Submitted:
05 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
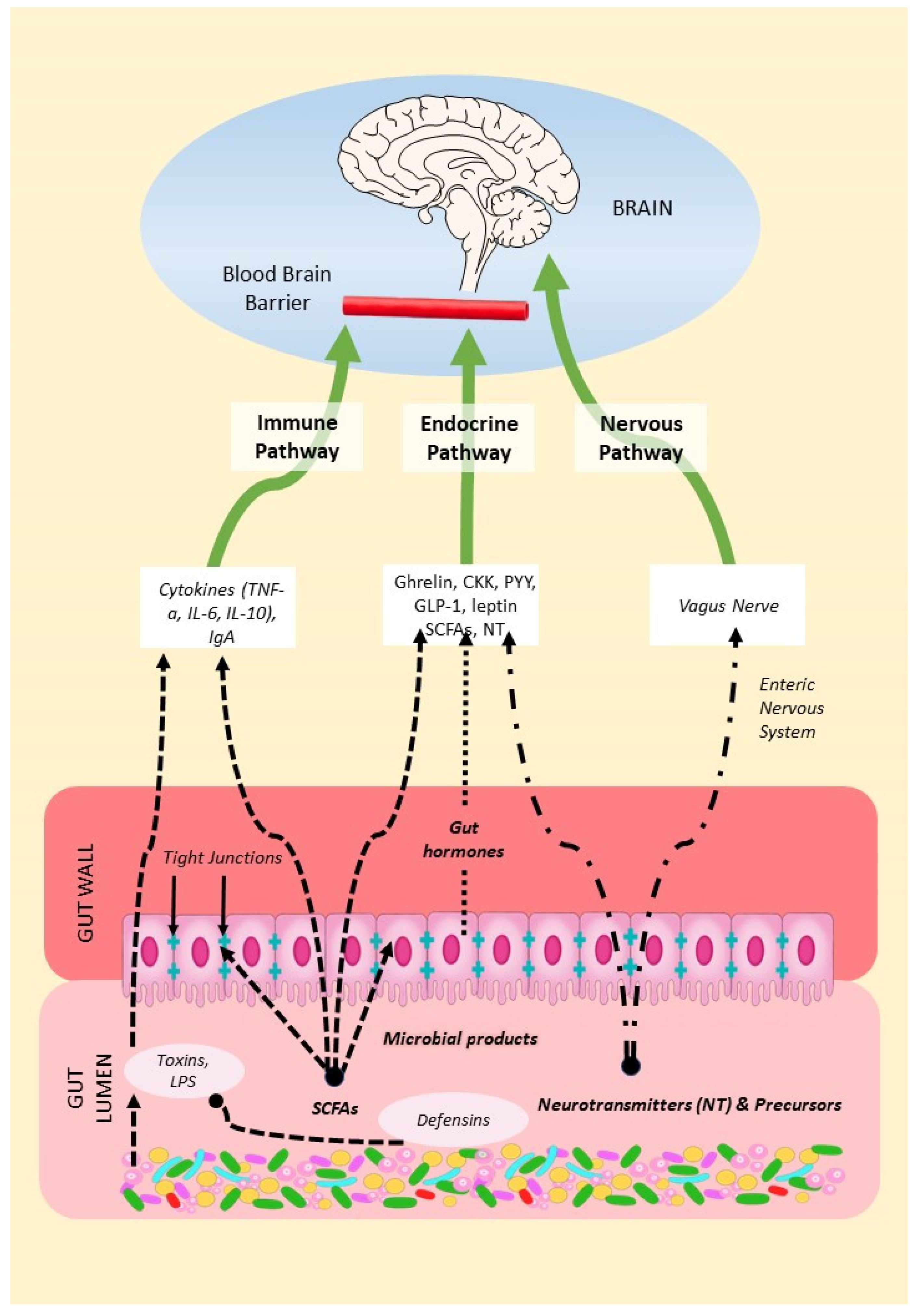
2. Mechanisms of the Microbiota-Gut-Brain Axis (MGBA)
2.1. The Endocrine Pathway
2.2. The Nervous Pathway
2.3. The Immune Pathway
2.3.1. From Gut to Host Immune System
2.3.2. From Host Immune System to Gut Microbiota
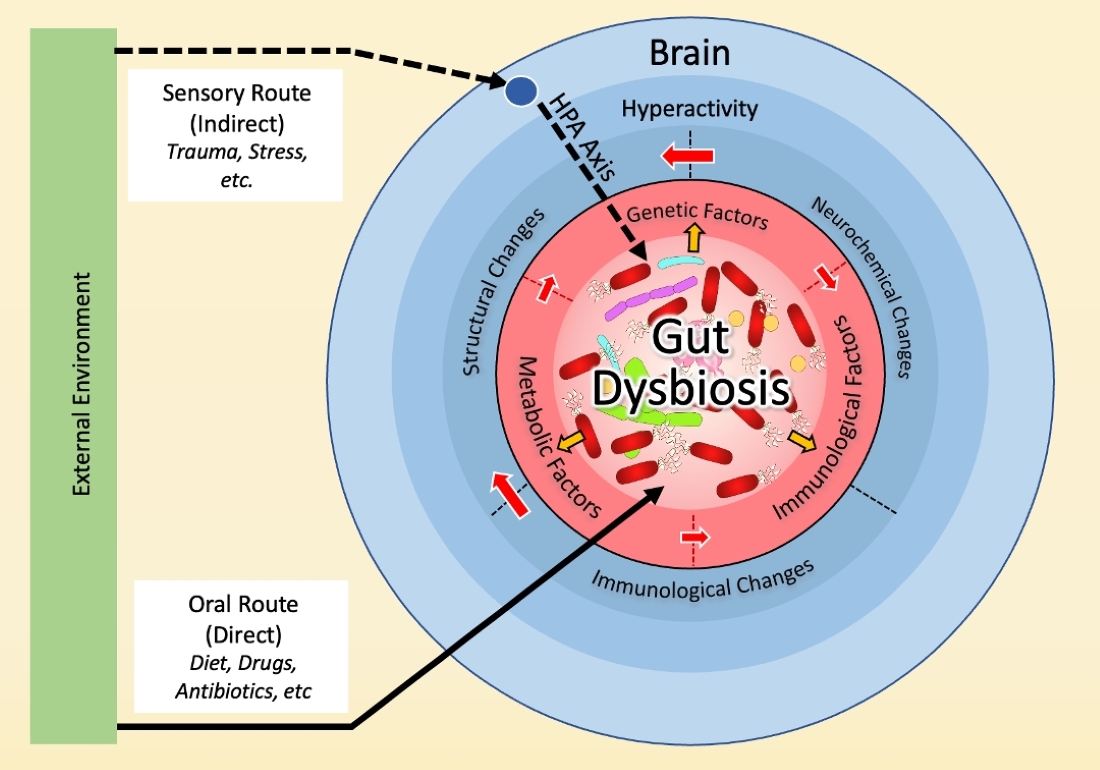
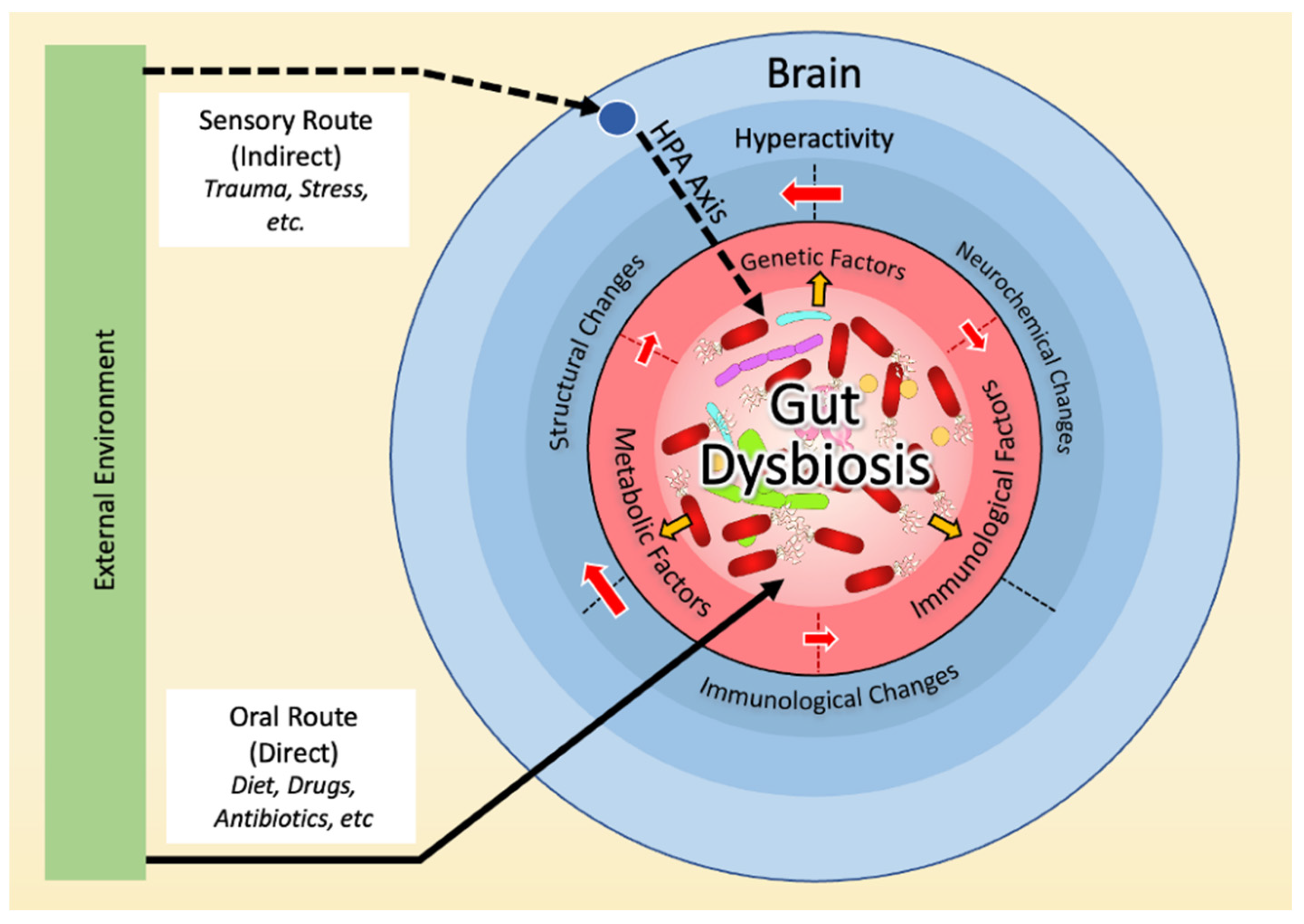
3. Dysbiosis and the Neurobiology Basis of OCD
3.1. Dysbiosis in OCD
3.2. Dysbiosis and Hyperactivity in the Cortico-Striato-Thalamo-Cortical Circuit (CSTC)
3.2.1. Serotonin
3.2.2. Glutamate
3.2.3. Dopamine
3.3. Dysbiosis and the Immune Basis of OCD
3.4. Dysbiosis and the Genetic Basis of OCD
3.5. Dysbiosis and the Environmental Basis of OCD
4. Microbial Reprogramming Strategies
4.1. Prebiotics, Probiotics and Postbiotics
4.2. Fecal Microbiota Transplants
5. Discussion
6. Conclusions
Author Contributions
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Published online May 22, 2013. [CrossRef]
- Oren E, Dar R, Liberman N. Obsessive-Compulsive tendencies are related to a maximization strategy in making decisions. Front Psychol. 2018, 9, 778. [CrossRef]
- Nestadt G, Kamath V, Maher BS, et al. Doubt and the Decision-making Process in Obsessive-Compulsive Disorder. Med Hypotheses. 2016, 96, 1. [CrossRef]
- Pushkarskaya H, Tolin D, Ruderman L, et al. Decision-Making Under Uncertainty in Obsessive-Compulsive Disorder. J Psychiatr Res. 2015, 69:166. [CrossRef]
- Murphy DL, Timpano KR, Wheaton MG, Greenberg BD, Miguel EC. Obsessive-compulsive disorder and its related disorders: a reappraisal of obsessive-compulsive spectrum concepts. Dialogues Clin Neurosci. 2010, 12, 131. [CrossRef]
- Jalal B, Chamberlain SR, Sahakian BJ. Obsessive-compulsive disorder: Etiology, neuropathology, and cognitive dysfunction. Brain Behav. Published online 2023, e3000. [CrossRef]
- Johnson PM, Kenny PJ. Addiction-like reward dysfunction and compulsive eating in obese rats: Role for dopamine D2 receptors. Nat Neurosci. 2010, 13, 635. [CrossRef]
- Mattheisen M, Samuels JF, Wang Y, et al. Genome-wide association study in obsessive-compulsive disorder: results from the OCGAS. Mol Psychiatry. 2015, 20, 337–344. [CrossRef]
- Frick L, Pittenger C. Microglial Dysregulation in OCD, Tourette Syndrome, and PANDAS. J Immunol Res. 2016, 2016. [CrossRef]
- Mahjani B, Klei L, Mattheisen M, et al. The genetic architecture of obsessive-compulsive disorder: Alleles across the frequency spectrum contribute liability to OCD. medRxiv. Published online 2021. [CrossRef]
- Purty A, Nestadt G, Samuels J, Viswanath B. Genetics of obsessive-compulsive disorder. Indian J Psychiatry. 2019, 61, S37–S43. [CrossRef]
- Fawcett EJ, Power H, Fawcett JM. Women are at greater risk of OCD than men: A meta-analytic review of OCD prevalence worldwide. Journal of Clinical Psychiatry. 2020, 81. [CrossRef]
- Grassi G, Cecchelli C, Vignozzi L, Pacini S. Investigational and Experimental Drugs to Treat Obsessive-Compulsive Disorder. J Exp Pharmacol. 2020, 12, 695. [CrossRef]
- Allen A. Cognitive behavioral therapy of obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010, 12, 199. [CrossRef]
- Kellner M. Drug treatment of obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010, 12, 187. [CrossRef]
- Fineberg NA, Gale TM, Sivakumaran T. A Review of Antipsychotics in the Treatment of Obsessive Compulsive Disorder. 2007, 5, 354–360. [CrossRef]
- Eisen JL, Goodman WK, Keller MB, et al. Patterns of Remission and Relapse in Obsessive-Compulsive Disorder: A 2-Year Prospective Study. J Clin Psychiatry. 1999, 60, 14024. [CrossRef]
- Ferguson JM. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim Care Companion J Clin Psychiatry. 2001, 3, 22. [CrossRef]
- Thursby E, Juge N. Introduction to the human gut microbiota. Biochemical Journal. 2017, 474, 1823. [CrossRef]
- Benjamin JL, Hedin CRH, Koutsoumpas A, et al. Smokers with active Crohnʼs disease have a clinically relevant dysbiosis of the gastrointestinal microbiota*. Inflamm Bowel Dis. 2012, 18, 1092–1100. [CrossRef]
- Kang S, Denman SE, Morrison M, et al. Dysbiosis of fecal microbiota in Crohnʼs disease patients as revealed by a custom phylogenetic microarray. Inflamm Bowel Dis. 2010, 16, 2034–2042. [CrossRef]
- Hou M, Xu G, Ran M, Luo W, Wang H. APOE-ε4 Carrier Status and Gut Microbiota Dysbiosis in Patients With Alzheimer Disease. Front Neurosci. 2021, 15. [CrossRef]
- Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev. 2016, 74, 624–634. [CrossRef]
- Shabbir U, Arshad MS, Sameen A, Oh DH. Crosstalk between gut and brain in alzheimer’s disease: The role of gut microbiota modulation strategies. Nutrients. 2021, 13, 1–23. [CrossRef]
- Scheperjans F, Aho V, Pereira PAB, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Movement Disorders. 2015, 30, 350–358. [CrossRef]
- Madore C, Leyrolle Q, Lacabanne C, et al. Neuroinflammation in Autism: Plausible Role of Maternal Inflammation, Dietary Omega 3, and Microbiota. Neural Plast. 2016, 2016:3597209. [CrossRef]
- De Angelis M, Francavilla R, Piccolo M, De Giacomo A, Gobbetti M. Autism spectrum disorders and intestinal microbiota. Gut Microbes. 2015, 6, 207–213. [CrossRef]
- Li Q, Zhou JM. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience. 2016, 324(March):131-139. [CrossRef]
- Ding HT, Taur Y, Walkup JT. Gut Microbiota and Autism: Key Concepts and Findings. J Autism Dev Disord. 2017, 47, 480–489. [CrossRef]
- Zhu F, Guo R, Wang W, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2020, 25, 2905–2918. [CrossRef]
- Frazier TH, Dibaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011, 35(5 Suppl):14S-20S. [CrossRef]
- Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010, 33, 2277–2284. [CrossRef]
- Phimister EG, Jess T. Microbiota, Antibiotics, and Obesity. New England Journal of Medicine. 2014, 371, 2526–2528. [CrossRef]
- Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin Pract. 2017, 7, 987. [CrossRef]
- Iannone LF, Preda A, Blottière HM, et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev Neurother. 2019, 19, 1037–1050. [CrossRef]
- Sommer F, Bäckhed F. The gut microbiota-masters of host development and physiology. Nat Rev Microbiol. 2013, 11, 227–238. [CrossRef]
- Sudo N. Microbiome, HPA axis and production of endocrine hormones in the gut. Adv Exp Med Biol. 2014, 817:177-194. [CrossRef]
- de Weerth C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci Biobehav Rev. 2017, 83:458-471. [CrossRef]
- Sudo N. Role of microbiome in regulating the HPA axis and its relevance to allergy. Chem Immunol Allergy. 2012, 98:163-175. [CrossRef]
- Sudo N. Role of Microbiome in Regulating the HPA Axis and Its Relevance to Allergy. Published online 2012:163-175. [CrossRef]
- Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J Lipid Res. 2016, 57, 943–954. [CrossRef]
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proceedings of the Nutrition Society. 2003, 62, 67–72. [CrossRef]
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019, 16, 461–478. [CrossRef]
- van der Hee B, Wells JM. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [CrossRef]
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019, 16, 461–478. [CrossRef]
- Vijay N, Morris M. Role of Monocarboxylate Transporters in Drug Delivery to the Brain. Curr Pharm Des. 2014, 20, 1487–1498. [CrossRef]
- Bolognini D, Tobin AB, Milligan G, Moss CE. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol. 2016, 89, 388–398. [CrossRef]
- Asbjornsdottir B, Snorradottir H, Andresdottir E, et al. Zonulin-Dependent Intestinal Permeability in Children Diagnosed with Mental Disorders: A Systematic Review and Meta-Analysis. Nutrients. 2020, 12, 1–27. [CrossRef]
- Caviglia GP, Rosso C, Ribaldone DG, et al. Physiopathology of intestinal barrier and the role of zonulin. Minerva Biotecnol. 2019, 31, 83–92. [CrossRef]
- Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009, 294, 1–8. [CrossRef]
- Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol. 2016, 7:979. [CrossRef]
- Szentirmai É, Millican NS, Massie AR, Kapás L. Butyrate, a metabolite of intestinal bacteria, enhances sleep. Sci Rep. 2019, 9(1). [CrossRef]
- Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016, 99:110-132. [CrossRef]
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: The role of butyrate on colonic function. Aliment Pharmacol Ther. 2008, 27, 104–119. [CrossRef]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015, 9:392. [CrossRef]
- Doney E, Dion-Albert L, Coulombe-Rozon F, et al. Chronic stress exposure alters the gut barrier: sex-specific effects on microbiota and jejunum tight junctions. Biological Psychiatry Global Open Science. Published online May 2023. [CrossRef]
- Viggiano D, Ianiro G, Vanella G, et al. Gut barrier in health and disease: focus on childhood. Eur Rev Med Pharmacol Sci. 2015, 19, 1077–1085. Accessed March 13, 2017. http://www.ncbi.nlm.nih.gov/pubmed/25855935.
- Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014, 6(263). [CrossRef]
- Bien-Ly N, Watts RJ. The blood-brain barrier’s gut check. Sci Transl Med. 2014, 6(263). [CrossRef]
- Smith O. The gut microbiota and the blood-brain barrier. Sci Signal. 2014, 7(353). [CrossRef]
- Oldendorf WH. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. 1973, 224, 1450–1453. [CrossRef]
- Bachmann C, Colombo JP, Berüter J. Short chain fatty acids in plasma and brain: Quantitative determination by gas chromatography. Clinica Chimica Acta. 1979, 92, 153–159. [CrossRef]
- Liu J, Sun J, Wang F, et al. Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed Res Int. 2015, 2015. [CrossRef]
- Sun J, Ling Z, Wang F, et al. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett. 2016, 613:30-35. [CrossRef]
- Hoyles L, Snelling T, Umlai UK, et al. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome. 2018, 6, 1–13. [CrossRef]
- Jin X, Chen D, Wu F, et al. Hydrogen Sulfide Protects Against Ammonia-Induced Neurotoxicity Through Activation of Nrf2/ARE Signaling in Astrocytic Model of Hepatic Encephalopathy. Front Cell Neurosci. 2020, 14:336. [CrossRef]
- Blachier F, Andriamihaja M, Larraufie P, Ahn E, Lan A, Kim E. Production of hydrogen sulfide by the intestinal microbiota and epithelial cells and consequences for the colonic and rectal mucosa. Am J Physiol Gastrointest Liver Physiol. 2021, 320, G125–G135. [CrossRef]
- Tomasova L, Konopelski P, Ufnal M. Gut Bacteria and Hydrogen Sulfide: The New Old Players in Circulatory System Homeostasis. Molecules. 2016, 21(11). [CrossRef]
- Kossewska J, Bierlit K, Trajkovski V. Personality, Anxiety, and Stress in Patients with Small Intestine Bacterial Overgrowth Syndrome. The Polish Preliminary Study. Int J Environ Res Public Health. 2023, 20(1). [CrossRef]
- Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014, 5. [CrossRef]
- Weis AM, Round JL. Microbiota-antibody interactions that regulate gut homeostasis. Cell Host Microbe. 2021, 29, 334–346. [CrossRef]
- Abokor AA, McDaniel GH, Golonka RM, et al. Immunoglobulin A, an Active Liaison for Host-Microbiota Homeostasis. Microorganisms. 2021, 9(10). [CrossRef]
- Lyte M. Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior. PLoS Pathog. 2013, 9(11). [CrossRef]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010, 170, 1179–1188. [CrossRef]
- Reigstad CS, Salmonson CE, Rainey JF, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. The FASEB Journal. 2015, 29, 1395–1403. [CrossRef]
- Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015, 161, 264–276. [CrossRef]
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nature Reviews Gastroenterology & Hepatology 2019 16:8. 2019, 16, 461–478. [CrossRef]
- Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015, 161, 264–276. [CrossRef]
- Reigstad CS, Salmonson CE, Rainey JF, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. The FASEB Journal. 2015, 29, 1395–1403. [CrossRef]
- Nankova BB, Agarwal R, MacFabe DF, La Gamma EF. Enteric Bacterial Metabolites Propionic and Butyric Acid Modulate Gene Expression, Including CREB-Dependent Catecholaminergic Neurotransmission, in PC12 Cells - Possible Relevance to Autism Spectrum Disorders. PLoS One. 2014, 9, e103740. [CrossRef]
- Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Molecular Endocrinology. 2014, 28, 1221–1238. [CrossRef]
- Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci. 2018, 12:49. [CrossRef]
- Breit S, Kupferberg A, Rogler G, Hasler G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry. 2018, 9:44. [CrossRef]
- Bravo JA, Forsythe P, Chew M V, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011, 108, 16050–16055. [CrossRef]
- Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. 2002, 8, 540–545. [CrossRef]
- Zhang L, Wang Y, Xiayu X, et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2017, 60, 1241–1257. [CrossRef]
- Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. 2018, 18, 83–90. [CrossRef]
- Yoo DY, Kim W, Nam SM, et al. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem Res. 2011, 36, 1850–1857. [CrossRef]
- Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020, 11:25. [CrossRef]
- Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science (1979). 2012, 336, 1262–1267. [CrossRef]
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. Journal of Clinical Investigation. 2011, 121, 2126–2132. [CrossRef]
- Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012, 150, 470–480. [CrossRef]
- Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015, 7, 2839–2849. [CrossRef]
- Moreira APB, Texeira TFS, Ferreira AB, Peluzio M do CG, Alfenas R de CG. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012, 108, 801–809. [CrossRef]
- Rosean CB, Bostic RR, Ferey JCM, et al. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor–positive breast cancer. Cancer Res. 2019, 79, 3662–3675. [CrossRef]
- Pai YC, Li YH, Turner JR, Yu LCH. Transepithelial Barrier Dysfunction Drives Microbiota Dysbiosis to Initiate Epithelial Clock-driven Inflammation. J Crohns Colitis. Published online April 2, 2023:1-18. [CrossRef]
- Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44:38-50. [CrossRef]
- Diamanti AP, Manuela Rosado M, Laganà B, D’Amelio R. Microbiota and chronic inflammatory arthritis: An interwoven link. J Transl Med. 2016, 14(1). [CrossRef]
- Schirmer M, Smeekens SP, Vlamakis H, et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 2016, 167, 1125. [CrossRef]
- Liu B, Ding Z, Xiong J, Heng X, Wang H, Chu W. Gut Microbiota and Inflammatory Cytokine Changes in Patients with Ankylosing Spondylitis. Biomed Res Int. 2022, 2022. [CrossRef]
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009, 461, 1282–1286. [CrossRef]
- An L, Wirth U, Koch D, et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. Journal of Gastrointestinal Surgery. 2022, 26, 671. [CrossRef]
- De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014, 156(1-2):84-96. [CrossRef]
- Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nature Neuroscience 2016 20:2. 2017, 20, 145–155. [CrossRef]
- Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013 504:7480. 2013, 504, 451–455. [CrossRef]
- Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science (1979). 2013, 341, 569–573. [CrossRef]
- Haghikia A, Jörg S, Duscha A, et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2015, 43, 817–829. [CrossRef]
- Erny D, De Angelis ALH, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience 2015 18:7. 2015, 18, 965–977. [CrossRef]
- Stanisavljević S, Čepić A, Bojić S, et al. Oral neonatal antibiotic treatment perturbs gut microbiota and aggravates central nervous system autoimmunity in Dark Agouti rats. Scientific Reports 2019 9:1. 2019, 9, 1–13. [CrossRef]
- Minter MR, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Scientific Reports 2016 6:1. 2016, 6, 1–12. [CrossRef]
- Minter MR, Hinterleitner R, Meisel M, et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1ΔE9 murine model of Alzheimer’s disease. Scientific Reports 2017 7:1. 2017, 7, 1–18. [CrossRef]
- Jang HM, Lee HJ, Jang SE, Han MJ, Kim DH. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018, 11, 1386–1397. [CrossRef]
- Patnala R, Arumugam T V., Gupta N, Dheen ST. HDAC Inhibitor Sodium Butyrate-Mediated Epigenetic Regulation Enhances Neuroprotective Function of Microglia During Ischemic Stroke. Mol Neurobiol. 2017, 54, 6391–6411. [CrossRef]
- Wang P, Zhang Y, Gong Y, et al. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol Dis. 2018, 111:12-25. [CrossRef]
- Sharon G, Cruz NJ, Kang DW, et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell. 2019, 177, 1600–1618.e17. [CrossRef]
- Soliman ML, Puig KL, Combs CK, Rosenberger TA. Acetate reduces microglia inflammatory signaling in vitro. J Neurochem. 2012, 123, 555–567. [CrossRef]
- Reddy DS, Wu X, Golub VM, Dashwood WM, Dashwood RH. Measuring Histone Deacetylase Inhibition in the Brain. Curr Protoc Pharmacol. 2018, 81, e41. [CrossRef]
- Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthésy B. Secretory component: A new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002, 17, 107–115. [CrossRef]
- Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science (1979). 2006, 314, 1157–1160. [CrossRef]
- Huus KE, Petersen C, Finlay BB. Diversity and dynamism of IgA−microbiota interactions. Nat Rev Immunol. 2021, 21, 514–525. [CrossRef]
- Senior BW, Dunlop JI, Batten MR, Kilian M, Woof JM. Cleavage of a recombinant human immunoglobulin A2 (IgA2)-IgA1 hybrid antibody by certain bacterial IgA1 proteases. Infect Immun. 2000, 68, 463–469. [CrossRef]
- Mantis NJ, Rol N, Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [CrossRef]
- Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011, 4, 590–597. [CrossRef]
- Bunker JJ, Erickson SA, Flynn TM, et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science (1979). 2017, 358(6361). [CrossRef]
- Fransen F, Zagato E, Mazzini E, et al. BALB/c and C57BL/6 Mice Differ in Polyreactive IgA Abundance, which Impacts the Generation of Antigen-Specific IgA and Microbiota Diversity. Immunity. 2015, 43, 527–540. [CrossRef]
- Sterlin D, Fadlallah J, Adams O, et al. Human IgA binds a diverse array of commensal bacteria. Journal of Experimental Medicine. 2020, 217(3). [CrossRef]
- Yang Y, Palm NW. Immunoglobulin A and the microbiome. Curr Opin Microbiol. 2020, 56:89-96. [CrossRef]
- Janzon A, Goodrich JK, Koren O, Waters JL, Ley RE. Interactions between the Gut Microbiome and Mucosal Immunoglobulins A, M, and G in the Developing Infant Gut. mSystems. 2019, 4(6). [CrossRef]
- Donaldson GP, Ladinsky MS, Yu KB, et al. Gut microbiota utilize immunoglobulin a for mucosal colonization. Science (1979). 2018, 360, 795–800. [CrossRef]
- Sutherland DB, Suzuki K, Fagarasan S. Fostering of advanced mutualism with gut microbiota by Immunoglobulin A. Immunol Rev. 2016, 270, 20–31. [CrossRef]
- Dixon BREA, Radin JN, Piazuelo MB, Contreras DC, Algood HMS. IL-17a and IL-22 Induce Expression of Antimicrobials in Gastrointestinal Epithelial Cells and May Contribute to Epithelial Cell Defense against Helicobacter pylori. PLoS One. 2016, 11, 148514. [CrossRef]
- Keir ME, Yi T, Lu TT, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med. 2020, 217(3). [CrossRef]
- Selsted ME, Miller SI, Henschen AH, Ouellette AJ. Enteric defensins: antibiotic peptide components of intestine host defense. J Cell Biol. 1992, 118, 929–936. [CrossRef]
- Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 2003 422:6931. 2003, 422, 522–526. [CrossRef]
- Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995, 13:61-92. [CrossRef]
- Rajabi M, De Leeuw E, Pazgier M, Li J, Lubkowski J, Lu W. The Conserved Salt Bridge in Human α-Defensin 5 Is Required for Its Precursor Processing and Proteolytic Stability*âTM¦. Published online 2008. [CrossRef]
- Turna J, Grosman Kaplan K, Anglin R, et al. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: a pilot study. Acta Psychiatr Scand. 2020, 142, 337–347. [CrossRef]
- Rees JC. Obsessive-compulsive disorder and gut microbiota dysregulation. Med Hypotheses. 2014, 82, 163–166. [CrossRef]
- Turna J, Grosman Kaplan K, Anglin R, Van Ameringen M. “wHAT’S BUGGING the GUT in OCD?” A REVIEW of the GUT MICROBIOME in OBSESSIVE-COMPULSIVE DISORDER. Depress Anxiety. 2016, 33, 171–178. [CrossRef]
- Domènech L, Willis J, Alemany-Navarro M, et al. Changes in the stool and oropharyngeal microbiome in obsessive-compulsive disorder. Sci Rep. 2022, 12(1). [CrossRef]
- Higher prevalence of irritable bowel syndrome and greater gastrointestinal symptoms in obsessive-compulsive disorder - PubMed. Accessed May 27, 2023. https://pubmed.ncbi.nlm.nih.gov/31437616/. [CrossRef]
- Troyer EA, Kohn JN, Ecklu-Mensah G, Aleti G, Rosenberg DR, Hong S. Searching for host immune-microbiome mechanisms in obsessive-compulsive disorder: A narrative literature review and future directions. Neurosci Biobehav Rev. 2021, 125:517-534. [CrossRef]
- Turna J, Grosman Kaplan K, Anglin R, et al. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: a pilot study. Acta Psychiatr Scand. 2020, 142, 337–347. [CrossRef]
- Quagliariello A, Del Chierico F, Russo A, et al. Gut microbiota profiling and gut-brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Front Microbiol. 2018, 9(APR):675. [CrossRef]
- Jiang H yin, Zhou Y yue, Zhou G ling, et al. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behavioural Brain Research. 2018, 347:408-413. [CrossRef]
- Wang LJ, Yang CY, Chou WJ, et al. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. Published online May 22, 2019. [CrossRef]
- Aarts E, Ederveen THA, Naaijen J, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One. 2017, 12, e0183509. [CrossRef]
- Axelsson PB, Clausen TD, Petersen AH, et al. Relation between Infant Microbiota and Autism?: Results from a National Cohort Sibling Design Study. Epidemiology. 2019, 30, 52–60. [CrossRef]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol. 2011, 77, 6718–6721. [CrossRef]
- Lu Bai HMXH. The Development of Native Chinese Affective Picture System--A pretest in 46 College Students. Chinese Mental Health Journal. 2005, 19, 719–722. Accessed August 3, 2021. https://psycnet.apa.org/record/2005-15454-001.
- Kang DW, Park JG, Ilhan ZE, et al. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS One. 2013, 8(7). [CrossRef]
- Rǎdulescu A, Herron J, Kennedy C, Scimemi A. Global and local excitation and inhibition shape the dynamics of the cortico-striatal-thalamo-cortical pathway. Sci Rep. 2017, 7(1). [CrossRef]
- Brem S, Grünblatt E, Drechsler R, Riederer P, Walitza S. The neurobiological link between OCD and ADHD. Atten Defic Hyperact Disord. 2014, 6, 175–202. [CrossRef]
- Gao J, Zhou Y, Yang X, et al. Abnormalities within and beyond the cortico-striato-thalamo-cortical circuitry in medication-free patients with OCD revealed by the fractional amplitude of low-frequency fluctuations and resting-state functional connectivity. Neurosci Lett. 2019, 712. [CrossRef]
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Research. 2015, 277:32-48. [CrossRef]
- Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015, 161, 264–276. [CrossRef]
- Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei - PubMed. Accessed May 31, 2023. https://pubmed.ncbi.nlm.nih.gov/15801987/. [CrossRef]
- Simpson CA, Mu A, Haslam N, Schwartz OS, Simmons JG. Feeling down? A systematic review of the gut microbiota in anxiety/depression and irritable bowel syndrome. J Affect Disord. 2020, 266:429-446. [CrossRef]
- Menzies RG, O’Brian S, Onslow M, Packman A, St. Clare T, Block S. An experimental clinical trial of a cognitive-behavior therapy package for chronic stuttering. J Speech Lang Hear Res. 2008, 51, 1451–1464. [CrossRef]
- Dougherty DM, Mathias CW, Marsh DM, Papageorgiou TD, Swann AC, Moeller FG. Laboratory Measured Behavioral Impulsivity Relates to Suicide Attempt History. Suicide Life Threat Behav. 2004, 34, 374–385. [CrossRef]
- Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. Journal of Physiology. 2004, 558, 263–275. [CrossRef]
- Dell’Osso L, Carmassi C, Mucci F, Marazziti D. Depression, Serotonin and Tryptophan. Curr Pharm Des. 2016, 22, 949–954. [CrossRef]
- Cangiano C, Cardelli-Cangiano P, Cascino A, et al. On the stimulation by insulin of tryptophan transport across the blood-brain barrier. Biochem Int. 1983, 7, 617–627. http://europepmc.org/abstract/MED/6091659.
- Waclawiková B, El Aidy S. Role of Microbiota and Tryptophan Metabolites in the Remote Effect of Intestinal Inflammation on Brain and Depression. Pharmaceuticals (Basel). 2018, 11, 63. [CrossRef]
- Morris G, Berk M, Carvalho A, et al. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol Neurobiol. Published online June 27, 2016. [CrossRef]
- Hsiao EY, McBride SW, Hsien S, et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013, 155, 1451–1463. [CrossRef]
- Rosenberg DR, Macmaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000, 39, 1096–1103. [CrossRef]
- Goddard AW, Shekhar A, Whiteman AF, McDougle CJ. Serotoninergic mechanisms in the treatment of obsessive-compulsive disorder. Drug Discov Today. 2008, 13(7-8):325-332. [CrossRef]
- Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S. Glutamatergic dysfunction in OCD. Neuropsychopharmacology. 2005, 30, 1735–1740. [CrossRef]
- Grant JE, Odlaug BL. Update on pathological skin picking. Curr Psychiatry Rep. 2009, 11, 283–288. [CrossRef]
- Grant JE, Chamberlain SR, Redden SA, Leppink EW, Odlaug BL, Kim SW. N-Acetylcysteine in the Treatment of Excoriation Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2016, 73, 490–496. [CrossRef]
- Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693:128-133.
- Strandwitz P, Kim KH, Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019, 4, 396–403. [CrossRef]
- Denys D, Fluitman S, Kavelaars A, Heijnen C, Westenberg H. Decreased TNF-α and NK activity in obsessive-compulsive disorder. Psychoneuroendocrinology. 2004, 29, 945–952. [CrossRef]
- Van Der Wee NJ, Stevens H, Hardeman JA, et al. Enhanced dopamine transporter density in psychotropic-naive patients with obsessive-compulsive disorder shown by [123I]{beta}-CIT SPECT. Am J Psychiatry. 2004, 161, 2201–2206. [CrossRef]
- Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011, 141(2). [CrossRef]
- Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterology & Motility. 2013, 25, 713–719. [CrossRef]
- Dinan TG, Cryan JF. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol Clin North Am. 2017, 46, 77–89. [CrossRef]
- Dinan TG, Cryan JF. The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr Opin Clin Nutr Metab Care. 2015, 18, 552–558. [CrossRef]
- Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012, 13, 701–712. [CrossRef]
- Marazziti D, Palermo S, Arone A, et al. Obsessive-Compulsive Disorder, PANDAS, and Tourette Syndrome: Immuno-inflammatory Disorders. Adv Exp Med Biol. 2023, 1411:275-300. [CrossRef]
- Williams K, Shorser-Gentile L, Sarvode Mothi S, et al. Immunoglobulin A Dysgammaglobulinemia Is Associated with Pediatric-Onset Obsessive-Compulsive Disorder. J Child Adolesc Psychopharmacol. 2019, 29, 268. [CrossRef]
- Westwell-Roper C, Williams KA, Samuels J, et al. Immune-Related Comorbidities in Childhood-Onset Obsessive Compulsive Disorder: Lifetime Prevalence in the Obsessive Compulsive Disorder Collaborative Genetics Association Study. J Child Adolesc Psychopharmacol. 2019, 29, 615. [CrossRef]
- Palm NW, de Zoete MR, Flavell RA. Immune-microbiota interactions in health and disease. Clinical Immunology. 2015, 159, 122–127. [CrossRef]
- McCusker RH, Kelley KW. Immune-neural connections: How the immune system’s response to infectious agents influences behavior. Journal of Experimental Biology. 2013, 216, 84–98. [CrossRef]
- Lazar V, Ditu LM, Pircalabioru GG, et al. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018, 9(AUG). [CrossRef]
- Robbins TW, Vaghi MM, Banca P. Obsessive-Compulsive Disorder: Puzzles and Prospects. Neuron. 2019, 102, 27–47. [CrossRef]
- Iervolino AC, Rijsdijk F V., Cherkas L, Fullana MA, Mataix-Cols D. A multivariate twin study of obsessive-compulsive symptom dimensions. Arch Gen Psychiatry. 2011, 68, 637. [CrossRef]
- Mahjani B, Bey K, Boberg J, Burton C. Genetics of obsessive-compulsive disorder. Psychol Med. 2021, 51, 2247–2259. [CrossRef]
- Schiele MA, Thiel C, Kollert L, et al. Oxytocin Receptor Gene DNA Methylation: A Biomarker of Treatment Response in Obsessive-Compulsive Disorder? Psychother Psychosom. 2020, 90, 57–63. [CrossRef]
- Zilhão NR, Smit DJ, Boomsma DI, Cath DC. Cross-disorder genetic analysis of tic disorders, obsessive-compulsive, and hoarding symptoms. Front Psychiatry. 2016, 7(JUN). [CrossRef]
- Rotge JY, Aouizerate B, Tignol J, Bioulac B, Burbaud P, Guehl D. The glutamate-based genetic immune hypothesis in obsessive-compulsive disorder. An integrative approach from genes to symptoms. Neuroscience. 2010, 165, 408–417. [CrossRef]
- Gassó P, Ortiz AE, Mas S, et al. Association between genetic variants related to glutamatergic, dopaminergic and neurodevelopment pathways and white matter microstructure in child and adolescent patients with obsessive-compulsive disorder. J Affect Disord. 2015, 186:284-292. [CrossRef]
- Mohammadi AH, Karimian M, Mirzaei H, Milajerdi A. Epigenetic modifications and obsessive–compulsive disorder: what do we know? Brain Struct Funct. Published online May 19, 2023:1-11. [CrossRef]
- Dondu A, Caliskan M, Orenay-Boyacioglu S. Link between obsessive-compulsive disorder and polymorphisms in HDAC genes. Brazilian Journal of Psychiatry. 2022, 44, 156. [CrossRef]
- Kratsman N, Getselter D, Elliott E. Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology. 2016, 102:136-145. [CrossRef]
- Cappi C, Diniz JB, Requena GL, et al. Epigenetic evidence for involvement of the oxytocin receptor gene in obsessive-compulsive disorder. BMC Neurosci. 2016, 17, 1–8. [CrossRef]
- D’Addario C, Pucci M, Bellia F, et al. Regulation of oxytocin receptor gene expression in obsessive–compulsive disorder: a possible role for the microbiota-host epigenetic axis. Clin Epigenetics. 2022, 14(1). [CrossRef]
- D’Addario C, Pucci M, Bellia F, et al. Regulation of oxytocin receptor gene expression in obsessive–compulsive disorder: a possible role for the microbiota-host epigenetic axis. Clin Epigenetics. 2022, 14(1). [CrossRef]
- Bey K, Campos-Martin R, Klawohn J, et al. Hypermethylation of the oxytocin receptor gene (OXTR) in obsessive-compulsive disorder: further evidence for a biomarker of disease and treatment response. Epigenetics. 2022, 17, 642–652. [CrossRef]
- Schiele MA, Thiel C, Deckert J, Zaudig M, Berberich G, Domschke K. Monoamine Oxidase A Hypomethylation in Obsessive-Compulsive Disorder: Reversibility By Successful Psychotherapy? Int J Neuropsychopharmacol. 2020, 23, 319–323. [CrossRef]
- D’Addario C, Bellia F, Benatti B, et al. Exploring the role of BDNF DNA methylation and hydroxymethylation in patients with obsessive compulsive disorder. J Psychiatr Res. 2019, 114:17-23. [CrossRef]
- Nissen JB, Hansen CS, Starnawska A, et al. DNA Methylation at the Neonatal State and at the Time of Diagnosis: Preliminary Support for an Association with the Estrogen Receptor 1, Gamma-Aminobutyric Acid B Receptor 1, and Myelin Oligodendrocyte Glycoprotein in Female Adolescent Patients with OCD. Front Psychiatry. 2016, 7(MAR). [CrossRef]
- Frisch A, Michaelovsky E, Rockah R, et al. Association between obsessive-compulsive disorder and polymorphisms of genes encoding components of the serotonergic and dopaminergic pathways. Eur Neuropsychopharmacol. 2000, 10, 205–209. [CrossRef]
- Hemmings SMJ, Kinnear CJ, Niehaus DJH, et al. Investigating the role of dopaminergic and serotonergic candidate genes in obsessive-compulsive disorder. European Neuropsychopharmacology. 2003, 13, 93–98. [CrossRef]
- Billett EA, Richter MA, Sam F, et al. Investigation of dopamine system genes in obsessive-compulsive disorder. Psychiatr Genet. 1998, 8, 163–169. [CrossRef]
- Miguita K, Cordeiro Q, Siqueira-Roberto J, et al. Association analysis between a VNTR intron 8 polymorphism of the dopamine transporter gene (SLC6A3) and obsessive- compulsive disorder in a Brazilian sample. Arq Neuropsiquiatr. 2007, 65(4A):936-941. [CrossRef]
- Cruz C, Orozco B. DRD2, DRD3 and 5HT2A receptor genes polymorphisms in Obsessive-Compulsive Disorder. Accessed May 28, 2023. https://www.researchgate.net/publication/14064809.
- Catalano M, Sciuto G, Di Bella D, Novelli E, Nobile M, Bellodi L. Lack of association between obsessive-compulsive disorder and the dopamine D3 receptor gene: some preliminary considerations. Am J Med Genet. 1994, 54, 253–255. [CrossRef]
- Rosin S, Xia K, Azcarate-Peril MA, et al. A Preliminary Study of Gut Microbiome Variation and HPA Axis Reactivity in Healthy Infants. Psychoneuroendocrinology. 2021, 124:105046. [CrossRef]
- Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018, 555, 210–215. [CrossRef]
- Diamanti T, Prete R, Battista N, Corsetti A, De Jaco A. Exposure to Antibiotics and Neurodevelopmental Disorders: Could Probiotics Modulate the Gut-Brain Axis? Antibiotics (Basel). 2022, 11(12). [CrossRef]
- Njotto LL, Simin J, Fornes R, et al. Maternal and Early-Life Exposure to Antibiotics and the Risk of Autism and Attention-Deficit Hyperactivity Disorder in Childhood: a Swedish Population-Based Cohort Study. Drug Saf. Published online May 1, 2023. [CrossRef]
- Slob EMA, Brew BK, Vijverberg SJH, et al. Early-life antibiotic use and risk of attention-deficit hyperactivity disorder and autism spectrum disorder: results of a discordant twin study. Int J Epidemiol. 2021, 50, 475–484. [CrossRef]
- Ahmed S, Travis SD, Díaz-Bahamonde F V., et al. Early Influences of Microbiota on White Matter Development in Germ-Free Piglets. Front Cell Neurosci. 2021, 15:520. [CrossRef]
- Hoban AE, Stilling RM, Ryan FJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016, 6, e774. [CrossRef]
- Tette FM, Kwofie SK, Wilson MD. Therapeutic Anti-Depressant Potential of Microbial GABA Produced by Lactobacillus rhamnosus Strains for GABAergic Signaling Restoration and Inhibition of Addiction-Induced HPA Axis Hyperactivity. Curr Issues Mol Biol. 2022, 44, 1434. [CrossRef]
- Bravo JA, Forsythe P, Chew M V, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011, 108, 16050–16055. [CrossRef]
- Chávez-Castillo M. Depression as a neuroendocrine disorder: Emerging neuropsychopharmacological approaches beyond monoamines. Adv Pharmacol Pharm Sci. 2019, 2019:7943481.
- Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome | Scientific Reports. Accessed May 31, 2023. https://www.nature.com/articles/s41598-022-05756-0. [CrossRef]
- Laswi I, Shafiq A, Al-Ali D, et al. A comparative pilot study of bacterial and fungal dysbiosis in neurodevelopmental disorders and gastrointestinal disorders: Commonalities, specificities and correlations with lifestyle. Microorganisms. 2021, 9, 741. [CrossRef]
- Bendriss G, Al-Ali D, Shafiq A, et al. Targeting the gut microbiome: A brief report on the awareness, practice, and readiness to engage in clinical interventions in Qatar. Qatar Med J. 2021, 2020(3). [CrossRef]
- Sanders ME. Probiotics: Definition, Sources, Selection, and Uses. Clinical Infectious Diseases. 2008, 46(s2):S58-S61. [CrossRef]
- Skott E, Yang LL, Stiernborg M, et al. Effects of a synbiotic on symptoms, and daily functioning in attention deficit hyperactivity disorder – A double-blind randomized controlled trial. Brain Behav Immun. 2020, 89:9-19. [CrossRef]
- Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science (1979). 2018, 359, 1151–1156. [CrossRef]
- Wu Z, Xu Q, Wang Q, et al. The impact of dietary fibers on Clostridioides difficile infection in a mouse model. Front Cell Infect Microbiol. 2022, 12. [CrossRef]
- Chen K, Chen H, Faas MM, et al. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol Nutr Food Res. 2017, 61, 1601006. [CrossRef]
- Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the National Academy of Sciences. 2013, 110, 9066–9071. [CrossRef]
- Demirci M, Tokman HB, Uysal HK, et al. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol (Madr). 2019, 47, 365–371. [CrossRef]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol. 2011, 77, 6718–6721. [CrossRef]
- Klaenhammer TR, Altermann E, Pfeiler E, et al. Functional Genomics of Probiotic Lactobacilli. J Clin Gastroenterol. 2008, 42(September):S160-S162. [CrossRef]
- Maassen CBM, Claassen E. Strain-dependent effects of probiotic lactobacilli on EAE autoimmunity. Vaccine. 2008, 26, 2056–2057. [CrossRef]
- Martín R, Delgado S, Maldonado A, et al. Isolation of lactobacilli from sow milk and evaluation of their probiotic potential. Journal of Dairy Research. 2009, 76, 418–425. [CrossRef]
- Moorthy G, Murali MR, Devaraj SN. Lactobacilli facilitate maintenance of intestinal membrane integrity during Shigella dysenteriae 1 infection in rats. Nutrition. 2009, 25, 350–358. [CrossRef]
- Alghamdi MA, Al-Ayadhi L, Hassan WM, Bhat RS, Alonazi MA, El-Ansary A. Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders. Metabolites. 2022, 12(6). [CrossRef]
- Kantak PA, Bobrow DN, Nyby JG. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behavioural Pharmacology. 2014, 25, 71–79. [CrossRef]
- Sanikhani NS, Modarressi MH, Jafari P, et al. The Effect of Lactobacillus casei Consumption in Improvement of Obsessive–Compulsive Disorder: an Animal Study. Probiotics Antimicrob Proteins. 2020, 12, 1409–1419. [CrossRef]
- Kobliner V, Mumper E, Baker SM. Reduction in obsessive compulsive disorder and self-injurious behavior with saccharomyces boulardii in a child with autism: A case report. Integrative Medicine (Boulder). 2018, 17, 38–41.
- Pochakom A, Mu C, Rho JM, Tompkins TA, Mayengbam S, Shearer J. Selective Probiotic Treatment Positively Modulates the Microbiota-Gut-Brain Axis in the BTBR Mouse Model of Autism. Brain Sci. 2022, 12(6). [CrossRef]
- Sen P, Sherwin E, Sandhu K, et al. The live biotherapeutic Blautia stercoris MRx0006 attenuates social deficits, repetitive behaviour, and anxiety-like behaviour in a mouse model relevant to autism. Brain Behav Immun. 2022, 106:115-126. [CrossRef]
- Skott E, Yang LL, Stiernborg M, et al. Effects of a synbiotic on symptoms, and daily functioning in attention deficit hyperactivity disorder - A double-blind randomized controlled trial. Brain Behav Immun. 2020, 89:9-19. [CrossRef]
- Enrichment of Gut Ecosystem by Daily Supplementation of Selective Probiotic Strains and Probiotic Complex in Dysbiosis Condition of Autism. International Journal of Pharmaceutical Research. 2021, 13(01). [CrossRef]
- Szklany K, Wopereis H, de Waard C, et al. Supplementation of dietary non-digestible oligosaccharides from birth onwards improve social and reduce anxiety-like behaviour in male BALB/c mice. Nutr Neurosci. 2020, 23, 896–910. [CrossRef]
- Tabouy L, Getselter D, Ziv O, et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav Immun. 2018, 73:310-319. [CrossRef]
- Al-Ali D, Ahmed A, Shafiq A, et al. Fecal microbiota transplants: A review of emerging clinical data on applications, efficacy, and risks (2015-2020). Qatar Med J. 2021, 2021(1). [CrossRef]
- Kang DW, Adams JB, Coleman DM, et al. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019, 9, 5821. [CrossRef]
- Ananthaswamy A. Faecal transplant eases symptoms of Parkinson’s disease. New Sci (1956). 2011, 209, 8–9. [CrossRef]
- Ianiro G, Segal JP, Mullish BH, et al. Fecal microbiota transplantation in gastrointestinal and extraintestinal disorders. Future Microbiol. 2020, 15, 1173–1186. [CrossRef]
- Li N, Chen H, Cheng Y, et al. Fecal Microbiota Transplantation Relieves Gastrointestinal and Autism Symptoms by Improving the Gut Microbiota in an Open-Label Study. Front Cell Infect Microbiol. 2021, 11. [CrossRef]
- Wang J, Liu X, Li Q. Interventional strategies for ischemic stroke based on the modulation of the gut microbiota. Front Neurosci. 2023, 17:1158057. [CrossRef]
- Khanna S, Vazquez-Baeza Y, González A, et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome. 2017, 5(1). [CrossRef]
- Kang DW, Adams JB, Gregory AC, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome. 2017, 5, 1–16. [CrossRef]
- Kilinçarslan S. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with inflammatory bowel disease: an experimental study. Actas Esp Psiquiatr. 2020, 48, 1–7.
- Wang J, Cao Y, Hou W, et al. Fecal microbiota transplantation improves VPA-induced ASD mice by modulating the serotonergic and glutamatergic synapse signaling pathways. Transl Psychiatry. 2023, 13(1). [CrossRef]
- Wilkins LJ, Monga M, Miller AW. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci Rep. 2019, 9, 12918. [CrossRef]
- Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014, 16, 1024–1033. [CrossRef]
- Mirsepasi-Lauridsen HC, Vrankx K, Engberg J, et al. Disease-Specific Enteric Microbiome Dysbiosis in Inflammatory Bowel Disease. Front Med (Lausanne). 2018, 5:304. [CrossRef]
- Coker OO, Nakatsu G, Dai RZ, et al. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut. 2019, 68, 654–662. [CrossRef]
- Li L, Wang F, Liu Y, Gu F. Intestinal microbiota dysbiosis in children with recurrent respiratory tract infections. Microb Pathog. 2019, 136:103709. [CrossRef]
- Hou M, Xu G, Ran M, Luo W, Wang H. APOE-ε4 Carrier Status and Gut Microbiota Dysbiosis in Patients With Alzheimer Disease. Front Neurosci. 2021, 15. [CrossRef]
- Cassani E, Barichella M, Cancello R, et al. Increased urinary indoxyl sulfate (indican): New insights into gut dysbiosis in Parkinson’s disease. Parkinsonism Relat Disord. 2015, 21, 389–393. [CrossRef]
- Wood H. New models show gut–brain transmission of Parkinson disease pathology. Nat Rev Neurol. 2019, 15, 491. [CrossRef]
- CR Martin VOAKEM. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018, 6:133-148. [CrossRef]
- O’ Mahony SM, Stilling RM, Dinan TG, Cryan JF. The microbiome and childhood diseases: Focus on brain-gut axis. Birth Defects Res C Embryo Today. 2015, 105, 296–313. [CrossRef]
- Kim N, Yun M, Oh YJ, Choi HJ. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. Journal of Microbiology. 2018, 56, 172–182. [CrossRef]
- Shao T, Hsu R, Hacein-Bey C, et al. The Evolving Landscape of Fecal Microbial Transplantation. Clin Rev Allergy Immunol. Published online 2023. [CrossRef]
- Nicco C, Paule A, Konturek P, Edeas M. From Donor to Patient: Collection, Preparation and Cryopreservation of Fecal Samples for Fecal Microbiota Transplantation. Diseases. 2020, 8, 9. [CrossRef]
- Nøhr MK, Egerod KL, Christiansen SH, et al. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015, 290:126-137. [CrossRef]
- Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014, 40, 128–139. [CrossRef]
- Oleskin A V., Shenderov BA. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb Ecol Health Dis. 2016, 27(0). [CrossRef]
- Billeci L, Callara AL, Guiducci L, et al. A randomized controlled trial into the effects of probiotics on electroencephalography in preschoolers with autism. Autism. 2023, 27, 117. [CrossRef]
- Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose-response efficacy of a proprietary probiotic formula of lactobacillus acidophilus CL1285 and lactobacillus casei LBC80R for antibiotic-associated diarrhea and clostridium difficile-associated diarrhea prophylaxis in adult patients. American Journal of Gastroenterology. 2010, 105, 1636–1641. [CrossRef]
- Ruszczyński M, Radzikowski A, Szajewska H. Clinical trial: effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment Pharmacol Ther. 2008, 28, 154–161. [CrossRef]
- Canani RB, Cirillo P, Terrin G, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007, 335, 340. [CrossRef]
- Kruis W, Frič P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004, 53, 1617–1623. [CrossRef]
- Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019, 25, 716–729. [CrossRef]
- Dietrich CG, Kottmann T, Alavi M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J Gastroenterol. 2014, 20, 15837–15844. [CrossRef]
- Pereg D, Kimhi O, Tirosh A, Orr N, Kayouf R, Lishner M. The effect of fermented yogurt on the prevention of diarrhea in a healthy adult population. Am J Infect Control. 2005, 33, 122–125. [CrossRef]
- Lichtman JS, Sonnenburg JL, Elias JE. Monitoring host responses to the gut microbiota. ISME J. 2015, 9, 1908–1915. [CrossRef]
- Carmody RN, Gerber GK, Luevano JM, et al. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015, 17, 72–84. [CrossRef]
- Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes. J Clin Immunol. 2001, 21, 264–271. [CrossRef]
- Chuong KH, Mack DR, Stintzi A, O’Doherty KC. Human microbiome and learning healthcare systems: Integrating research and precision medicine for inflammatory bowel disease. OMICS. 2018, 22, 119–126. [CrossRef]
- Han S, Lu Y, Xie J, et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front Cell Infect Microbiol. 2021, 11. [CrossRef]
- Pelto L, Isolauri E, Lillus EM, Nuutila J, Salminen S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clinical & Experimental Allergy. 1998, 28, 1474–1479. [CrossRef]
- Roessler A, Friedrich U, Vogelsang H, et al. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin Exp Allergy. 2008, 38, 93–102. [CrossRef]
- Zmora N, Zilberman-Schapira G, Suez J, et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell. 2018, 174, 1388–1405.e21. [CrossRef]
- Hod K, Dekel R, Aviv Cohen N, et al. The effect of a multispecies probiotic on microbiota composition in a clinical trial of patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2018, 30(12). [CrossRef]
- Suwal S, Wu Q, Liu W, et al. The probiotic effectiveness in preventing experimental colitis is correlated with host gut microbiota. Front Microbiol. 2018, 9(NOV):2675. [CrossRef]
- Abildgaard A, Kern T, Pedersen O, Hansen T, Wegener G, Lund S. The antidepressant-like effect of probiotics and their faecal abundance may be modulated by the cohabiting gut microbiota in rats. Eur Neuropsychopharmacol. 2019, 29, 98–110. [CrossRef]
- Ferrario C, Taverniti V, Milani C, et al. Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus paracasei DG Varies among Healthy Adults. J Nutr. 2014, 144, 1787–1796. [CrossRef]
- Johnson KVA. Gut microbiome composition and diversity are related to human personality traits. Hum Microb J. 2020, 15:None. [CrossRef]
- He F, Ouwehand AC, Isolauri E, Hosoda M, Benno Y, Salminen S. Differences in composition and mucosal adhesion of bifidobacteria isolated from healthy adults and healthy seniors. Curr Microbiol. 2001, 43, 351–354. [CrossRef]
- Andriantsoanirina V, Teolis AC, Xin LX, Butel MJ, Aires J. Bifidobacterium longum and Bifidobacterium breve isolates from preterm and full term neonates: Comparison of cell surface properties. Anaerobe. 2014, 28:212-215. [CrossRef]
- Aceti A, Maggio L, Beghetti I, et al. Probiotics Prevent Late-Onset Sepsis in Human Milk-Fed, Very Low Birth Weight Preterm Infants: Systematic Review and Meta-Analysis. Nutrients. 2017, 9, 904. [CrossRef]
- He L, Chen R, Zhang B, et al. Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes mellitus. Front Immunol. 2022, 13. [CrossRef]
- Biazzo M, Deidda G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J Clin Med. 2022, 11(14). [CrossRef]
- Kang DW, Adams JB, Gregory AC, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome. 2017, 5(1). [CrossRef]
- Goodswen SJ, Barratt JLN, Kennedy PJ, Kaufer A, Calarco L, Ellis JT. Machine learning and applications in microbiology. FEMS Microbiol Rev. 2021, 45, 1–19. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




