Submitted:
05 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract

Keywords:
Introduction
Motivation
Gulf Cooperation Council
Misconceptions
- ❖ “Regulatory bodies need scientists to evaluate a dossier. Others suggested that the region is plenty of scientists, pharmacists, and academicians to serve this duty.”
- “Discussion between national and international regulatory bodies is needed to ensure biosimilar approval is consistent worldwide.”
- “The limitations faced by recently established regulatory bodies must be recognized and addressed by mature regulatory bodies worldwide.”
- “Countries with greater experience must support countries with less experience of biosimilars.”
- “Action should be taken to ensure that all biosimilar products globally are traceable at batch level to ensure adequate pharmacovigilance is upheld. Biosimilar naming will be key to this.”
- Strong governmental regulators should be in place to ensure drug products can be tracked.
- “The long-term effects of switching and multi-switching between biosimilars and/or reference products need to be understood and addressed. This requires a concerted international effort to develop an optimal methodological approach.”
- “Biosimilar patient registries could be established and implemented to gather further data on switching.”
- “Electronic healthcare records need to be developed and implemented to facilitate pharmacovigilance and gather further data on switching.”
- “Encourage meeting with clinicians to explain what biosimilars are and what they are not, to enable them how to decide whether to prescribe biosimilars.”
- “Physicians, pharmacists, regulators, patients, and all stakeholders must communicate and share their experiences–challenges, and successes–with biosimilars.”
- A lack of agreement exists among the Arab states regarding regulatory approval issues, particularly regarding interchangeability and switching. In Saudi Arabia, biosimilars are not automatically interchangeable. For example, ten biosimilars have been approved, of which only two are regarded as interchangeable. It is evident that, in Saudi Arabia, biosimilarity alone is not sufficient for substitution or switching. However, biosimilars approved by EMA are considered interchangeable. Additionally, a clinical trial that involves switching must be run to approve switching- this must happen before the biosimilar is approved.
- The Egyptian Drug Authority (EDA) “Guideline for registration of Biosimilar products in Egypt” is in place as of March 2020. The applicant must exhibit and compare the biosimilarity of their product to the innovator/reference product by completing and comparing pre-clinical and clinical studies and quality exercises. The EDA adopts the EMA guidelines and refers to the U.S. FDA’s safety and quality considerations, the WHO guidelines for evaluating similar biotherapeutic products, and relevant ICH guidelines. In Egypt, however, the Ministry of Health will make interchangeability decisions, where the patient will not be given a choice.
- The Jordanian FDA’s guidelines are based on the EMA, where the EMA model has been implemented for quality assessment and comparability. It also authorizes the approval of manufacturing sites as a prerequisite to product approval and filing. Currently, six products have been approved according to Jordan’s biosimilar guidelines. Jordan’s approach to biosimilar regulation can be considered vigilant and strict. Nonetheless, biosimilars manufactured and marketed in reference countries, including but not limited to the UK, USA, Germany, France, Netherlands, Sweden, Australia, Austria, and Japan, are usually given more privileges.
- Medicines in Tunisia are obtained by centralized pharmacy purchase (PCP). The Biosimilar Specialized Committee makes decisions on a case-by-case basis regarding interchangeability. The committee comprises representatives of pharmaceutical inspection, national control laboratory, regulatory authorities, various clinicians, and experts who utilize biosimilars.
Biosimilars
Proposed Guideline
SRA Sourcing
Non-SRA Country Biosimilars
Scope
Definition
Reference Product
Characterization
Impurities
Functional Assays
Test Methods
The number of batches
Data Evaluation
Expression System
Post-translation Modifications
Release Specification
Formulation
Reference Standards
Stability
Process Qualification
Animal Toxicology
Clinical Pharmacology
Immunogenicity
Clinical Efficacy
Naming
Label
Substitution
Pediatrics
Human Factor Studies
Risk Management Plan
Conclusions
Conflicts of Interest
References
- https://www.statista.com/statistics/280572/medicine-spending-worldwide/.
- C.P. Gross and K.A. Sepkowitz, “The myth of the medical breakthrough: smallpox, vaccination, and Jenner reconsidered,” Int J Infect Dis, 3(1):54-60, 1998.
- A. von Schwerin et al., “Biologics: an introduction,” in Biologics, a History of Agents Made From Living Organisms in the Twentieth Century, A. von Schwerin, H. Stoff, B. Wahrig, ed., London: Pickering & Chatto, 2013, pp. 1-33.
- A. von Schwerin et al., “Biologics: an introduction,” in Biologics, a History of Agents Made From Living Organisms in the Twentieth Century, A. von Schwerin, H. Stoff, B. Wahrig, ed., London: Pickering & Chatto, 2013, pp. 1-33.
- Kalkan AK, Palaz F, Sofija S, Elmousa N, Ledezma Y, Cachat E, Rios-Solis L. Improving recombinant protein production in CHO cells using the CRISPR-Cas system. Biotechnol Adv. 2023 May-Jun;64:108115. [CrossRef] [PubMed]
- L. Andrews et al., “A snapshot of biologic drug development: Challenges and opportunities,” Hum Exp Toxicol, 34(12):1279-1285, 2015.
- N. Casadevall et al., “Evolution of biological agents: how established drugs can become less safe,” BMJ. 357:j1707, 2017.
- Wouters OJ, et. Al. , Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020 Mar 3;323(9):844-853. Erratum in: JAMA. 2022 Sep 20;328(11):1110. Erratum in: JAMA. 2022 Sep 20;328(11):1111. [CrossRef]
- https://www.fda.gov/drugs/development-approval-process-drugs/frequently-asked-questions-patents-and-exclusivity#howlongexclusivity.
- Schlander, M. , Hernandez-Villafuerte, K., Cheng, CY. et al. How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment. PharmacoEconomics 39, 1243–1269 (2021). [CrossRef]
- www.pharmaceutical-technology.com/features/most-expensive-drugs-us/.
- Kronfol, N. Eastern Mediterranean Health Journal. Vol. 18 No. 11, 2012. https://apps.who.int/iris/bitstream/handle/10665/118493/EMHJ_2012_18_11_1151_1156.pdf;jsessionid=A6C226BFF2849E550596A7F50848641E?sequence=1.
- McCall SJ, Semaan A, Altijani N, Opondo C, Abdel-Fattah M, Kabakian-Khasholian T. Trends, wealth inequalities and the role of the private sector in caesarean section in the Middle East and North Africa: A repeat cross-sectional analysis of population-based surveys. PLoS One. 2021 Nov 16;16(11):e0259791. [CrossRef]
- Goffman, LF. Medicine and Health in the Modern Middle East and North Africa. 2019. https://www.jadaliyya.com/Details/40332.
- Mate K, Bryan C, Deen N, McCall J. Review of Health Systems of the Middle East and North Africa Region. International Encyclopedia of Public Health. 2017:347–56. [CrossRef]
- WHO. The global health observatory. https://www.who.int/data/gho/publications/world-health-statistics.
- https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS?locations=1A.
- https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nhe-fact-sheet#:~:text=NHE%20grew%202.7%25%20to%20%244.3,Gross%20Domestic%20Product%20(GDP).
- https://www.reutersevents.com/pharma/commercial/middle-east-pharma-market-making#:~:text=The%20total%20regional%20market%20is,imported%20generics%20and%20branded%20drugs.
- https://arabcenterdc.org/resource/advancement-and-inequity-in-the-arab-worlds-medical-and-pharmaceutical-sectors/.
- https://arabcenterdc.org/resource/health-care-in-the-arab-world-outcomes-of-a-broken-social-contract/.
- Arab Language International Council. https://alarabiah.org/.
- https://www.emergobyul.com/news.
- https://www.pharmasolutions-int.com/rise-growth-of-pharma-distributors-in-the-middle-east/.
- https://www.drugdiscoverytrends.com/50-of-2022s-best-selling-pharmaceuticals/.
- https://www.who.int/news-room/articles-detail/call-for-consultant-on-monoclonal-antibodies-for-infectious-diseases#:~:text=The%20cost%20of%20goods%20to,US%2095%2D200%20per%20gram.
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q13-continuous-manufacturing-drug-substances-and-drug-products.
- https://www.coherentmarketinsights.com/market-insight/mena-biologics-and-biosimilars-market-4393#:~:text=The%20Arab World%20biologics%20%26%20biosimilars%20market,period%20(2020%2D2027).
- https://www.coherentmarketinsights.com/market-insight/saudi-arabia-pharmaceuticals-market-3557.
- https://www.vision2030.gov.sa/.
- https://tia.gov.tn/storage/app/media/ARGUMENTAIRES/TIA_TUNISIA_PHARMA/AG%20PHARMA%20ANG.pdf.
- https://www.jordantimes.com/news/local/jordans-pharmaceutical-exports-reach-jd1b-%E2%80%94-japm.
- https://www.marketdataforecast.com/market-reports/mea-biosimilars-market.
- Bassil N, Sasmaz S, M El S, Akalankam A. Realizing biosimilar potential in the Middle East & Africa. the Middle East and Africa perspective white paper [Internet]. IQVIA; 2020 [cited August 24, 2022]: 28. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/mea/white-paper/biosimilar_iqvia-whitepaper_final.pdf. Accessed September 20, 2022.
- Market Data Forecast. MEA biosimilars market size, trends, growth | 2022 to 2027 https://www.marketdataforecast. com/market-reports/mea-biosimilars-market. Accessed September 20, 2022.
- https://www.refworld.org/docid/3ae6b3ab18.html.
- Market Data Forecast. MEA biosimilars market size, trends, growth | 2022 to 2027 https://www.marketdataforecast. com/market-reports/mea-biosimilars-market. Accessed September 20, 2022.
- https://www.sfda.gov.sa/en/news/88233.
- http://gabi-journal.net/2nd-mena-stakeholder-meeting-on-biosimilars-2018-report.html.
- https://www.fda.gov/science-research/about-science-research-fda/fda-science-forum#:~:text=The%20Forum%20offers%20an%20exciting,efforts%20of%20FDA’s%2011%2C000%20scientists.
- https://www.lee.senate.gov/2022/11/sen-lee-introduces-biosimilar-red-tape-elimination-act. 2022.
- https://www.ema.europa.eu/en/news/biosimilar-medicines-can-be-interchanged.
- Niazi, SK. Biosimilars: Harmonizing the Approval Guidelines. Biologics. 2022; 2(3):171-195. [CrossRef]
- de Mora, F. Biosimilars: a value proposition. BioDrugs. 2019;33(4):353–356. [CrossRef]
- Bassil N, Sasmaz S, M El S, Akalankam A. Realizing biosimilar potential in the Middle East & Africa. the Middle East and Africa perspective white paper [Internet]. IQVIA; 2020 [cited August 24, 2022]: 28. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/mea/white-paper/biosimilar_iqvia-whitepaper_final.pdf. Accessed September 20, 2022.
- Niazi, SK. Molecular Biosimilarity-An AI-Driven Paradigm Shift. Int J Mol Sci. 2022 Sep 14;23(18):10690. [CrossRef]
- Beygmoradi A, Homaei A, Hemmati R, Fernandes P. Recombinant protein expression: Challenges in production and folding related matters. Int J Biol Macromol. 2023 Apr 1;233:123407. [CrossRef] [PubMed]
- EMA Centrally Approved Biosimilars. https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_field/Human/ema_group_types/ema_medicine/field_ema_med_status/authorised-36/ema_medicine_types/field_ema_med_biosimilar/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar; (Accessed on 8 June 2022).
- Inxight Drug Database. https://drugs.ncats.io/substances?facet=Development%20Status%2FUS%20Approved%20Rx&facet=Substance%20Class%2Fprotein&facet=Substance%20Form%2FPrincipal%20Form&page=1 (accessed on 25 April 2023).
- WHO Guidelines on Evaluation of Biosimilars. Replacement of Annex 2 of WHO Technical Report Series, No. 977 https://www.who.int/publications/m/item/guidelines-on-evaluation-of-biosimilars WHO. https://www.who.int/publications/i/item/9789240021853; (Accessed on 8 June 2022).
- Niazi, S. The WHO Biosimilar guidance is based on weak science. https://www.centerforbiosimilars.com/view/who-biosimilar-guidance-is-based-on-weak-science.
- Niazi, S. Opinion: One step forward, half step back. https://www.centerforbiosimilars.com/view/opiniononestepforwardhalfastepbackforwhobiosimilarguidance; (Accessed on 8 June 2022).
- CDSCO, India. https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadAlertsFiles/BiosimilarGuideline2016.pdf; (Accessed on 8 June 2022).
- https://www.fda.gov/files/drugs/published/Labeling-for-Biosimilar-Products-Guidance-for-Industry.pdf.
- https://www.forbes.com/sites/nicolefisher/2018/07/25/one-mans-mission-to-fix-the-fdas-biosimilar-problem/?sh=2386ed9d2380.
- Niazi, SK. End animal testing for biosimilar approval. Science. 2022 Jul 8;377(6602):162-163. [CrossRef] [PubMed]
- Li J, Florian J, Campbell E, et al. Advancing Biosimilar Development Using Pharmacodynamic Biomarkers in Clinical Pharmacology Studies. Clin Pharmacol Ther. 2020;107(1):40-42. [CrossRef]
- Niazi, S. Scientific Rationale for Waiving Clinical Efficacy Testing of Biosimilars. Drug Des Devel Ther. 2022 Aug 24;16:2803-2815. [CrossRef]
- MHRA. Biosimilar Guidance. https://www.gov.uk/government/publications/guidance-on-the-licensing-of-biosimilar-products/guidance-on-the-licensing-of-biosimilar-products; (Accessed on 8 June 2022).
- Global Biosimilar Guidelines. https://www.gabionline.net/reports/Guidelines-for-biosimilars-around-the-world.
- Niazi, SK. Biosimilars: Harmonizing the Approval Guidelines. Biologics. 2022; 2(3):171-195. [CrossRef]
- Garcia-A, A, et al., J Pharm Pharm Sci (www.cspsCanada.org). 2021; 24, 113–126.
- Niazi, S.K. The Coming of Age of Biosimilars: A Personal Perspective. Biologics 2022, 2, 107–127, https://www.mdpi.com/2673-8449/2/2/9. [Accessed 8 June 2022]. [Google Scholar] [CrossRef]
- Niazi, SK. (2022) Biosimilars: A futuristic fast-to-market advice to developers, Expert Opinion on Biological Therapy, 22:2, 149-155. [CrossRef]
- MHRA. Biosimilar Guidance. https://www.gov.uk/government/publications/guidance-on-the-licensing-of-biosimilar-products/guidance-on-the-licensing-of-biosimilar-products; (Accessed on 8 June 2022).
- Niazi, SK. Biosimilars: Harmonizing the Approval Guidelines. Biologics. 2022; 2(3):171-195. [CrossRef]
- https://www.fda.gov/drugs/development-resources/advancing-real-world-evidence-program.
- Niazi, S.K. The Coming of Age of Biosimilars: A Personal Perspective. Biologics 2022, 2, 107–127 https://wwwmdpicom/2673. [Google Scholar] [CrossRef]
- Niazi, SK. (2022) Biosimilars: A futuristic fast-to-market advice to developers, Expert Opinion on Biological Therapy, 22:2, 149-155. [CrossRef]
- Chen Y, Monnard A, Jorge Santos Da S, An inflection point for biosimilars, McKinsey & Co. [cited 2021 Oct 12]. Available from: https://www.mckinsey.com/industries/life-sciences/our-insights/an- inflection-point-for-biosimilars; (Accessed on 8 June 2022).
- Niazi, SK. End animal testing for biosimilar approval. Science. 2022 Jul 8;377(6602):162-163. [CrossRef] [PubMed]
- Niazi, S. Scientific Rationale for Waiving Clinical Efficacy Testing of Biosimilars. Drug Des Devel Ther. 2022 Aug 24;16:2803-2815. [CrossRef]
- https://www.congress.gov/bill/117th-congress/senate-bill/5002.
- MHRA. Biosimilar Guidance. https://www.gov.uk/government/publications/guidance-on-the-licensing-of-biosimilar-products/guidance-on-the-licensing-of-biosimilar-products; (Accessed on 8 June 2022).
- https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/division-applied-regulatory-science.
- Chiu, K. , et al., (2023). New science, drug regulation, and emergent public health issues: The work of FDA’s division of applied regulatory science. Frontiers in Medicine, 9. [CrossRef]
- US Food and Drug Administration. FDA Guidance: Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product https://www.fda.gov/media/88622/ download (2016).
- Chiu K, et al., New science, drug regulation, and emergent public health issues: The work of FDA’s division of applied regulatory science. Front Med (Lausanne). 2023 Jan 19;9:1109541. [CrossRef]
- Li, L. et al. Quantitative relationship between AUEC of absolute neutrophil count and duration of severe neutropenia for GCSF in breast cancer patients. Clin Pharmacol Ther 104, 742–748 (2018).
- Li J, Florian J, Campbell E, Schrieber S, Bai J, Weaver J, et al. Advancing biosimilar development using pharmacodynamic biomarkers in clinical pharmacology studies. Clin Pharmacol Ther. (2020) 107:40–2. [CrossRef]
- Sheikhy M, Schrieber S, Sun Q, Gershuny V, Matta M, Bai J, et al. Considerations for use of pharmacodynamic biomarkers to support biosimilar development- (I) a randomized trial with PCSK9 inhibitors. Clin Pharmacol Ther. (2022). [CrossRef]
- Gershuny V, Sun Q, Schrieber S, Matta M, Weaver J, Ji P, et al. Considerations for use of pharmacodynamic biomarkers to support biosimilar development–(II) a randomized trial with IL-5 antagonists. Clin Pharmacol Ther. (2022). [CrossRef]
- Florian JG, Sun Q, Schrieber S, Matta M, Hazel A, Sheikhy M, et al. Considerations for use of pharmacodynamic biomarkers to support biosimilar development–(III) a randomized trial with interferon beta-1a products. Clin Pharmacol Ther. (2022). [CrossRef]
- Hyland P, Chekka L, Samarth D, Rosenzweig B, Decker E, Mohamed E, et al. Evaluating the utility of proteomics for the identification of circulating pharmacodynamic biomarkers of IFNbeta-1a biologics. Clin Pharmacol Ther. (2022). [CrossRef]
- Niazi, S.K. Functional Biosimilarity to Replace Clinical Efficacy Testing of mAb Biosimilars: Advancing the FDA Perspective. Preprints.org 2023, 2023041141. [Google Scholar] [CrossRef]
- Woodcock. https://www.biopharmadive.com/news/fdas-woodcock-the-clinical-trial-system-is-broken/542698/. 5426.
- US Congress. https://www.govinfo.gov/content/pkg/STATUTE-98/pdf/STATUTE-98-Pg1585.pdf.
- https://www.fda.gov/drugs/biosimilars/biosimilars-science-and-research.
- EMA Biosimilar EPAR Program. https://www.ema.europa.eu/en/search/search/field_ema_web_categories%253Aname_field/Human/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar?search_api_views_fulltext=epar%20biosimilar.
- FDA. Biosimilars Approval Documents. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
- Clinical Trial Database. https://clinicaltrials.gov/ct2/results?term=biosimilar&age_v=&gndr=&type=&rslt=With&Search=Apply.
- Biosimilar Clinical Trials @PubMed. https://pubmed.ncbi.nlm.nih.gov/?term=biosimilar+clinical+trial).
- US Congress. BPCIA. https://www.congress.gov/bill/111th-congress/house-bill/3590.
- https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/division-applied-regulatory-science.
- Chiu, K. , et al., (2023). New science, drug regulation, and emergent public health issues: The work of FDA’s division of applied regulatory science. Frontiers in Medicine, 9. [CrossRef]
- US Food and Drug Administration. FDA Guidance: Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product https://www.fda.gov/media/88622/ download (2016).
- Chiu K, et al., New science, drug regulation, and emergent public health issues: The work of FDA’s division of applied regulatory science. Front Med (Lausanne). 2023 Jan 19;9:1109541. [CrossRef]
- Li, L. et al. Quantitative relationship between AUEC of absolute neutrophil count and duration of severe neutropenia for GCSF in breast cancer patients. Clin Pharmacol Ther 104, 742–748 (2018).
- Li J, Florian J, Campbell E, Schrieber S, Bai J, Weaver J, et al. Advancing biosimilar development using pharmacodynamic biomarkers in clinical pharmacology studies. Clin Pharmacol Ther. (2020) 107:40–2. [CrossRef]
- Sheikhy M, Schrieber S, Sun Q, Gershuny V, Matta M, Bai J, et al. Considerations for use of pharmacodynamic biomarkers to support biosimilar development- (I) a randomized trial with PCSK9 inhibitors. Clin Pharmacol Ther. (2022). [CrossRef]
- Gershuny V, Sun Q, Schrieber S, Matta M, Weaver J, Ji P, et al. Considerations for use of pharmacodynamic biomarkers to support biosimilar development–(II) a randomized trial with IL-5 antagonists. Clin Pharmacol Ther. (2022). [CrossRef]
- Florian JG, Sun Q, Schrieber S, Matta M, Hazel A, Sheikhy M, et al. Considerations for use of pharmacodynamic biomarkers to support biosimilar development–(III) a randomized trial with interferon beta-1a products. Clin Pharmacol Ther. (2022). [CrossRef]
- Hyland P, Chekka L, Samarth D, Rosenzweig B, Decker E, Mohamed E, et al. Evaluating the utility of proteomics for the identification of circulating pharmacodynamic biomarkers of IFNbeta-1a biologics. Clin Pharmacol Ther. (2022). [CrossRef]
- Florian JS, et al., Pharmacodynamic biomarkers for biosimilar development and approval: a workshop summary. Clin Pharmacol Ther. (2022). [CrossRef]
- Wang YM, Strauss DG. Advancing Innovations in Biosimilars. Clin Pharmacol Ther. 2023 Jan;113(1):11-15. [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Guidance: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product (2015). https://www.fda.gov/media/82647/download. (accessed on 25 April 2023).
- US Food and Drug Administration. FDA Guidance: Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product (2016). https://www.fda.gov/media/88622/download. (accessed on 25 April 2023).
- Li, L. et al. Quantitative relationship between AUEC of absolute neutrophil count and duration of severe neutropenia for G-CSF in breast cancer patients. Clin Pharmacol Ther 104, 742–748 (2018).
- US Food and Drug Administration. FDA draft guidance: Biomarker Qualification: Evidentiary Framework (2018). https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM628118.pdf. (accessed on 25 April 2023).
- Strauss DG, Wang YM, Florian J, Zineh I. Pharmacodynamic Biomarkers Evidentiary Considerations for Biosimilar Development and Approval. Clin Pharmacol Ther. 2023 Jan;113(1):55-61. [CrossRef]
- Hotzel, I. , et al., A strategy for risk mitigation of antibodies with fast clearance mAbs, 4 (2012), pp. 753-760.
- Sharma, TW. , et al., In silico selection of therapeutic antibodies for development: viscosity, clearance, and chemical stability. Proc Natl Acad Sci, 111 (2014), pp. 18601-18606.
- Cymera F, Becka H, Rohde A, Reusch D. Therapeutic monoclonal antibody N-glycosylation—structure, function and therapeutic potential. Biologicals. 2018;52:1–11.
- Prior S, et al., International standards for monoclonal antibodies to support pre- and post-marketing product consistency: evaluation of a candidate international standard for the bioactivities of rituximab. MAbs. 2018;10(1):129–42.
- Ryding J, Stahl M, Ullmann M. Demonstrating biosimilar and originator antidrug antibody binding comparability in antidrug antibody assays: a practical approach. Bioanalysis. 2017 Sep;9(18):1395-1406. [CrossRef] [PubMed]
- Wang X, An Z, Luo W, Xia N, Zhao Q. Molecular and functional analysis of monoclonal antibodies in support of biologics development. Protein Cell. 2018 Jan;9(1):74-85. [CrossRef]
- Cymera F, Becka H, Rohde A, Reusch D. Therapeutic monoclonal antibody N-glycosylation—structure, function and therapeutic potential. Biologicals. 2018;52:1–11.
- Prior S, et al., International standards for monoclonal antibodies to support pre- and post-marketing product consistency: evaluation of a candidate international standard for the bioactivities of rituximab. MAbs. 2018;10(1):129–42.
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/generally-accepted-scientific-knowledge-applications-drug-and-biological-products-nonclinical.
- https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence.
- Corrigan-Curay J, Sacks L, Woodcock J. Real-World Evidence and Real-World Data for Evaluating Drug Safety and Effectiveness. JAMA. 2018;320(9):867–868. [CrossRef]
- Chiu, K. , et al., (2023). New science, drug regulation, and emergent public health issues: The work of FDA’s division of applied regulatory science. Frontiers in Medicine, 9. [CrossRef]
- https://www.congress.gov/bill/117th-congress/senate-bill/5002.
- https://www.congress.gov/bill/110th-congress/senate-bill/1695.
- https://www.who.int/initiatives/who-listed-authority-reg-authorities/SRAs.
- World Health Organization & WHO Expert Committee on Specifications for Pharmaceutical Preparations (2018). Fifty-second report of the WHO Expert Committee on Specifications for Pharmaceutical Preparations (PDF). Geneva, Switzerland: World Health Organization. p. 355–6. ISBN 978-92-4-121019-5. OCLC 1039407367.
- No authors listed. “WHO | List of Stringent Regulatory Authorities (SRAs)”. World Health Organization. Retrieved 2022-08-30.
- https://www.outlookindia.com/business/biocon-biologics-biocon-biologics-bribery-cbi-arrests-joint-drug-controller-for-alleged-bribery-to-favour-biocon-biologics-medicine-news-203753.
- https://www.financierworldwide.com/bribery-and-corruption-in-the-pharmaceutical-sector#.Y1AWiOzMKlM.
- https://www.fda.gov/files/drugs/published/Data-Integrity-and-Compliance-With-Current-Good-Manufacturing-Practice-Guidance-for-Industry.pdf.
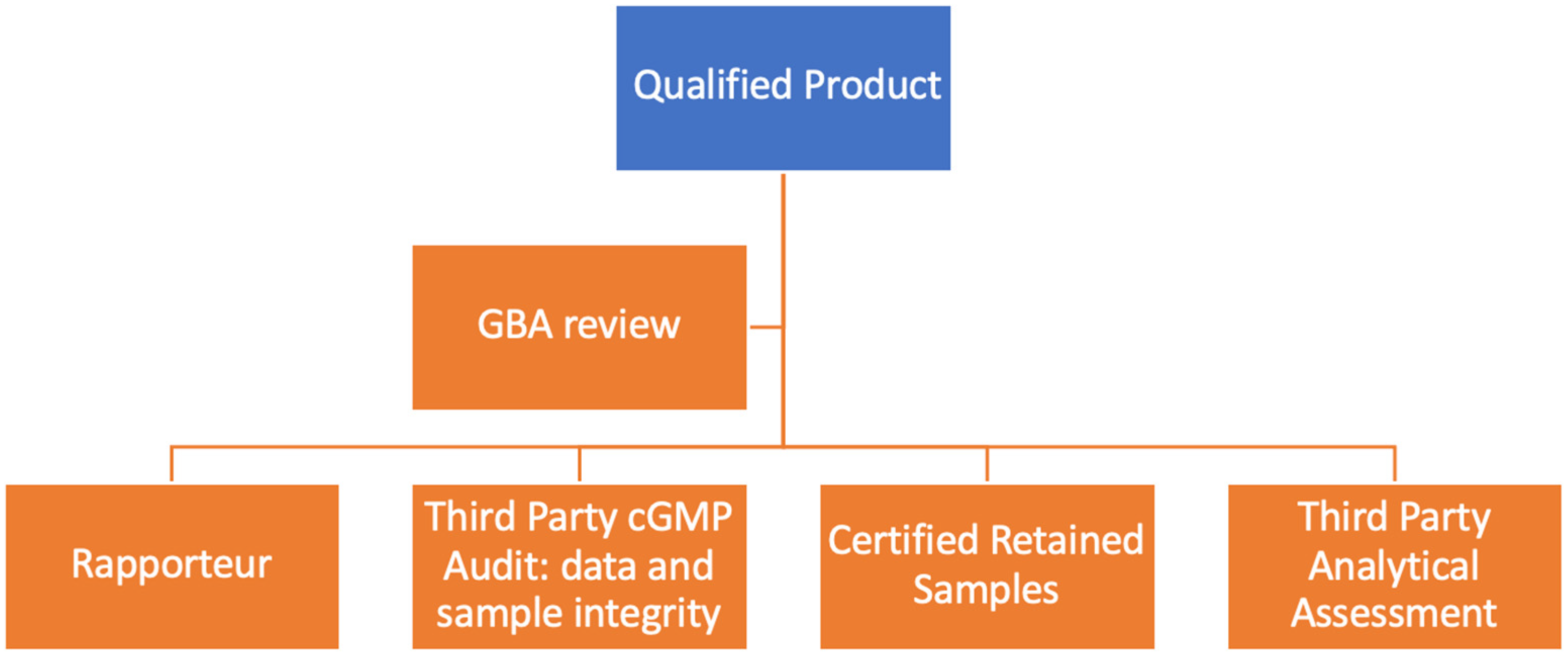
- https://www.ema.europa.eu/en/search/search/field_ema_web_categories%253Aname_field/Human/field_ema_web_topics%253Aname_field/Biosimilars?search_api_views_fulltext=rapporteur.
- Niazi, SK. Molecular Biosimilarity-An AI-Driven Paradigm Shift. Int J Mol Sci. 2022 Sep 14;23(18):10690. [CrossRef]
- Niazi, SK. No two classes of biosimilars: Urgent advice to the US Congress and the FDA. J Clin Pharm Ther. 2022 Sep;47(9):1352-1361. [CrossRef] [PubMed]
- Niazi, SK. BioRationality. https://www.centerforbiosimilars.com/view/biorationality-a-dr-sarfaraz-niazi-column-the-ema-declares-biosimilars-interchangeable.
- https://www.avmi-africa.org/projects/current-projects/#1513773628836-0f0598a8-ec17.
- Niazi, SK. Making COVID-19 mRNA vaccines accessible: challenges resolved. Expert Rev Vaccines. 2022 Sep;21(9):1163-1176. [CrossRef] [PubMed]
- Niazi SK, Al-Shaqha WM, Mirza Z. Proposal of International Council for Harmonization (ICH) Guideline for the Approval of Biosimilars. J Mark Access Health Policy. 2022 Nov 17;11(1):2147286. [CrossRef]
| Drug | Active | Indication | Cost, USD |
|---|---|---|---|
| Actemra | Tocilizumab | Rheumatoid arthritis and cytokine release syndrome | 6,000–9,000 per month. |
| Acthar Gel | Repository corticotropin | Multiple sclerosis, infantile spasms, and nephrotic syndrome | 40,000–60,000 per vial. |
| Actimmune | Interferon gamma-1b | Chronic granulomatous disease and severe, malignant osteopetrosis | 157,000/yr |
| Alecensa | Alectinib | Non-small cell lung cancer (NSCLC) | 159,000–178,000/yr |
| Almita | Olorinab | Breast cancer | 150,000/yr |
| Amondys 45 | Casimersen | Duchenne muscular dystrophy | 300,000/yr |
| Blenrep | Belantamab mafodotin | Multiple myeloma | 400,000/yr |
| Blincyto | Blinatumomab | Acute lymphoblastic leukemia (ALL) | 178,000/trt |
| Braftovi | Encorafenib | Melanoma | 174,000/yr |
| Brineura | Cerliponase alfa | Late infantile neuronal ceroid lipofuscinosis type 2 (CLN2) | 350,000/yr |
| Bylvay | Lumasiran | Bile acid synthesis disorders | 300,000/yr |
| Calquence | Acalabrutinib | Chronic lymphocytic leukemia (CLL) | 175,000/yr |
| Ceredase | Alglucerase | Gaucher disease | 200,000–300,000/yr |
| Cerezyme | Imiglucerase | Gaucher disease | 350,000/yr |
| Darzalex | Daratumumab | Multiple myeloma | 150,000–170,000/yr |
| Elaprase | Idursulfase | Hunter syndrome (MPS II) | 375,000/yr |
| Erwinaze | Asparaginase Erwinia chrysanthemi | Acute lymphoblastic leukemia (ALL) | 14,000–28,000 per vial. |
| Evrysdi | Risdiplam | Spinal muscular atrophy | 340,000/yr |
| Firdapse | Amifampridine | Lambert- Eaton myasthenic syndrome (LEMS) | $140,000/yr |
| Gattex | Teduglutide | Short bowel syndrome | 350,000–400,000/yr |
| Gilenya | Fingolimod | Multiple sclerosis | 90,000–100,000/yr |
| Glybera | Alipogene tiparvovec | Lipoprotein lipase deficiency | 1,000,000/trt |
| Harvoni | Ledipasvir + Sofosbuvir | Chronic hepatitis C | 94,500/trt |
| Hemlibra | Emicizumab | Prevention of bleeding episodes in hemophilia A with factor VIII inhibitors | 482,000/yr |
| Ilaris | Canakinumab | Cryopyrin- associated periodic syndromes (CAPS) and systemic juvenile idiopathic arthritis | 200,000–300,000/yr |
| Imfinzi | Durvalumab | Certain types of cancer, including lung cancer | 150,000–170,000/yr |
| Isturisa | Osilodrostat | Cushing’s disease | 295,000/yr |
| Jakafi | Ruxolitinib | Myelofibrosis and polycythemia vera | 12,000–14,000 per month. |
| Kalydeco | Ivacaftor | Cystic fibrosis | 330,000–360,000/yr |
| Keytruda | Pembrolizumab | Various types of cancer, including melanoma and lung cancer | 150,000–170,000/yr |
| Kymriah | Tisagenlecleucel | Certain types of non-Hodgkin lymphoma and acute lymphoblastic leukemia | $475,000/trt |
| Kyprolis | Carfilzomib | Multiple myeloma | 180,000–200,000/yr |
| Lumakras | Sotorasib | Non- small cell lung cancer (NSCLC) | $17,000 per month. |
| Luxturna | Voretigene neparvovec | Inherited retinal diseases causing blindness | 850,000/trt |
| Mavenclad | Cladribine | Multiple sclerosis | 99,000/yr |
| Monjuvi | Tafasitamab | Diffuse large B- cell lymphoma | $160,000/yr |
| Myalept | Metreleptin | Leptin deficiency in generalized lipodystrophy | 700,000/yr |
| Naglazyme | Galsulfase | Mucopolysaccharidosis VI (MPS VI) | 375,000/yr |
| Nerlynx | Neratinib | Breast cancer | 150,000/yr |
| Ocrevus | Ocrelizumab | Multiple sclerosis | 65,000/yr |
| Olysio | Simeprevir | Chronic hepatitis C | 66,000–84,000/trt |
| Opdivo | Nivolumab | Various types of cancer, including melanoma and lung cancer | 150,000–170,000/yr |
| Opdivo plus | Nivolumab + Ipilimumab | Certain types of cancer, including melanoma and lung cancer | 250,000–270,000/yr |
| Opsumit | Macitentan | Pulmonary arterial hypertension (PAH) | 200,000–220,000/yr |
| Orfadin | Nitisinone | Hereditary tyrosinemia type 1 | 275,000/yr |
| Orkambi | Lumacaftor + Ivacaftor | Cystic fibrosis | 260,000–300,000/yr |
| Orladeyo | Berotralstat | Hereditary angioedema | 470,000/yr |
| Orlissa | Elagolix | Endometriosis and uterine fibroids | 30,000/yr |
| Padcev | Enfortumab vedotin | Urothelial cancer | 16,000 per month. |
| Pomalyst | Pomalidomide | Multiple myeloma | 160,000–180,000/yr |
| Pulmozyme | Dornase alfa | Cystic fibrosis | 311,000/yr |
| Ravicti | Glycerol phenylbutyrate | Chronic management of urea cycle disorders | 350,000–400,000/yr |
| Remicade | Infliximab | Rheumatoid arthritis, Crohn’s disease, and other autoimmune conditions | 30,000–40,000/yr |
| Rozlytrek | Entrectinib | Solid tumors with NTRK gene fusion | 450,000/yr |
| Sandostatin LAR | Octreotide | Acromegaly and neuroendocrine tumors | 15,000–20,000 per month. |
| Signifor | Pasireotide | Cushing’s disease and acromegaly | 200,000–300,000/yr |
| Soliris | Eculizumab | Paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (ahus) | 500,000–700,000/yr |
| Sovaldi | Sofosbuvir | Chronic hepatitis C | 84,000/trt |
| Spinraza | Nusinersen | Spinal muscular atrophy | 375,000 for the first year and 375,000/yr after that. |
| Sprycel | Dasatinib | Chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) | 120,000–130,000/yr |
| Stelara | Ustekinumab | Psoriasis, psoriatic arthritis, and Crohn’s disease | 30,000–40,000/yr |
| Strensiq | Asfotase alfa | Hypophosphatasia | 300,000/yr |
| Synagis | Palivizumab | Prevention of respiratory syncytial virus (RSV) in infants | 9,000–15,000 per month during RSV season. |
| Takhzyro | Lanadelumab | Hereditary angioedema | 488,000/yr |
| Tecentriq | Atezolizumab | Certain types of cancer, including bladder cancer | 150,000–170,000/yr |
| Translarna | Ataluren | Duchenne muscular dystrophy | 262,000/yr |
| Trikafta | Elexacaftor + Tezacaftor + Ivacaftor | Cystic fibrosis | 311,000/yr |
| Ultomiris | Ravulizumab | Paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (ahus) | 498,000/yr |
| Vimizim | Elosulfase alfa | Morquio A syndrome | 375,000/yr |
| Vitrakvi | Larotrectinib | Solid tumors with NTRK gene fusion | 400,000/yr |
| Xalkori | Crizotinib | Small cell lung cancer (NSCLC) | $149,000–167,000/yr |
| Xolair | Omalizumab | Severe asthma and chronic idiopathic urticaria | 32,500/yr |
| Xpovio | Selinexor | Multiple myeloma | 160,000/yr |
| Xtandi | Enzalutamide | Prostate cancer | 129,000–144,000/yr |
| Xyrem | Sodium oxybate | Narcolepsy with cataplexy | 50,000–75,000/yr |
| Yervoy | Ipilimumab | Certain types of cancer, including melanoma | 150,000–170,000/yr |
| Yescarta | Axicabtagene ciloleucel | Certain types of non-Hodgkin lymphoma | $373,000/trt |
| Zolgensma | Onasemnogene abeparvovec | Spinal muscular atrophy | 2,100,000/trt |
| Hemgenix | Viral gene therapy | Hemophilia B gene | 4,3000,000/dose |
| Drug name | 2022 Sales, USD B |
|---|---|
| Actemra/RoActemra (tocilizumab) | USD 2.58 |
| Darzalex (daratumumab) | USD 7.98 |
| Dupixent (dupilumab) | USD 17.42 |
| Enbrel (etanercept) | USD 4.12 |
| Eylea (aflibercept) | USD 12.72 |
| Hemlibra (emicizumab) | USD 3.65 |
| Humira (adalimumab) | USD 21.24 |
| Imfinzi (durvalumab) | USD 2.78 |
| Lantus (insulin glargine) | USD 2.38 |
| Ocrevus (ocrelizumab) | USD 5.76 |
| Opdivo (nivolumab) | USD 8.25 |
| Perjeta (pertuzumab) | USD 3.90 |
| Prolia (denosumab) | USD 3.63 |
| Remicade (infliximab) | USD 2.34 |
| Skyrizi (risankizumab) | USD 5.17 |
| Stelara (ustekinumab) | USD 9.72 |
| Taltz (ixekizumab) | USD 2.48 |
| Tecentriq (atezolizumab) | USD 3.55 |
| Tremfya (guselkumab) | USD 2.67 |
| Trulicity (dulaglutide) | USD 7.44 |
| Drug | Patent Expiry |
|---|---|
| Interferon beta-1b | 2004 |
| Parathyroid hormone | 2004 |
| Interferon alfa-2b | 2004 |
| Chorionic gonadotropin | 2007 |
| Interferon alfa-n3 | 2011 |
| Etanercept | 2012 |
| Menotropins | 2015 |
| Urofollitropin | 2015 |
| Peginterferon alfa-2b | 2015 |
| Interferon beta-1a | 2020 |
| Insulin regular | 2025 |
| Insulin lispro | 2014 |
| Country | Authority | The criterion for consideration as SRA |
|---|---|---|
| Australia | Therapeutic Goods Administration | Mutual recognition agreement with ICH members |
| Austria | Austrian Agency for Health and Food Safety (AGES) | EC member |
| Belgium | Federal Agency for Medicines and Health Products (FAMHP) | EC member |
| Bulgaria | Bulgarian Drug Agency | EC member |
| Canada | Health Canada | ICH observer |
| Croatia | Agency for Medicinal Products and Medical Devices of Croatia (HALMED) | EC member |
| Cyprus | Ministry of Health — Pharmaceutical Services | EC member |
| Czech Republic | State Institute for Drug Control (SUKL) | EC member |
| Denmark | Danish Medicines Agency | EC member |
| Estonia | State Agency of Medicines (Ravimiamet) | EC member |
| Finland | Finnish Medicines Agency (Fimea) | EC member |
| France | National Agency for the Safety of Medicine and Health Products (ANSM) | EC member |
| Germany | Federal Institute for Drugs and Medical Devices | EC member |
| Greece | National Organization for Medicines | EC member |
| Hungary | National Institute of Pharmacy and Nutrition (OGYEI) | EC member |
| Iceland | Icelandic Medicines Agency | EFTA member/mutual recognition agreement |
| Ireland | Health Products Regulatory Authority | EC member |
| Italy | Italian Medicines Agency (AIFA) | EC member |
| Japan | Ministry of Health, Labour and Welfare/Pharmaceuticals and Medical Devices Agency | ICH member |
| Latvia | State Agency of Medicines | EC member |
| Liechtenstein | Office of Health / Department of Pharmaceuticals | EFTA member/mutual recognition agreement |
| Lithuania | State Medicines Control Agency (VVKT) | EC member |
| Luxembourg | Ministry of Health | EC member |
| Malta | Medicines Authority | EC member |
| Netherlands | Health and Youth Care Inspectorate (IGZ) | EC member |
| Norway | Norwegian Medicines Agency | EFTA member/mutual recognition agreement |
| Poland | Chief Pharmaceutical Inspectorate | EC member |
| Portugal | National Authority of Medicines and Health Products (Infarmed) | EC member |
| Romania | National Agency for Medicines and Medical Devices | EC member |
| Slovakia | State Institute for Drug Control (SIDC) | EC member |
| Slovenia | Agency for Medicinal Products and Medical Devices (JAZMP) | EC member |
| Spain | Spanish Agency of Medicines and Medical Devices (AEMPS) | EC member |
| Sweden | Medical Products Agency | EC member |
| Switzerland | Swiss Agency for Therapeutic Products (Swissmedic) | ICH observer/EFTA member |
| United Kingdom | Medicines and Healthcare Products Regulatory Agency (MHRA) | EC member (as of 23 October 2015) |
| United States of America | Food and Drug Administration | ICH member |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





