Submitted:
30 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Osteoarthritis
2.1. Osteoarthritis Epidemiology in Horse
2.2. Osteoarthritis Epidemiology in Human
2.3. Pathogenesis
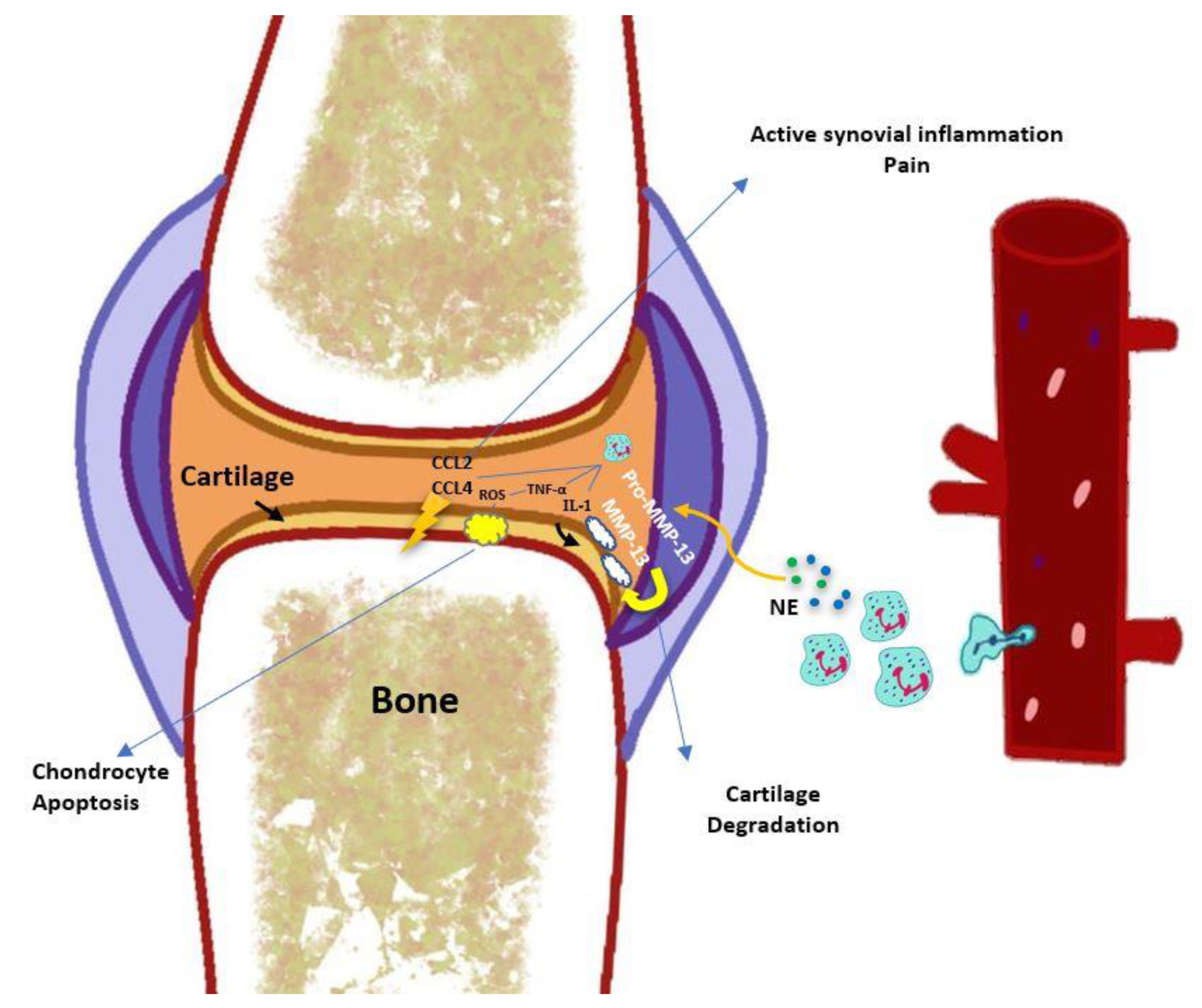
3. Involvement of Neutrophils in the Pathophysiology of Osteoarthritis
3.1. Immune Cells in Joint Injury and Repair
3.2. Trafficking of Circulating Blood Neutrophils to the Synovial Cavity
3.3. Neutrophil-Derived Cytokines, Chemokines, and Enzymes
3.4. Neutrophil Elastase
3.5. MicroRNAs Expression by Neutrophils
3.6. Neutrophils in Cartilage Degradation
3.7. Synovium, Cartilage, Subchondral Bone, and Innate Immunity
4. Neutrophil Biology, Recruitment, and Function in Inflammation
4.1. Neutrophil Life Cycle (Neutrophil Mobilization, Clearance, and Circulation)
4.2. Neutrophils in Acute and Chronic Inflammation
4.3. Neutrophil Crosstalk/Interaction with other Circulation Cells (Platelets, Adaptative Immune Cells, Monocytes/Macrophages)
4.4. Neutrophil Heterogeneity in Chronic Inflammation
4.5. New Insights into Neutrophil Extracellular Traps in Inflammation
5. Neutrophils as a Target for Osteoarthritis Treatment
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, K.; Thoma, L.; Golightly, Y. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Chaney, S.; Vergara, R.; Qiryaqoz, Z.; Suggs, K.; Akkouch, A. The Involvement of Neutrophils in the Pathophysiology and Treatment of Osteoarthritis. Biomedicines 2022, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Sacitharan, P.K. Ageing and osteoarthritis. Biochemistry and cell biology of ageing: part II clinical science, 2019: p. 123-159.
- Garbin, L.C.; Morris, M.J. A Comparative Review of Autologous Conditioned Serum and Autologous Protein Solution for Treatment of Osteoarthritis in Horses. Front. Veter- Sci. 2021, 8, 602978. [Google Scholar] [CrossRef]
- Ramos, S.; Pinto, A.; Cardoso, M.; Alexandre, N.; Bettencourt, E.; Monteiro, S.; Gama, L.T. Prevalence of Radiographic Signs of Osteoarthritis in Lusitano Purebred Horses. J. Equine Veter- Sci. 2020, 94, 103196. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, P.A.; Meireles, M.A.D.; Ribeiro, L.M.F.; de Lannes, S.T.; Meireles, N.F.T.; Viana, I.S.; Hokamura, H.K. Influence of Exercise, Age, Body weight, and Growth on the Development of Tarsal Osteoarthritis in Young Mangalarga Marchador Horses. J. Equine Veter- Sci. 2019, 80, 36–39. [Google Scholar] [CrossRef]
- Balamurugan, K.; Shammi, M.; George, R.S.; Kannan, T.; Siva, R.; Kar, S. A Retrospective Study on Equine Lameness and Influence of Age, Breed and Joint in Osteoarthritis. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3391–3393. [Google Scholar] [CrossRef]
- McIlwraith, C.W. , et al., Principles of Musculoskeletal Disease: Joint Injuries And Disease And Osteoarthritis. Adams and Stashak’s Lameness in Horses, 2020: p. 801-874.
- Ireland, J.; Clegg, P.; McGowan, C.; Platt, L.; Pinchbeck, G. Factors associated with mortality of geriatric horses in the United Kingdom. Prev. Veter- Med. 2011, 101, 204–218. [Google Scholar] [CrossRef]
- Neundorf, R.H.; Lowerison, M.B.; Cruz, A.M.; Thomason, J.J.; McEwen, B.J.; Hurtig, M.B. Determination of the prevalence and severity of metacarpophalangeal joint osteoarthritis in Thoroughbred racehorses via quantitative macroscopic evaluation. Am. J. Vet. Res. 2010, 71, 1284–1293. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012, 1, 297–309. [Google Scholar] [CrossRef]
- Arden, N. and M.C. Nevitt, Osteoarthritis: epidemiology. Best practice & research Clinical rheumatology, 2006. 20(1): p. 3-25.
- Curry, Z.A.; Beling, A.; Borg-Stein, J. Knee osteoarthritis in midlife women: unique considerations and comprehensive management. Menopause 2022, 29, 748–755. [Google Scholar] [CrossRef]
- Tu, C.; He, J.; Wu, B.; Wang, W.; Li, Z. An extensive review regarding the adipokines in the pathogenesis and progression of osteoarthritis. Cytokine 2019, 113, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ashkavand, Z.; Malekinejad, H.; Vishwanath, B.S. The pathophysiology of osteoarthritis. J. Pharm. Res. 2013, 7, 132–138. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, W.; Yong, H.; He, M.; Yang, Y.; Deng, Z.; Li, Y. Macrophages in osteoarthritis: pathophysiology and therapeutics. Am. J. Transl. Res 2020, 12, 261–268. [Google Scholar] [PubMed]
- Grässel, S.; Aszodi, A. Osteoarthritis and Cartilage Regeneration: Focus on Pathophysiology and Molecular Mechanisms. Int. J. Mol. Sci. 2019, 20, 6156. [Google Scholar] [CrossRef]
- Vincent, T.L. IL-1 in osteoarthritis: time for a critical review of the literature. F1000Research 2019, 8, 934. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef]
- Liu-Bryan, R. Synovium and the Innate Inflammatory Network in Osteoarthritis Progression. Curr. Rheumatol. Rep. 2013, 15, 1–7. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2021, 140, 23–42. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.E.; Pezzanite, L.; Goodrich, L.; Santangelo, K.; Chow, L.; Dow, S.; Wheat, W. Role of Innate Immunity in Initiation and Progression of Osteoarthritis, with Emphasis on Horses. Animals 2021, 11, 3247. [Google Scholar] [CrossRef] [PubMed]
- Arve-Butler, S.; Schmidt, T.; Mossberg, A.; Berthold, E.; Gullstrand, B.; Bengtsson, A.A.; Kahn, F.; Kahn, R. Synovial fluid neutrophils in oligoarticular juvenile idiopathic arthritis have an altered phenotype and impaired effector functions. Arthritis Res. Ther. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Fattori, V.; Amaral, F.A.; Verri, W.A. Neutrophils and arthritis: Role in disease and pharmacological perspectives. Pharmacol. Res. 2016, 112, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Zdziennicka, J.; Szponder, T.; Wessely-Szponder, J. Application of Natural Neutrophil Products for Stimulation of Monocyte-Derived Macrophages Obtained before and after Osteochondral or Bone Injury. Microorganisms 2021, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Fujie, K.; Shinguh, Y.; Inamura, N.; Yasumitsu, R.; Okamoto, M.; Okuhara, M. Release of neutrophil elastase and its role in tissue injury in acute inflammation: effect of the elastase inhibitor, FR134043. Eur. J. Pharmacol. 1999, 374, 117–125. [Google Scholar] [CrossRef]
- Gong, Y.; Koh, D.-R. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 2009, 339, 437–448. [Google Scholar] [CrossRef]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Carames, B.; Martín, M.A.; Cillero-Pastor, B.; Lires-Dean, M.; Fuentes-Boquete, I.; Arenas, J.; Blanco, F.J. Mitochondrial activity is modulated by TNFalpha and IL-1beta in normal human chondrocyte cells. Osteoarthr. Cartil. 2006, 14, 1011–1022. [Google Scholar] [CrossRef]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive oxygen species and superoxide dismutases: Role in joint diseases. Jt. Bone Spine 2007, 74, 324–329. [Google Scholar] [CrossRef]
- Wagner, G.; Lehmann, C.; Bode, C.; Miosge, N.; Schubert, A. High Mobility Group Box 1 Protein in Osteoarthritic Knee Tissue and Chondrogenic Progenitor Cells: An Ex Vivo and In Vitro Study. CARTILAGE 2019, 12, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Joos, H.; Wildner, A.; Hogrefe, C.; Reichel, H.; Brenner, R.E. Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis Res. Ther. 2013, 15, R119–R119. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.B.; van der Kraan, P.M.; Berg, W.B.v.D. Cytokine Targeting in Osteoarthritis. Curr. Drug Targets 2007, 8, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Pyrillou, K., L. C. Burzynski, and M.C. Clarke, Alternative pathways of IL-1 activation, and its role in health and disease. Frontiers in immunology, 2020. 11: p. 3288.
- Wilkinson, D.J. , et al., Matrix metalloproteinase-13 is fully activated by neutrophil elastase and inactivates its serpin inhibitor, alpha-1 antitrypsin: Implications for osteoarthritis. The FEBS journal, 2022. 289(1): p. 121-139.
- Carrión, M. , et al., IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatology, 2013. 52(12): p. 2177-2186.
- Deligne, C.; Casulli, S.; Pigenet, A.; Bougault, C.; Campillo-Gimenez, L.; Nourissat, G.; Berenbaum, F.; Elbim, C.; Houard, X. Differential expression of interleukin-17 and interleukin-22 in inflamed and non-inflamed synovium from osteoarthritis patients. Osteoarthr. Cartil. 2015, 23, 1843–1852. [Google Scholar] [CrossRef]
- Grevers, L.C. , et al., Different amplifying mechanisms of interleukin-17 and interferon-γ in Fcγ receptor–mediated cartilage destruction in murine immune complex–mediated arthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 2009. 60(2): p. 396-407.
- Wang, G.; Jing, W.; Bi, Y.; Li, Y.; Ma, L.; Yang, H.; Zhang, Y. Neutrophil Elastase Induces Chondrocyte Apoptosis and Facilitates the Occurrence of Osteoarthritis via Caspase Signaling Pathway. Front. Pharmacol. 2021, 12, 666162. [Google Scholar] [CrossRef]
- Muley, M.M.; Reid, A.R.; Botz, B.; Bölcskei, K.; Helyes, Z.; McDougall, J.J. Neutrophil elastase induces inflammation and pain in mouse knee joints via activation of proteinase-activated receptor-2. Br. J. Pharmacol. 2016, 173, 766–777. [Google Scholar] [CrossRef]
- Kaneva, M.K. Neutrophil elastase and its inhibitors—overlooked players in osteoarthritis. FEBS J. 2022, 289, 113–116. [Google Scholar] [CrossRef]
- Prajzlerová, K.; Kryštůfková, O.; Hánová, P.; Horváthová, V.; Gregová, M.; Pavelka, K.; Vencovský, J.; Šenolt, L.; Filková, M. High miR-451 expression in peripheral blood mononuclear cells from subjects at risk of developing rheumatoid arthritis. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- guila, S. , et al., Micrornas as new regulators of neutrophil extracellular trap formation. International Journal of Molecular Sciences, 2021. 22(4): p. 2116.
- Gurol, T., W. Zhou, and Q. Deng, Micro RNA s in neutrophils: potential next generation therapeutics for inflammatory ailments. Immunological reviews, 2016. 273(1): p. 29-47.
- de Almeida, R.C. , et al., RNA sequencing data integration reveals an miRNA interactome of osteoarthritis cartilage. Annals of the rheumatic diseases, 2019. 78(2): p. 270-277.
- Okuhara, A. , et al., Changes in microRNA expression in peripheral mononuclear cells according to the progression of osteoarthritis. Modern rheumatology, 2012. 22(3): p. 446-457.
- McClurg, O.; Tinson, R.; Troeberg, L. Targeting Cartilage Degradation in Osteoarthritis. Pharmaceuticals 2021, 14, 126. [Google Scholar] [CrossRef]
- Majtnerová, P.; Roušar, T. An overview of apoptosis assays detecting DNA fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef]
- Wang, H. , et al., Histomorphology and innate immunity during the progression of osteoarthritis: Does synovitis affect cartilage degradation? Journal of cellular physiology, 2018. 233(2): p. 1342-1358.
- Wilson, M.E.; Mccandless, E.E.; Olszewski, M.A.; Robinson, N.E. Alveolar macrophage phenotypes in severe equine asthma. Vet. J. 2020, 256, 105436. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, M.; Zhang, X.; Wellman, S.S.; Bolognesi, M.; Kraus, V.B. Synergistic Roles of Macrophages and Neutrophils in Osteoarthritis Progression. Arthritis Rheumatol. 2020, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Haraden, C.A.; Huebner, J.L.; Hsueh, M.-F.; Li, Y.-J.; Kraus, V.B. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res. Ther. 2019, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.; McDaniel, G.; Huebner, J.; Stabler, T.; Pieper, C.; Shipes, S.; Petry, N.; Low, P.; Shen, J.; McNearney, T.; et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hunter, D.; Jin, X.; Ding, C. The importance of synovial inflammation in osteoarthritis: current evidence from imaging assessments and clinical trials. Osteoarthr. Cartil. 2017, 26, 165–174. [Google Scholar] [CrossRef]
- Thomson, A. and C.M. Hilkens, Synovial Macrophages in Osteoarthritis: The Key to Understanding Pathogenesis? Frontiers in Immunology, 2021: p. 1831.
- McIlwraith, C.W. , Traumatic arthritis and posttraumatic osteoarthritis in the horse, in Joint disease in the horse. 2016, Elsevier. p. 33-48.
- Donell, S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019, 4, 221–229. [Google Scholar] [CrossRef]
- Benigni, G. , et al., CXCR3/CXCL10 axis regulates neutrophil–NK cell cross-talk determining the severity of experimental osteoarthritis. The Journal of Immunology, 2017. 198(5): p. 2115-2124.
- Furman, B.D.; Kent, C.L.; Huebner, J.L.; Kraus, V.B.; McNulty, A.L.; Guilak, F.; Olson, S.A. CXCL10 is upregulated in synovium and cartilage following articular fracture. J. Orthop. Res. 2018, 36, 1220–1227. [Google Scholar] [CrossRef]
- Jung, Y.-K.; Han, M.-S.; Park, H.-R.; Lee, E.-J.; Jang, J.-A.; Kim, G.-W.; Lee, S.-Y.; Moon, D.; Han, S. Calcium-phosphate complex increased during subchondral bone remodeling affects earlystage osteoarthritis. Sci. Rep. 2018, 8, 487. [Google Scholar] [CrossRef]
- Hidalgo, A.; Chilvers, E.R.; Summers, C.; Koenderman, L. The Neutrophil Life Cycle. Trends Immunol. 2019, 40, 584–597. [Google Scholar] [CrossRef]
- Capucetti, A.; Albano, F.; Bonecchi, R. Multiple Roles for Chemokines in Neutrophil Biology. Front. Immunol. 2020, 11, 1259. [Google Scholar] [CrossRef]
- V Lerman, Y. and M. Kim, Neutrophil migration under normal and sepsis conditions. Cardiovascular & Haematological Disorders-Drug Targets (Formerly Current Drug Targets-Cardiovascular & Hematological Disorders), 2015. 15(1): p. 19-28.
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-Derived Cytokines: Facts Beyond Expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed]
- Mócsai, A.; Walzog, B.; Lowell, C.A. Intracellular signalling during neutrophil recruitment. Cardiovasc. Res. 2015, 107, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016, 28, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Burn, G.L. , et al., The neutrophil. Immunity, 2021. 54(7): p. 1377-1391.
- Christoffersson, G. and M. Phillipson, The neutrophil: one cell on many missions or many cells with different agendas? Cell and tissue research, 2018. 371(3): p. 415-423.
- Furze, R.C.; Rankin, S.M. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008, 22, 3111–3119. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. More friend than foe: the emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef]
- Tamassia, N. , et al., Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. European journal of clinical investigation, 2018. 48: p. e12952.
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef]
- Costa, S.; Bevilacqua, D.; Cassatella, M.A.; Scapini, P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology 2019, 156, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Hidalgo, A.; Soehnlein, O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 2016, 127, 2173–2181. [Google Scholar] [CrossRef]
- Scapini, P.; Marini, O.; Tecchio, C.; Cassatella, M.A. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol. Rev. 2016, 273, 48–60. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Edwards, S.W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 593–601. [Google Scholar] [CrossRef]
- Gupta, K.; Shukla, M.; Cowland, J.B.; Malemud, C.J.; Haqqi, T.M. Neutrophil gelatinase–associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007, 56, 3326–3335. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.M.; Betz, T.V.; Lamont, D.J.; Kim, M.B.; Shaw, S.K.; Froio, R.M.; Baleux, F.; Arenzana-Seisdedos, F.; Alon, R.; Luscinskas, F.W. Elastase Release by Transmigrating Neutrophils Deactivates Endothelial-bound SDF-1α and Attenuates Subsequent T Lymphocyte Transendothelial Migration. J. Exp. Med. 2004, 200, 713–724. [Google Scholar] [CrossRef]
- Hacbarth, E. and A. Kajdacsy-Balla, Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 1986. 29(11): p. 1334-1342.
- Gierlikowska, B. , et al., Phagocytosis, Degranulation and Extracellular Traps Release by Neutrophils—The Current Knowledge, Pharmacological Modulation and Future Prospects. Frontiers in Pharmacology, 2021. 12: p. 666732.
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Liberale, L.; Carbone, F.; Vecchié, A.; Diaz-Cañestro, C.; Camici, G.G.; Montecucco, F.; Dallegri, F. The Pathophysiological Role of Neutrophil Extracellular Traps in Inflammatory Diseases. Arthritis Res. Ther. 2018, 118, 006–027. [Google Scholar] [CrossRef] [PubMed]
- Fousert, E.; Toes, R.; Desai, J. Neutrophil Extracellular Traps (NETs) Take the Central Stage in Driving Autoimmune Responses. Cells 2020, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2017, 18, 134–147. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40–178ra40. [Google Scholar] [CrossRef]
- Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef]
- Headland, S.E.; Jones, H.R.; Norling, L.V.; Kim, A.; Souza, P.R.; Corsiero, E.; Gil, C.D.; Nerviani, A.; Dell’accio, F.; Pitzalis, C.; et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med. 2015, 7, 315ra190–315ra190. [Google Scholar] [CrossRef]
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef]
- Filep, J.G. Targeting Neutrophils for Promoting the Resolution of Inflammation. Front. Immunol. 2022, 13, 866747. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.-P.; Martel-Pelletier, J.; Rannou, F.; Cooper, C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2015, 45, S22–S27. [Google Scholar] [CrossRef]
- Paglia, M.D.G.; Silva, M.T.; Lopes, L.C.; Barberato-Filho, S.; Mazzei, L.G.; Abe, F.C.; Bergamaschi, C.d.C. Use of corticoids and non-steroidal anti-inflammatories in the treatment of rheumatoid arthritis: Systematic review and network meta-analysis. PLOS ONE 2021, 16, e0248866. [Google Scholar] [CrossRef]
- Bertolotto, M.; Contini, P.; Ottonello, L.; Pende, A.; Dallegri, F.; Montecucco, F. Neutrophil migration towards C5a and CXCL8 is prevented by non-steroidal anti-inflammatory drugs via inhibition of different pathways. Br. J. Pharmacol. 2014, 171, 3376–3393. [Google Scholar] [CrossRef] [PubMed]
- Marsolais, D.; Côté, C.H.; Frenette, J. Nonsteroidal Anti-Inflammatory Drug Reduces Neutrophil and Macrophage Accumulation but Does Not Improve Tendon Regeneration. Lab. Investig. 2003, 83, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Ricci, E.; Migliorati, G.; Gentili, M.; Riccardi, C. How Glucocorticoids Affect the Neutrophil Life. Int. J. Mol. Sci. 2018, 19, 4090. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Cidlowski, J.A. One Hormone, Two Actions: Anti- and Pro-Inflammatory Effects of Glucocorticoids. Neuroimmunomodulation 2014, 22, 20–32. [Google Scholar] [CrossRef]
- Jin, L.; Xu, K.; Liang, Y.; Du, P.; Wan, S.; Jiang, C. Effect of hyaluronic acid on cytokines and immune cells change in patients of knee osteoarthritis. BMC Musculoskelet. Disord. 2022, 23, 1–9. [Google Scholar] [CrossRef]
- A Nicholls, M.; Fierlinger, A.; Niazi, F.; Bhandari, M. The Disease-Modifying Effects of Hyaluronan in the Osteoarthritic Disease State. Clin. Med. Insights: Arthritis Musculoskelet. Disord. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C. , et al., Hyaluronic acid injection reduces inflammatory and apoptotic markers through modulation of AKT by repressing the oxidative status of neutrophils from osteoarthritic synovial fluid. International Journal of Biological Macromolecules, 2020. 165: p. 2765-2772.
- Chisari, E., K. Yaghmour, and W. Khan, The effects of TNF-alpha inhibition on cartilage: A systematic review of preclinical studies. Osteoarthritis and Cartilage, 2020. 28(5): p. 708-718.
- Kim, J.-R.; Yoo, J.J.; Kim, H.A. Therapeutics in Osteoarthritis Based on an Understanding of Its Molecular Pathogenesis. Int. J. Mol. Sci. 2018, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, N.; Diao, Z.; Chen, Y.; Zhang, Y. Therapeutic potential of TNFα inhibitors in chronic inflammatory disorders: Past and future. Genes Dis. 2020, 8, 38–47. [Google Scholar] [CrossRef]
- Hastings, R.; Ding, T.; Butt, S.; Gadsby, K.; Zhang, W.; Moots, R.J.; Deighton, C. Neutropenia in patients receiving anti–tumor necrosis factor therapy. Arthritis Care Res. 2010, 62, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shu, W.; Zhou, G.; Lin, J.; Chu, F.; Wu, H.; Liu, Z. Anti-TNF-α Therapy Suppresses Proinflammatory Activities of Mucosal Neutrophils in Inflammatory Bowel Disease. Mediat. Inflamm. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef]
- Potera, R.M.; Jensen, M.J.; Hilkin, B.M.; South, G.K.; Hook, J.S.; A Gross, E.; Moreland, J.G. Neutrophil azurophilic granule exocytosis is primed by TNF-α and partially regulated by NADPH oxidase. J. Endotoxin Res. 2016, 22, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Capsoni, F.; Sarzi-Puttini, P.; Atzeni, F.; Minonzio, F.; Bonara, P.; Doria, A.; Carrabba, M. Effect of adalimumab on neutrophil function in patients with rheumatoid arthritis. Thromb. Haemost. 2005, 7, R250–R255. [Google Scholar] [CrossRef]
- Taylor, P.C.; Peters, A.M.; Paleolog, E.; Chapman, P.T.; Elliott, M.J.; McCloskey, R.; Feldmann, M.; Maini, R.N. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor α blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000, 43, 38–47. [Google Scholar] [CrossRef]
- Chevalier, X. and F. Eymard, Anti-IL-1 for the treatment of OA: dead or alive? Nature Reviews Rheumatology, 2019. 15(4): p. 191-192.
- Guma, M. , et al., Caspase 1–independent activation of interleukin-1β in neutrophil-predominant inflammation. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 2009. 60(12): p. 3642-3650.
- Kay, J.; Calabrese, L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology 2004, 43 (Suppl. S3), iii2–iii9. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Prince, L.R.; Allen, L.; Jones, E.C.; Hellewell, P.G.; Dower, S.K.; Whyte, M.K.; Sabroe, I. The Role of Interleukin-1β in Direct and Toll-Like Receptor 4-Mediated Neutrophil Activation and Survival. Am. J. Pathol. 2004, 165, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- zcan, A. and O. Boyman, Mechanisms regulating neutrophil responses in immunity, allergy, and autoimmunity. Allergy, 2022.
- Mehta, S.; Akhtar, S.; Porter, R.M.; Önnerfjord, P.; Bajpayee, A.G. Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Res. Ther. 2019, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Koenders, M.; Kalabokis, V.; Kim, J.; Tan, A.C.; Garlanda, C.; Mantovani, A.; Dagna, L.; Joosten, L.A.B.; Dinarello, C.A. Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation. Rheumatology 2016, 55, 2220–2229. [Google Scholar] [CrossRef]
- Mistry, P.; Carmona-Rivera, C.; Ombrello, A.K.; Hoffmann, P.; Seto, N.L.; Jones, A.; Stone, D.L.; Naz, F.; Carlucci, P.; Dell’orso, S.; et al. Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome. Rheumatology 2018, 77, 1825–1833. [Google Scholar] [CrossRef]
- Cai, X.; Yuan, S.; Zeng, Y.; Wang, C.; Yu, N.; Ding, C. New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 2021, 12, 645842. [Google Scholar] [CrossRef]
- Cheleschi, S.; Cantarini, L.; Pascarelli, N.A.; Collodel, G.; Lucherini, O.M.; Galeazzi, M.; Fioravanti, A. Possible chondroprotective effect of canakinumab: An in vitro study on human osteoarthritic chondrocytes. Cytokine 2014, 71, 165–172. [Google Scholar] [CrossRef]
- Ghouri, A.; Conaghan, P.G. Prospects for Therapies in Osteoarthritis. Calcif. Tissue Int. 2020, 109, 339–350. [Google Scholar] [CrossRef]
- Ohyama, A.; Osada, A.; Kawaguchi, H.; Kurata, I.; Nishiyama, T.; Iwai, T.; Ishigami, A.; Kondo, Y.; Tsuboi, H.; Sumida, T.; et al. Specific Increase in Joint Neutrophil Extracellular Traps and Its Relation to Interleukin 6 in Autoimmune Arthritis. Int. J. Mol. Sci. 2021, 22, 7633. [Google Scholar] [CrossRef]
- Yu, X.-M.; Meng, H.-Y.; Yuan, X.-L.; Wang, Y.; Guo, Q.-Y.; Peng, J.; Wang, A.-Y.; Lu, S.-B. MicroRNAs’ Involvement in Osteoarthritis and the Prospects for Treatments. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Malemud, C.J. MicroRNAs and Osteoarthritis. Cells 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. , et al., miR-335-5P contributes to human osteoarthritis by targeting HBP1. Experimental and therapeutic medicine, 2021. 21(2): p. 1-1.
- Kmiołek, T.; Paradowska-Gorycka, A. miRNAs as Biomarkers and Possible Therapeutic Strategies in Rheumatoid Arthritis. Cells 2022, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, C.; Zhang, C.; Luo, C.; Zhong, B.; Yu, X. MiRNA-132 regulates the development of osteoarthritis in correlation with the modulation of PTEN/PI3K/AKT signaling. BMC Geriatr. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Castro-Villegas, C.; Pérez-Sánchez, C.; Escudero, A.; Filipescu, I.; Verdu, M.; Ruiz-Limón, P.; Aguirre, M.A.; Jiménez-Gomez, Y.; Font, P.; Rodriguez-Ariza, A.; et al. Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFα. Arthritis Res. Ther. 2015, 17, 1–15. [Google Scholar] [CrossRef]
- De la Rosa, I.A. , et al., Impaired microRNA processing in neutrophils from rheumatoid arthritis patients confers their pathogenic profile. Modulation by biological therapies. Haematologica, 2020. 105(9): p. 2250.
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments (Review). Mol. Med. Rep. 2022, 25, 1–15. [Google Scholar] [CrossRef]
- Muley, M.M.; Krustev, E.; Reid, A.R.; McDougall, J.J. Prophylactic inhibition of neutrophil elastase prevents the development of chronic neuropathic pain in osteoarthritic mice. J. Neuroinflammation 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Micheli, L.; Cinci, L.; Maresca, M.; Vergelli, C.; Pacini, A.; Quinn, M.T.; Giovannoni, M.P.; Ghelardini, C. Effects of the neutrophil elastase inhibitor EL-17 in rat adjuvant-induced arthritis. Rheumatology 2016, 55, 1285–1294. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, L.; Yu, Z.; Yu, C.; Bi, J.; Sun, B.; Cong, H. Sivelestat sodium hydrate improves post-traumatic knee osteoarthritis through nuclear factor-κB in a rat model. Exp. Ther. Med. 2017, 14, 1531–1537. [Google Scholar] [CrossRef]
- Crocetti, L.; Giovannoni, M.P.; Cantini, N.; Guerrini, G.; Vergelli, C.; Schepetkin, I.A.; Khlebnikov, A.I.; Quinn, M.T. Novel Sulfonamide Analogs of Sivelestat as Potent Human Neutrophil Elastase Inhibitors. Front. Chem. 2020, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Tadie, J.-M.; Bae, H.-B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.-F.; Tracey, K.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, L342–L349. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ming, B.; Dong, L. The Role of HMGB1 in Rheumatic Diseases. Front. Immunol. 2022, 13, 815257. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-L.; Duan, H.-X.; Liang, Q.; Huang, Y.-L.; Wang, L.-Y.; Zhang, Q.; Wu, C.-J.; Liu, S.-Q.; Peng, W. Targeting matrix metalloproteases: A promising strategy for herbal medicines to treat rheumatoid arthritis. Front. Immunol. 2022, 13, 1046810. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E. and C. Libert, Is there new hope for therapeutic matrix metalloproteinase inhibition? Nature reviews Drug discovery, 2014. 13(12): p. 904-927.
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next generation matrix metalloproteinase inhibitors — Novel strategies bring new prospects. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2017, 1864, 1927–1939. [Google Scholar] [CrossRef]
- Clarke, J. NETs directly injure cartilage in RA. Nat. Rev. Rheumatol. 2020, 16, 410–410. [Google Scholar] [CrossRef]
- Yang, F.; Luo, X.; Luo, G.; Zhai, Z.; Zhuang, J.; He, J.; Han, J.; Zhang, Y.; Zhuang, L.; Sun, E.; et al. Inhibition of NET formation by polydatin protects against collagen-induced arthritis. Int. Immunopharmacol. 2019, 77, 105919. [Google Scholar] [CrossRef]
- Larkins, N.; King, C.M. Effectiveness of apocynin-paeonol (APPA) for the management of osteoarthritis in dogs: comparisons with placebo and meloxicam in client-owned dogs. 2017, 3. 3. [CrossRef]
- Cross, A.L.; Hawkes, J.; Wright, H.L.; Moots, R.J.; Edwards, S.W. APPA (apocynin and paeonol) modulates pathological aspects of human neutrophil function, without supressing antimicrobial ability, and inhibits TNFα expression and signalling. Inflammopharmacology 2020, 28, 1223–1235. [Google Scholar] [CrossRef]
- Han, S. Osteoarthritis year in review 2022: biology. Osteoarthr. Cartil. 2022, 30, 1575–1582. [Google Scholar] [CrossRef]
- Semerad, C.L.; Liu, F.; Gregory, A.D.; Stumpf, K.; Link, D.C. G-CSF Is an Essential Regulator of Neutrophil Trafficking from the Bone Marrow to the Blood. Immunity 2002, 17, 413–423. [Google Scholar] [CrossRef]
- Campbell, I.K.; Leong, D.; Edwards, K.M.; Rayzman, V.; Ng, M.; Goldberg, G.L.; Wilson, N.J.; Scalzo-Inguanti, K.; Mackenzie-Kludas, C.; Lawlor, K.E.; et al. Therapeutic Targeting of the G-CSF Receptor Reduces Neutrophil Trafficking and Joint Inflammation in Antibody-Mediated Inflammatory Arthritis. J. Immunol. 2016, 197, 4392–4402. [Google Scholar] [CrossRef]
- Alam, M.J. , et al. Therapeutic blockade of CXCR2 rapidly clears inflammation in arthritis and atopic dermatitis models: demonstration with surrogate and humanized antibodies. in MAbs. 2020. Taylor & Francis.
- Freitag, J.; Bates, D.; Boyd, R.; Shah, K.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy – a review. BMC Musculoskelet. Disord. 2016, 17, 1–13. [Google Scholar] [CrossRef]
- Maumus, M.; Guérit, D.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res. Ther. 2011, 2, 14–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C., W. Wu, and X. Qu, Mesenchymal stem cells in osteoarthritis therapy: A review. American journal of translational research, 2021. 13(2): p. 448.
- Le Blanc, K.; Davies, L.C. Mesenchymal stromal cells and the innate immune response. Immunol. Lett. 2015, 168, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M. , et al., The immune properties of mesenchymal stem cells. International journal of biomedical science: IJBS, 2007. 3(2): p. 76.
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2019, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Joel, M.D.M.; Yuan, J.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. MSC: immunoregulatory effects, roles on neutrophils and evolving clinical potentials. 2019, 11, 3890–3904.
- Rosales, C. , Neutrophil: a cell with many roles in inflammation or several cell types? Frontiers in physiology, 2018. 9: p. 113.
- Molnar, V.; Pavelić, E.; Vrdoljak, K.; Čemerin, M.; Klarić, E.; Matišić, V.; Bjelica, R.; Brlek, P.; Kovačić, I.; Tremolada, C.; et al. Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review. Genes 2022, 13, 949. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Varin, A.; Casteilla, L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front. Immunol. 2021, 12, 626755. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Volarevic, V. The Cross-Talk between Mesenchymal Stem Cells and Immune Cells in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 2472. [Google Scholar] [CrossRef] [PubMed]
- Li, Z., Z. Huang, and L. Bai, Cell interplay in osteoarthritis. Frontiers in Cell and Developmental Biology, 2021: p. 2132.
- Dai, H.; Chen, R.; Gui, C.; Tao, T.; Ge, Y.; Zhao, X.; Qin, R.; Yao, W.; Gu, S.; Jiang, Y.; et al. Eliminating senescent chondrogenic progenitor cells enhances chondrogenesis under intermittent hydrostatic pressure for the treatment of OA. Stem Cell Res. Ther. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, C.; Zheng, H.; Wang, Q.; Song, H.; Buckwalter, J.A.; Martin, J.A. Migrating Progenitor Cells Derived From Injured Cartilage Surface Respond to Damage-Associated Molecular Patterns. CARTILAGE 2021, 13, 755S–765S. [Google Scholar] [CrossRef]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef]
- Liu, L.; Pan, D.; Chen, S.; Martikainen, M.-V.; Kårlund, A.; Ke, J.; Pulkkinen, H.; Ruhanen, H.; Roponen, M.; Käkelä, R.; et al. Systematic design of cell membrane coating to improve tumor targeting of nanoparticles. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Narain, A.; Asawa, S.; Chhabria, V.; Patil-Sen, Y.; Cottam, E.; Pierini, R.; Roberts, R.; Wileman, T.; Kim, S.-W.; Kim, H.; et al. Cell membrane coated nanoparticles: next-generation therapeutics. Nanomedicine 2017, 12, 2677–2692. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, B.; Guo, J.; Zheng, L.; Guo, Q.; Peng, J.; Xu, J.; Qin, L. Nanoparticle–Cartilage Interaction: Pathology-Based Intra-articular Drug Delivery for Osteoarthritis Therapy. Nano-Micro Lett. 2021, 13, 1–48. [Google Scholar] [CrossRef]
- Collison, J. Nanoparticles in neutrophil clothing. Nat. Rev. Rheumatol. 2018, 14, 622–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Pang, Y.; Zhou, H. The interaction between nanoparticles and immune system: application in the treatment of inflammatory diseases. J. Nanobiotechnology 2022, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gong, H.; Gao, W.; Zhang, L. Recent Progress in Capturing and Neutralizing Inflammatory Cytokines. CCS Chem. 2020, 2, 376–389. [Google Scholar] [CrossRef]
- Yu, Q.; Huang, Y.; Chen, X.; Chen, Y.; Zhu, X.; Liu, Y.; Liu, J. A neutrophil cell membrane-biomimetic nanoplatform based on l-arginine nanoparticles for early osteoarthritis diagnosis and nitric oxide therapy. Nanoscale 2022, 14, 11619–11634. [Google Scholar] [CrossRef]
- Wang, H.; Zang, J.; Zhao, Z.; Zhang, Q.; Chen, S. The Advances of Neutrophil-Derived Effective Drug Delivery Systems: A Key Review of Managing Tumors and Inflammation. Int. J. Nanomed. 2021, ume 16, 7663–7681. [Google Scholar] [CrossRef]
- Su, Y.; Gao, J.; Kaur, P.; Wang, Z. Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases. Pharmaceutics 2020, 12, 1222. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef] [PubMed]
- A Marino, A.; Waddell, D.D.; Kolomytkin, O.V.; Meek, W.D.; Wolf, R.; Sadasivan, K.K.; A Albright, J. Increased Intercellular Communication through Gap Junctions May Contribute to Progression of Osteoarthritis. Clin. Orthop. Relat. Res. 2004, 422, 224–232. [Google Scholar] [CrossRef]
- Carpintero-Fernandez, P.; Gago-Fuentes, R.; Wang, H.Z.; Fonseca, E.; Caeiro, J.R.; Valiunas, V.; Brink, P.R.; Mayan, M.D. Intercellular communication via gap junction channels between chondrocytes and bone cells. Biochim. et Biophys. Acta (BBA) - Biomembr. 2018, 1860, 2499–2505. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Sánchez, G.B.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular vesicles: mediators of intercellular communication in tissue injury and disease. Cell Commun. Signal. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2021, 11, 604274. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X.; Zhao, J.; Yang, Y.; Cai, X.; Xu, J.; Cao, P. Exosomes: A Novel Strategy for Treatment and Prevention of Diseases. Front. Pharmacol. 2017, 8, 300–300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bréchard, S. Neutrophil Extracellular Vesicles: A Delicate Balance between Pro-Inflammatory Responses and Anti-Inflammatory Therapies. Cells 2022, 11, 3318. [Google Scholar] [CrossRef]
- Rondelli, V.; Helmy, S.; Passignani, G.; Parisse, P.; Di Silvestre, D. Integrated Strategies for a Holistic View of Extracellular Vesicles. ACS Omega 2022, 7, 19058–19069. [Google Scholar] [CrossRef]
- Johnson, L.B.; Kuethe, J.W.; Caldwell, C.C. Neutrophil derived microvesicles: emerging role of a key mediator to the immune response. In Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets - Immune, Endocrine & Metabolic Disorders); 2014; Volume 14, pp. 210–217.
- Zhang, L.; Qin, Z.; Sun, H.; Chen, X.; Dong, J.; Shen, S.; Zheng, L.; Gu, N.; Jiang, Q. Nanoenzyme engineered neutrophil-derived exosomes attenuate joint injury in advanced rheumatoid arthritis via regulating inflammatory environment. Bioact. Mater. 2022, 18, 1–14. [Google Scholar] [CrossRef]
- Hong, C.-W. Extracellular Vesicles of Neutrophils. Immune Netw. 2018, 18, e43. [Google Scholar] [CrossRef] [PubMed]
- Zhan, D.; Cross, A.; Wright, H.L.; Moots, R.J.; Edwards, S.W.; Honsawek, S. Internalization of Neutrophil-Derived Microvesicles Modulates TNFα-Stimulated Proinflammatory Cytokine Production in Human Fibroblast-Like Synoviocytes. Int. J. Mol. Sci. 2021, 22, 7409. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Miller, R.J.; Malfait, A.-M. Osteoarthritis joint pain: the cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




