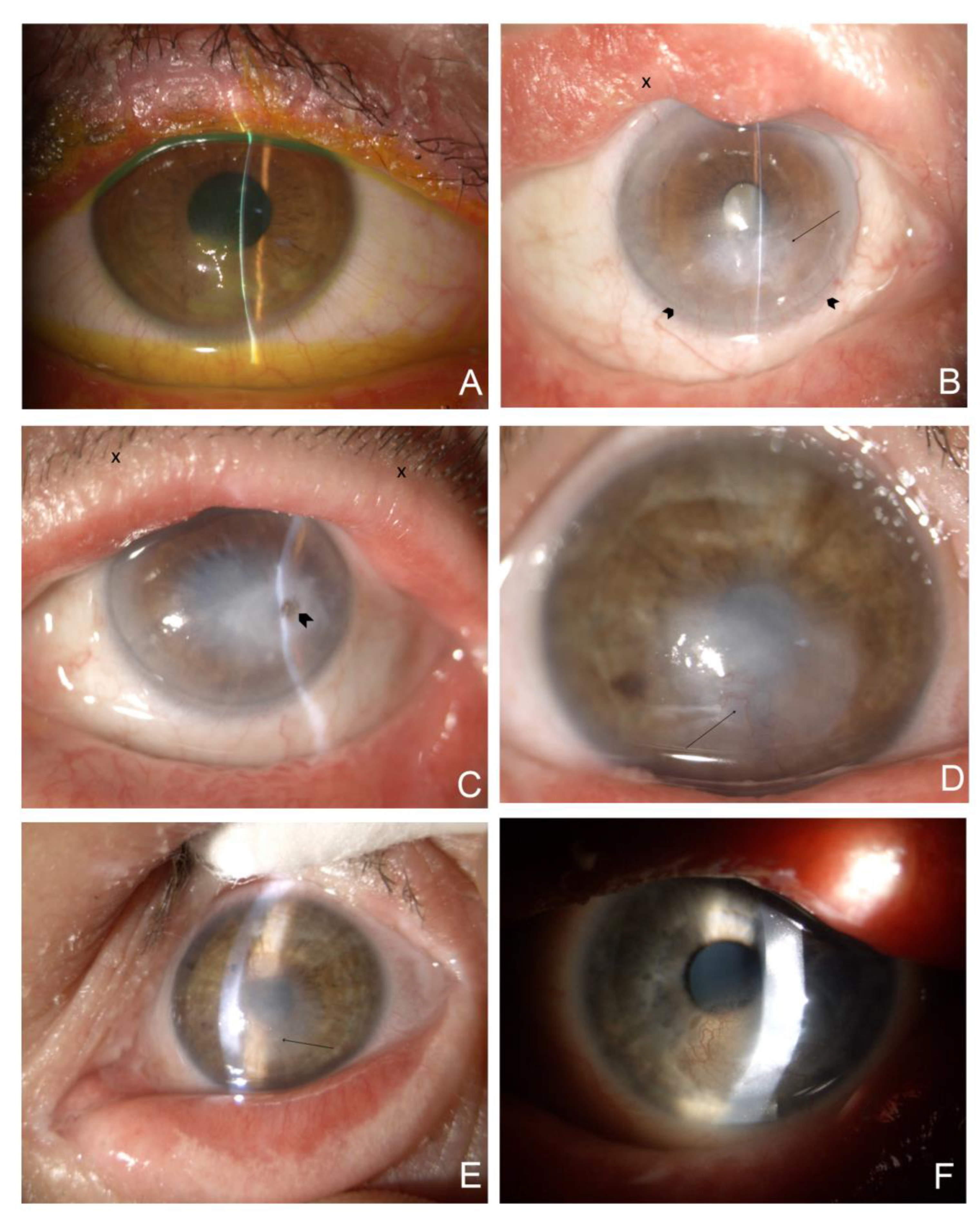
Figure 2.
Representative results of swept source OCT (SS-OCT); Ectasia Screening Index (ESI) report of affected eyes from the study group (a, anterior; p, posterior). The report includes the following data: APK (axial power keratometric); APP (axial power posterior); Sph.6: spherical component of FI at a 6 mm diameter (D), Reg.6: regular astigmatism component at a 6 mm diameter (D), Asy.6: asymmetric component at a 6 mm diameter (D), and Hio.6: higher-order irregular astigmatism component at a 6 mm diameter (D); P (pachymetry), the corneal thickness (μm) of the thinnest part and the location related to the corneal apex (coordinates: X, Y) (mm); IPP (instantaneous power posterior), Steepest@6 (mm): the value of the steepest instantaneous posterior power and its location related to the corneal apex ( coordinates: X, Y) (mm). ASM (anterior eye segment morphology) line scan. 2A. P2, RE (LI); Note the high degree of irregularity on the anterior corneal surface (arrow). 6 mm k Asymmetry FI is 10,39 D, which reflects the significant difference between the upper and lower anterior corneal surface. The summarized similarity of ectasia corneal pattern is 95% (aESI 95%; pESI 66%). 2B. P3, RE (IV); Note the high degree of irregularity on the posterior corneal surface (arrow), CTT decreased to 356 µm, Sph@6 increased to 53,55 D. The summarized similarity of ectasia corneal pattern is 95% (aESI 95%; pESI 95%).
Figure 2.
Representative results of swept source OCT (SS-OCT); Ectasia Screening Index (ESI) report of affected eyes from the study group (a, anterior; p, posterior). The report includes the following data: APK (axial power keratometric); APP (axial power posterior); Sph.6: spherical component of FI at a 6 mm diameter (D), Reg.6: regular astigmatism component at a 6 mm diameter (D), Asy.6: asymmetric component at a 6 mm diameter (D), and Hio.6: higher-order irregular astigmatism component at a 6 mm diameter (D); P (pachymetry), the corneal thickness (μm) of the thinnest part and the location related to the corneal apex (coordinates: X, Y) (mm); IPP (instantaneous power posterior), Steepest@6 (mm): the value of the steepest instantaneous posterior power and its location related to the corneal apex ( coordinates: X, Y) (mm). ASM (anterior eye segment morphology) line scan. 2A. P2, RE (LI); Note the high degree of irregularity on the anterior corneal surface (arrow). 6 mm k Asymmetry FI is 10,39 D, which reflects the significant difference between the upper and lower anterior corneal surface. The summarized similarity of ectasia corneal pattern is 95% (aESI 95%; pESI 66%). 2B. P3, RE (IV); Note the high degree of irregularity on the posterior corneal surface (arrow), CTT decreased to 356 µm, Sph@6 increased to 53,55 D. The summarized similarity of ectasia corneal pattern is 95% (aESI 95%; pESI 95%).
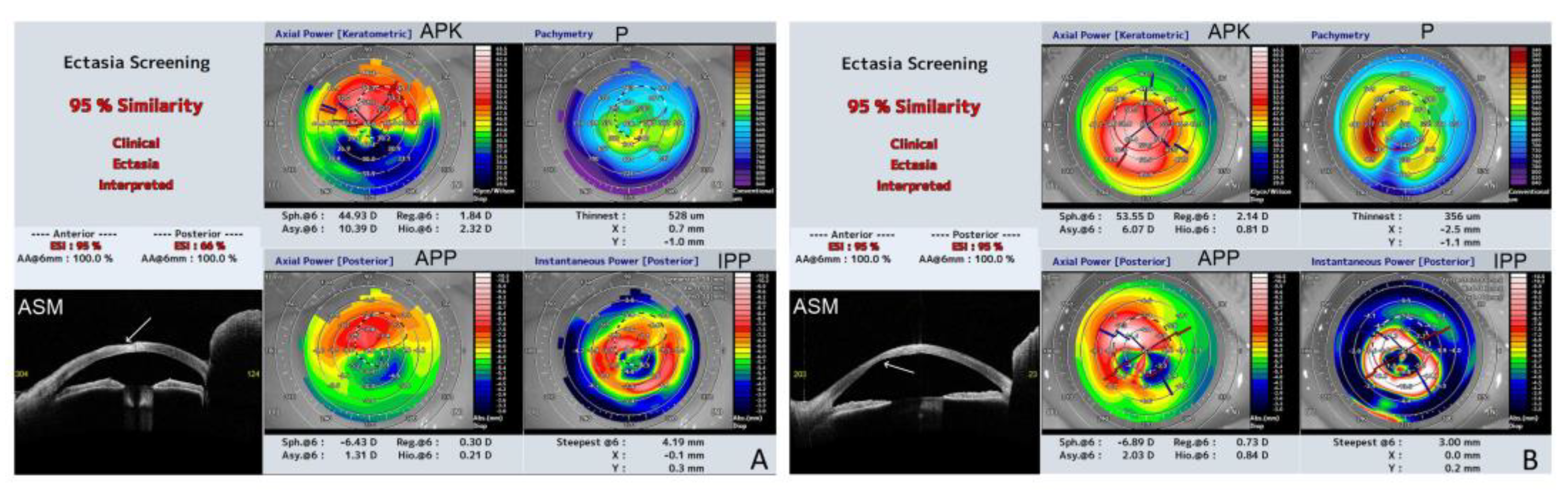
Figure 3.
Representative high-definition morphology SS-OCT scans of the most severely affected patients. 3A. P2.RE.(LI). Line 0-180
o. Irregularity of the anterior corneal surface (arrow). Increased reflectivity of the corneal stroma corresponding with the area of corneal scar (
Figure 1B). 3B. P2.LE. (LI). Line 0-180
o. Generalized anterior iris synechiae (arrows). The significant irregularity on both corneal surfaces. Increased reflectivity of the stroma due to diffuse, dense corneal scar subsequent to exposure keratopathy (
Figure 1C). Corneal edema with different local severity (X). The area of Descemet membrane detachment paracentrally (arrowhead). Increased lens reflectivity due to cataract. 3C. P3. RE. (IV). Line 80-260
o. Irregular anterior and posterior corneal surface. Central hyperreflectivity corresponding to the central corneal scar visible in
Figure 1D. Anterior iris synechiae (arrow). The localized Descemet membrane rupture (arrowhead) and local corneal edema (X). 3D. P4. LE. (HI). Line 150-330
o. Marked hyperreflectivity in the anterior, central corneal part (arrows). Additionally, the diffuse region of hyperreflectivity extends to the paracentral part at the axis of 330
o (arrowheads). This region of increased reflectivity corresponds to the central lipid keratopathy with a reticular pattern of vascularization (
Figure 1F).
Figure 3.
Representative high-definition morphology SS-OCT scans of the most severely affected patients. 3A. P2.RE.(LI). Line 0-180
o. Irregularity of the anterior corneal surface (arrow). Increased reflectivity of the corneal stroma corresponding with the area of corneal scar (
Figure 1B). 3B. P2.LE. (LI). Line 0-180
o. Generalized anterior iris synechiae (arrows). The significant irregularity on both corneal surfaces. Increased reflectivity of the stroma due to diffuse, dense corneal scar subsequent to exposure keratopathy (
Figure 1C). Corneal edema with different local severity (X). The area of Descemet membrane detachment paracentrally (arrowhead). Increased lens reflectivity due to cataract. 3C. P3. RE. (IV). Line 80-260
o. Irregular anterior and posterior corneal surface. Central hyperreflectivity corresponding to the central corneal scar visible in
Figure 1D. Anterior iris synechiae (arrow). The localized Descemet membrane rupture (arrowhead) and local corneal edema (X). 3D. P4. LE. (HI). Line 150-330
o. Marked hyperreflectivity in the anterior, central corneal part (arrows). Additionally, the diffuse region of hyperreflectivity extends to the paracentral part at the axis of 330
o (arrowheads). This region of increased reflectivity corresponds to the central lipid keratopathy with a reticular pattern of vascularization (
Figure 1F).
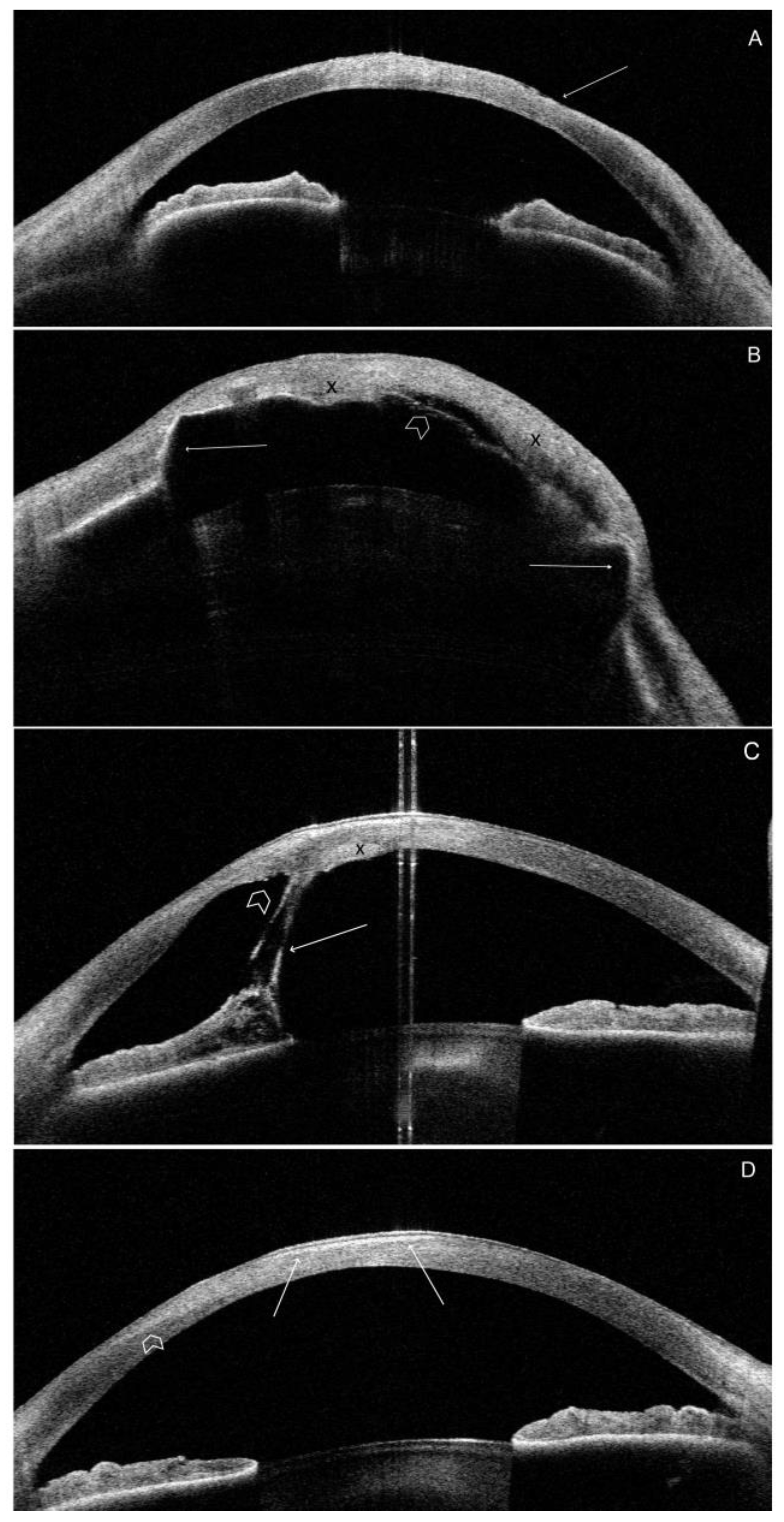
Figure 4.
Representative IVCM images at different scanning depths. A-D; P1.RE. A. Confocal image at a depth of 21 µm. Corneal epithelium. The irregular shape of cells, and hyperreflectivity pattern of cells with hyperreflective patches (*), and multiple highly reflective irregular deposits (arrow). B. Confocal image at a depth of 55 µm. Irregularity of cells with hyperreflective patches (*), subepithelial fibrosis with the absent nerve plexus (arrow). C. Confocal image at a depth of 330 µm. Stroma. Confluent group of abnormally hyperreflective keratocyte nuclei with visible cytoplasmic processes (arrow). D. Confocal image at a depth of 530 µm. Endothelium. Hyperreflective, small precipitates (*), a few hyporeflective spots containing a central highlight (arrow). E-H; P2. RE. E. Confocal image at a depth of 48 µm. Corneal epithelium. Marked irregularity of cells. Notable, hyperreflective patches (arrow). F. Confocal image at a depth of 175 µm. Stromal haze and scaring. Numerous small hyperreflective dots (arrow). G. Confocal image at a depth of 300 µm. Non-homogenous area of massive scarring and fibrosis. H. Confocal image at a depth of 400 µm. Dark differently orientated striae (*). Stromal haze. Keratocyte nuclei are barely distinguishable. I-L; P3. RE. I. Confocal image at a depth of 15 µm. Epithelial squamous metaplasia. Enlarged, hyperreflective cells, irregularly arranged, and decreased cell density. J. Confocal image at a depth of 54 µm. Loss of nerves increased tortuosity. Hyperreflective, non-homogenous patches (*). K. Confocal image at a depth of 320 µm. Hyperreflective scarring with neovascularization (arrow). L. Confocal image at a depth of 566 µm. Cell pleomorphism. Hyperreflective, small precipitates (arrow). M-P. P4. LE. M. Confocal image at a depth of 30 µm. Marked irregularity of cells. Hyperreflective areas covering epithelium (arrow). N. Confocal image at a depth of 80 µm. Homogenous, hyperreflective, distinctive area of calcification (arrow). Stromal haze and keratocyte nuclei are barely distinguishable. Multiple, small microdots. O. Confocal image at a depth of 310 µm. Multiple, hyperreflective, needle-like opacities differently orientated. Crystalline lipid keratopathy P. Confocal image at a depth of 520 µm. Scaring with vessels (arrow). Dark stromal striae (*). R-U. P5. LE. R. Confocal image at a depth of 20 µm. Corneal epithelium. S. Confocal image at a depth of 60 µm. Nerve-plexus. T. Confocal image at a depth of 340 µm. Multiple stromal microdots (arrow). U. Confocal image at a depth of 550 µm. Posterior stroma and endothelium. Image quality is compromised due to the patient's age and poor cooperation during the examination.
Figure 4.
Representative IVCM images at different scanning depths. A-D; P1.RE. A. Confocal image at a depth of 21 µm. Corneal epithelium. The irregular shape of cells, and hyperreflectivity pattern of cells with hyperreflective patches (*), and multiple highly reflective irregular deposits (arrow). B. Confocal image at a depth of 55 µm. Irregularity of cells with hyperreflective patches (*), subepithelial fibrosis with the absent nerve plexus (arrow). C. Confocal image at a depth of 330 µm. Stroma. Confluent group of abnormally hyperreflective keratocyte nuclei with visible cytoplasmic processes (arrow). D. Confocal image at a depth of 530 µm. Endothelium. Hyperreflective, small precipitates (*), a few hyporeflective spots containing a central highlight (arrow). E-H; P2. RE. E. Confocal image at a depth of 48 µm. Corneal epithelium. Marked irregularity of cells. Notable, hyperreflective patches (arrow). F. Confocal image at a depth of 175 µm. Stromal haze and scaring. Numerous small hyperreflective dots (arrow). G. Confocal image at a depth of 300 µm. Non-homogenous area of massive scarring and fibrosis. H. Confocal image at a depth of 400 µm. Dark differently orientated striae (*). Stromal haze. Keratocyte nuclei are barely distinguishable. I-L; P3. RE. I. Confocal image at a depth of 15 µm. Epithelial squamous metaplasia. Enlarged, hyperreflective cells, irregularly arranged, and decreased cell density. J. Confocal image at a depth of 54 µm. Loss of nerves increased tortuosity. Hyperreflective, non-homogenous patches (*). K. Confocal image at a depth of 320 µm. Hyperreflective scarring with neovascularization (arrow). L. Confocal image at a depth of 566 µm. Cell pleomorphism. Hyperreflective, small precipitates (arrow). M-P. P4. LE. M. Confocal image at a depth of 30 µm. Marked irregularity of cells. Hyperreflective areas covering epithelium (arrow). N. Confocal image at a depth of 80 µm. Homogenous, hyperreflective, distinctive area of calcification (arrow). Stromal haze and keratocyte nuclei are barely distinguishable. Multiple, small microdots. O. Confocal image at a depth of 310 µm. Multiple, hyperreflective, needle-like opacities differently orientated. Crystalline lipid keratopathy P. Confocal image at a depth of 520 µm. Scaring with vessels (arrow). Dark stromal striae (*). R-U. P5. LE. R. Confocal image at a depth of 20 µm. Corneal epithelium. S. Confocal image at a depth of 60 µm. Nerve-plexus. T. Confocal image at a depth of 340 µm. Multiple stromal microdots (arrow). U. Confocal image at a depth of 550 µm. Posterior stroma and endothelium. Image quality is compromised due to the patient's age and poor cooperation during the examination.
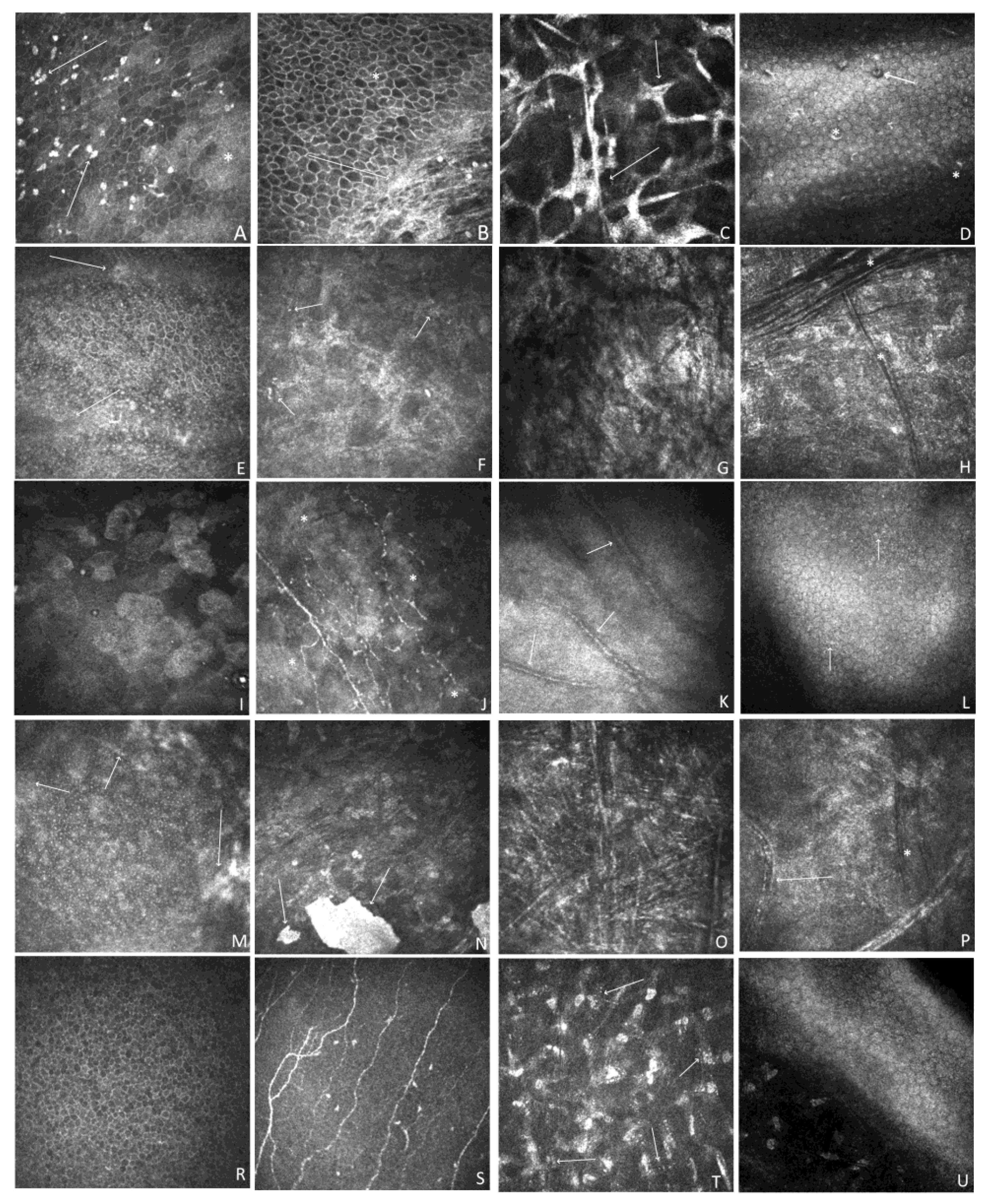
Table 1.
Demographic data and medical history data of the study group.
Table 1.
Demographic data and medical history data of the study group.
| |
Patient 1 (P1) |
Patient 2 (P2) |
Patient 3 (P3) |
Patient 4 (P4) |
Patient 5 (P5) |
| Age |
20 |
66 |
44 |
23 |
13 |
| Gender |
Male |
Female |
Female |
Female |
Male |
| Family history |
Female sibling affected |
|
|
A male sibling died shortly after birth due to an ichthyosis complication |
|
| Scaling at birth |
Present |
Present |
No |
Present |
Present |
| Type and distribution of scaling |
Large, rhomboid, dark brown; generalized
|
Large, light. Brownish; generalized |
Small, white, and gray; generalized |
Coarse and platelike, white; generalized
|
Small white, grey on the face, brownish on the trunk; the disparity between trunk and extremities and face |
| Extremities |
Skin deprived of sweat glands, hairless;
|
Skin deprived of sweat glands, hairless; |
Sweat glands present;
Skin hairless; |
Synechiae of digits; skin deprived of sweat glands, hairless;
|
Excessive scale in armpits, in the bends of the elbows and knees; sweat glands and hair present |
| Scalp abnormalities, eyebrows, lashes |
Scarring alopecia, no eyebrows, lashes on the upper and lower eyelid present |
Localized temporal alopecia, otherwise hair on the head, brows, and lashes on the upper eyelid present |
Hair on the head, brows, and lashes on the upper and lower eyelid present |
Synchiae of auricles, scarring alopecia, brows, and lashes on the upper eyelid present, but very brittle |
Hair on head, eyebrows, and lashes present, but fair and brittle |
| Symptoms general |
Cryptorchidism at birth,
Hypercholesterolemia |
The short statue, failure to thrive,
Hypercholesterolemia |
None |
Hearing affected, short statue, failure to thrive |
Sepsis at birth, intraventricular leakage of open foramen ovale |
| Type of ichthyosis |
ARCI
LI
(genetic confirmation)
|
ARCI
LI
|
IV
|
ARCI
HI
|
ARCI
BSI (LI minor variant)
(genetic confirmation) |
| ARCI autosomal recessive congenital ichthyosis; LI, lamellar ichthyosis; IV, ichthyosis vulgaris; BSI, bathing suit ichthyosis HI, harlequin ichthyosis. |
Table 2.
Summarized BCVA, ocular surface examination, and corneal topography and thickness map results. The detailed results are presented in
Table S2.
Table 2.
Summarized BCVA, ocular surface examination, and corneal topography and thickness map results. The detailed results are presented in
Table S2.
| Parameter |
P1 |
P2 |
P3 |
P4 |
P5 |
min |
max |
median |
| BCVA |
RE 0,8
LE 0,7 |
RE 0,2
LE LP |
RE 0,05
LE 0,1 |
RE 0,3
LE 0,2 |
RE 1,0
LE 0,4 |
LP |
1,0 |
0,25 |
| TBUT [s] |
RE 6
LE 8 |
RE 4
LE 4 |
RE 7
LE 8 |
RE 5
LE 5 |
RE 12
LE 8 |
4 |
10 |
6,5 |
| Fluorescein staining [Oxforrd scale] |
RE IV
LE II |
RE III
LE IV |
RE IV
LE III |
RE II
LE II |
RE 0
LE I |
0 |
IV |
2,0 |
| kAvgK [D] |
RE 42,1
LE 41,8 |
RE 47,0
LE 66,2 |
RE 55,4
LE 49,8 |
RE 48,9
LE 47,0 |
RE 43,9
LE 43,5 |
41,8 |
66,2 |
47 |
| pAvgK [D] |
RE -6,0
LE -6,2 |
RE -6,5
LE -10,1 |
RE -7,6
LE -6,5 |
RE -7,4
LE -6,7 |
RE -6,4
LE -6,3 |
-10,1 |
-6 |
-6,5 |
| rAvgK [D] |
RE 41,0
LE 40,5 |
RE 46,1
LE 64,4 |
RE 55,3
LE 49,2 |
RE 47,3
LE 45,7 |
RE 42,6
LE 42,2 |
40,5 |
64,4 |
45,9 |
| rCYL [D] |
RE 2,0
LE 0,8 |
RE 2,4
LE 1,9 |
RE 5,4
LE 7,3 |
RE 2,6
LE 1,0 |
RE 2,1
LE 2,1 |
0,8 |
7,3 |
2,1 |
| CAT[µm] |
RE 539
LE 529 |
RE 606
LE 734 |
RE 573
LE 552 |
RE 480
LE 505 |
RE 545
LE 548 |
480 |
734 |
546,5 |
| CTT [µm] |
RE 520
LE 509 |
RE 528
LE 395 |
RE 356
LE 459 |
RE 462
LE 498 |
RE 540
LE 540 |
356 |
540 |
503,5 |
| CAT-CTT [µm] |
RE 10
LE 20 |
RE 78
LE 375 |
RE 217
LE 93 |
RE 18
LE 7 |
RE 5
LE 8 |
5 |
375 |
19 |
| ACD [mm] |
RE 2,97
LE 2,94 |
RE 2,27
LE 1,9 |
RE 2,86
LE 2,81 |
RE 3,03
LE 2,95 |
RE 3,02
LE 2,91 |
1,9 |
3,03 |
2,925 |
| ESI [%] |
RE 0
LE 0 |
RE 95
LE 95 |
RE 95
LE 50 |
RE 61
LE 7 |
RE 0
LE 0 |
0 |
95 |
28,5 |
Table 3.
Fourier Indices summarized results presenting parameters, which showed abnormalities in at least four patients. The detailed results are presented in
Table S2.
Table 3.
Fourier Indices summarized results presenting parameters, which showed abnormalities in at least four patients. The detailed results are presented in
Table S2.
| Parameter |
P1 |
P2 |
P3 |
P4 |
P5 |
min |
max |
median |
| 6 mm k Reg. Astigmatism |
RE 1,08*
LE 0,3 |
RE 1,84*
LE 4,86* |
RE 2,14*
LE 3,32* |
RE 1,48*
LE 0,6 |
RE 1,21*
LE 1,31* |
0,3 |
4,86 |
1,39 |
| 6 mm a Reg. Astigmatism |
RE 1,2*
LE 0,33 |
RE 2,06*
LE 5,41* |
RE 2,38*
LE 3,69* |
RE 1,64*
LE 0,67 |
RE 1,35*
LE 1,46* |
0,33 |
5,41 |
1,55 |
| 3 mm p Reg. Astigmatism |
RE 0,14
LE 0,14 |
RE 0,38*
LE 1,93* |
RE 0,88*
LE 0,23 |
RE 0,34*
LE 0,19 |
RE 0,35*
LE 0,33 |
0,14 |
1,93 |
0,34 |
| 3 mm p Asymmetry |
RE 0,12
LE 0,15* |
RE 1,25*
LE 0,41* |
RE 1,97*
LE 0,86* |
RE 1,09*
LE 0,14* |
RE 0,07
LE 0,03 |
0,03 |
1,97 |
0,15 |
| 3 mm p Higher Order |
RE 0,04*
LE 0,06* |
RE 0,23*
LE 1,81* |
RE 0,93*
LE 0,16* |
RE 0,07*
LE 0,03 |
RE 0,02
LE 0,03 |
0,02 |
1,81 |
0,06 |
| 6 mm p Reg. Astigmatism |
RE 0,13
LE 0,12 |
RE 0,3
LE 1,74* |
RE 0,73*
LE 0,29 |
RE 0,34*
LE 0,18 |
RE 0,32*
LE 0,31* |
0,12 |
1,74 |
0,31 |
| 6 mm p Higher Order |
RE 0,05
LE 0,06* |
RE 0,21*
LE 1,81* |
RE 0,84*
LE 0,15* |
RE 0,06*
LE 0,03 |
RE 0,02
LE 0,04 |
0,02 |
1,81 |
0,06 |
| *out of reference range (normative database); RE right eye; LE left eye; (k), keratometric; (a) anterior; (p) postetrior; Reg., regular. |









