Submitted:
07 June 2023
Posted:
08 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Physical characterization of grain
2.2. Flowered grain quality
2.3. Effect of type of flowering
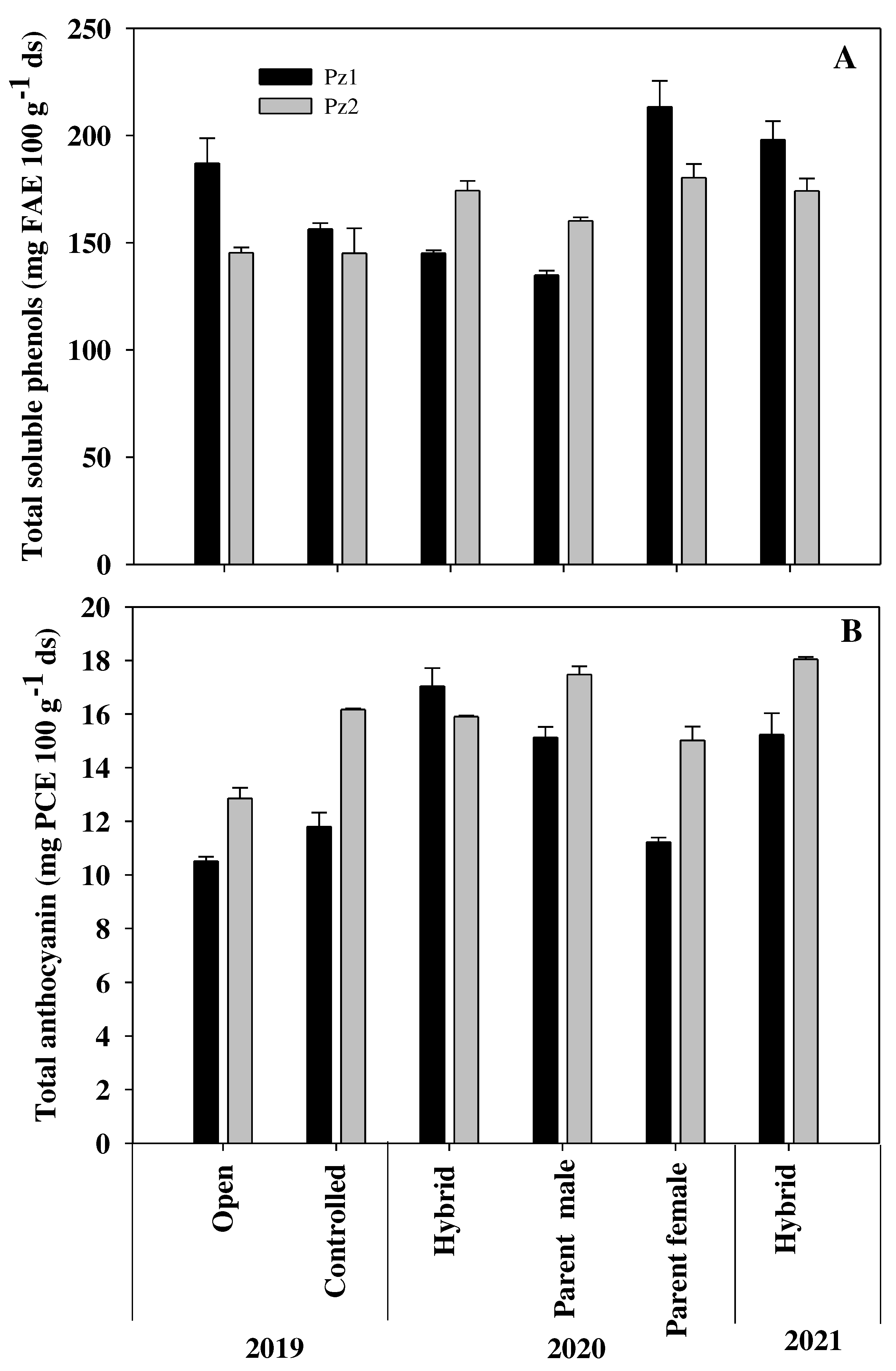
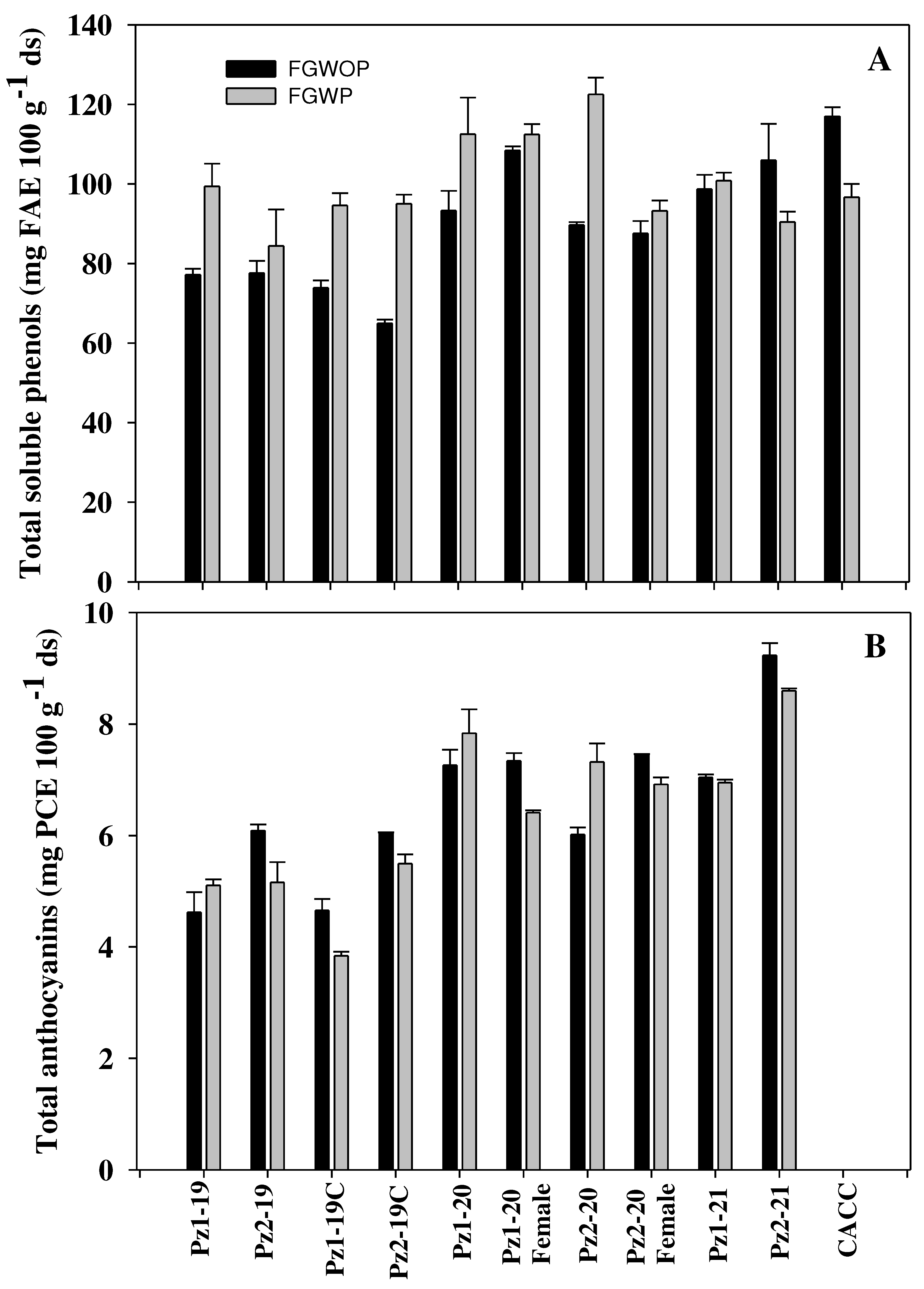
2.5. Phytochemical components
2.5.1. Total soluble phenols (TSP) and total anthocyanins (TA) in whole grain
3. Materials and Methods
3.1. Genotypes.
3.2. Physical characteristics of grain
3.3. Nixtamalization and obtaining flowered grain
3.4. Quality of flowered grain
3.5. Phytochemical components
3.5.1. Obtaining the extract.
3.5.2. Total soluble phenol content.
3.5.3. Total anthocyanin content.
3.6. Statistical analysis
4. Conclusions
Author Contributions
Funding
Data Availability
Acknowledgments
Conflicts of Interest
References
- ReferencesCONABIO (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). Proyecto global de maíces nativos. https://biodiversidad.gob.mx/diversidad/proyectoMaices (accessed 2023-04-23).
- Vázquez-Carrillo, M. G.; Santiago-Ramos, D.; Palacios-Rojas, N. Calidad Industrial y Nutricional de Razas Mexicanas de Maíz Pozolero, 1st ed.; Biblioteca Básica de Agricultura: México, 2016. [Google Scholar]
- Ballesteros Martínez, G.; Zarazúa Villaseñor, P.; Salinas Moreno, Y.; De la Cruz Larios, L. Fijación del color en grano y características físicas, tecnológicas y nutracéuticas en maíz Elotes Occidentales. Rev. Mex. Ciencias Agrícolas 2019, 10 (3), 585–599. [Google Scholar] [CrossRef]
- Preciado-Ortiz, R. E.; Ochoa-Centeno, N. J.; Vázquez-Carrillo, M. G.; Santiago-Ramos, D.; Terrón-Ibarra, A. D. Grain yield, physical and pasting properties, and anthocyanins of non-conventional pigmented corn hybrids for pozole end-use adapted to subtropical regions. Appl. Food Res. 2022, 2, 100180. [Google Scholar] [CrossRef]
- Gómez-Montiel, N. O.; Cantú-Almaguer, M. Á.; Hernández-Galeno, C. del Á.; Vázquez Carrillo, M. G.; Aragón Cuevas, F.; Palemón Alberto, F. V-237 AN, Cultivar mejorado de maíz “Ancho Pozolero” para la región semicálida de Guerrero. Rev. Mex. Ciencias Agrícolas 2014, 5 (7), 1315–1319. [Google Scholar] [CrossRef]
- Kim, H. Y.; Lee, K. Y.; Kim, M.; Hong, M.; Deepa, P.; Kim, S. A review of the biological properties of purple corn ( Zea Mays L.). Sci. Pharm. 2023, 91 (1), 6. [Google Scholar] [CrossRef]
- Secretaría de Economía, S. Productos Alimenticios Para Uso Humano No Industrializados-Cereales-Maíz-Parte1: Granos Para Tortillas y Productos Nixtamalizados-Especificaciones y Métodos de Prueba, NMX-FF-034/1-SCFI-2020; Dirección General de Normas; Ciudad de México, México. 2020.
- Salinas-Moreno, Y.; Aragón-Cuevas, F.; Ybarra-Moncada, C.; Aguilar-Villarreal, J.; Altunar-López, B.; Sosa-Montes, E. Caracterización física y composición química de razas de maíz de grano azul/morado de las regiones tropicales y subtropicales de Oaxaca. Rev. Fitotec. Mex. 2013, 36 (1), 23–31. [Google Scholar] [CrossRef]
- Vázquez-Carrillo, M. G.; Santiago-Ramos, D.; Domínguez-Rendón, E.; Audelo-Benites, M. A. Effects of Two different pozole preparation processes on quality variables and pasting properties of processed maize grain. J. Food Qual. 2017, 8627363. [Google Scholar] [CrossRef]
- Vázquez-Carrillo, M. G.; Pérez-Camarrilo, J. P.; Hernández-Casillas, J. M.; Marrufo-Díaz, M. de la L.; Martínez-Ruiz, E. Calidad de grano y tortillas de maíces criollos del Altiplano y Valle del Mezquital, México. Rev. Fitotec. Mex. 2010, 33 (4), 49–56. [Google Scholar]
- Santiago-ramos, D.; Figueroa-Cárdenas, J. D. D.; Mariscal-Moreno, R. M.; Escalante-aburto, A.; Ponce-García, N.; Véles-Medina, J. J. Physical and chemical changes undergone by pericarp and endosperm during corn nixtamalization-A review. J. Cereal Sci. 2018, 81, 108–117. [Google Scholar] [CrossRef]
- Méndez-Lagunas, L. L.; Cruz-Gracida, M.; Barriada-Bernal, L. G.; Rodríguez-Méndez, L. I. Profile of phenolic acids, antioxidant activity and total phenolic compounds during blue corn tortilla processing and its bioaccessibility. J. Food Sci. Technol. 2020, 57 (12), 4688–4696. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernández, L. A.; Castillo-González, F.; Vázquez-Carrillo, M. G.; Livera-Muñoz, M.; Benítez-Riquelme, I.; Nieto-Sotelo, J.; Ramírez-Hernández, A. Composition and flowering quality of Cacahuacintle maize populations from the High Valleys of Mexico. Plant Foods Hum. Nutr. 2023. [Google Scholar] [CrossRef] [PubMed]
- Dorantes-Campuzano, M. F.; Cabrera-Ramírez, A. H.; Rodríguez-García, M. E.; Palacios-Rojas, N.; Preciado-Ortíz, R. E.; Luzardo-Ocampo, I.; Gaytán Martínez, M. Effect of maize processing on amylose-lipid complex in pozole, a traditional mexican dish. Appl. Food Res. 2022, 2 (1). [Google Scholar] [CrossRef]
- Kahrıman, F.; Egesel, C. Ö.; Aydın, T.; Subaşı, S. The Role of artificial pollination and pollen effect on ear development and kernel structure of different maize genotypes. J. Pollinat. Ecol. 2015, 15 (2), 6–14. [Google Scholar] [CrossRef]
- Darrah, L. L.; McMullen, M. D.; Zuber, M. S. Breeding, genetics and seed corn production. In Corn: Chemistry and Technology; Serna-Saldívar, S. O., Ed.; Elsevier Inc., 2018; pp 19–41. [CrossRef]
- Das, A. K.; Singh, V. Antioxidative free and bound phenolic constituents in botanical fractions of indian specialty maize (Zea Mays L.) genotypes. Food Chem. 2016, 201, 298–306. [Google Scholar] [CrossRef]
- Ruiz-Torres, N. A.; Rincón-Sánchez, F.; Hernández-López, V. M.; Figueroa-Cárdenas, J. D. D.; Loarca-Piña, M. G. F. Determinación de compuestos fenólicos y su actividad antioxidante en granos de maíz. Rev. Fitotec. Mex. 2008, 31 (3), 29–34. [Google Scholar] [CrossRef]
- Paulsmeyer, M. N.; Juvik, J. A. Increasing aleurone layer number and pericarp yield for elevated nutrient content in maize. G3/Genes/Genomics/Genetics 2023, 00. [Google Scholar] [CrossRef]
- Espinosa Trujillo, E.; Mendoza Castillo, M. D. C.; Castillo González, F.; Ortiz Cereceres, J.; Delgado Alvarado, A.; Carrillo Salazar, A. Anthocyanin accumulation in pericarp and aleurone layer of maize kernel and their genetic effects on native pigmented varieties. Rev. Fitotec. Mex. 2009, 32 (4), 303–309. [Google Scholar]
- Rocandio-Rodríguez, M.; Torres-Castillo, J. A.; Juárez-Aragón, M. C.; Chacón-Hernández, J. C.; Moreno-Ramírez, Y. D. R.; Mora-Ravelo, S. G.; Delgado-Martínez, R.; Hernández-Juárez, A.; Heinz-Castro, R. T. Q.; Reyes-Zepeda, F. Evaluation of resistance of eleven maize races (Zea Mays L.) to the red spider mite (Tetranychus Merganser, Boudreaux). Plants 2022, 11 (11). [Google Scholar] [CrossRef] [PubMed]
- Broa Rojas, E.; Vázquez Carrillo, M. G.; Estrella Chulím, N. G.; Hernández Salgado, J. H.; Ramírez Valverde, B.; Bahena Delgado, G. Características fisicoquímicas y calidad de la proteína de maíces nativos pigmentados de Morelos en dos años de cultivo. Rev. Mex. Ciencias Agrícolas 2019, 10 (3), 683–697. [Google Scholar] [CrossRef]
- Mansilla, P. S.; Bongianino, N. F.; Nazar, M. C.; Pérez, G. T. Agronomic and chemical description of open-pollinated varieties of opaque-2 and purple maize (Zea Mays L.) adapted to semiarid region of Argentina. Genet. Resour. Crop Evol. 2021, 68 (6), 2351–2366. [Google Scholar] [CrossRef]
- Liu, T.; Yang, L.; Liu, B.; Tan, L. Hydroxycinnamic acids release during bioconversion of corn stover and their effects on lignocellulolytic enzymes. Bioresour. Technol. 2019, 294, 122116. [Google Scholar] [CrossRef]
- Peralta-Veran, L.; Espinosa-Leal, C.; Escalante-Aburto, A.; Preciado-Ortiz, R. E.; Puente-Garza, C. A.; Serna-Saldivar, S. O.; García-Lara, S. Effects of pozole broth production on phenolic acids and antioxidant activity of specialty maize landraces. J. Cereal Sci. 2022, 107 (June), 1–8. [Google Scholar] [CrossRef]
- Das, A. K.; Singh, V. Antioxidative free and bound phenolic constituents in pericarp, germ and endosperm of indian dent (Zea Mays var. indentata) and flint (Zea Mays var. indurata) maize. J. Funct. Foods 2015, 13, 363–374. [Google Scholar] [CrossRef]
- Razgonova, M.; Zinchenko, Y.; Pikula, K.; Tekutyeva, L.; Son, O.; Zakharenko, A.; Kalenik, T.; Golokhvast, K. Spatial distribution of polyphenolic compounds in corn grains (Zea Mays L. var. pioneer) studied by laser confocal microscopy and high-Resolution mass spectrometry. Plants 2022, 11, 630. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Soria-Ruiz, J.; Espinosa Trujillo, E. Aprovechamiento y Distribución de Maíz Azul En El Estado de México, 1ra ed.; Impresos Lebam: Texcoco, Edo. México. México, 2010. [Google Scholar]
- Gutiérrez-Uribe, J. A.; Rojas-García, C.; García-Lara, S.; Serna-Saldivar, S. O. Phytochemical analysis of wastewater (nejayote) obtained after lime-cooking of different types of maize kernels processed into masa for tortillas. J. Cereal Sci. 2010, 52 (3), 410–416. [Google Scholar] [CrossRef]
- Petroni, K.; Pilu, R.; Tonelli, C. Anthocyanins in corn: a wealth of genes for human health. Planta 2014, 240 (5), 901–911. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Gurak, P. D.; Ferreira Marczak, L. D.; Tessaro, I. C. Tracking bioactive compounds with colour changes in foods – a review. Dye. Pigment. 2013, 98 (3), 601–608. [Google Scholar] [CrossRef]
- AACC Approved Methods of Analysis. Method 44-15.02. Moisture-Air Oven Methods, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 2020. [Google Scholar]
- Singleton, V. L.; Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16 (3), 144–158. [Google Scholar] [CrossRef]
- López-Vásquez, V. M.; Salinas-Moreno, Y.; Alemán-de la Torre, I.; Morales-Hernández, N.; Bautista-Ramírez, E. Effect of adding anthocyanins to blue maize dough on color, texture and antioxidant capacity of maize tortillas. Ing. Agrícola y Biosist. 2020, 12 (2), 183–200. [Google Scholar] [CrossRef]
- Peniche-Pavía, H. A.; Tiessen, A. Anthocyanin profiling of maize grains using DIESI-MSQD reveals that cyanidin-based derivatives predominate in purple corn, whereas pelargonidin-based molecules occur in red-pink varieties from Mexico. J. Agric. Food Chem. 2020, 68 (21), 5980–5994. [Google Scholar] [CrossRef]
- Hernández Galeno, C. del Á.; Salinas Moreno, Y.; Antonio López, P.; Santacruz Varela, A.; Castillo González, F.; Corona Torres, T. Calidad pozolera en poblaciones de maíz Cacahuacintle de Los Valles Altos de Puebla, México. Rev. Mex. Ciencias Agrícolas 2014, 5 (4), 703–716. [Google Scholar] [CrossRef]

| Genotype | Year | |||
|---|---|---|---|---|
| 2019 | 2020 | 2021 | HSD | |
| Test weight (kg hL-1) | ||||
| Pz1 | 74.75 ± 0.7 Aa | 71.65 ± 0.35 Ba | 68.10 ±7.35 Aa | 17.763 |
| Pz2 | 73.05 ±0.07 Bb | 73.25 ± 0.35 Aab | 73.95 ± 0.07 Aa | 0.886 |
| HSD | 0.304 | 1.521 | 22.375 | |
| Hundred grain weight (g) | ||||
| Pz1 | 41.92 ± 0.21 Ba | 41.04 ± 0.01 Ab | 36.38 ± 0.01 Bc | 0.512 |
| Pz2 | 44.51 ± 0.30 Aa | 38.59 ± 0.02 Bc | 43.24 ± 0.01 Ab | 0.736 |
| HSD | 1.13 | 0.068 | 0.0481 | |
| Flotation index(%) | ||||
| Pz1 | 58.0 ± 1.4 Bb | 88.5 ± 0.7 Aa | 93.0 ± 4.2 Aa | 10.923 |
| Pz2 | 68.0 ± 1.4 Aa | 79.0 ± 7.1 Aa | 82.0 ± 2.8 Aa | 18.688 |
| HSD | 6.085 | 21.62 | 15.513 | |
| Pedicel (%) | ||||
| Pz1 | 1.32 ± 0.05 Aa | 0.85 ± 0.05 Ba | 1.37 ± 0.29 Aa | 0.719 |
| Pz2 | 1.23 ± 0.67 Aa | 1.23 ± 0.04 Aa | 1.19 ± 0.002 Aa | 0.199 |
| HSD | 0.263 | 0.198 | 0.882 | |
| Pericarp (%) | ||||
| Pz1 | 4.3 ± 0.06 Aa | 4.08 ± 0.13 Aa | 4.51 ± 0.13 Aa | 0.467 |
| Pz2 | 4.0 ± 0.13 Aa | 4.51 ± 0.23 Aa | 4.12 0.21 Aa | 0.828 |
| HSD | 0.444 | 0.838 | 0.734 | |
| Germ (%) | ||||
| Pz1 | 11.35 ± 0.04 Ba | 11.49 ± 0.23 Aa | 10.39 ± 0.01 Ab | 0.572 |
| Pz2 | 11.77 ± 0.07 Aa | 10.99 ± 0.49 Aa | 11.13 ± 0.69 Aa | 2.072 |
| HSD | 0.251 | 1.684 | 2.108 | |
| Floury endosperm (%) | ||||
| Pz1 | 42.5 ± 0.31 Ab | 62.26 ± 5.43 Aa | 70.67 ± 0.74 Aa | 13.245 |
| Pz2 | 44.13 ± 0.86 Ab | 53.11 ± 5.71 Aab | 64.78 ± 0.57 Ba | 13.992 |
| HSD | 2.79 | 23.967 | 2.853 | |
| Vitreous endosperm (%) | ||||
| Pz1 | 40.53 Aa | 16.34 Ab | 13.07 Bb | 13.559 |
| Pz2 | 38.88 Aa | 30.16 Aab | 18.78 Ab | 13.776 |
| HSD | 3.732 | 23.412 | 5.668 | |
| Lightness (%) | ||||
| Pz1 | 48.90 ± 0.04 Aa | 41.87 ± 0.28 Bb | 48.18 ± 0.82 Aa | 2.095 |
| Pz2 | 46.64 ± 0.35 Bb | 46.67 ± 0.16 Ab | 50.43 ± 0.74 Aa | 1.997 |
| HSD | 1.059 | 0.982 | 3.352 | |
| Hue (°) | ||||
| Pz1 | 51.41 ± 0.63 Bc | 61.16 ± 0.64 Ab | 65.0 ± 1.27 Aa | 3.753 |
| Pz2 | 61.21 ± 0.92 Ab | 55.59 ± 1.97 Ab | 69.1 ± 2.12 Aa | 7.327 |
| HSD | 3.372 | 6.306 | 7.527 | |
| Chroma | ||||
| Pz1 | 11.76 ± 0.19 Bb | 12.08 ± 10.43 Ab | 14.20 ± 0.28 Aa | 1.346 |
| Pz2 | 14.21 ± 0.51 Aa | 13.33 ± 0.41 Aa | 13.45 ± 0.35 Aa | 1.793 |
| HSD | 1.662 | 1.826 | 1.378 | |
| Mean squares of technological variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variation source |
gl | RGF | VGF | PMS | TF | HGF | Dureza | L | Hue | Croma |
| Genotype | 10 | 0.044* | 1607.95* | 0.29 | 2042.49* | 25.46* | 0.35* | 158.17* | 838.74* | 4.41* |
| Flowered | 1 | 0.014 | 205.11 | 0.22 | 9.09 | 12.767* | 0.039 | 92.10* | 217.78* | 63.62* |
| FXG | 10 | 0.0037 | 121.36 | 0.24 | 409.84 | 7.61* | 0.099* | 12.36* | 35.21* | 4.56* |
| Error | 22 | 0.0043 | 116.48 | 0.23 | 209.95 | 2.94 | 0.024 | 0.26 | 3.87 | 0.35 |
| Mean | 2.4 | 354.43 | 7.33 | 171.14 | 61.62 | 1.56 | 46.59 | 54.37 | 15.49 | |
| CV(%) | 2.75 | 3.05 | 6.48 | 8.47 | 2.78 | 9.99 | 1.09 | 3.62 | 3.82 | |
| R | 0.84 | 0.87 | 0.52 | 0.84 | 0.84 | 0.89 | 0.99 | 0.99 | 0.95 | |
| FGY (kg kg-1maize) | VFG (cm3) |
FT (min) |
FGM (%) |
DML (%) |
FGPF (N) |
L (%) |
Hue (°) |
Chroma | |
|---|---|---|---|---|---|---|---|---|---|
| Pz1-19 | 2.26 ± 0.08* | 332.5 ± 6.45* | 147.5 ± 5.0* | 58.93 ± 1.2 | 7.23 ± 0.23 | 1.84 ± 0.07* | 51.92 ± 3.6* | 63.17 ± 3.4* | 16.72 ±2.9 |
| Pz2-19 | 2.32 ± 0.07 | 338.8 ± 33.15* | 151.3 ± 10.3* | 57.71 ± 2.9* | 7.71 ± 0.43 | 1.99 ± 0.2* | 47.53 ± 1.9* | 63.19 ± 3.3* | 14.54 ± 1.9* |
| Pz1-19C | 2.24 ± 0.11* | 320.0 ± 10.8* | 153.8 ± 7.5* | 57.61 ± 2.5* | 7.32 ± 0.53 | 1.91 ± 0.15* | 46.21 ± 3.2* | 61.08 ± 3.5* | 15.77 ± 0.5* |
| Pz2-19C | 2.32 ± 0.07 | 345.0 ± 10.8* | 150.0 ± 0.0* | 60.29 ± 2.0 | 7.21 ± 0.49 | 1.59 ± 0.12 | 47.52 ± 0.4* | 56.77 ± 3.2* | 15.34 ± 2.4* |
| Pz1-20 | 2.50 ± 0.05 | 375.0 ± 19.6 | 196.8 ± 22.6 | 62.71 ± 0.5 | 6.88 ± 0.49 | 1.19 ± 0.11 | 42.58 ± 1.2* | 40.83 ± 1.9* | 15.11 ± 0.6* |
| ♀Pz1-20 | 2.55 ± 0.05 | 385.0 ± 12.9 | 194.8 ± 17.04 | 63.55 ± 0.8 | 7.70 ± 0.20 | 1.14 ± 0.21 | 43.85 ± 1.2* | 46.68 ± 4.1* | 15.74 ± 1.3* |
| Pz2-20 | 2.51 ± 0.07 | 352.5 ± 8.7* | 161.3 ± 26.3* | 63.73 ± 1.9 | 7.23 ± 0.48 | 1.23 ± 0.12 | 46.76 ±1.1* | 49.34 ± 9.6* | 15.34 ± 0.5* |
| ♀Pz2-20 | 2.43 ± 0.02 | 365.0 ± 10.0 | 205.0 ± 13.21 | 62.79 ± 1.2 | 7.56 ± 0.42 | 1.61 ± 0.24 | 40.85 ± 0.4* | 39.08 ± 6.4* | 15.82 ± 0.3* |
| Pz1-21 | 2.38 ± 0.07 | 351.3 ± 2.5* | 160.0± 14.1* | 63.97 ± 4.0 | 7.32 ± 0.53 | 1.52 ± 0.30 | 42.38 ± 2.3* | 47.68 ± 2.7* | 15.55 ± 0.3* |
| Pz2-21 | 2.41 ± 0.06 | 356.3 ± 8.5 | 163.5 ± 16.3* | 64.17 ± 2.9 | 6.96 ± 0.80 | 1.76 ± 0.41* | 40.4 ± 5.6* | 41.53 ± 1.9* | 13.21 ± 2.5* |
| CACC | 2.45 ± 0.05 | 377.5 ± 8.7 | 198.8 ± 22.5 | 62.38 ± 1.0 | 7.50 ± 0.39 | 1.41 ± 0.19 | 62.48 ± 2.4 | 88.72 ± 2.8 | 17.21 ± 2.6 |
| Raw grain | Flowered grain wihtout pedicel | Flowered grain wiht pedicel | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FI | L | Hue | Chroma | TA | L | Hue | Chroma | TA | L | Hue | Chroma | TA | |
| FI | 1 | 0.304 | 0.750* | -0.279 | 0.748* | -0.757* | -0.819* | -0.053 | 0.754* | -0.506* | -0.748* | -0.150 | 0.730* |
| L | 1 | 0.274 | 0.202 | 0.015 | 0.022 | -0.125 | -0.264 | 0.225 | -0.192 | -0.027 | -0.150 | 0.052 | |
| Hue | 1 | -0.317 | 0.734* | -0.678* | -0.539* | -0.603* | 0.896* | -0.612* | -0.692* | -0.243 | 0.776* | ||
| Chroma | 1 | -0.252 | -0.066 | 0.222 | 0.323 | -0.278 | -0.064 | 0.263 | -0.253 | -0.542* | |||
| TA | 1 | 0.760* | -0.830* | -0.214 | 0.860* | -0.658* | -0.840* | -0.488* | 0.831* | ||||
| LWOP | 1 | 0.722* | -0.034 | -0.719* | 0.586* | 0.716* | 0.554* | -0.562* | |||||
| HueWOP | 1 | -0.142 | -0.747* | 0.683* | 0.829* | 0.434 | -0.804* | ||||||
| ChromaWOP | 1 | -0.428 | 0.166 | 0.082 | -0.184 | -0.343 | |||||||
| ATWOP | 1 | -0.793* | -0.882* | -0.437 | 0.902* | ||||||||
| LWP | 1 | 0.833* | 0.477* | -0.713* | |||||||||
| HueWP | 1 | 0.431 | -0.852* | ||||||||||
| ChromaWP | 1 | -0.351 | |||||||||||
| TAWP | 1 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




