Submitted:
31 May 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2.1. COVID 19 and Obesity
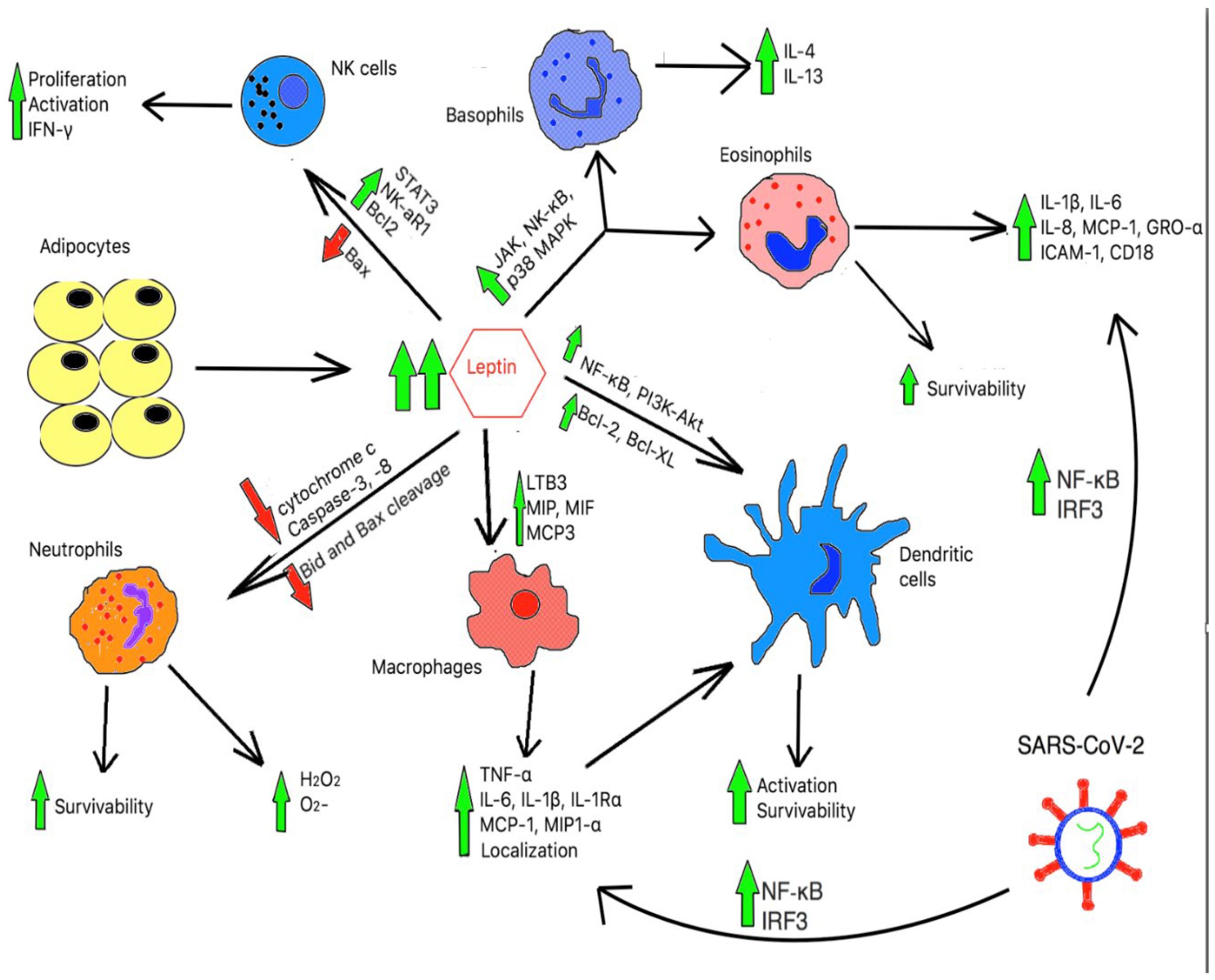
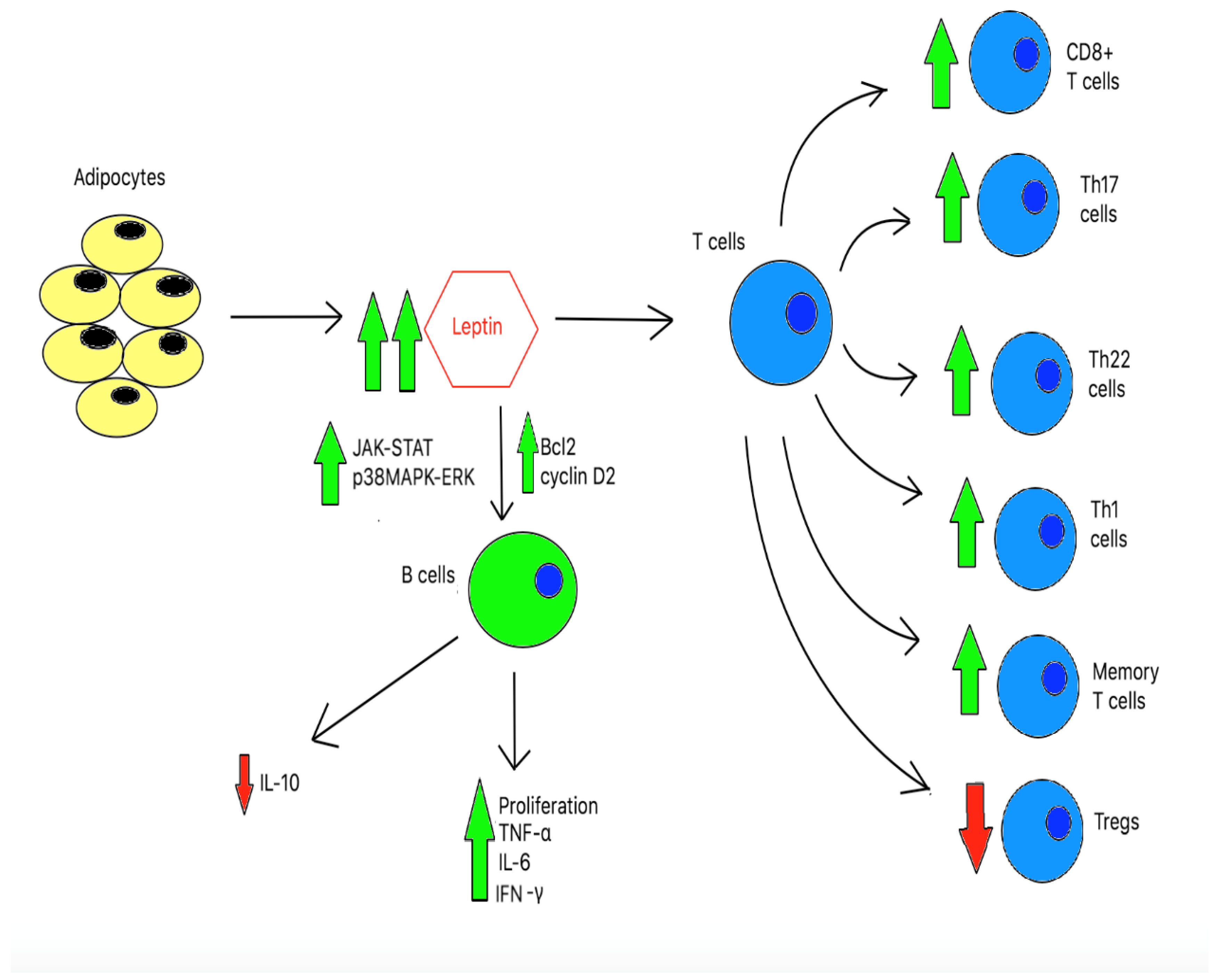
2.2. Immune Function in Obesity
2.3. Covid19- the virus and how it affects immunity:
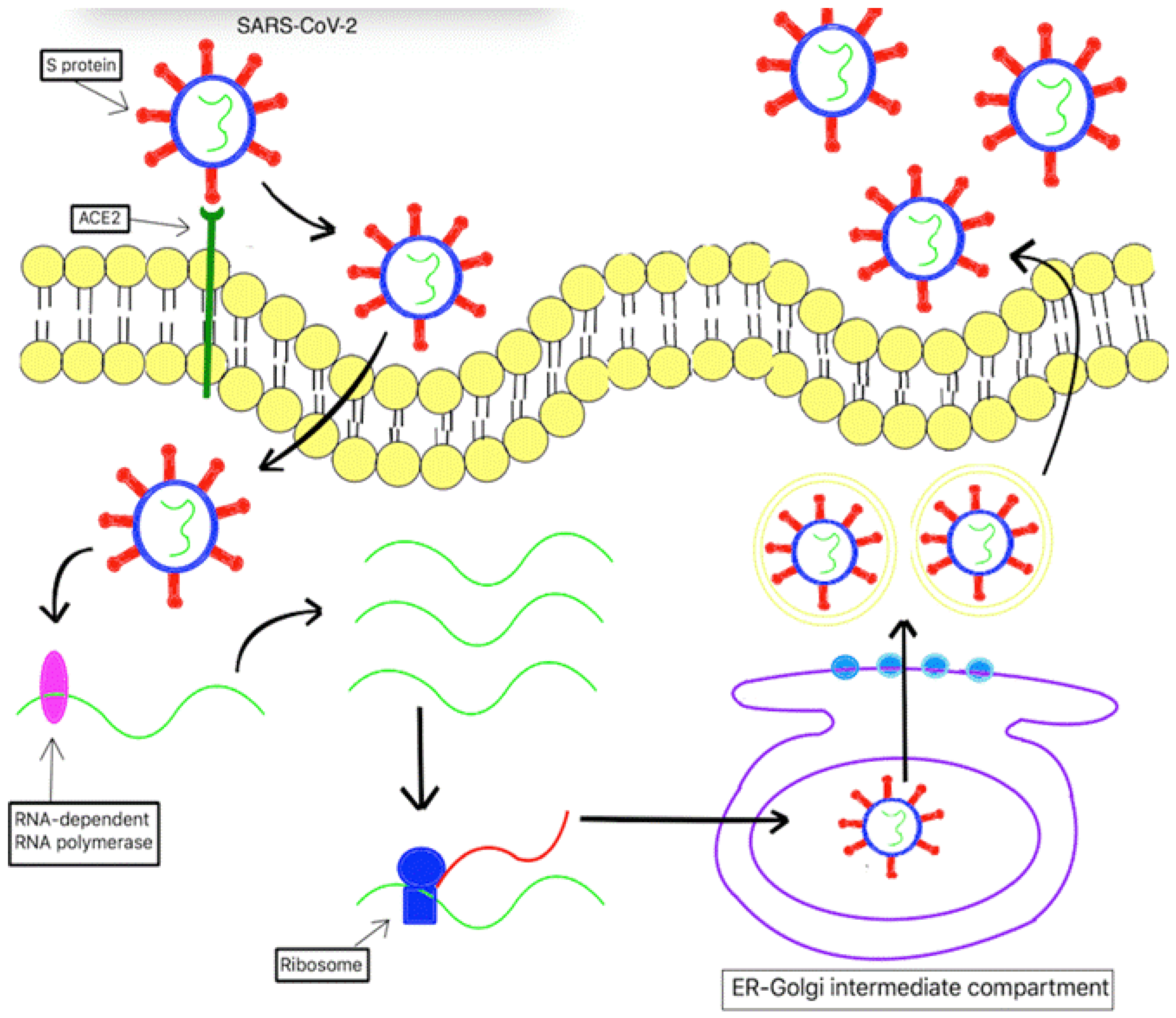
3. The Cytokine Storm
4. Discussion
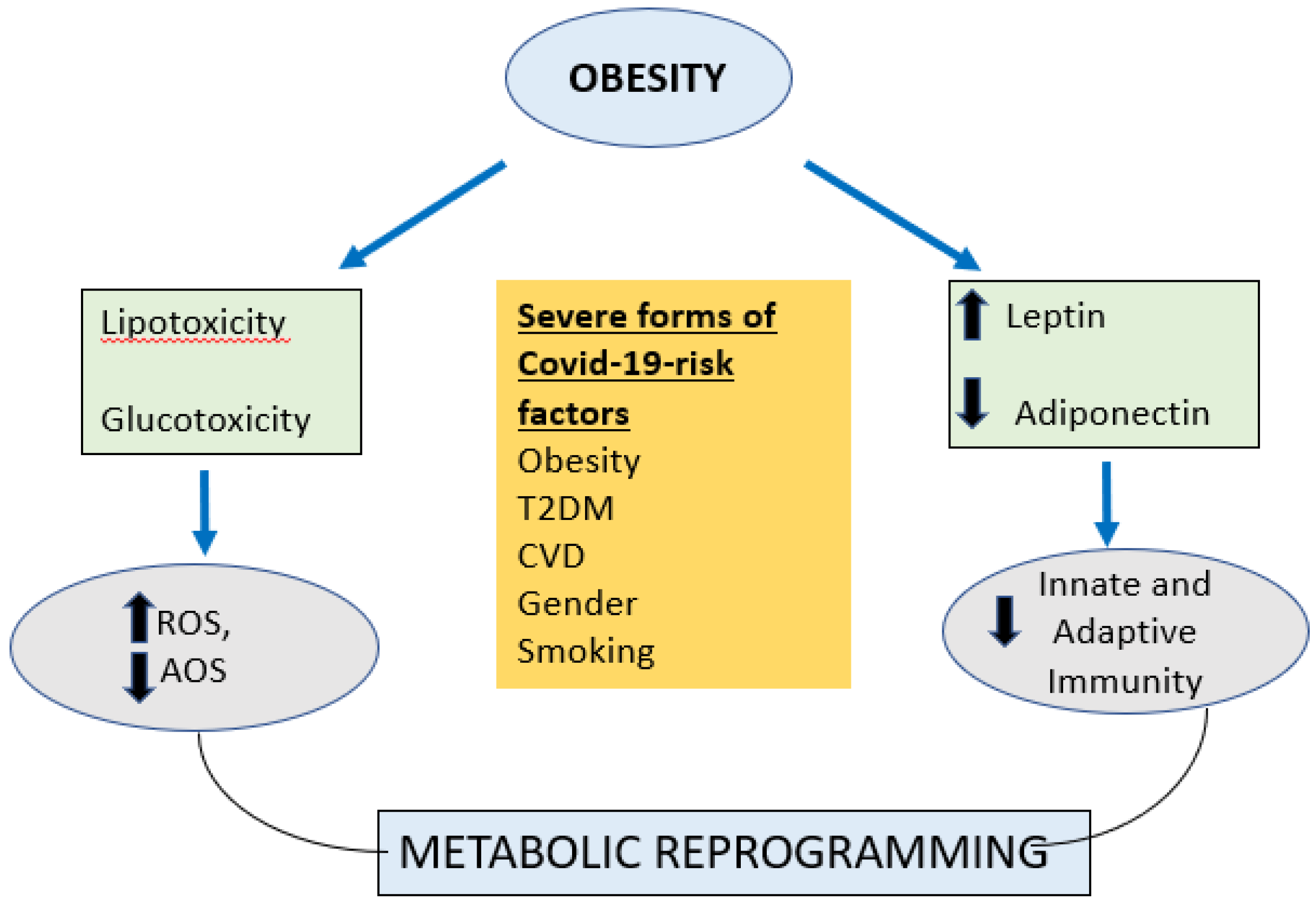
5. CONCLUSIONS
Author Contributions
Funding
References
- Coronavirus disease (COVID-19). Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201012-weekly-epi-update-9.pdf. (Accessed: 14th October 2020).
- Morgan, O. W. et al. Morbid Obesity as a Risk Factor for Hospitalization and Death Due to 2009 Pandemic Influenza A(H1N1) Disease. PLoS ONE 5, (2010). [CrossRef]
- Gupta, R., Ghosh, A., Singh, A. K. & Misra, A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14, 211–212 (2020). [CrossRef]
- Obesity and overweight. World Health Organization Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. (Accessed: 14th October 2020).
- Ni, Y.-N. et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Critical Care 21, (2017). [CrossRef]
- Tamara, A. & Tahapary, D. L. Obesity as a predictor for a poor prognosis of COVID-19: A systematic review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14, 655–659 (2020). [CrossRef]
- Simonnet, A. et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 28, 1195–1199 (2020).
- Seidu, S. et al. The impact of obesity on severe disease and mortality in people with SARS-CoV-2: A systematic review and meta-analysis. Endocrinology, Diabetes & Metabolism (2020). doi:10.1002/edm2.176. [CrossRef]
- Cai, Q. et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care 43, 1392–1398 (2020). [CrossRef]
- Gao, F. et al. Obesity Is a Risk Factor for Greater COVID-19 Severity. Diabetes Care 43, (2020). [CrossRef]
- Petrilli, C. M. et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. Bmj m1966 (2020). doi:10.1136/bmj.m1966. [CrossRef]
- Lighter, J. et al. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clinical Infectious Diseases 71, 896–897 (2020). [CrossRef]
- Kass, D. A., Duggal, P. & Cingolani, O. Obesity could shift severe COVID-19 disease to younger ages. The Lancet 395, 1544–1545 (2020).
- Onder, G., Rezza, G. & Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. Jama (2020). doi:10.1001/jama.2020.4683. [CrossRef]
- Hu, C. & Jia, W. Diabetes in China: Epidemiology and Genetic Risk Factors and Their Clinical Utility in Personalized Medication. Diabetes 67, 3–11 (2017). [CrossRef]
- Caspar-Bauguil, S. et al. Adipose tissues as an ancestral immune organ: Site-specific change in obesity. FEBS Letters 579, 3487–3492 (2005). [CrossRef]
- Saely, C. H., Geiger, K. & Drexel, H. Brown versus White Adipose Tissue: A Mini-Review. Gerontology 58, 15–23 (2012). [CrossRef]
- Poglio, S. et al. Adipose Tissue as a Dedicated Reservoir of Functional Mast Cell Progenitors. Stem Cells 28, 2065–2072 (2010). [CrossRef]
- Wensveen, F. M., Valentić, S., Šestan, M., Wensveen, T. T. & Polić, B. Interactions between adipose tissue and the immune system in health and malnutrition. Seminars in Immunology 27, 322–333 (2015).
- Brotfain, E. et al. Neutrophil functions in morbidly obese subjects. Clinical & Experimental Immunology 181, 156–163 (2015). [CrossRef]
- Bruno, A., Conus, S., Schmid, I. & Simon, H.-U. Apoptotic Pathways Are Inhibited by Leptin Receptor Activation in Neutrophils. The Journal of Immunology 174, 8090–8096 (2005). [CrossRef]
- Surmi, B. K. & Hasty, A. H. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascular Pharmacology 52, 27–36 (2010). [CrossRef]
- Torres-Castro, I. et al. Corrigendum to ‘Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose’ [Immunol. Lett. 176 (2016) 81–89]. Immunology Letters 192, 106 (2017).
- Acedo, S. C., Gambero, S., Cunha, F. G. P., Lorand-Metze, I. & Gambero, A. Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. In Vitro Cellular & Developmental Biology - Animal 49, 473–478 (2013). [CrossRef]
- Santos-Alvarez, J., Goberna, R. & Sánchez-Margalet, V. Human Leptin Stimulates Proliferation and Activation of Human Circulating Monocytes. Cellular Immunology 194, 6–11 (1999). [CrossRef]
- Mattioli, B., Giordani, L., Quaranta, M. G. & Viora, M. Leptin exerts an anti-apoptotic effect on human dendritic cells via the PI3K-Akt signaling pathway. FEBS Letters 583, 1102–1106 (2009). [CrossRef]
- Mattioli, B. et al. Leptin as an immunological adjuvant: enhanced migratory and CD8 + T cell stimulatory capacity of human dendritic cells exposed to leptin. The FASEB Journal 22, 2012–2022 (2008). [CrossRef]
- Al-Hassi, H. O. et al. A mechanistic role for leptin in human dendritic cell migration: differences between ileum and colon in health and Crohn’s disease. Mucosal Immunology 6, 751–761 (2012). [CrossRef]
- Lam, Q. L. K., Liu, S., Cao, X. & Lu, L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. European Journal of Immunology 36, 3118–3130 (2006). [CrossRef]
- Bertola, A. et al. Identification of Adipose Tissue Dendritic Cells Correlated With Obesity-Associated Insulin-Resistance and Inducing Th17 Responses in Mice and Patients. Diabetes 61, 2238–2247 (2012). [CrossRef]
- Conus, S., Bruno, A. & Simon, H. Leptin is an eosinophil survival factor. Journal of Allergy and Clinical Immunology 116, 1228–1234 (2005).
- Suzukawa, M. et al. Leptin Enhances Survival and Induces Migration, Degranulation, and Cytokine Synthesis of Human Basophils. The Journal of Immunology 186, 5254–5260 (2011). [CrossRef]
- Wong, C. K., Cheung, P. F.-Y. & Lam, C. W. K. Leptin-mediated cytokine release and migration of eosinophils: Implications for immunopathophysiology of allergic inflammation. European Journal of Immunology 37, 2337–2348 (2007). [CrossRef]
- Wensveen, F. M. et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nature Immunology 16, 376–385 (2015). [CrossRef]
- Lee, B.-C. et al. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metabolism 23, 685–698 (2016). [CrossRef]
- Lo, C. K. C. et al. Leptin Signaling Protects NK Cells from Apoptosis During Development in Mouse Bone Marrow. Cellular & Molecular Immunology 6, 353–360 (2009). [CrossRef]
- Zhao, Y., Sun, R., You, L., Gao, C. & Tian, Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochemical and Biophysical Research Communications 300, 247–252 (2003). [CrossRef]
- Wrann, C. D. et al. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. American Journal of Physiology-Endocrinology and Metabolism 302, (2012). [CrossRef]
- Laue, T. et al. Altered NK cell function in obese healthy humans. BMC Obesity 2, (2015). [CrossRef]
- Nave, H. et al. Resistance of Janus Kinase-2 Dependent Leptin Signaling in Natural Killer (NK) Cells: A Novel Mechanism of NK Cell Dysfunction in Diet-Induced Obesity. Endocrinology 149, 3370–3378 (2008). [CrossRef]
- Papathanassoglou, E. et al. Leptin Receptor Expression and Signaling in Lymphocytes: Kinetics During Lymphocyte Activation, Role in Lymphocyte Survival, and Response to High Fat Diet in Mice. The Journal of Immunology 176, 7745–7752 (2006). [CrossRef]
- Procaccini, C. et al. Leptin as immune mediator: Interaction between neuroendocrine and immune system. Developmental & Comparative Immunology 66, 120–129 (2017). [CrossRef]
- Xia, C., Rao, X. & Zhong, J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. Journal of Diabetes Research 2017, 1–6 (2017). [CrossRef]
- Martín-Romero, C., Santos-Alvarez, J., Goberna, R. & Sánchez-Margalet, V. Human Leptin Enhances Activation and Proliferation of Human Circulating T Lymphocytes. Cellular Immunology 199, 15–24 (2000). [CrossRef]
- Misumi, I. et al. Obesity Expands a Distinct Population of T Cells in Adipose Tissue and Increases Vulnerability to Infection. Cell Reports 27, (2019). [CrossRef]
- Rosa, V. D. et al. A Key Role of Leptin in the Control of Regulatory T Cell Proliferation. Immunity 26, 241–255 (2007).
- Plitas, G. & Rudensky, A. Y. Regulatory T Cells: Differentiation and Function. Cancer Immunology Research 4, 721–725 (2016). [CrossRef]
- Kucharska, A. M., Pyrżak, B. & Demkow, U. Regulatory T Cells in Obesity. Advances in Experimental Medicine and Biology Noncommunicable Diseases 35–40 (2015). doi:10.1007/5584_2015_147. [CrossRef]
- Milner, J. J. et al. Diet-Induced Obese Mice Exhibit Altered Heterologous Immunity during a Secondary 2009 Pandemic H1N1 Infection. The Journal of Immunology 191, 2474–2485 (2013). [CrossRef]
- Milner, J. J. et al. Obesity Increases Mortality and Modulates the Lung Metabolome during Pandemic H1N1 Influenza Virus Infection in Mice. The Journal of Immunology 194, 4846–4859 (2015). [CrossRef]
- Lam, Q. L. K., Wang, S., Ko, O. K. H., Kincade, P. W. & Lu, L. Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and Cyclin D1. Proceedings of the National Academy of Sciences 107, 13812–13817 (2010). [CrossRef]
- Agrawal, S., Gollapudi, S., Su, H. & Gupta, S. Leptin Activates Human B Cells to Secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 Signaling Pathway. Journal of Clinical Immunology 31, 472–478 (2011).
- Defuria, J. et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences 110, 5133–5138 (2013). [CrossRef]
- Chan, J. F.-W. et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes & Infections 9, 221–236 (2020). [CrossRef]
- Fehr, A. R. & Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. Coronaviruses Methods in Molecular Biology 1–23 (2015). doi:10.1007/978-1-4939-2438-7_1. [CrossRef]
- Yin, C. Genotyping coronavirus SARS-CoV-2: methods and implications. Genomics 112, 3588-3596 (2020).
- Shah, A., Kashyap, R., Tosh, P., Sampathkumar, P. & O’Horo, J. C. Guide to Understanding the 2019 Novel Coronavirus. Mayo Clinic Proceedings 95, 646–652 (2020). [CrossRef]
- Hoffmann, M. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, (2020).
- Hamming, I. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology 203, 631–637 (2004). [CrossRef]
- Xu, H. et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science 12, (2020). [CrossRef]
- Donoghue, M. et al. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circulation Research 87, (2000). [CrossRef]
- Wrapp, D. et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263 (2020). [CrossRef]
- Wang, Q. et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 181, (2020).
- Rabaan, A. et al. SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview. Infez Med 28, 174-184 (2020).
- Wang, K. et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. (2020). doi:10.1101/2020.03.14.988345. [CrossRef]
- Sawicki, S. G. & Sawicki, D. L. Coronavirus Transcription: A Perspective. Current Topics in Microbiology and Immunology Coronavirus Replication and Reverse Genetics 31–55 (2005). doi:10.1007/3-540-26765-4_2. [CrossRef]
- Hussain, S. et al. Identification of Novel Subgenomic RNAs and Noncanonical Transcription Initiation Signals of Severe Acute Respiratory Syndrome Coronavirus. Journal of Virology 79, 5288–5295 (2005). [CrossRef]
- Perrier, A. et al. The C-terminal domain of the MERS coronavirus M protein contains a trans-Golgi network localization signal. Journal of Biological Chemistry 294, 14406–14421 (2019). [CrossRef]
- Tang, T., Bidon, M., Jaimes, J. A., Whittaker, G. R. & Daniel, S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Research 178, 104792 (2020). [CrossRef]
- Guo, Y.-R. et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Medical Research 7, (2020). [CrossRef]
- Thaiss, C. A., Levy, M., Itav, S. & Elinav, E. Integration of Innate Immune Signaling. Trends in Immunology 37, 84–101 (2016). [CrossRef]
- Prompetchara, E., Ketloy, C. & Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific Journal of Allergy and Immunology (2020). doi:10.12932/ap-200220-0772. [CrossRef]
- Felsenstein, S., Herbert, J. A., Mcnamara, P. S. & Hedrich, C. M. COVID-19: Immunology and treatment options. Clinical Immunology 215, 108448 (2020). [CrossRef]
- Yamato, M. et al. Role of adaptor TRIF in the MyD88-indepenent toll-like receptor signaling pathway. Science 301, 640-643 (2003).
- Akira, S., Uematsu, S. & Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 124, 783–801 (2006). [CrossRef]
- Kell, A. M. & Gale, M. RIG-I in RNA virus recognition. Virology 479-480, 110–121 (2015). [CrossRef]
- Loo, Y.-M., Galem M. Immune signaling by RIG-I-like receptors. Immunity 34, 680-692 (2011).
- Wit, E. D., Doremalen, N. V., Falzarano, D. & Munster, V. J. SARS and MERS: recent insights into emerging coronaviruses. Nature Reviews Microbiology 14, 523–534 (2016).
- Gabriella, D. M., Cristina, S., Concetta, R., Francesco, R. & Annalisa, C. SARS-Cov-2 infection: Response of human immune system and possible implications for the rapid test and treatment. International Immunopharmacology 84, 106519 (2020).
- Mehta, P. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet 395, 1033–1034 (2020). [CrossRef]
- Jamilloux, Y. et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmunity Reviews 19, 102567 (2020). [CrossRef]
- Decroly, E. et al. Coronavirus Nonstructural Protein 16 Is a Cap-0 Binding Enzyme Possessing (Nucleoside-2′O)-Methyltransferase Activity. Journal of Virology 82, 8071–8084 (2008). [CrossRef]
- Züst, R. et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nature Immunology 12, 137–143 (2011). [CrossRef]
- Sun, L. et al. Coronavirus Papain-like Proteases Negatively Regulate Antiviral Innate Immune Response through Disruption of STING-Mediated Signaling. PLoS ONE 7, (2012). [CrossRef]
- Menachery, V. D. et al. Pathogenic Influenza Viruses and Coronaviruses Utilize Similar and Contrasting Approaches To Control Interferon-Stimulated Gene Responses. mBio 5, (2014). [CrossRef]
- Frieman, M. et al. Severe Acute Respiratory Syndrome Coronavirus ORF6 Antagonizes STAT1 Function by Sequestering Nuclear Import Factors on the Rough Endoplasmic Reticulum/Golgi Membrane. Journal of Virology 81, 9812–9824 (2007). [CrossRef]
- Lokugamage, K. G. et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. (2020). doi:10.1101/2020.03.07.982264. [CrossRef]
- Hadjadj, J. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020). [CrossRef]
- Diao, B. et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Frontiers in Immunology 11, (2020). [CrossRef]
- Qin, C. et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clinical Infectious Diseases 71, 762–768 (2020). [CrossRef]
- Wang, F. et al. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. The Journal of Infectious Diseases 221, 1762–1769 (2020). [CrossRef]
- Wang, D. et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. Jama 323, 1061 (2020). [CrossRef]
- Zheng, H.-Y. et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cellular & Molecular Immunology 17, 541–543 (2020). [CrossRef]
- Wang, X. et al. Retraction Note to: SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cellular & Molecular Immunology 17, 894–894 (2020).
- Tan, Y.-X. et al. Induction of Apoptosis by the Severe Acute Respiratory Syndrome Coronavirus 7a Protein Is Dependent on Its Interaction with the Bcl-XL Protein. Journal of Virology 81, 6346–6355 (2007).
- Yue, Y. et al. SARS-Coronavirus Open Reading Frame-3a drives multimodal necrotic cell death. Cell Death & Disease 9, (2018). [CrossRef]
- Mehta, A. K., Gracias, D. T. & Croft, M. TNF activity and T cells. Cytokine 101, 14–18 (2018). [CrossRef]
- Brooks, D. G. et al. Interleukin-10 determines viral clearance or persistence in vivo. Nature Medicine 12, 1301–1309 (2006). [CrossRef]
- Andersen, C. J., Murphy, K. E. & Fernandez, M. L. Impact of Obesity and Metabolic Syndrome on Immunity. Advances in Nutrition 7, 66–75 (2016). [CrossRef]
- Hunsche, C., Hernandez, O. & Fuente, M. D. L. Impaired Immune Response in Old Mice Suffering from Obesity and Premature Immunosenescence in Adulthood. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 71, 983–991 (2015). [CrossRef]
- Painter, S. D., Ovsyannikova, I. G. & Poland, G. A. The weight of obesity on the human immune response to vaccination. Vaccine 33, 4422–4429 (2015). [CrossRef]
- Park, H.-L. et al. Obesity-induced chronic inflammation is associated with the reduced efficacy of influenza vaccine. Human Vaccines & Immunotherapeutics 10, 1181–1186 (2014). [CrossRef]
- Backhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences 101, 15718–15723 (2004). [CrossRef]
- Membrez, M. et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. The FASEB Journal 22, 2416–2426 (2008). [CrossRef]
- Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006). [CrossRef]
- Maier, H. E. et al. Obesity Increases the Duration of Influenza A Virus Shedding in Adults. The Journal of Infectious Diseases 218, 1378–1382 (2018). [CrossRef]
- Dhurandhar, N. V., Bailey, D. & Thomas, D. Interaction of obesity and infections. Obesity Reviews 16, 1017–1029 (2015). [CrossRef]
- Panic A, Stanimirovic J, Sudar-Milovanovic E, Isenovic ER. Oxidative stress in obesity and insulin resistance. Explor Med. 2022;3:58–70. [CrossRef]
- Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–62.
- National Institutes of Health (NIH). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. http://www.covid29treatmentguidelines.nih.gov/. Accessed 8/3/2022.
- Andrews N, Stowe N, Kirsebom F, et al. Covid-19 Vaccine Effectiveness against the Omicron (B1.1.529) Variant. N Engl J Med. 2022;2119451(March):•••. doi:10.1056/NEJMoa. [CrossRef]
- Ray Yutani, Viswanath Venketaraman. The COVID-19 Illness: Addressing the Current Treatment Limitations and Care Gaps with a Novel Alternative and Complementary Agent-the Glutathione-Cyclodextrin Complex. Altern Ther Health Med. 2023 May;29(4):28-35.
- Khanfar A, Al Qaroot B. Could glutathione depletion be the Trojan horse of COVID-19 mortality? Eur Ver Med Pharmacol Sci. 2020;202012:24046. 10.26355’eurrev.
- 7. Ghezzi P. Role of glutathione in immunity and inflammation in the lung. Int J Gen Med. 2011;4:105-113. doi:10.2147/IJGM.S15618. [CrossRef]
- Pena LR, Hill DB, McClain CJ. Treatment with glutathione precursor decreases cytokine activity. JPEN J Parenter Enteral Nutr. 1999;23(1):1-6. doi:10.1177/014860719902300101. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




