Submitted:
08 June 2023
Posted:
09 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
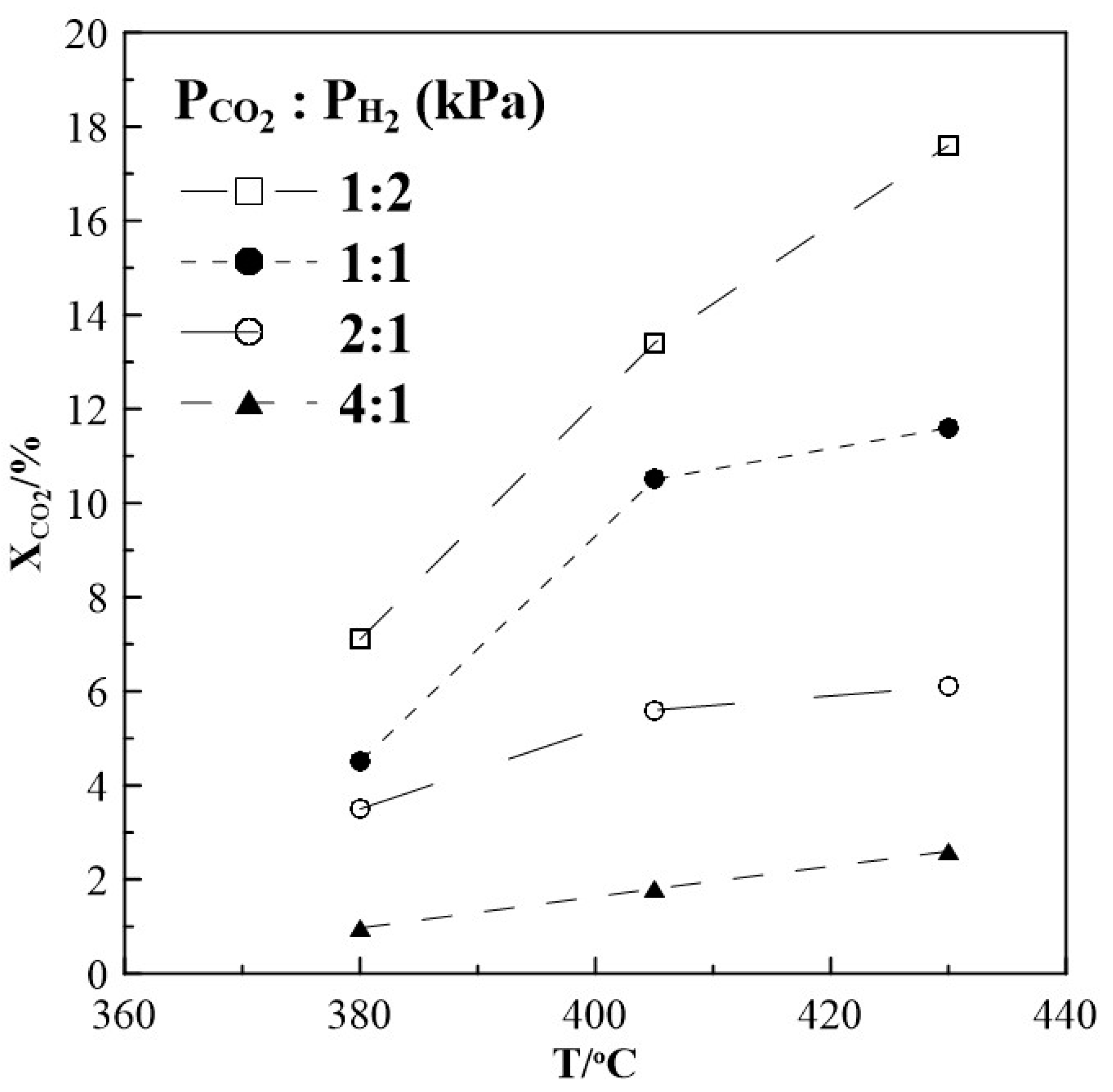
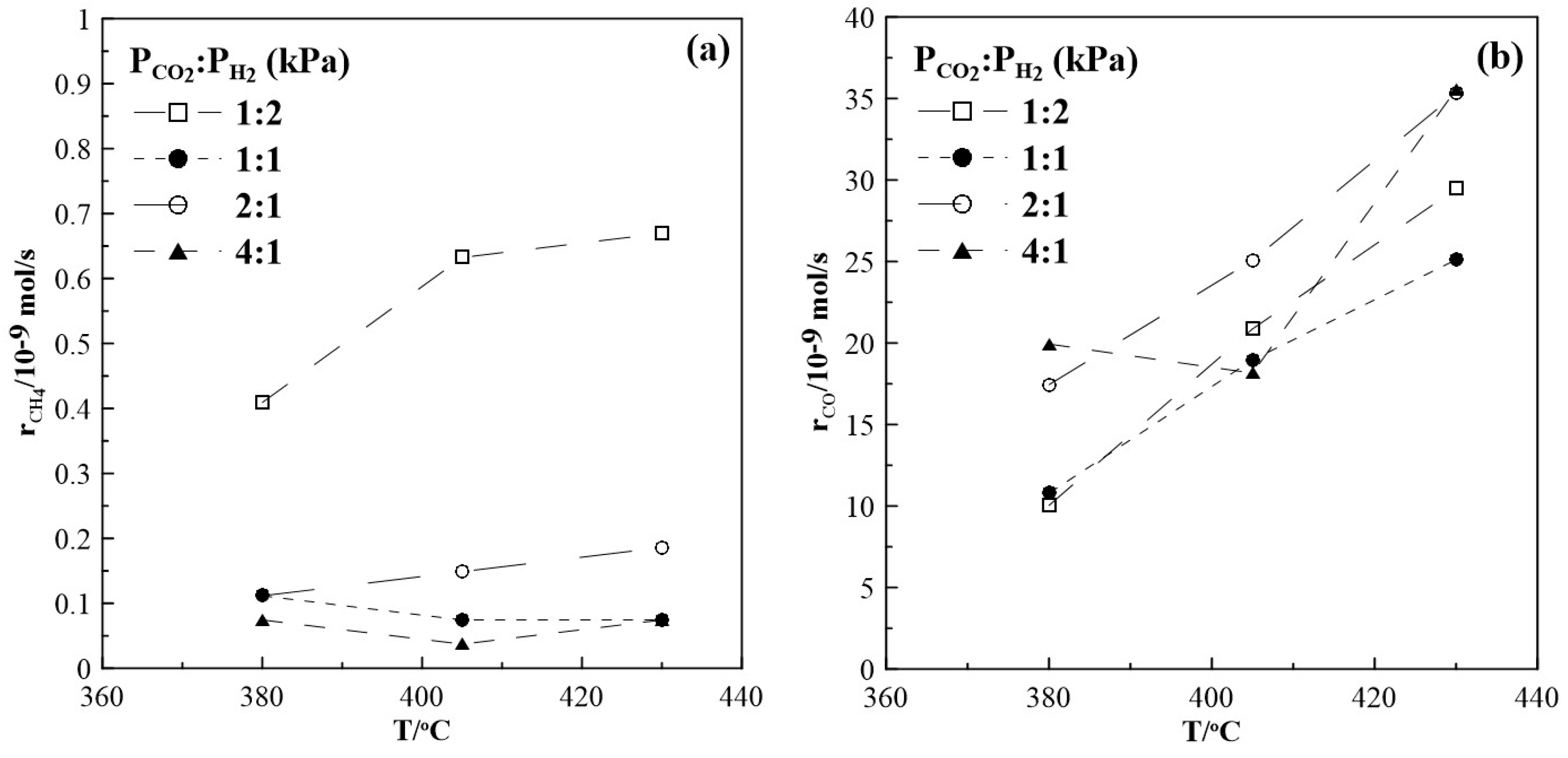
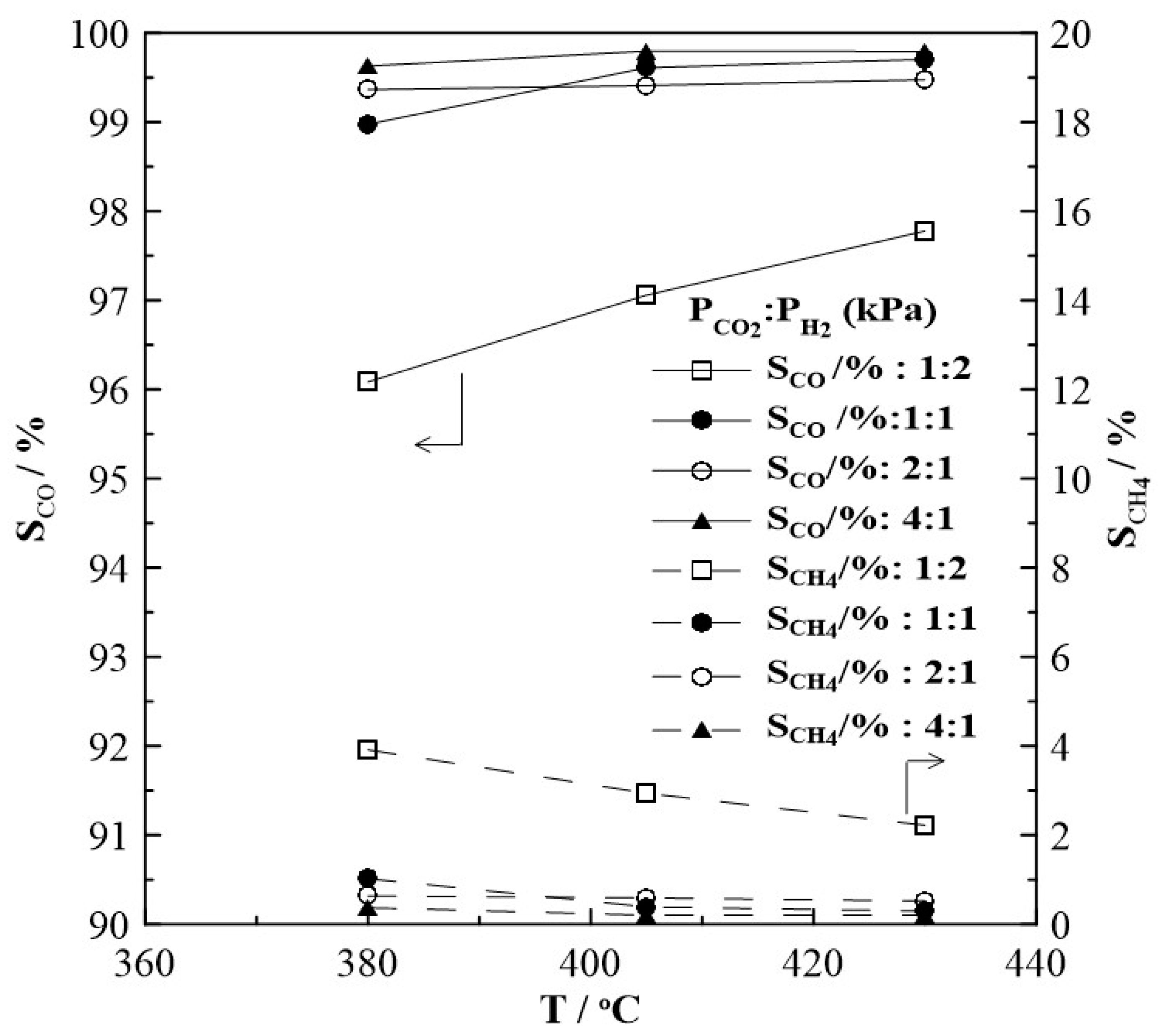
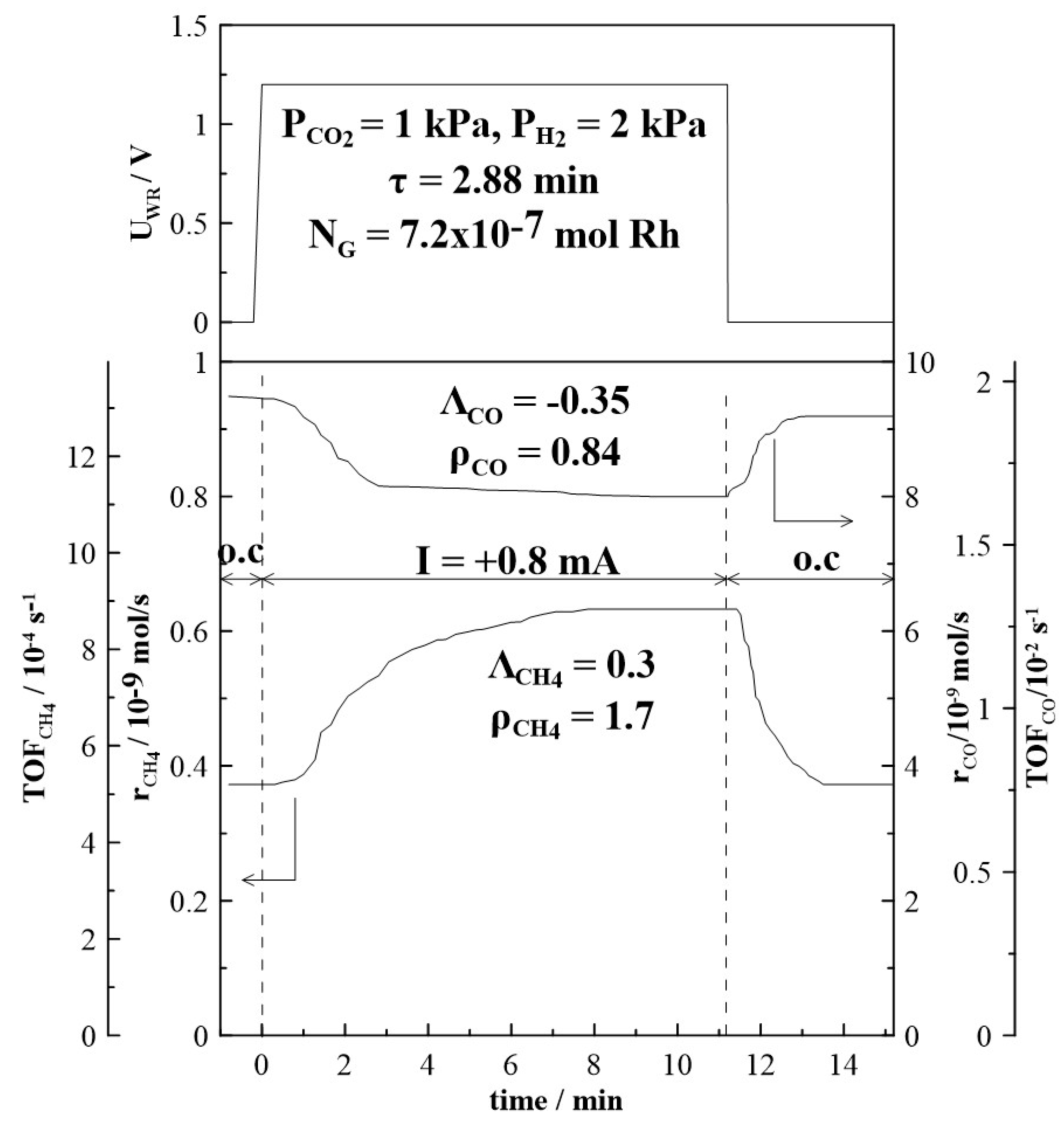
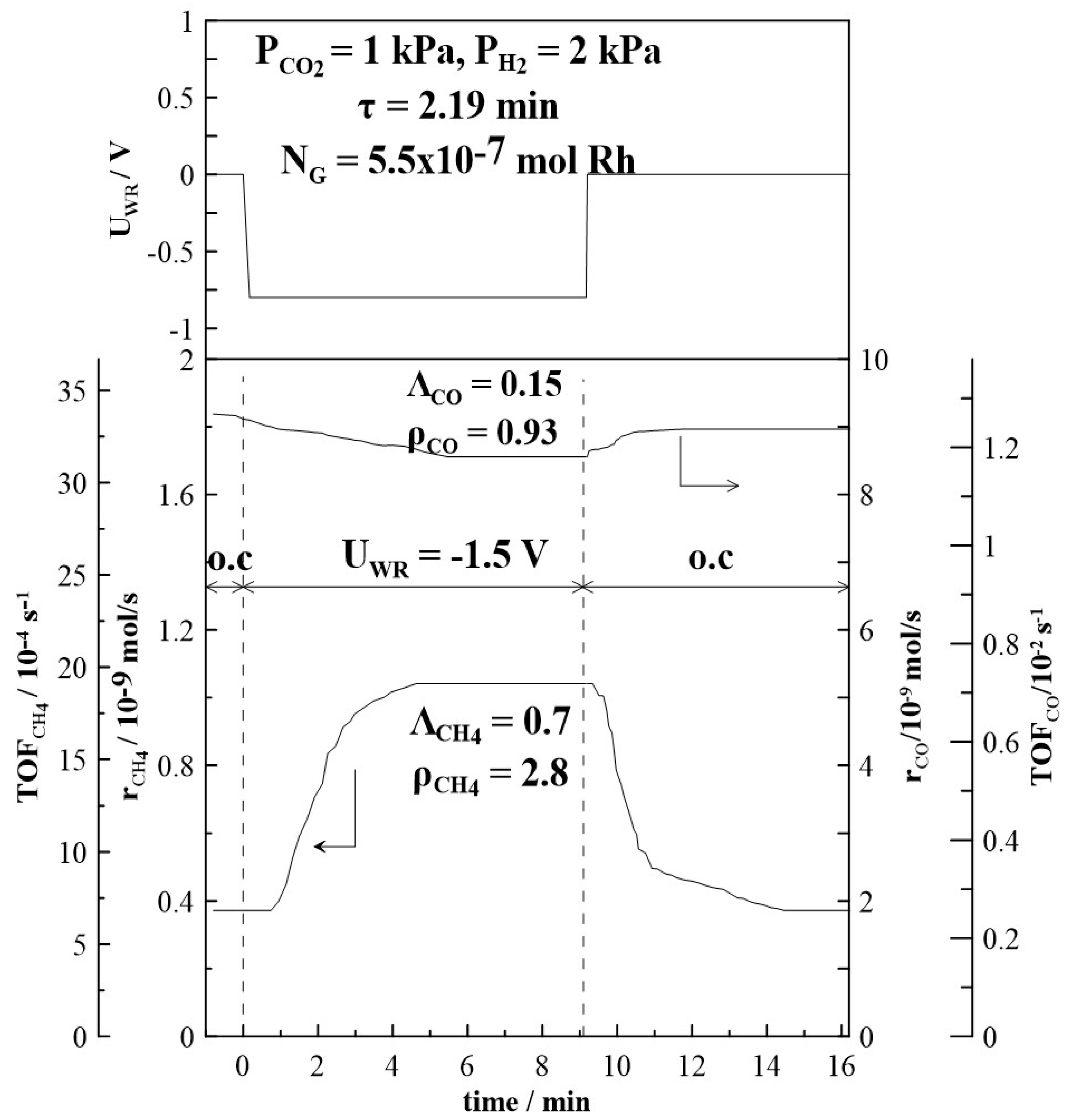
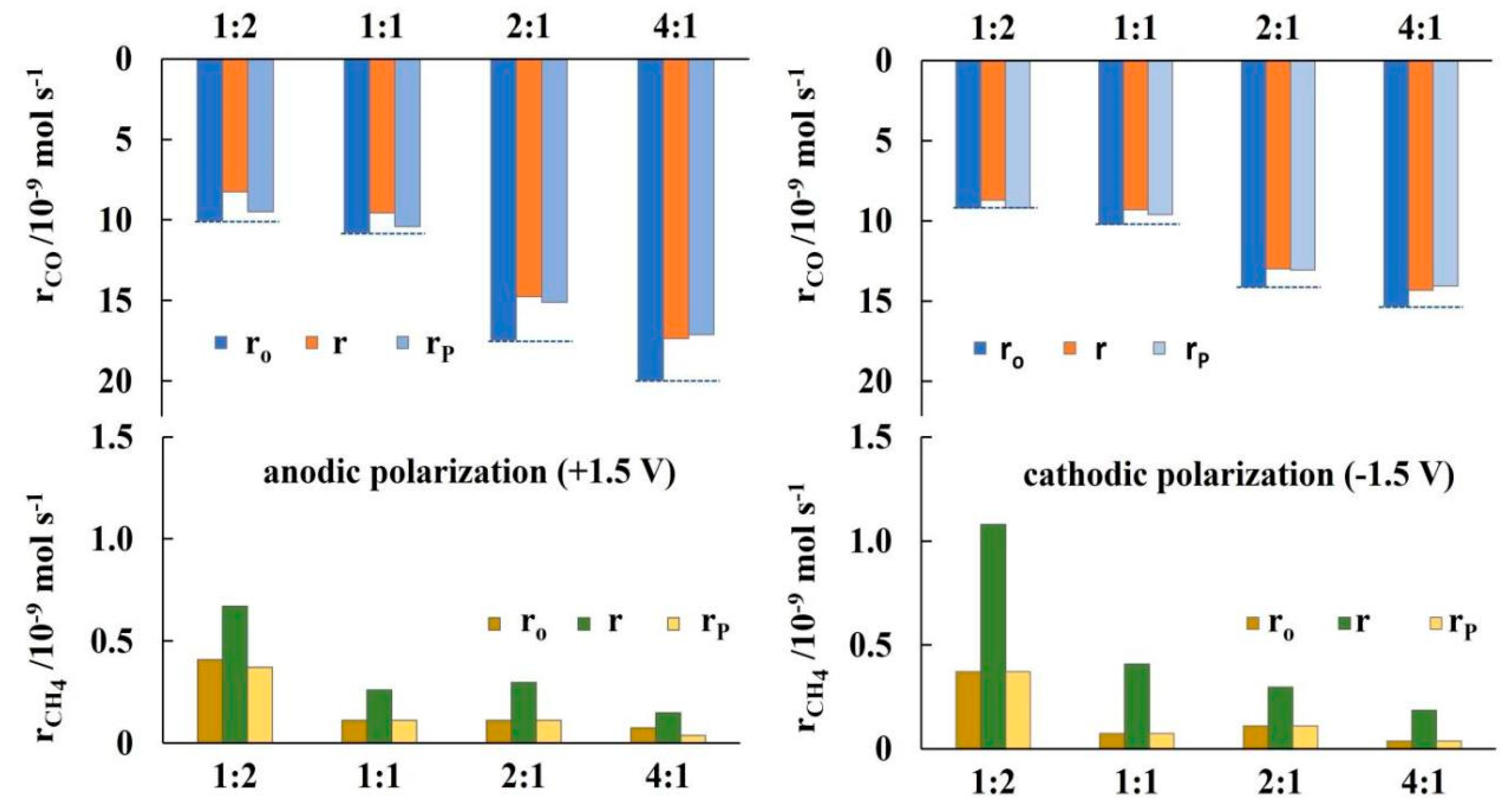
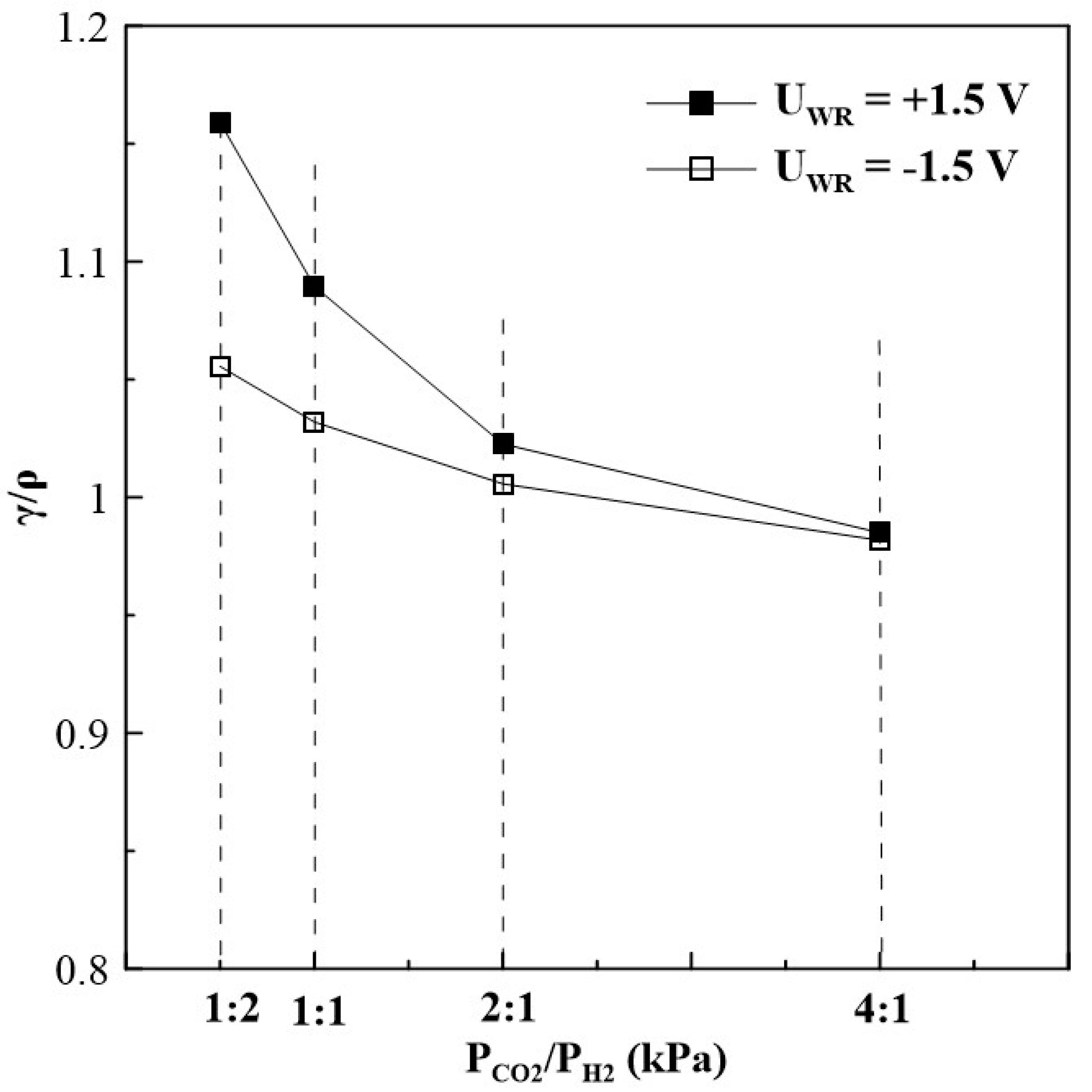
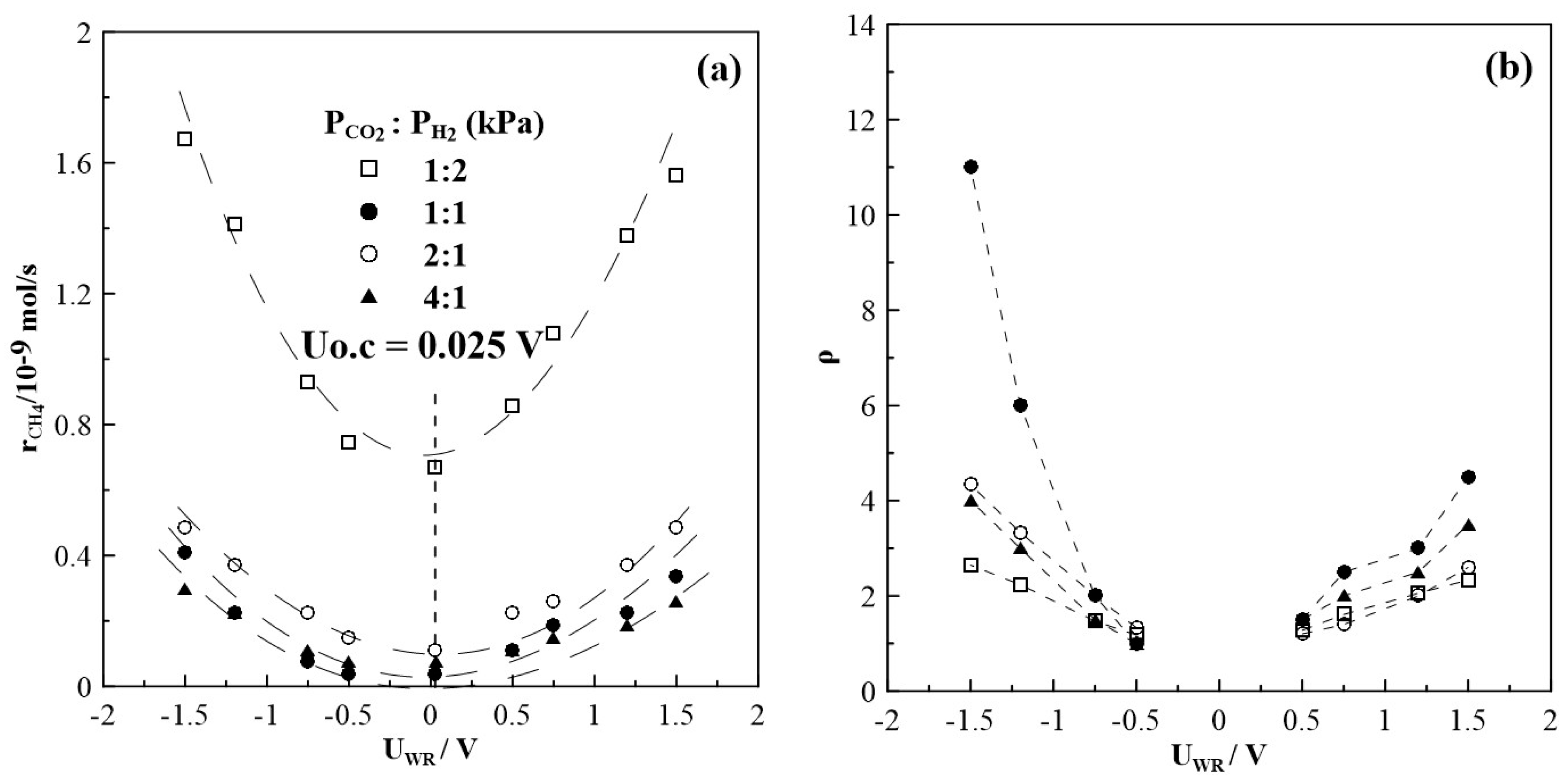
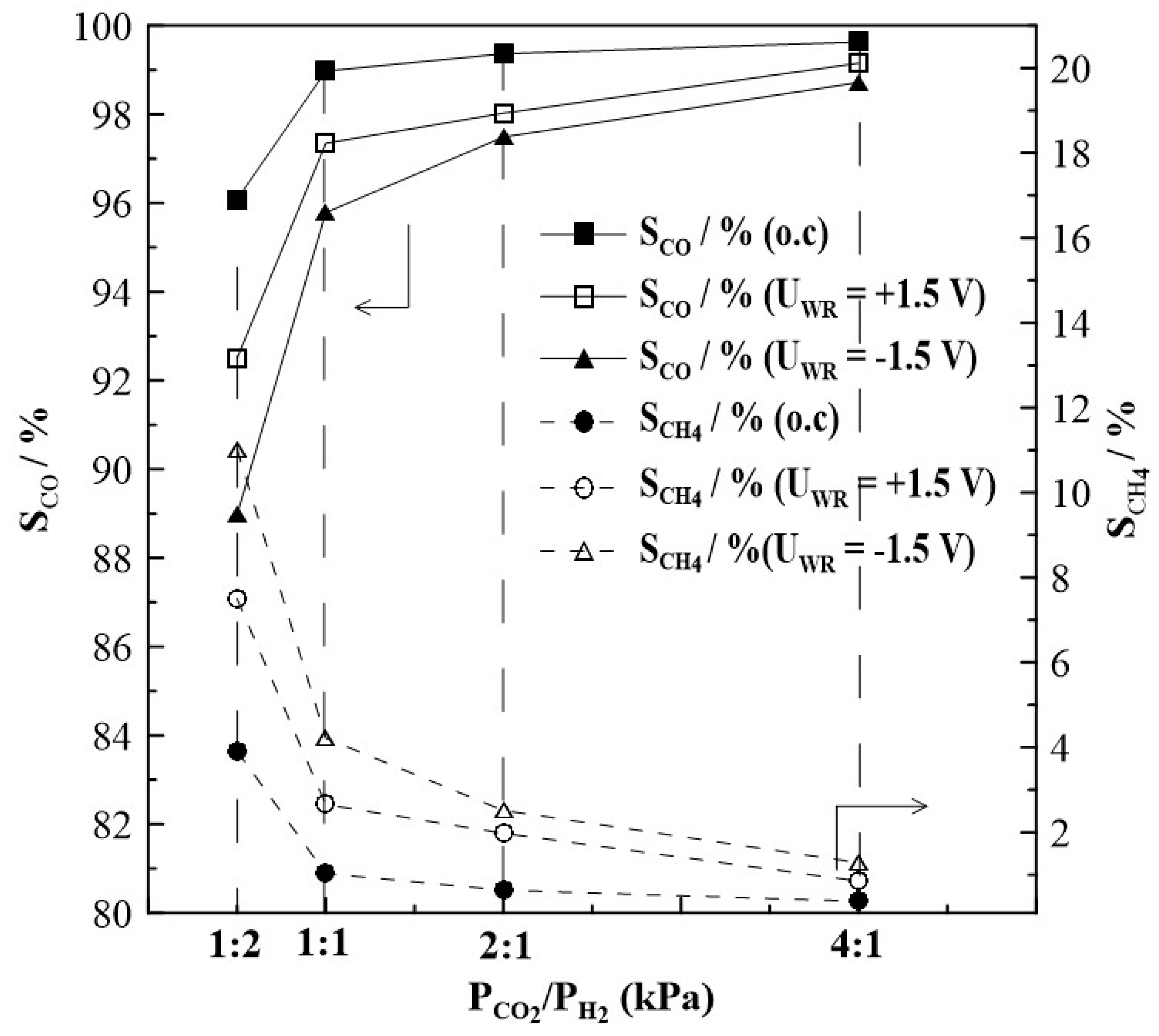
2.1. Hydrogenation Activity Measurements
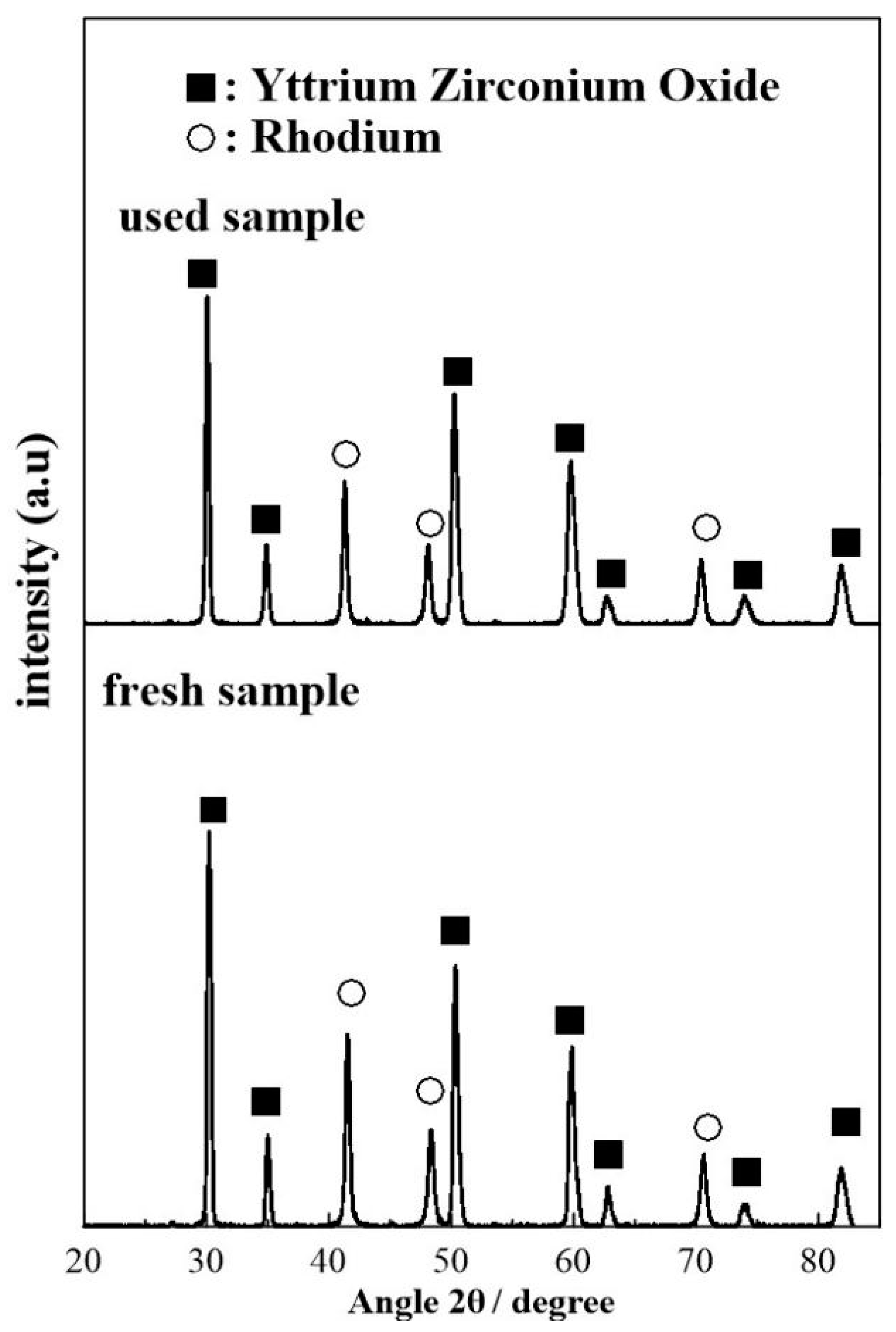
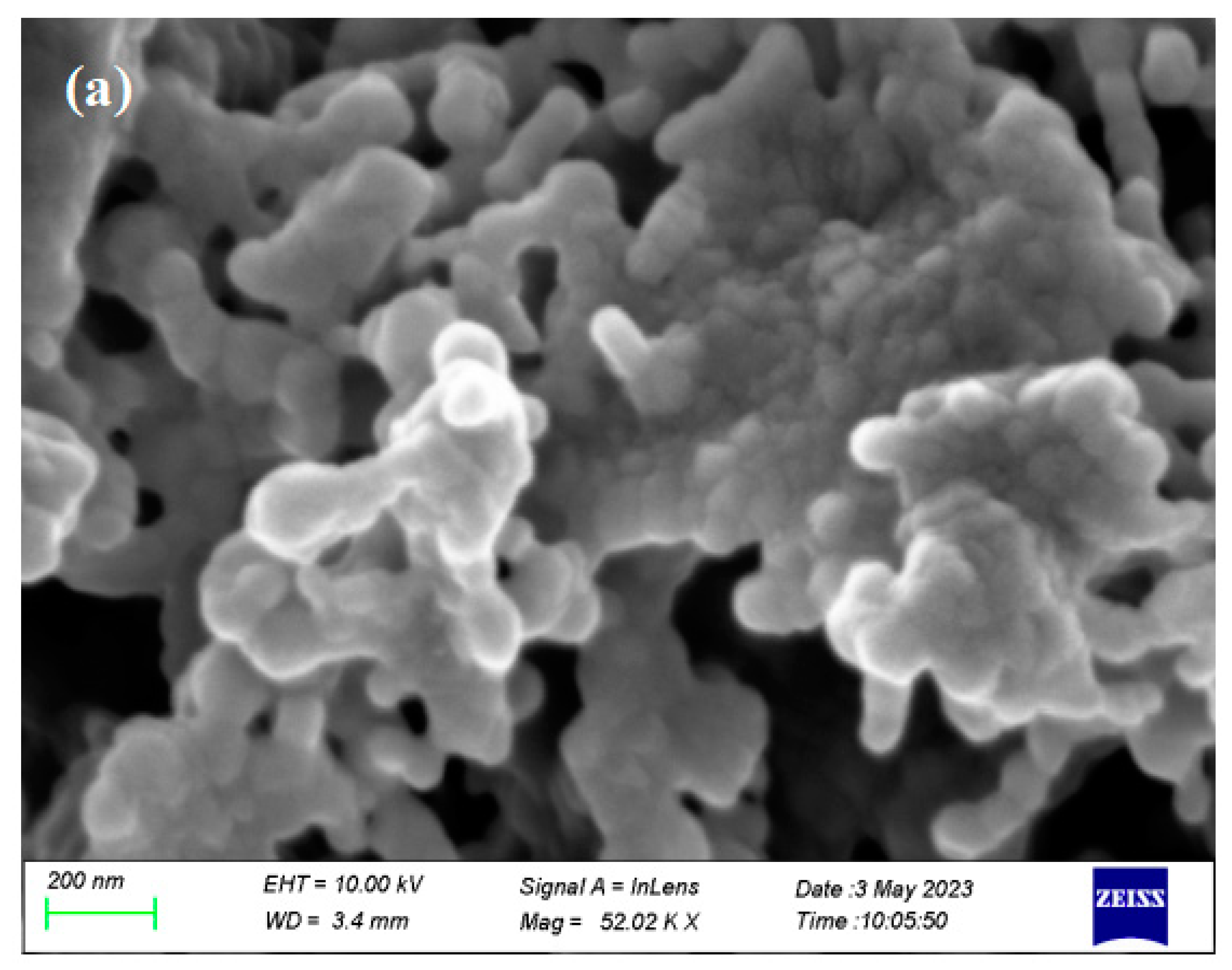
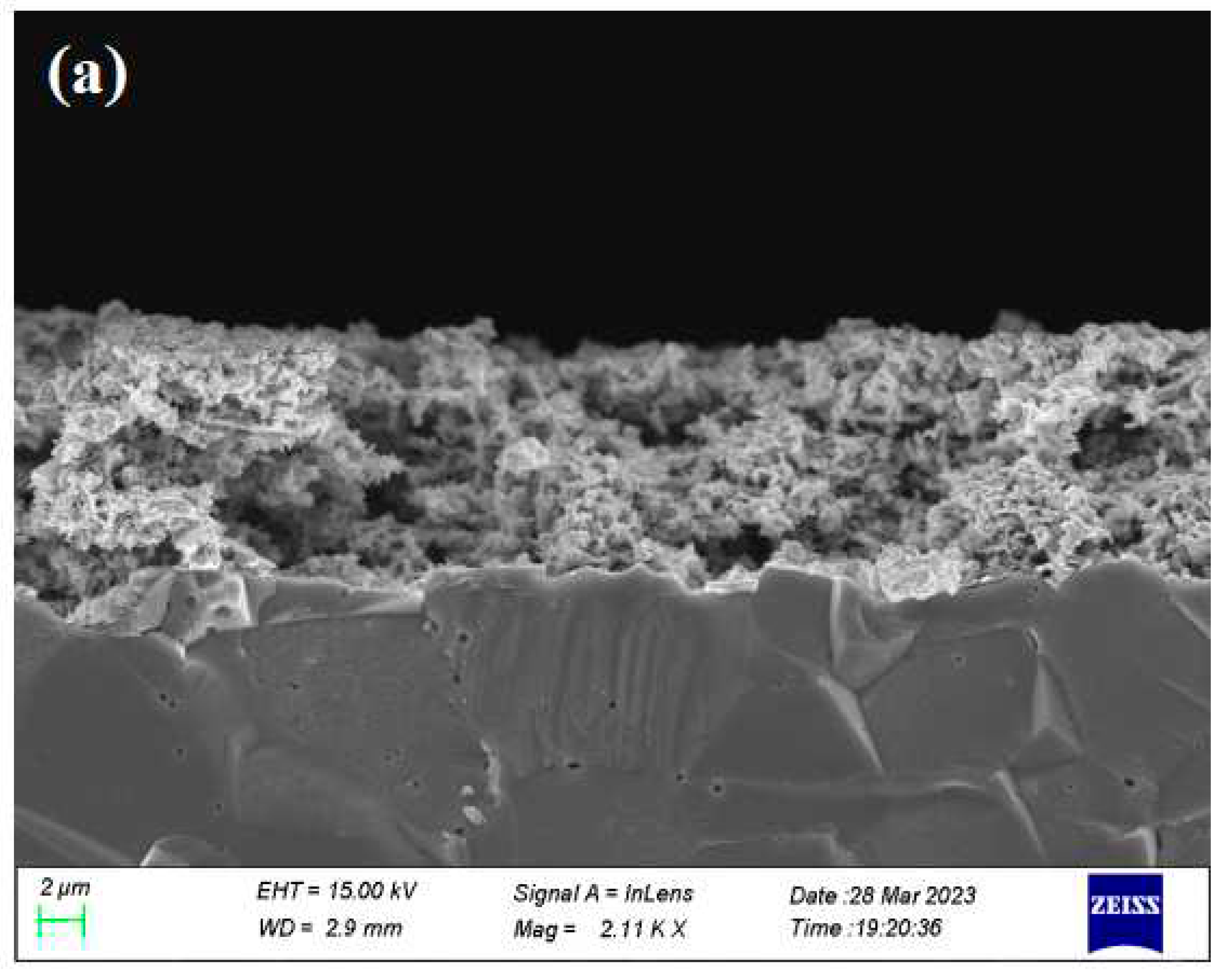
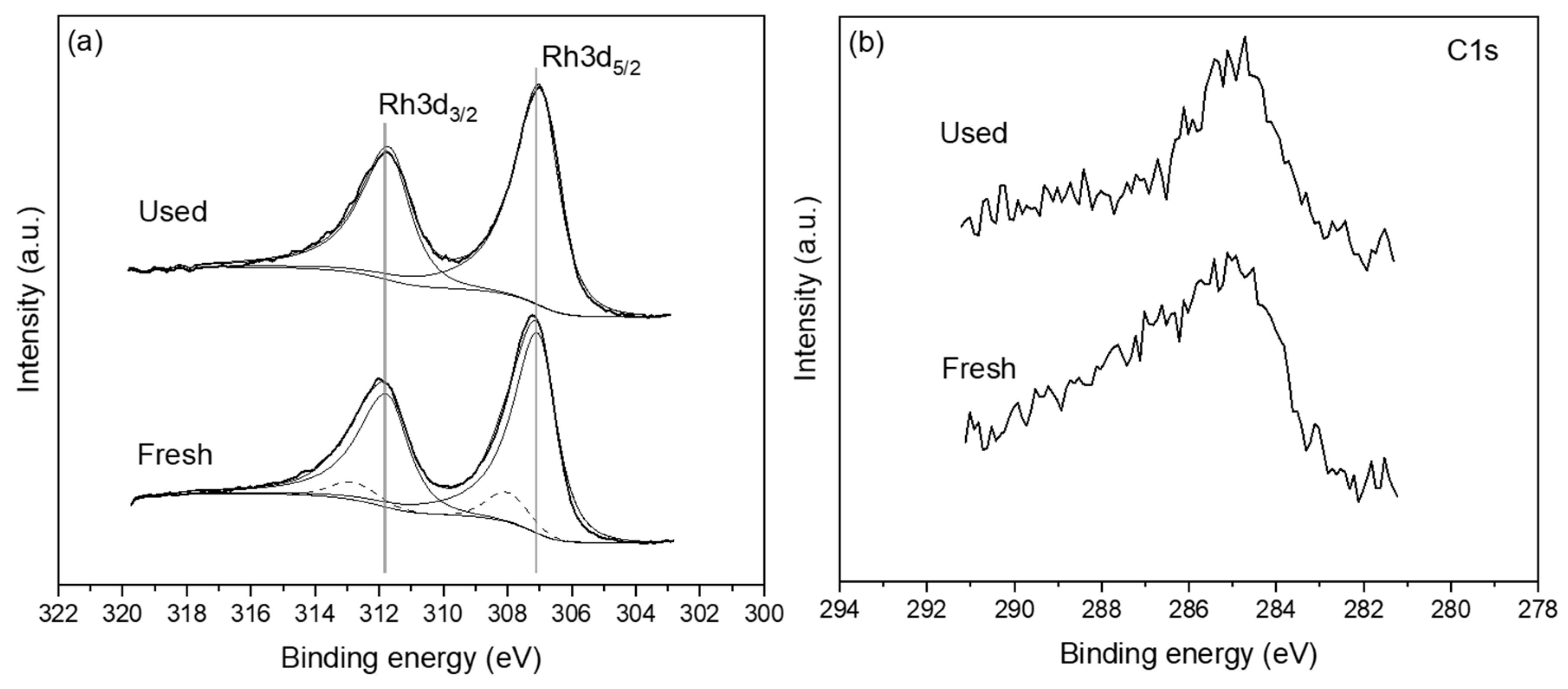
2.2. Catalyst Characterization
3. Experimental
3.1. Catalyst Preparation
3.2. Reactor Operation
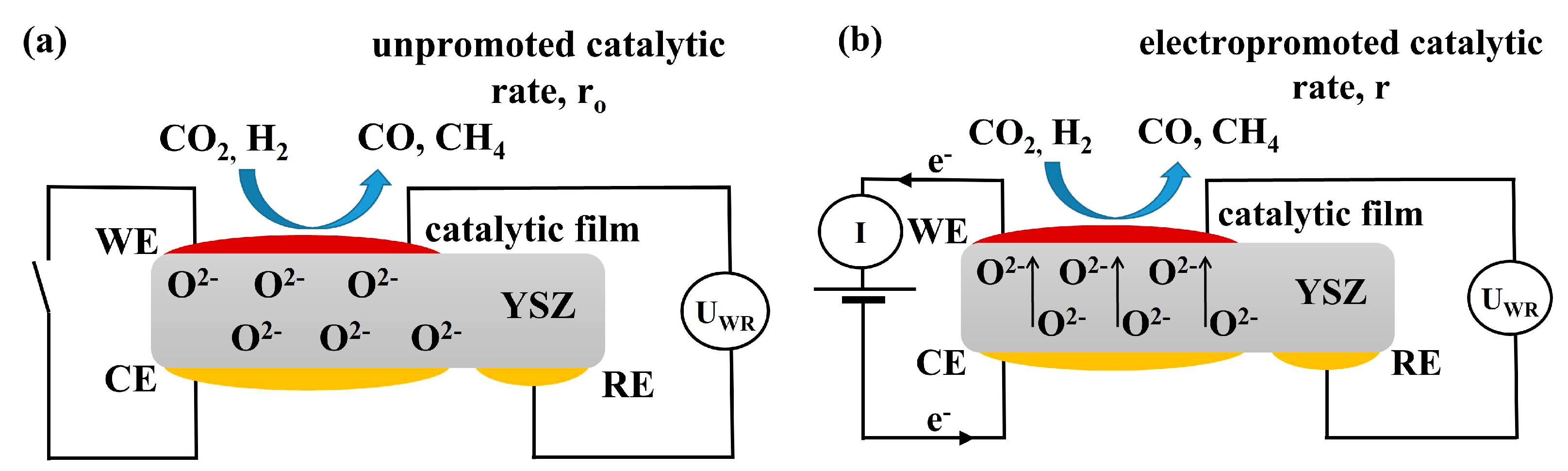
3.3. Electrochemical Promotion Parameters Computation
3.4. Catalyst Characterization
4. Conclusions
Acknowledgments
References
- Kinney, P.L. Interactions of Climate Change, Air Pollution, and Human Health. Curr Environ Health Rep 2018, 5, 179–186. [Google Scholar] [CrossRef]
- Berry, H.L.; Waite, T.D.; Dear, K.B.G.; Capon, A.G.; Murray, V. The Case for Systems Thinking about Climate Change and Mental Health. Nat Clim Chang 2018, 8, 282–290. [Google Scholar] [CrossRef]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning Selectivity of CO2 Hydrogenation Reactions at the Metal/Oxide Interface. J Am Chem Soc 2017, 139, 9739–9754. [Google Scholar] [CrossRef]
- Knutson, T.R.; Tuleya, R.E. Impact of CO2-Induced Warming on Simulated Hurricane Intensity and Precipitation: Sensitivity to the Choice of Climate Model and Convective Parameterization; J. of Climate 2004, 17/18, 3477-3495.
- Schwiderowski, P.; Ruland, H.; Muhler, M. Current Developments in CO2 Hydrogenation towards Methanol: A Review Related to Industrial Application. Curr Opin Green Sustain Chem 2022, 38. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Ding, M.; Hong, X.; Liu, G.; Tsang, S.C.E. Advances in Higher Alcohol Synthesis from CO2 Hydrogenation. Chem 2021, 7, 849–881. [Google Scholar] [CrossRef]
- Chatzilias, C.; Martino, E.; Zagoraios, D.; Kyriakou, G.; Katsaounis, A. Electrochemical Promotion of Catalysis for CO2 Valorization. In: Vernoux, P., Vayenas, C.G. (eds) Recent Advances in Electrochemical Promotion of Catalysis. Modern Aspects of Electrochemistry, 2022, Vol 61, Springer, Cham.
- Nozaki, F.; Sodesawa, T.; Satoh, S.; Kimura, K. Hydrogenation of Carbon Dioxide into Light Hydrocarbons at Atmospheric Pressure over Rh/Nb205 or Cu/Si02-Rh/Nb205. J Catal 1987, 104, 339–346. [Google Scholar] [CrossRef]
- Solymosi, F.; Pasztor, M. Analysis of the R-Spectral Behavior of Adsorbed CO Formed in H2 + CO2 Surface Interaction over Supported Rhodium. J Catal 1987, 104, 312–322. [Google Scholar] [CrossRef]
- Lapidus, A.L.; Gaidai, N.A.; Nekrasov, N. V.; Tishkova, L.A.; Agafonov, Y.A.; Myshenkova, T.N. The Mechanism of Carbon Dioxide Hydrogenation on Copper and Nickel Catalysts. Petroleum Chemistry 2007, 47, 91–98. [Google Scholar] [CrossRef]
- Borodko, Y.; Somorjai, G.A. Catalytic Hydrogenation of Carbon Oxides-a 10-Year Perspective; 1999, 186, 1–2, 355-362.
- Kusama, H.; Kitamura Bando, K.; Okabe, K.; Arakawa, H. Effect of Metal Loading on CO 2 Hydrogenation Reactivity over Rh/SiO 2 Catalysts. Appl Catal 2000, 197, 255–268. [Google Scholar] [CrossRef]
- Gasser, D.; Baiker, A. Hydrogenation of Carbon Dioxide over Copper-Zirconia Catalysts Prepared by In-Situ Activation of Amorphous Copper-Zirconium Alloy; Appl Catal 1989, 48, 279–294.
- Amenomiya, Y. Methanol synthesis from CO+H2. II Copper-based binary and ternary catalysts. Appl Catal 1987, 30, 57–68.
- Sahibzada, M.; Chadwick, D.; Metcalfe, I.S. Hydrogenation of Carbon Dioxide to Methanol over Palladium-Promoted Cu/ZnO/A1203 Catalysts. Catalysis Today 1996, 29, 367–372. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdhelyi, A.; Bansagi, T. Methanation of CO, on Supported Rhodium Catalyst. J Catal 1981, 68, 371–382. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Tsubaki, N. Dual Catalysis Mechanism of Alcohol Solvent and Cu Catalyst for a New Methanol Synthesis Method. Catal Commun 2005, 6, 275–279. [Google Scholar] [CrossRef]
- Schild, C.; Wokaun, A.; Baiker, A. On the Mechanism of CO and CO2 Hydrogenation Reactions on Zirconia-Supported Catalysts: A Diffuse Reflectance FTIR Study Part II. Surface Species on Copper/Zirconia Catalysts: Implications for Methanol Synthesis Selectivity. J Mol Catal 1990, 63, 243–254. [Google Scholar] [CrossRef]
- Vannice, M.A. The Catalytic Synthesis of Hydrocarbons from HJCO Mixtures over the Group VIII Metals I. The Specific Activities and Product Distributions of Supported Metals. J Catal 1975, 31, 449–461. [Google Scholar] [CrossRef]
- Araki, R.; Ponec, V. Methanation of Carbon Monoxide on Nickel and Nickel-Copper Alloys. J Catal 1976, 44, 439–448. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Selective Methanation of CO over Supported Noble Metal Catalysts: Effects of the Nature of the Metallic Phase on Catalytic Performance. Appl Catal A Gen 2008, 344, 45–54. [Google Scholar] [CrossRef]
- Henderson, A.; Worley, S.D.; Peebles, D.E. An Infrared Study of the Hydrogenation of Carbon Dioxide on Supported Rhodium Catalys An inverse spillover effect. J. Phys. Chem. 1985, 89, 392–394. [Google Scholar] [CrossRef]
- Solymosi, F.; Tombacz, I.; Koszta, J. Effects of Variation of Electric Properties of TiOp Support on Hydrogenation of CO and CO2 over Rh Catalysts. J. Catal. 1985, 95, 578–586. [Google Scholar] [CrossRef]
- Brown Bourzutschky, J.A.; Homs, N.; Bell, A.A.T. Hydrogenation of 002 and 002/00 Mixtures over Copper-Containing Catalysts. J Catal 1990, 124, 73–85. [Google Scholar] [CrossRef]
- Chanchlani, K.G.; Hudgins, R.R.; Silveston, P.L. Methanol Synthesis from H2, CO, and 002 over Cu/ZnO Catalysts. J Catal 1992, 136, 59–75. [Google Scholar] [CrossRef]
- Nitta, Y.; Suwata, O.; Ikeda, Y.; Okamoto, Y.; Imanaka, T. Copper-Zirconia Catalysts for Methanol Synthesis from Carbon Dioxide: Effect of ZnO Addition to Cu-ZrO2 Catalysts. Catal Lett 1994, 26, 345–354. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, G.; Barbera, K.; Bordiga, S.; Bonura, G.; Spadaro, L.; Frusteri, F. Solid-State Interactions, Adsorption Sites and Functionality of Cu-ZnO/ZrO2 Catalysts in the CO2 Hydrogenation to CH3OH. Appl Catal A Gen 2008, 350, 16–23. [Google Scholar] [CrossRef]
- Reubroycharoen, P.; Vitidsant, T.; Yoneyama, Y.; Tsubaki, N. Development of a New Low-Temperature Methanol Synthesis Process. Catal Today 2004, 89, 447–454. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Adsorption of CO Accompanied with Simultaneous Charge Transfer on Copper Single Crystal Electrodes Related with Electrochemical Reduction of CO2 to Hydrocarbons. Surface Science 1995, 335, 258–260. [Google Scholar] [CrossRef]
- Ando, H.; Xu, Q.; Fujiwara, M.; Matsumura, Y.; Tanaka, M.; Souma, Y. Hydrocarbon Synthesis from CO 2 over Fe±Cu Catal Tod 1998, 45, 229–234.
- Trovarelli, A.; Mustazza, C.; Dolcetti, G.; Kagpar, J.; Graziani, M. Carbon dioxide hydrogenation on rhodium supported on transition metal oxides. Effect of reduction temperature on product distribution. Appl Catal 1990, 65, 129–142. [Google Scholar] [CrossRef]
- Marwood, M.; Doepper, R.; Renken, A. In-Situ Surface and Gas Phase Analysis for Kinetic Studies under Transient Conditions:The Catalytic Hydrogenation of CO2. Appl Catal 1997, 151, 223–246. [Google Scholar] [CrossRef]
- Falconer, J.L. and Zagli, A.E. Adsorption and Methanation of Carbon Dioxide on a Nickel/Silica Catalyst. J Catal 1980, 62, 280–285. [Google Scholar] [CrossRef]
- 34. Biloen,’, P.; Helle, J.N.; Van Den Berg, F.G.A.; Sachtler, W.M.H. On the Activity of Fischer-Tropsch and Methanation Catalysts: A Study Utilizing Isotopic Transients. J Catal 1983, 81, 450–463. [CrossRef]
- Coenen, J.W.E.; Van Nisselrooy, P.F.M.T.; De Croon, M.H.J.M.; Dooren, P.F.H.A. Van; Van Meerten, R.Z.C. The dynamics of methanation of carbon modoxide on Nickel catalysts. Appl Catal 1986, 25, 1–8. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Ma, Z.; Zhang, G.; Zhang, H.; Fu, X.; Ma, Y.; Liu, F.; Liu, M.; Huang, H. Seeded Growth of Gold–Copper Janus Nanostructures as a Tandem Catalyst for Efficient Electroreduction of CO2 to C2+ Products. Small 2022, 18, 2201695. [Google Scholar] [CrossRef]
- Zhong, Y.; Kong, X.; Song, Z.; Liu, Y.; Peng, L.; Zhang, L.; Luo, X.; Zeng, J.; Geng, Z. Adjusting Local CO Confinement in Porous-Shell Ag@Cu Catalysts for Enhancing C-C Coupling toward CO2Eletroreduction. Nano Lett 2022, 22, 2554–2560. [Google Scholar] [CrossRef]
- Theleritis, D.; Souentie, S.; Siokou, A.; Katsaounis, A.; Vayenas, C.G. Hydrogenation of CO 2 over Ru/YSZ Electropromoted Catalysts. ACS Catal 2012, 2, 770–780. [Google Scholar] [CrossRef]
- Theleritis, D.; Makri, M.; Souentie, S.; Caravaca, A.; Katsaounis, A.; Vayenas, C.G. Comparative Study of the Electrochemical Promotion of CO2 Hydrogenation over Ru-Supported Catalysts Using Electronegative and Electropositive Promoters. ChemElectroChem 2014, 1. [Google Scholar] [CrossRef]
- Kalaitzidou, I.; Katsaounis, A.; Norby, T.; Vayenas, C.G. Electrochemical Promotion of the Hydrogenation of CO2 on Ru Deposited on a BZY Proton Conductor. J Catal 2015, 331, 98–109. [Google Scholar] [CrossRef]
- Makri, M.; Katsaounis, A.; Vayenas, C.G. Electrochemical Promotion of CO2 Hydrogenation on Ru Catalyst-Electrodes Supported on a K-Β″-Al2O3 Solid Electrolyte. Electrochim Acta 2015, 179, 556–564. [Google Scholar] [CrossRef]
- Kalaitzidou, I.; Makri, M.; Theleritis, D.; Katsaounis, A.; Vayenas, C.G. Comparative Study of the Electrochemical Promotion of CO2 Hydrogenation on Ru Using Na+, K+, H+ and O2- Conducting Solid Electrolytes. Surf Sci 2016, 646, 194–203. [Google Scholar] [CrossRef]
- Makri, M.; Symillidis, A.; Grigoriou, D.; Katsaounis, A.; Vayenas, C.G. Electrochemical Promotion of CO 2 Reduction on a Dispersed Ru/YSZ Catalyst Supported on YSZ Solid Electrolyte; 2018, 5, 27617–27625.
- Panaritis, C.; Michel, C.; Couillard, M.; Baranova, E.A.; Steinmann, S.N. Elucidating the Role of Electrochemical Polarization on the Selectivity of the CO2 Hydrogenation Reaction over Ru. Electrochim Acta 2020, 350, 136405. [Google Scholar] [CrossRef]
- Panaritis, C.; Zgheib, J.; Ebrahim, S.A.H.; Couillard, M.; Baranova, E.A. Electrochemical In-Situ Activation of Fe-Oxide Nanowires for the Reverse Water Gas Shift Reaction. Appl Catal B 2020, 269, 118826. [Google Scholar] [CrossRef]
- Zagoraios, D.; Panaritis, C.; Krassakopoulou, A.; Baranova, E.A.; Katsaounis, A.; Vayenas, C.G. Electrochemical Promotion of Ru Nanoparticles Deposited on a Proton Conductor Electrolyte during CO2 Hydrogenation. Appl Catal B 2020, 276, 119148. [Google Scholar] [CrossRef]
- Chatzilias, C.; Martino, E.; Vayenas, C.G.; Kyriakou, G.; Katsaounis, A. A Low Temperature SOFC as a Self-Promoted Reactor for CO2 Catalytic Hydrogenation. Appl Catal B 2022, 317, 121778. [Google Scholar] [CrossRef]
- Chatzilias, C.; Martino, E.; Tsatsos, S.; Kyriakou, G.; Katsaounis, A.; Vayenas, C.G. Kinetic Study of CO2 Hydrogenation on Ru/ YSZ Catalyst Using a Monolithic Electropromoted Reactor (MEPR). Chemical Engineering Journal 2022, 430, 132967. [Google Scholar] [CrossRef]
- Chatzilias, C.; Martino, E.; Katsaounis, A.; Vayenas, C.G. Electrochemical Promotion of CO2 Hydrogenation in a Monolithic Electrochemically Promoted Reactor (MEPR). Appl Catal B 2021, 284, 119695. [Google Scholar] [CrossRef]
- Bebelis, S.; Karasali, H.; Vayenas, C.G. Electrochemical Promotion of CO2 Hydrogenation on Rh/YSZ Electrodes. J Appl Electrochem 2008, 38, 1127–1133. [Google Scholar] [CrossRef]
- Papaioannou, E.I.; Souentie, S.; Hammad, A.; Vayenas, C.G. Electrochemical Promotion of the CO2 Hydrogenation Reaction Using Thin Rh, Pt and Cu Films in a Monolithic Reactor at Atmospheric Pressure. Catal Today 2009, 146, 336–344. [Google Scholar] [CrossRef]
- Jiménez, V.; Jiménez-Borja, C.; Sánchez, P.; Romero, A.; Papaioannou, E.I.; Theleritis, D.; Souentie, S.; Brosda, S.; Valverde, J.L. Electrochemical Promotion of the Co2 Hydrogenation Reaction on Composite Ni or Ru Impregnated Carbon Nanofiber Catalyst-Electrodes Deposited on YSZ. Appl Catal B 2011, 107, 210–220. [Google Scholar] [CrossRef]
- Kotsiras, A.; Kalaitzidou, I.; Grigoriou, D.; Symillidis, A.; Makri, M.; Katsaounis, A.; Vayenas, C.G. Electrochemical Promotion of Nanodispersed Ru-Co Catalysts for the Hydrogenation of CO2. Appl Catal B 2018, 232, 60–68. [Google Scholar] [CrossRef]
- Grigoriou, D.; Zagoraios, D.; Katsaounis, A.; Vayenas, C.G. The Role of the Promoting Ionic Species in Electrochemical Promotion and in Metal-Support Interactions. Catal Today 2021, 363, 122–127. [Google Scholar] [CrossRef]
- Panaritis, C.; Zgheib, J.; Ebrahim, S.A.H.; Couillard, M.; Baranova, E.A. Electrochemical In-Situ Activation of Fe-Oxide Nanowires for the Reverse Water Gas Shift Reaction. Appl Catal B 2020, 269. [Google Scholar] [CrossRef]
- Zagoraios, D.; Tsatsos, S.; Kennou, S.; Vayenas, C.G.; Kyriakou, G.; Katsaounis, A. Tuning the RWGS Reaction via EPOC and in Situ Electro-Oxidation of Cobalt Nanoparticles. ACS Catal 2020, 14916–14927. [Google Scholar] [CrossRef]
- Zagoraios, D.; Kokkinou, N.; Kyriakou, G.; Katsaounis, A. Electrochemical Control of the RWGS Reaction over Ni Nanoparticles Deposited on Yttria Stabilized Zirconia. Catal Sci Technol 2022, 12, 1869–1879. [Google Scholar] [CrossRef]
- Nicole, J.; Comninellis, C.; Tsiplakides, D.; Pliangos, C.; Verykios, X.E.; Vayenas, C.G. Electrochemical Promotion and Metal-Support Interactions. J Catal 2001, 204, 23–34. [Google Scholar] [CrossRef]
- Janek, J. C.G. Vayenas, S. Bebelis, C. Pliangos, S. Brosda, D. Tsiplakides: Electrochemical Activation of Catalysis: Promotion, Electrochemical Promotion, and Metal-Support Interaction. Journal of Solid State Electrochemistry 2002, 7, 60–61. [Google Scholar] [CrossRef]
- Stoukides, M.; Vayenas, C.G. The Effect of Electrochemical Oxygen Pumping on the Rate and Selectivity of Ethylene Oxidation on Polycrystalline Silver. J Catal 1981, 70, 137–146. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Koutsodontis, C.G. Non-Faradaic Electrochemical Activation of Catalysis. Journal of Chemical Physics 2008, 128, 182506. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Bebelis, S.; Ladas, S. Dependence of Catalytic Rates on Catalyst Work Function. Nature 1990, 343, 625–627. [Google Scholar] [CrossRef]
- Bebelis, S.; Vayenas’, C.G. Non-Faradaic Electrochemical Modification of Catalytic Activity 1. The Case of Ethylene Oxidation on Pt; 1989, 118, 125–146.
- Vayenas, C.G.; Bebelis, S.; Despotopoulou, M. Non-Faradaic Electrochemical Modification of Catalytic Activity 4. The Use of Β″-Al2O3 as the Solid Electrolyte. J Catal 1991, 128, 415–435. [Google Scholar] [CrossRef]
- Ladas, S.; Bebelis, S.; Vayenas, C.G. Work Function Measurements on Catalyst Films Subject to in Situ Electrochemical Promotion. Surf Sci 1991, 251–252, 251–252. [Google Scholar]
- Neophytides, S.G.; Tsiplakides, D.; Stonehart, P.; Jaksic, M.M.; Vayenas, C.G. Electrochemical Enhancement of a Catalytic Reaction in Aqueous Solution. Nature 1994, 370, 45–47. [Google Scholar] [CrossRef]
- Pliangos, C.; Raptis, C.; Badas, T.; Tsiplakides, D.; Vayenas, C.G. Electrochemical Promotion of a Classically Promoted Rh Catalyst for the Reduction of NO. Electrochimica Acta 2000, 46, 331–339. [Google Scholar] [CrossRef]
- Vernoux, P.; Gaillard, F.; Bultel, L.; Siebert, E.; Primet, M. Electrochemical Promotion of Propane and Propene Oxidation on Pt/YSZ. J Catal 2002, 208, 412–421. [Google Scholar] [CrossRef]
- Katsaounis, A.; Teschner, D.; Zafeiratos, S. The Effect of Polarization and Reaction Mixture on the Rh/YSZ Oxidation State During Ethylene Oxidation Studied by Near Ambient Pressure XPS. Top Catal 2018, 61, 2142–2151. [Google Scholar] [CrossRef]
- C.G.Vayenas; S.Bebelis; C.Pliangos; S.Brosda; D.Tsiplakides Electrochemical Activation of Catalysis Promotion, Electrochemical Promotion, and Metal-Support Interactions. Springer New York, NY 2002.
- Constantinou, I.; Archonta, D.; Brosda, S.; Lepage, M.; Sakamoto, Y.; Vayenas, C.G. Electrochemical Promotion of NO Reduction by C3H6 on Rh Catalyst-Electrode Films Supported on YSZ and on Dispersed Rh/YSZ Catalysts. J Catal 2007, 251, 400–409. [Google Scholar] [CrossRef]
- Souentie, S.; Xia, C.; Falgairette, C.; Li, Y.D.; Comninellis, C. Investigation of the “Permanent” Electrochemical Promotion of Catalysis (P-EPOC) by Electrochemical Mass Spectrometry (EMS) Measurements. Electrochem commun 2010, 12, 323–326. [Google Scholar] [CrossRef]
- Falgairette, C.; Jaccoud, A.; Fóti, G.; Comninellis, C. The Phenomenon of “Permanent” Electrochemical Promotion of Catalysis (P-EPOC). J Appl Electrochem 2008, 38, 1075–1082. [Google Scholar] [CrossRef]
- Kokka, A.; Petala, A.; Panagiotopoulou, P. Support Effects on the Activity of Ni Catalysts for the Propane Steam Reforming Reaction. Nanomaterials 2021, 11. [Google Scholar] [CrossRef]
- Yentekakis, I. V.; Goula, G.; Panagiotopoulou, P.; Kampouri, S.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Stabilization of Catalyst Particles against Sintering on Oxide Supports with High Oxygen Ion Lability Exemplified by Ir-Catalyzed Decomposition of N2O. Appl Catal B 2016, 192, 357–364. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Alhanash, A.M.; Benaissa, M.; Alsalme, A.; Alharthi, F.A.; Al-Zaqri, N. Rhodium Nanoparticles Incorporated Mesoporous Silica as an Active Catalyst for Cyclohexene Hydrogenation under Ambient Conditions. Catalysts 2020, 10. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the “Debye-Scherrer Equation. ” Nat Nanotechnol 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Borja, C.; De Lucas-Consuegra, A.; Sapountzi, F.; Dorado, F.; Katsaounis, A.; Valverde, J.L. Oscillatory Behavior of Rh/YSZ under Electropromoted Conditions. Chem Phys Lett 2012, 519–520, 89–92. [Google Scholar] [CrossRef]
- Blomberg, S.; Lundgren, E.; Westerström, R.; Erdogan, E.; Martin, N.M.; Mikkelsen, A.; Andersen, J.N.; Mittendorfer, F.; Gustafson, J. Structure of the Rh2O3(0001) Surface. Surf Sci 2012, 606, 1416–1421. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




