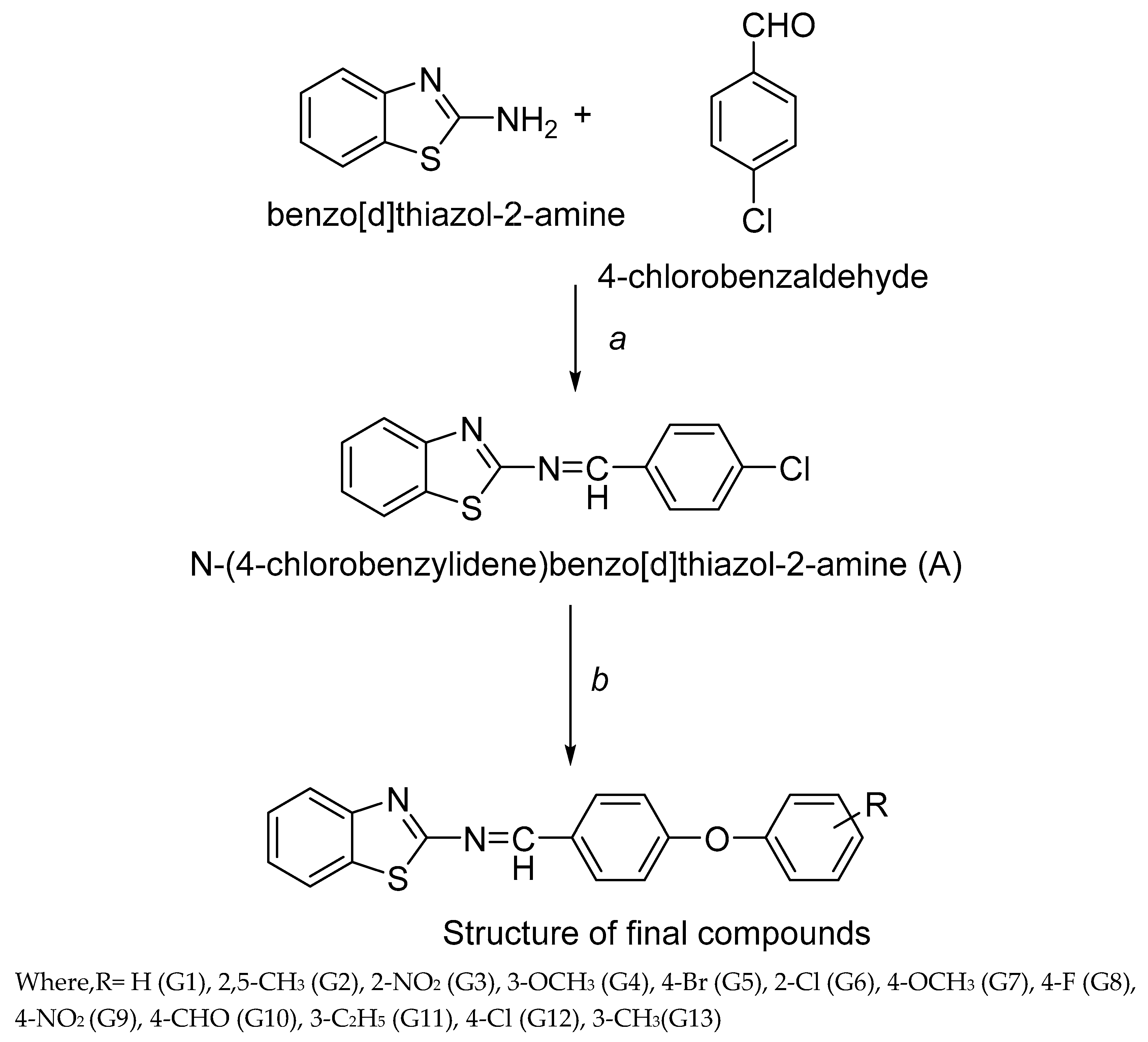
3.2. Synthesis
Synthesis of N-(4-chlorobenzylidene) benzo[d]thiazol-2-amine (A)
Equimolar of 2-aminobenzothiazole and 4-chlorobenzaldehyde was dissolved in ethanol (100ml) in 250ml round bottom flask (RBF) and 4-5 drops of glacial acetic acid was added in this. The reaction mixture was refluxed for 10 h. The reaction mixture was cooled to room temperature and set aside overnight for precipitation. The precipitated compounds were filtered, washed with water and recrystallized using ethanol. The reaction mixture was concentrated and filtered. Yellow colored product was obtained which was dried at room temperature. Then, melting point and TLC were observed.
M.P: 90-920C; Rf. Value: 0.74; IR (KBr) cm-1: 1488 (C-C Str. Aromatic), 1596 (C=C Str. Aromatic), 3054 (C-H Str. Aromatic), 1728 (C=N Str.), 1342 (C-N Str.), 718 (C-Cl Str.); 1HNMR (500 MHz) (ppm): 8.56 (s, 1H, CH), 8.17 (d, J = 10 Hz, 1H, benzothiazole), 7.99 (d, J = 5.14 Hz, 1H, benzothiazole), 7.75 (d, J = 4 Hz, 2H, Ar-H), 7.53 (t, J = 6.73 Hz, 2H, benzothiazole), 7.51 (d, J = 9.0 Hz, 2H, Ar-H); 13CNMR (500MHz) 121.3, 121.5, 124.3, 125.1, 125.5, 148.42, 174.3 (C, Benzothiazole), 159.83 (CH), 128.70, 128.73, 129.85, 130.10, 133.31, 135.32 (C, Ar-C.); MS (FAB) [M+1]+:(m/z) 273.01
General procedure for synthesis of Benzo[d]thiazol-2-amine derivatives
Equimolar of A and various substituted phenols were dissolved in 125 ml dry acetone in 250ml RBF. Thenequimolarof anhydrous potassium carbonate was added in reaction mixture and potassium iodide was added. The reaction mixture was refluxed for 21 h. After the completion of reaction, the reaction mixture was cooled and subjected to freeze overnight for precipitation. The reaction mixture was concentrated, filtered, washed with water and dried. Then, melting point and TLC was performed. In the reaction procedure, different phenols were used which resulted into newbenzo[d]thiazol-2-amine derivativesnamely G1-G13.
(E)-N-(4-Phenoxybenzylidene) benzo[d]thiazol-2-amine (G1)
IR (KBr) cm-1:3115 (C-H Aromatic), 1651 (C=N), 1613 (C-N), 1505 (C-C Str. Aromatic), 1482 (C=C Str. Aromatic), 1261 (C-S), 1128 (C-O str.); 1HNMR (500 MHz) (ppm): 8.53 (s, 1H, CH), 8.15 (d, J = 9.96 Hz,1H, benzothiazole), 7.98 (d, J = 5.37 Hz, 1H, benzothiazole), 7.87 (d, J = 4.12 Hz, 2H,.), 7.52 (t, J = 6.49 Hz, 2H, benzothiazole), 7.39 (d, J = 7.0 Hz, 2H,Ar-H), 7.21 (d, J = 7.5 Hz,2H, Ar-H), 7.18 (d,J = 8.27 Hz, 2H, Ar-H), 7.15 (t, J = 9.0 Hz,1H, Ar-H), 13CNMR (500MHz) 121.42, 121.60, 124.33, 125.21, 125.49, 148.53, 174.50 (C, Benzothiazole), 159.98 (CH), 117.12, 117.57, 118.52, 118.63, 120.83, 128.65, 128.75, 129.80, 129.92, 130.15, 156.49, 158.37 (C, Ar-C.);MS (FAB) [M+1]+:(m/z) 331.08
(E)-N-(4-(2,5-Dimethylphenoxy) benzylidene) benzo[d]thiazol-2-amine (G2)
IR (KBr) cm-1:3112 (C-H Aromatic), 1649 (C=N), 1614 (C-N), 1507 (C-C Str. Aromatic), 1485 (C=C Str. Aromatic), 1264 (C-S), 1130 (C-O str.); 1HNMR(500 MHz) (ppm): 8.53 (s, 1H, CH), 8.16 (d, J = 9.98 Hz,1H, benzothiazole), 7.96 (d, J = 5.71 Hz,1H, benzothiazole), 7.87 (d, J = 4.15 Hz,2H, Ar-H.), 7.51 (t, J = 6.43 Hz,2H, benzothiazole), 7.89 (d, J = 5.0 Hz,2H, Ar-H), 6.84 (d, J = 8.0 Hz,1H, Ar-H), 2.34 (s, 6H, CH3); 13CNMR (500MHz) 121.39, 121.55, 124.37, 125.29, 125.53, 148.46, 174.57 (C, Benzothiazole), 159.95 (CH), 117.09, 117.51, 118.49, 118.57, 120.78, 128.67, 128.71, 129.83, 129.95, 130.16, 156.51, 158.32(C, Ar-C.), 15.21 (CH3), 21.22 (CH3); MS (FAB) [M+1]+:(m/z) 359.11
(E)-N-(4-(2-Nitrophenoxy) benzylidene) benzo[d]thiazol-2-amine (G3)
IR (KBr) cm-1:3115 (C-H Aromatic), 1651 (C=N), 1617 (C-N), 1501 (C-C Str. Aromatic), 1479 (C=C Str. Aromatic), 1303 (N-O Str.), 1259 (C-S), 1128 (C-O str.); 1HNMR(500 MHz) (ppm): 8.58 (s, 1H, CH), 8.21 (d,J = 7.50 Hz, 1H, Ar-H.), 8.17 (d, J = 9.50 Hz, 1H, benzothiazole), 8.10 (d, J = 5.50 Hz, 1H, benzothiazole), 7.89 (d, J = 4.35 Hz, 2H, Ar-H), 7.76 (t, J = 6.50 Hz, 2H, Ar-H), 7.72 (d, J = 1.50 Hz, 1H, Ar-H), 7.50 (t, J = 4.98 Hz, 2H, benzothiazole), 7.20 (d, J = 5.0 Hz, 2H, Ar-H); 13CNMR (500MHz) 121.33, 121.54, 124.31, 125.22, 125.49, 148.43, 174.52 (C, Benzothiazole), 159.83 (CH), 117.31, 117.53, 118.43, 118.52, 120.65, 128.71, 128.73, 129.79, 129.98, 130.19, 149.63, 159.28 (C, Ar-C.); MS (FAB) [M+1]+:(m/z) 376.05
(E)-N-(4-(3-Methoxyphenoxy) benzylidene) benzo[d]thiazol-2-amine (G4)
IR (KBr) cm-1:3119 (C-H Aromatic), 1655 (C=N), 1619 (C-N), 1509 (C-C Str. Aromatic), 1481 (C=C Str. Aromatic), 1262 (C-S), 1130 (C-O str.); 1HNMR(500 MHz) (ppm): 8.56 (s, 1H, CH), 8.16 (d, J = 9.0 Hz, 1H, benzothiazole), 8.11 (d, J = 5.25 Hz, 1H, benzothiazole), 7.90 (d, J = 4.45 Hz, 2H, Ar-H), 7.52 (t, J = 6.0 Hz, 2H, benzothiazole), 7.18 (d, J = 5.50 Hz, 2H, Ar-H); 7.14 (t, J = 7.50 Hz, 1H, Ar-H), 6.35 (t, J = 4.58 Hz, 1H, Ar-H), 6.31 (d, J = 5.0Hz, 1H, Ar-H), 6.21 (d, J = 1.5 Hz, 1H, Ar-H), 3.81 (s, 3H, OCH3), 13CNMR (500MHz) 121.28, 121.49, 124.28, 125.19, 125.46, 148.39, 174.55 (C, Benzothiazole), 160.31 (CH), 117.30, 117.52, 118.40, 118.57, 120.59, 128.67, 128.75, 129.81, 129.92, 130.17, 157.65, 159.28 (C, Ar-C.), 55.31 (C, OCH3); MS (FAB) [M+1]+:(m/z) 361.08
(E)-N-(4-(4-Bromophenoxy) benzylidene) benzo[d]thiazol-2-amine (G5)
IR (KBr) cm-1:3114 (C-H Aromatic), 1653 (C=N), 1616 (C-N), 1510 (C-C Str. Aromatic), 1475 (C=C Str. Aromatic), 1260 (C-S), 1132 (C-O str.), 705 (C-Br Str.); 1HNMR(500 MHz) (ppm): 8.50 (s, 1H, CH), 8.13 (d,J = 9.25 Hz, 1H, benzothiazole), 8.02 (d, J = 5.34 Hz, 1H, benzothiazole), 7.88 (d, J = 4.36 Hz, 2H, Ar-H), 7.55 (d, J = 6.5 Hz, 2H, Ar-H), 7.50 (t, J = 7.0 Hz,2H, benzothiazole), 7.46 (d, J = 6.0 Hz, 2H, Ar-H), 7.20 (d, J = 7.05 Hz, 2H, Ar-H), 13CNMR (500MHz) 121.29, 121.50, 124.31, 125.24, 125.51, 148.47, 174.61 (C, Benzothiazole), 159.88 (CH), 117.29, 117.49, 118.43, 118.54, 120.48, 128.63, 128.71, 129.84, 129.96, 130.13, 157.62, 159.30 (C, Ar-C.); MS (FAB) [M+1]+:(m/z) 410.99
(E)-N-(4-(4-Chlorophenoxy)benzylidene)benzo[d]thiazol-2-amine (G6)
IR (KBr) cm-1:3116 (C-H Aromatic), 1655 (C=N), 1618 (C-N), 1512 (C-C Str. Aromatic), 1478 (C=C Str. Aromatic), 1259 (C-S), 1129 (C-O str.), 705 (C-Cl Str.); 1HNMR (500 MHz) (ppm): 8.58 (s, 1H, CH), 8.14 (d, J = 9.33 Hz, 1H, benzothiazole), 7.95 (d, J = 4.98 Hz, 1H, benzothiazole), 7.90 (d, J = 4.45 Hz, 2H, Ar-H), 7.55 (d, J = 6.0 Hz, 2H, Ar-H), 7.50 (t, J = 7.09 Hz, 2H, benzothiazole), 7.41 (d, J = 6.12 Hz, 2H, Ar-H), 7.20 (d, J = 7.10 Hz, 2H, Ar-H), 13CNMR (500MHz) 121.27, 121.55, 124.34, 125.29, 125.54, 148.43, 174.57 (C, Benzothiazole), 159.79 (CH), 117.26, 117.45, 118.38, 118.50, 120.46, 128.57, 128.73, 129.88, 129.94, 130.21, 157.58, 159.42 (C, Ar-C.); MS (FAB) [M+1]+:(m/z) 365.05
(E)-N-(4-(4-Methoxyphenoxy) benzylidene) benzo[d]thiazol-2-amine (G7)
IR (KBr) cm-1:3120 (C-H Aromatic), 1650 (C=N), 1615 (C-N), 1514(C-C Str. Aromatic), 1469 (C=C Str. Aromatic), 1250 (C-S), 1125 (C-O str.); 1HNMR (500 MHz) (ppm): 8.55 (s, 1H, CH), 8.12 (d, J = 9.12 Hz, 1H, benzothiazole), 7.90 (d, J = 4.50 Hz, 1H, benzothiazole), 7.88 (d, J = 7.5 Hz, 2H, Ar-H), 7.53 (d, J = 6.25 Hz, 2H, Ar-H), 7.49 (t, J = 7.05 Hz, 2H, benzothiazole), 6.97 (d, J = 7.42 Hz,2H, Ar-H), 3.85 (s, 3H, OCH3); 13CNMR (500 MHz) 121.39, 121.48, 124.45, 125.19, 125.73, 148.62, 174.46 (C, Benzothiazole), 159.83 (CH), 113.85, 114.32, 117.11, 117.39, 119.46, 128.32, 128.69, 129.83, 129.91, 131.21, 154.32, 158.76 (C, Ar-C.), 54.82 (C, OCH3); MS (FAB) [M+1]+:(m/z) 361.08
(E)-N-(4-(4-Fluorophenoxy) benzylidene) benzo[d]thiazol-2-amine (G8)
IR (KBr) cm-1:3119 (C-H Aromatic), 1658 (C=N), 1621 (C-N), 1515 (C-C Str. Aromatic), 1477 (C=C Str. Aromatic), 1255 (C-S), 1131 (C-O str.), 687 (C-F Str.); 1HNMR(500 MHz) (ppm): 8.57 (s, 1H, CH), 8.11 (d, J = 10 Hz, 1H, benzothiazole), 8.01 (d, J = 5.0 Hz, 1H, benzothiazole), 7.90 (d, J = 7.0 Hz,2H, Ar-H), 7.52 (t, J = 6.50 Hz,2H, benzothiazole), 7.43 (d, J = 7.0 Hz, 2H, Ar-H), 7.20 (d, J = 7.45 Hz, 4H, Ar-H); 13CNMR (500 MHz) 121.40, 121.51, 124.39, 125.23, 125.78, 148.65, 174.51 (C, Benzothiazole), 159.76 (CH), 113.71, 114.29, 117.19, 117.42, 119.53, 128.35, 128.62, 129.78, 129.95, 131.24, 154.36, 158.51 (C, Ar-C.);MS (FAB) [M+1]+:(m/z) 349.08
(E)-N-(4-(4-Nitrophenoxy)benzylidene)benzo[d]thiazol-2-amine (G9)
IR (KBr) cm-1:3116 (C-H Aromatic), 1653 (C=N), 1612 (C-N), 1507 (C-C Str. Aromatic), 1472 (C=C Str. Aromatic), 1305 (N-O Str.), 1251 (C-S), 1129 (C-O str.); 1HNMR (500 MHz) (ppm): 8.57 (s, 1H, CH), 8.21 (d, J = 9.98 Hz, 2H, Ar-H), 8.17 (d, J = 7.0 Hz, 1H, benzothiazole), 8.02 (d, J = 5.12 Hz, 1H, benzothiazole), 7.89 (d, J = 7.5 Hz, 2H, Ar-H), 7.49 (t, J = 6.25 Hz, 2H, benzothiazole), 7.21 (d, J = 7.25 Hz,4H, Ar-H); 13CNMR (500 MHz) 121.38, 121.54, 124.37, 125.26, 125.69, 148.77, 174.56 (C, Benzothiazole), 159.68 (CH), 113.66, 114.31, 117.23, 117.45, 119.48, 128.39, 128.59, 129.72, 129.89, 131.27, 154.39, 158.48 (C, Ar-C.);MS (FAB) [M+1]+:(m/z) 376.05
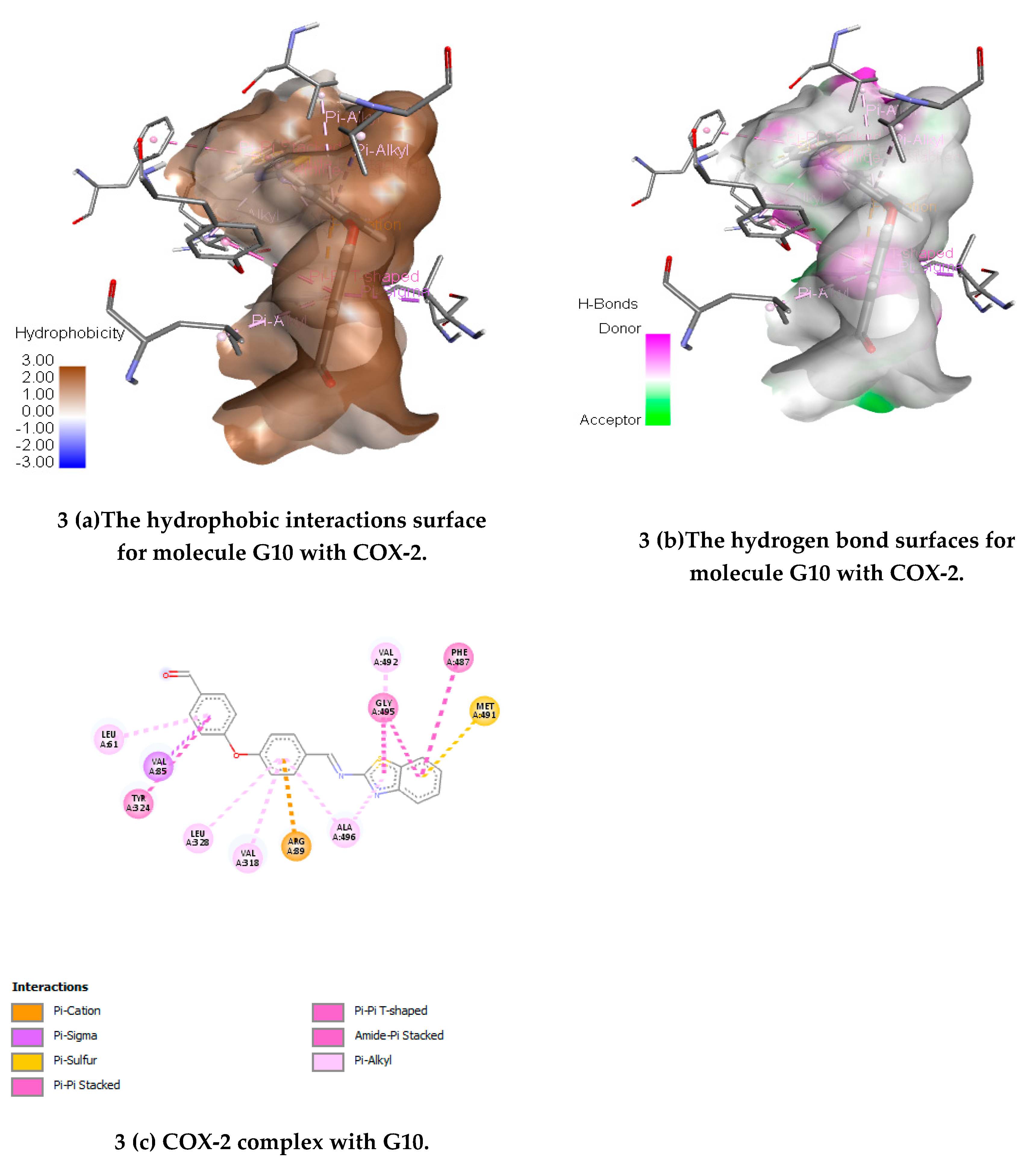
(E)-4-(4-((Benzo[d]thiazol-2-ylimino) methyl) phenoxy) benzaldehyde(G10)
IR (KBr) cm-1:3118 (C-H Aromatic), 1656 (C=N), 1618 (C-N), 1510 (C-C Str. Aromatic), 1475 (C=C Str. Aromatic), 1257 (C-S), 1125 (C-O str.); 1HNMR (500 MHz) (ppm): 9.85 (s, 1H, CHO), 8.52 (s, 1H, CH), 8.17 (d, J = 9.75 Hz, 1H, benzothiazole), 7.99 (d, J = 5.25 Hz,1H, benzothiazole), 7.89 (d, J = 7.75 Hz, 2H, Ar-H), 7.46 (t, J = 6.50 Hz, 2H, benzothiazole), 7.21 (d, J = 7.0 Hz, 2H, Ar-H), 6.79 (d, J = 5.50 Hz, 2H, Ar-H), 6.71 (d, J = 7.0 Hz, 2H, Ar-H); 13CNMR (500 MHz) 121.29, 121.59, 124.28, 125.21, 125.65, 148.69, 174.48 (C, Benzotriazole), 159.65 (CH), 113.71, 114.42, 117.31, 117.48, 119.46, 128.41, 128.53, 129.69, 129.85, 131.23, 154.33, 158.51 (C, Ar-C.); MS (FAB) [M+1]+:(m/z) 359.06
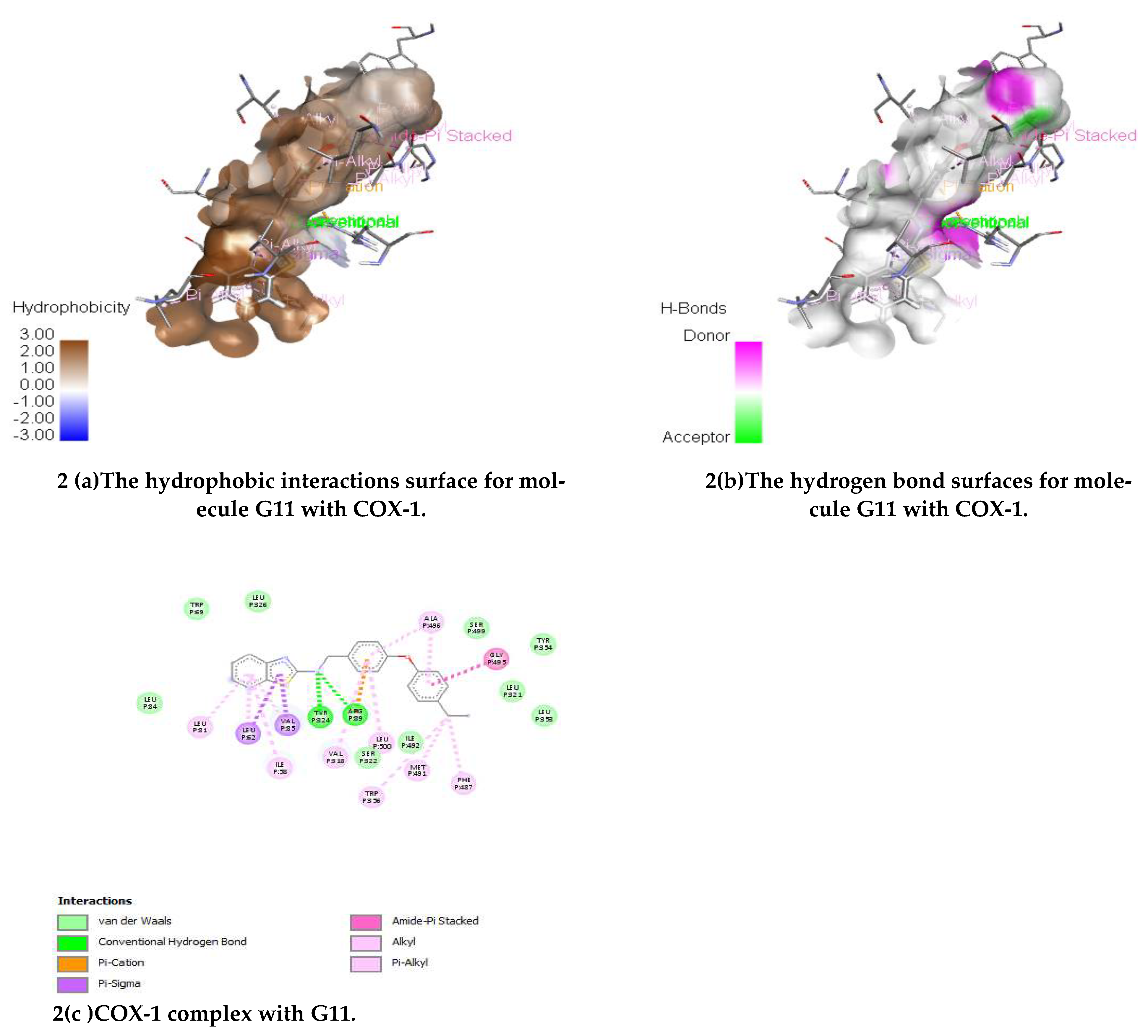
(E)-N-(4-(4-Ethylphenoxy)benzylidene)benzo[d]thiazol-2-amine (G11)
IR (KBr) cm-1:3114 (C-H Aromatic), 1652 (C=N), 1614 (C-N), 1509 (C-C Str. Aromatic), 1481 (C=C Str. Aromatic), 1260 (C-S), 1129 (C-O str.); 1HNMR (500 MHz) (ppm): 8.58 (s, 1H, CH), 8.18 (d, J = 9.83 Hz, 1H, benzothiazole), 8.01 (d, J = 5.50 Hz, 1H, benzothiazole), 7.91 (d, J = 8.0 Hz, 2H, Ar-H), 7.50 (t, J = 6.75 Hz, 2H, benzothiazole), 7.35 (d, J = 4.50 Hz, 2H, Ar-H), 7.25 (d, J = 7.25 Hz,2H, Ar-H), 6.98 (d, J = 6.0 Hz, 2H, Ar-H); 2.55 (s, 2H, CH2), 121 (s, 3H, CH3); 13CNMR (500 MHz) 121.31, 121.60, 124.31, 125.23, 125.68, 148.65, 174.41 (C, Benzothiazole), 159.52 (CH), 113.51, 114.46, 117.38, 117.51, 119.62, 128.43, 128.55, 129.63, 129.80, 131.28, 154.29, 158.48 (C, Ar-C.), 28.11 (C, CH2), 14.23 (C, CH3); MS (FAB) [M+1]+:(m/z) 359.10
(E)-N-(4-(4-Chlorophenoxy) benzylidene) benzo[d]thiazol-2-amine (G12)
IR (KBr) cm-1:3119 (C-H Aromatic), 1655 (C=N), 1618 (C-N), 1514 (C-C Str. Aromatic), 1471 (C=C Str. Aromatic), 1264 (C-S), 1135 (C-O str.), 708 (C-Cl Str.); 1HNMR(500 MHz) (ppm): 8.57 (s, 1H, CH), 8.15 (d, J = 10 Hz, 1H, benzothiazole), 7.93 (d, J = 7.95 Hz, 1H, benzothiazole), 7.88 (d, J = 8.25 Hz, 2H, Ar-H), 7.53 (d, J = 6.50 Hz,2H, Ar-H), 7.48 (t, J = 7.0 Hz,2H, benzothiazole), 7.38 (d, J = 7.50 Hz, 2H, Ar-H), 7.18 (d, J = 7.25 Hz, 2H, Ar-H), 13CNMR (500 MHz) 121.27, 121.63, 124.38, 125.29, 125.75, 148.59, 174.39 (C, Benzothiazole), 159.61 (CH), 113.42, 114.53, 117.42, 117.55, 119.58, 128.46, 128.52, 129.66, 129.83, 131.32, 154.26, 158.46 (C, Ar-C.); MS (FAB) [M+1]+:(m/z) 365.03
(E)-N-(Benzo[d]thiazol-2-yl)-1-(4-(m-tolyloxy) phenyl) methanimine (G13)
IR (KBr) cm-1:3111 (C-H Aromatic), 1643 (C=N), 1617 (C-N), 1501 (C-C Str. Aromatic), 1480 (C=C Str. Aromatic), 1265 (C-S), 1132 (C-O str.); 1HNMR(500 MHz) (ppm): 8.55 (s, 1H, CH), 8.18 (d, J = 9.50 Hz, 1H, benzothiazole), 7.93 (d, J = 7.83 Hz, 1H, benzothiazole), 7.83 (d, J = 8.0 Hz, 2H, Ar-H), 7.52 (t, J = 6.25 Hz, 2H, benzothiazole), 7.05 (d, J = 4.50 Hz, 1H, Ar-H), 7.20 (d, J = 7.25 Hz, 2H, Ar-H), 6.89 (d, J = 5.0 Hz, 1H, Ar-H), 6.75 (d, J = 4.0 Hz,1H, Ar-H); 1.23 (3H, CH3); 13CNMR (500 MHz) 121.19, 121.58, 124.41, 125.32, 125.68, 148.54, 174.35 (C, Benzothiazole), 159.58 (CH), 113.45, 114.48, 117.36, 117.58, 119.53, 128.39, 128.55, 129.61, 129.78, 131.30, 154.32, 158.53 (C, Ar-C.), 21.33 (C, CH3); MS (FAB) [M+1]+:(m/z) 359.11