Submitted:
30 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Cosmetics – Products intended to clean the body (except for soap).
- Household items/Other - Fragrance products, like scented candles, household cleaners, and air fresheners.
- Herbal medicinal products (HMPs), both for human and veterinary use.
- Traditional herbal medicinal products (THMPs) for human use.
2. Results
2.1. Antibacterial and Antibiofilm Activity on S. aureus
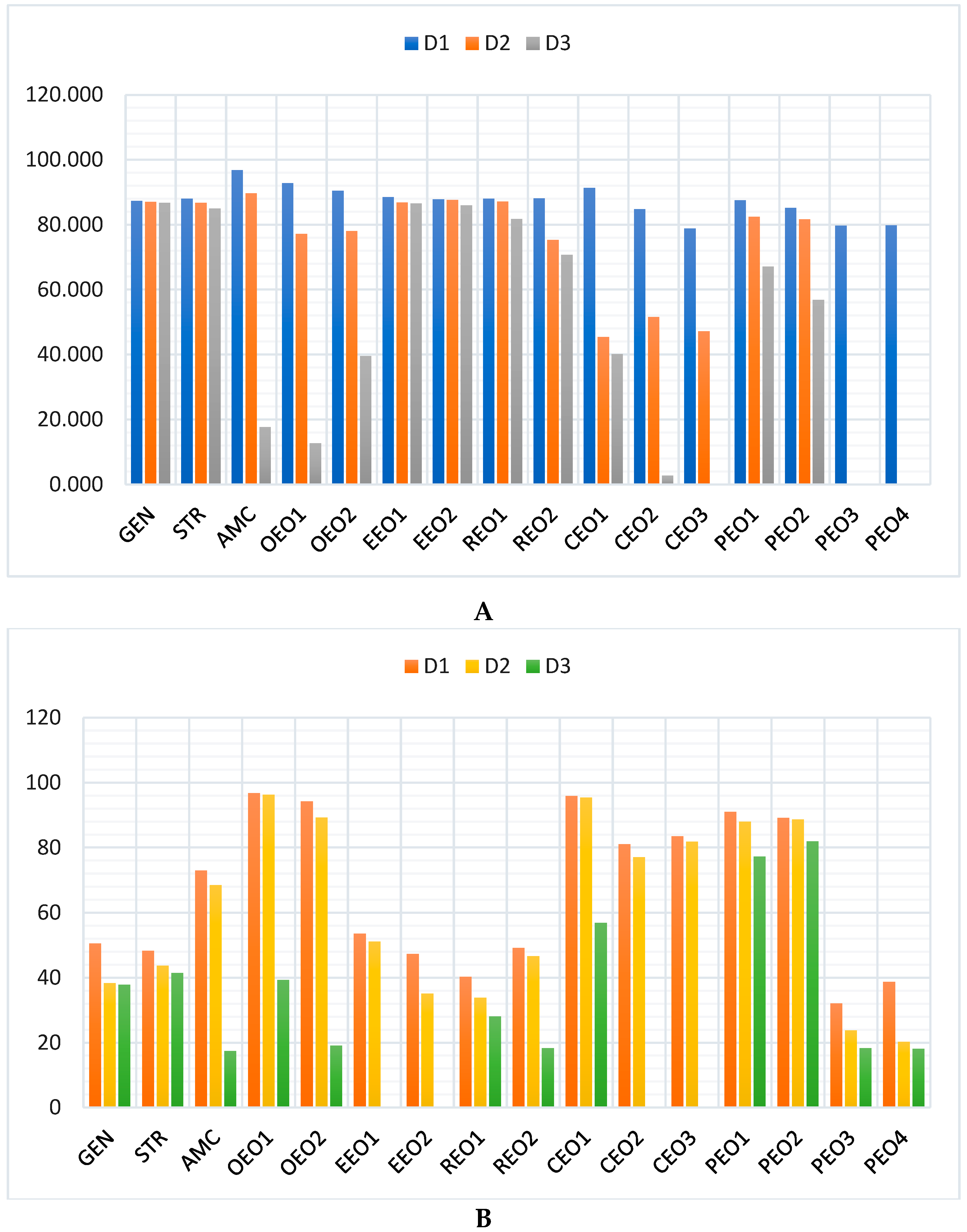
2.2. Antibacterial and Antibiofilm Activity on E. coli
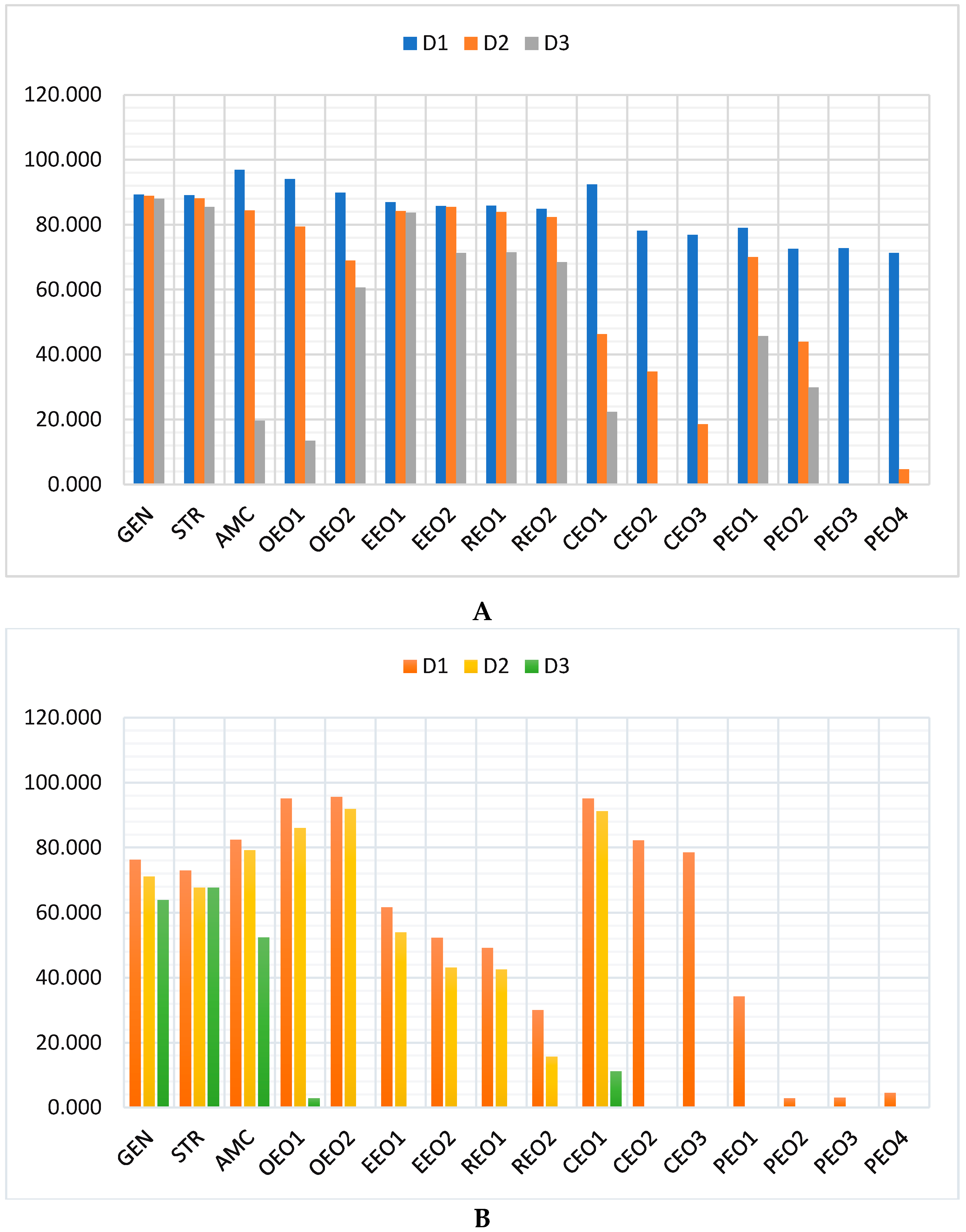
2.3. Antibacterial and Antibiofilm Activity on P. aeruginosa
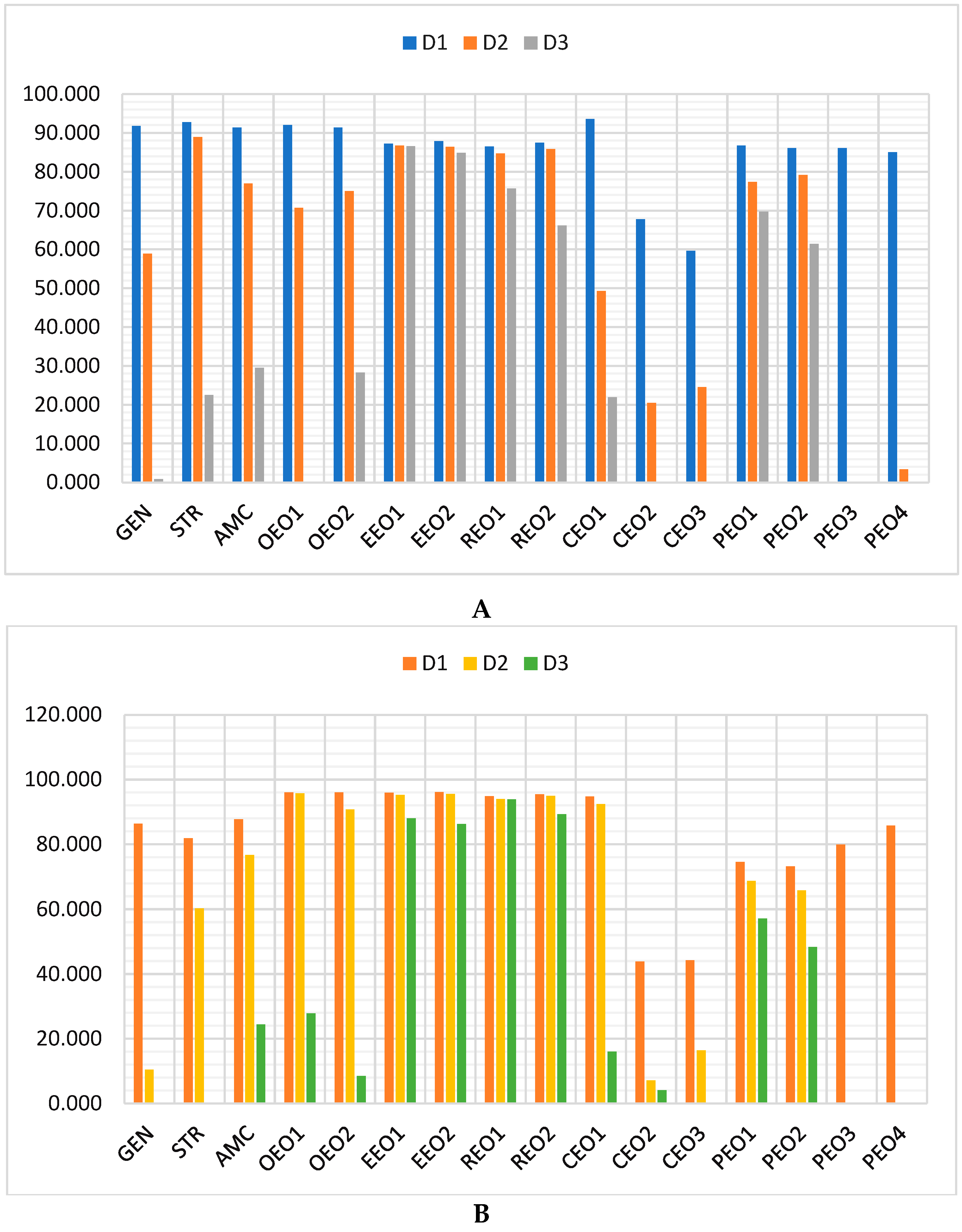
2.4. Data Analysis
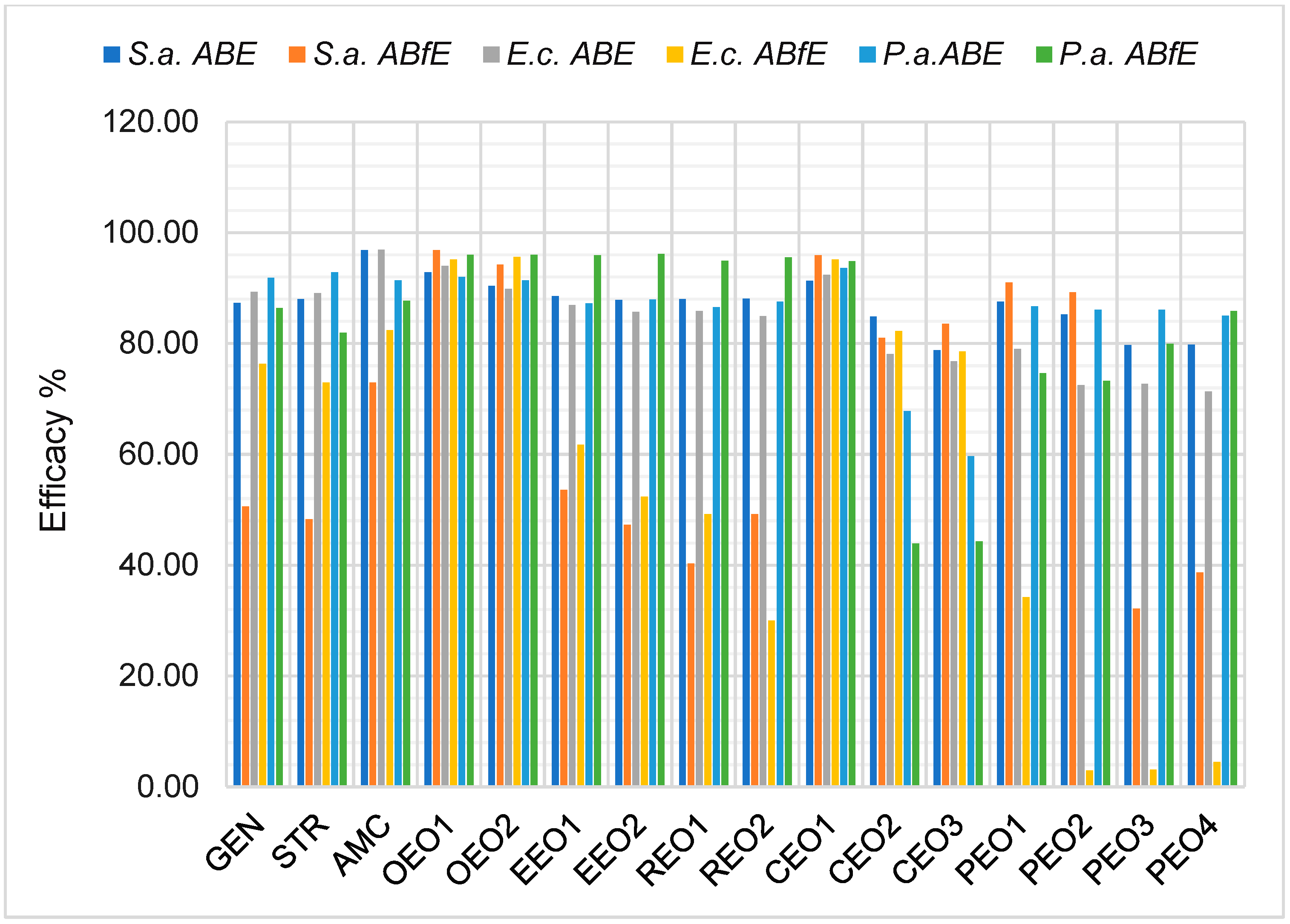
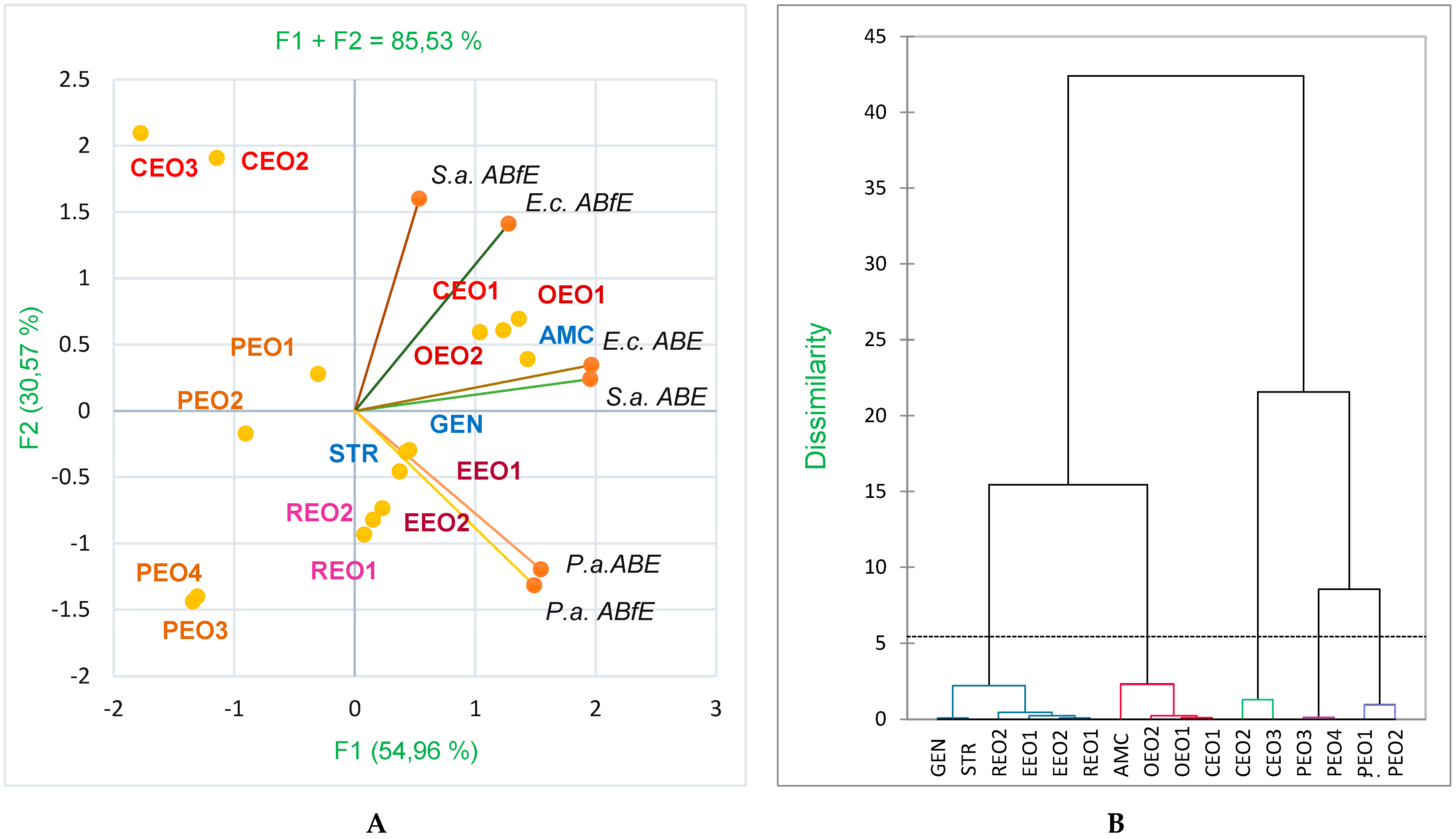
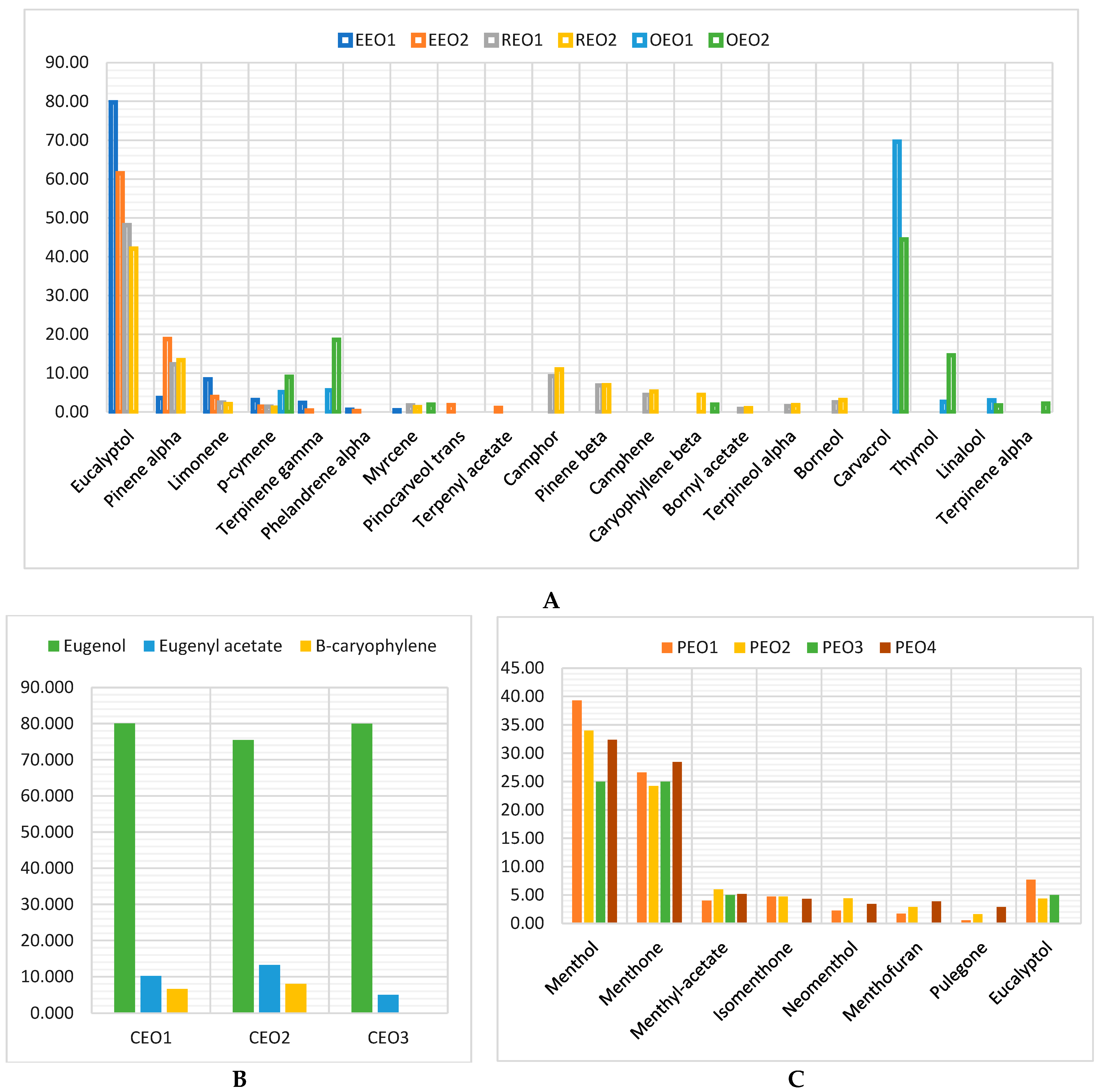
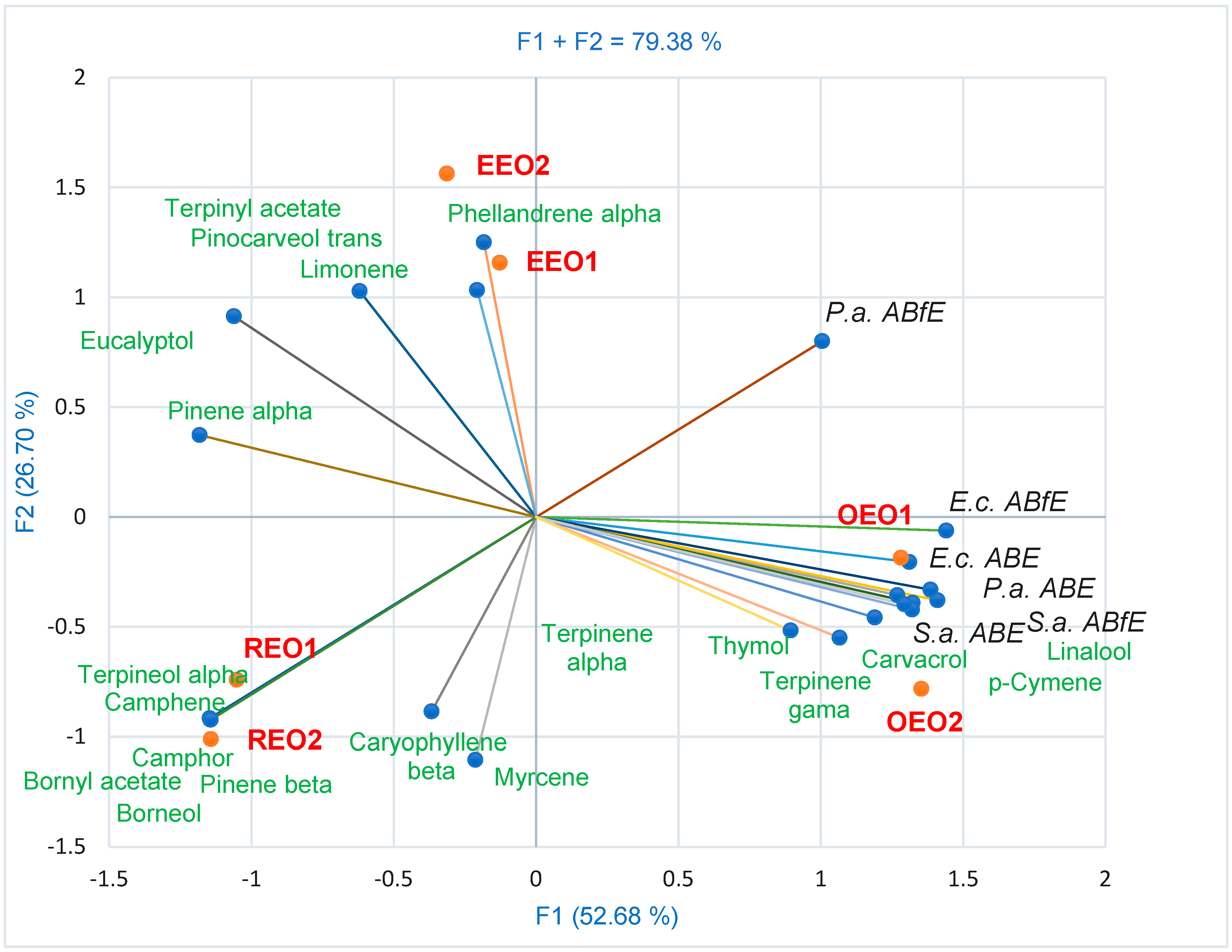
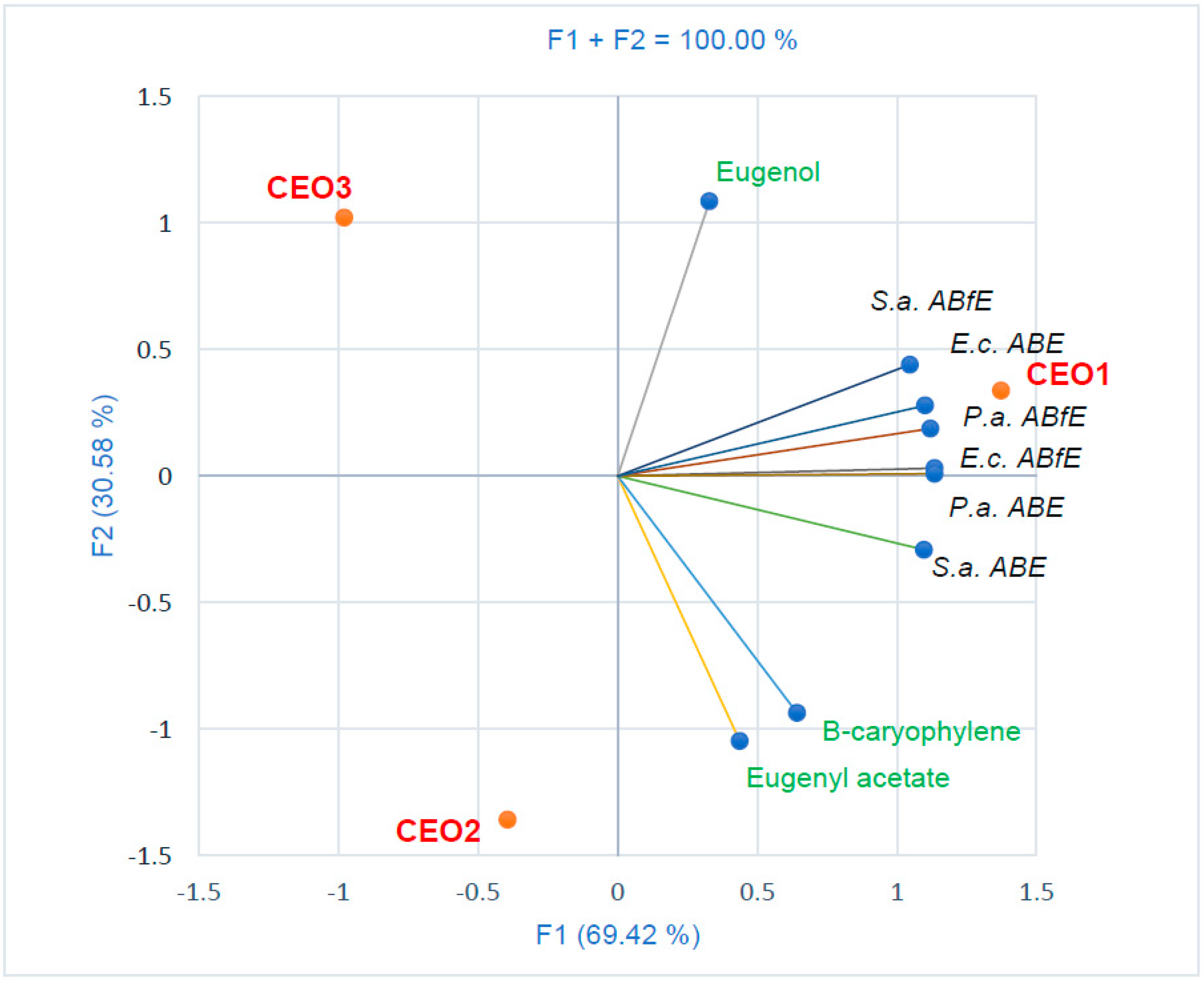
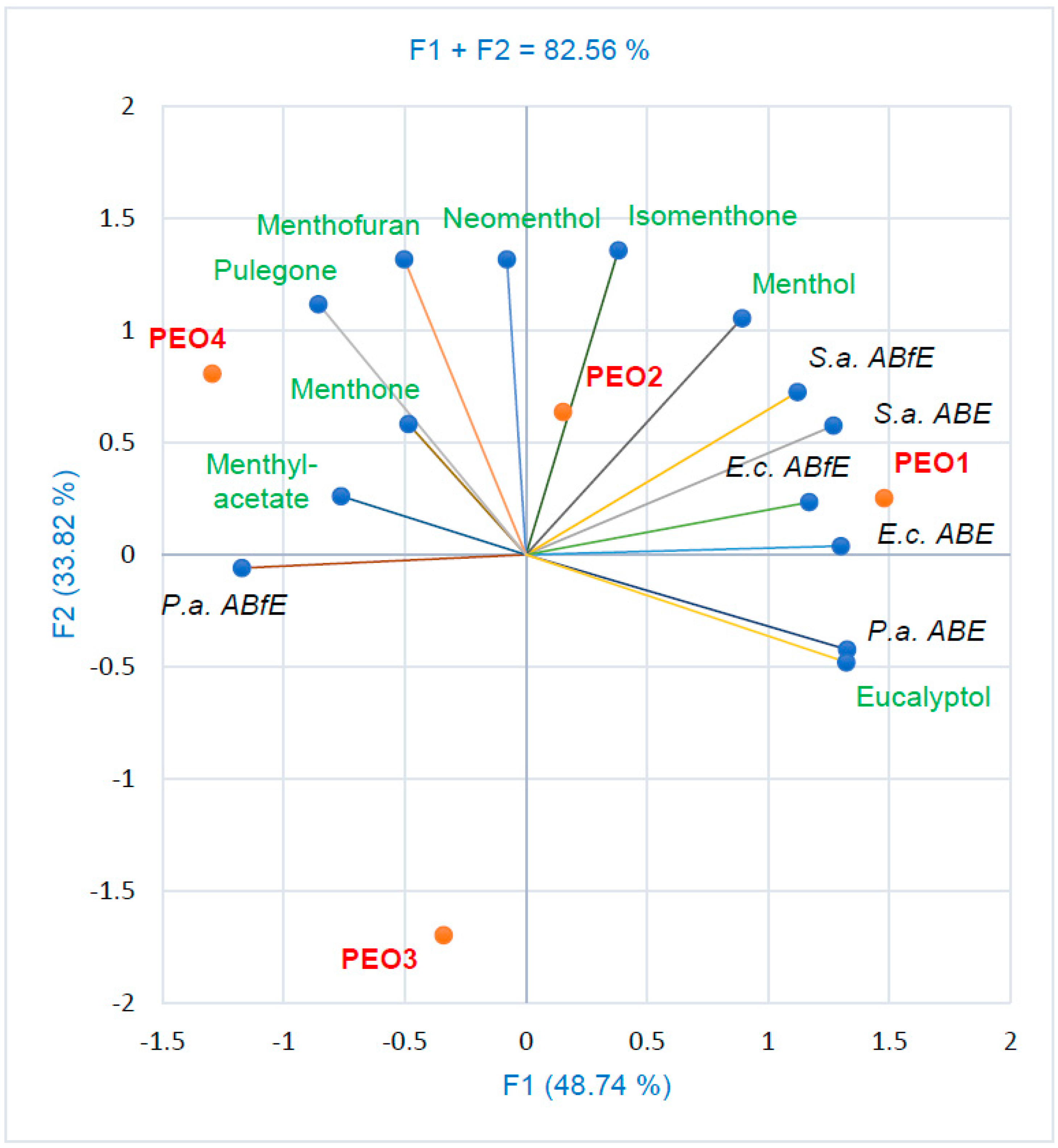
3. Discussion
| Essential oil name |
Botanical name |
Main phytoconstituents |
Therapeutic area / Applications |
|
|---|---|---|---|---|
| 1. |
Caryophylli floris aetheroleum (Clove oil) |
Syzygium aromaticum (L.) Merr. et L.M. Perry, syn. Eugenia caryophyllus (Spreng.) Bullock et S.G. Harrison |
|
Mouth and throat disorders [53] |
| 2. |
Menthae piperitae aetheroleum (Peppermint oil) |
Mentha x piperita L. |
|
Pain and inflammation; Skin disorders and minor wounds; Cough and cold; Gastrointestinal disorders [54] |
| 3. |
Eucalypti aetheroleum (Eucalyptus oil) |
Eucalyptus globulus Labill. Eucalyptus polybractea R.T. Baker. Eucalyptus smithii R.T. Baker. |
[55] |
Pain and inflammation Cough and cold [56] |
| 4. |
Rosmarini aetheroleum (Rosemary oil) |
Rosmarinus officinalis L. |
|
Circulatory disorders; Gastrointestinal disorders [57] |
| 7. |
Origani aetheroleum (Oregano oil) |
Origanum vulgare ssp. hirtum (Link) Ietsw. |
|
Feed additive for certain animal species [48] |
3.1. Antibacterial and Antibiofilm Activity of Oregano Oils
3.2. Antibacterial and Antibiofilm Activity of Eucalyptus Oils
3.3. Antibacterial and Antibiofilm Activity of Rosemary Oils
3.4. Antibacterial and Antibiofilm Activity of Clove Oils
3.5. Antibacterial and Antibiofilm Activity of Peppermint Oils
4. Materials and Methods
4.1. Materials
- ○
- Origani aetheroleum 1, 2 (Oregano essential oil, OEO);
- ○
- Eucalypti aetheroleum 1, 2 (Eucalyptus essential oil, EEO);
- ○
- Rosmarini aetheroleum 1, 2 (Rosemary essential oil, REO);
- ○
- Caryophylli aetheroleum 1. 2, 3 (Clove essential oil, CEO);
- ○
- Menthae aetheroleum 1, 2, 3, 4 (Peppermint essential oil, PEO).
4.2. Antibacterial Activity
4.2.1. Inoculum Preparation
4.2.2. Sample Preparation
4.2.3. Standard Antibiotic Solutions Preparation
4.2.4. Microdillution Method
4.3. Antibiofilm Activity
4.4. Quantification and Interpreting of Antibacterial and Antibiofilm Activities
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T. Bin; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia Aetherolea. Europeian Pharmacopoeia 2008.
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Manion, C.R.; Widder, R.M. Essentials of Essential Oils. American Journal of Health-System Pharmacy 2017, 74, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2876. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Xu, N.; Lei, H.; Li, X.; Wang, Q.; Liu, M.; Wang, M. Protective Effects of Ginger Essential Oil (Geo) against Chemically-Induced Cutaneous Inflammation. Food Science and Technology (Brazil) 2019, 39, 371–377. [Google Scholar] [CrossRef]
- Pandur, E.; Balatinácz, A.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Anti-Inflammatory Effect of Lavender (Lavandula Angustifolia Mill.) Essential Oil Prepared during Different Plant Phenophases on THP-1 Macrophages. BMC Complement Med Ther 2021, 21, s12906. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, L.; Li, Y.; Soo Khoo, C.; Han, D.; Liu, Q.; Li, P.; Zhang, X. Antioxidant and Immunomodulatory Activities of Essential Oil Isolated from Anti-Upper Respiratory Tract Infection Formulation and Their Chemical Analysis. Evidence-based Complementary and Alternative Medicine 2022, 2022, 7297499. [Google Scholar] [CrossRef]
- Pelvan, E.; Karaoğlu, Ö.; Önder Fırat, E.; Betül Kalyon, K.; Ros, E.; Alasalvar, C. Immunomodulatory Effects of Selected Medicinal Herbs and Their Essential Oils: A Comprehensive Review. J Funct Foods 2022, 94, 105108. [Google Scholar] [CrossRef]
- González-Velasco, H.E.; Pérez-Gutiérrez, M.S.; Alonso-Castro, Á.J.; Zapata-Morales, J.R.; Niño-Moreno, P.D.C.; Campos-Xolalpa, N.; González-Chávez, M.M. Anti-Inflammatory and Antinociceptive Activities of the Essential Oil of Tagetes Parryi A. Gray (Asteraceae) and Verbenone. Molecules 2022, 27, 2612. [Google Scholar] [CrossRef]
- Gómez-Betancur, I.; Benjumea, D.; Gómez, J.E.; Mejía, N.; León, J.F. Antinociceptive Activity of Essential Oils from Wild Growing and Micropropagated Plants of Renealmia Alpinia (Rottb.) Maas. Records of Natural Products 2019, 13, 10–17. [Google Scholar] [CrossRef]
- Eftekhari, M.; Hoseinsalari, A.; Mansourian, M.; Farjadmand, F.; Shams Ardekani, M.R.; Sharifzadeh, M.; Hassanzadeh, G.; Khanavi, M.; Gholami, M. Trachyspermum Ammi (L.) Sprague, Superb Essential Oil and Its Major Components on Peptic Ulcers: In Vivo Combined in Silico Studies. DARU, Journal of Pharmaceutical Sciences 2019, 27, 317–327. [Google Scholar] [CrossRef]
- Abu Bakar, N.A.; Hakim Abdullah, M.N.; Lim, V.; Yong, Y.K. Essential Oils Derived from Momordica Charantia Seeds Exhibited Antiulcer Activity against Hydrogen Chloride/Ethanol and Indomethacin. Evidence-based Complementary and Alternative Medicine 2021, 2021, 5525584. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential Oils as Anticancer Agents: Potential Role in Malignancies, Drug Delivery Mechanisms, and Immune System Enhancement. Biomedicine and Pharmacotherapy 2022, 146, 112514. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Yousefpoor, Y.; Alipanah, H.; Ghanbariasad, A.; Jalilvand, M.; Amani, A. In-Vitro Assessment of Essential Oils as Anticancer Therapeutic Agents: A Systematic Literature Review. Jordan Journal of Pharmaceutical Sciences 2022, 15, 173–203. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Aragão, C.E. de C.; Araújo, P.J.P. de; Benatto, A.; Chaaban, A.; Martins, C.E.N.; Amaral, W. do; Cipriano, R.R.; Zawadneak, M.A.C. Insecticide Activity and Toxicity of Essential Oils against Two Stored-Product Insects. Crop Protection 2021, 144, 105575. [Google Scholar] [CrossRef]
- Rants’o, T.A.; Koekemoer, L.L.; Panayides, J.L.; van Zyl, R.L. Potential of Essential Oil-Based Anticholinesterase Insecticides against Anopheles Vectors: A Review. Molecules 2022, 27, 7026. [Google Scholar] [CrossRef]
- Huong, L.T.T.; Huong, T.T.T.; Huong, N.T.T.; Hung, N.H.; Dat, P.T.T.; Luong, N.X.; Ogunwande, I.A. Mosquito Larvicidal Activity of the Essential Oil of Zingiber Collinsii against Aedes Albopictus and Culex Quinquefasciatus. J Oleo Sci 2020, 69, 153–160. [Google Scholar] [CrossRef]
- Budiman; Ishak, H.; Stang; Ibrahim, E.; Daud, A.; Amiruddin, R. Essential Oil as a New Tool for Larvicidal Aedes Aegypti: A Systematic Review. Gac Sanit 2021, 35, S459–S462. [CrossRef]
- Castro, L.M.; Pinto, N.B.; Moura, M.Q.; Villela, M.M.; Capella, G.A.; Freitag, R.A.; Berne, M.E.A. Antihelminthic Action of the Anethum Graveolens Essential Oil on Haemonchus Contortus Eggs and Larvae. Brazilian Journal of Biology 2021, 81, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R. Essential Oils: Antimicrobial, Antihelminthic, Antiviral, Anticancer and Antiinsect Properties. J Appl Biosci 2010, 36, 1–22. [Google Scholar]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals 2021, 14, 1210. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and Therapy with Essential Oils Having Antiviral, Anti-Inflammatory, and Immunomodulatory Properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Trindade, H. Review on Essential Oil, Extracts Composition, Molecular and Phytochemical Properties of Thymus Species in Iran. Ind Crops Prod 2019, 134, 89–99. [Google Scholar] [CrossRef]
- Garzoli, S. Chemical Composition and Antimicrobial Activity of Essential Oils. Plants 2023, 12, 800. [Google Scholar] [CrossRef]
- Bollyky, T.J.; Kesselheim, A.S. Reputation and Authority: The FDA and the Fight over U.S. Prescription Drug Importation. Vanderbilt Law Rev 2020, 73. [Google Scholar]
- Darrow, J.J.; Avorn, J.; Kesselheim, A.S. FDA Approval and Regulation of Pharmaceuticals, 1983-2018. JAMA - Journal of the American Medical Association 2020, 323, 164–176. [Google Scholar] [CrossRef]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nursing Clinics of North America 2020, 55, 489–504. [Google Scholar] [CrossRef]
- doTerra DoTerra.
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha Spp. Essential Oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC) Reflection Paper on Quality of Essential Oils as Active Substances in Herbal Medicinal Products / Traditional Herbal Medicinal Products. EMA/HMPC/84789/2013 2014, 44.
- Peschel, W. The Use of Community Herbal Monographs to Facilitate Registrations and Authorisations of Herbal Medicinal Products in the European Union 2004-2012. J Ethnopharmacol 2014, 158, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Petrović, S. Herbal and Traditional Herbal Medicinal Products, EU Herbal Monographs and EU List. Arh Farm (Belgr) 2019, 69, 221–269. [Google Scholar] [CrossRef]
- European Medicines Agency Guideline on Similar Biological Medicinal Products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf 2014, 44, 1–7.
- Iordache, A.M.; Nechita, C.; Voica, C.; Roba, C.; Botoran, O.R.; Ionete, R.E. Assessing the Health Risk and the Metal Content of Thirty-Four Plant Essential Oils Using the ICP-MS Technique. Nutrients 2022, 14, 2363. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Gualpa, F.; Ramos, L.A.; Ciobotă, V. Adulteration of Clove Essential Oil: Detection Using a Handheld Raman Spectrometer. Flavour Fragr J 2018, 33, 184–190. [Google Scholar] [CrossRef]
- Pierson, M.; Fernandez, X.; Antoniotti, S. Type and Magnitude of Non-Compliance and Adulteration in Neroli, Mandarin and Bergamot Essential Oils Purchased online: Potential Consumer Vulnerability. Sci Rep 2021, 11, 11096. [Google Scholar] [CrossRef]
- De Clerck, C.; Maso, S.D.; Parisi, O.; Dresen, F.; Zhiri, A.; Haissam Jijakli, M. Screening of Antifungal and Antibacterial Activity of 90 Commercial Essential Oils against 10 Pathogens of Agronomical Importance. Foods 2020, 9, 1418. [Google Scholar] [CrossRef]
- Thomsen, P.S.; Jensen, T.M.; Hammer, K.A.; Carson, C.F.; Mølgaard, P.; Riley, T. V. Survey of the Antimicrobial Activity of Commercially Available Australian Tea Tree (Melaleuca Alternifolia) Essential Oil Products in Vitro. Journal of Alternative and Complementary Medicine 2011, 17, 835–841. [Google Scholar] [CrossRef]
- Ogbaini-Emovon, E.; Schuster, H.; Waze, J.; Iche, K. In Vitro Antimicrobial Activity of Commercially Available Melaleuca Alternifolia (Tea Tree) Oil on Some Selected Clinical Pathogens. Br J Pharm Res 2015, 5, 202–208. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic Interactions of Plant Essential Oils with Antimicrobial Agents: A New Antimicrobial Therapy. Crit Rev Food Sci Nutr 2022, 62, 1740–1751. [Google Scholar] [CrossRef]
- Brun, P.; Bernabè, G.; Filippini, R.; Piovan, A. In Vitro Antimicrobial Activities of Commercially Available Tea Tree (Melaleuca Alternifolia) Essential Oils. Curr Microbiol 2019, 76, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Anquez-Traxler, C. The Legal and Regulatory Framework of Herbal Medicinal Products in the European Union: A Focus on the Traditional Herbal Medicines Category. Ther Innov Regul Sci 2011, 45, 15–23. [Google Scholar] [CrossRef]
- Peschel, W.; Alvarez, B.M. Harmonised European Standards as a Basis for the Safe Use of Herbal Medicinal Products and Their Marketing Authorisation in European Union Member States. Pharmaceut Med 2018, 32, 275–293. [Google Scholar] [CrossRef]
- Steinhoff, B. Harmonised Assessment Criteria for Efficacy and Safety of Herbal Medicinal Products. Revista de Fitoterapia 2002, 2, 47. [Google Scholar]
- Yevale, R.; Khan, N.; Kalamkar, P. Overview on “Regulations of Herbal Medicine. ” J Pharmacogn Phytochem 2018, 7, 61–63. [Google Scholar] [CrossRef]
- Commission, T.H.E.E. COMMISSION IMPLEMENTING REGULATION (EU) 2022/1248 of 19 July 2022 Concerning the Authorisation of Essential Oil from Origanum Vulgare Ssp. Hirtum (Link) Ietsw. as a Feed Additive for Certain Animal Species. 2022, 1248, 18–20. [Google Scholar]
- Bejar, E. Adulteration of Oregano Herb and Essential Oil. Botanical Adulterants Prevention Bulletin 2019, 10, 1–10. [Google Scholar]
- Mohammadi Gheisar, M.; Kim, I.H. Phytobiotics in Poultry and Swine Nutrition–a Review. Ital J Anim Sci 2018, 17. [Google Scholar] [CrossRef]
- Fonseca-García, I.; Escalera-Valente, F.; Martínez-González, S.; Carmona-Gasca, C.A.; Gutiérrez-Arenas, D.A.; Ramos, F. Effect of Oregano Oil Dietary Supplementation on Production Parameters, Height of Intestinal Villi and the Antioxidant Capacity in the Breast of Broiler. Austral J Vet Sci 2017, 49, 200083. [Google Scholar] [CrossRef]
- European Pharmacopoeia (Ph. Eur.) 10th Edition | EDQM - European Directorate for the Quality of Medicines Available Online: Https://Www.Edqm.Eu/En/European-Pharmacopoeia-Ph-Eur-10th-Edition accessed on 17 May 2023. 17 May.
- Committee, on H.M.P. Assessment Report on Syzygium Aromaticum (L .) Merill et L . M . Perry, Flos and Syzygium Aromaticum (L .) Merill Et. European Medicines Agency (EMA) 2011, 44, 26.
- Medicines Agency, E. Peppermint Oil - Herbal Summary for the Public. EMEA European Medicines Agency 2020, 31, 1–3. [Google Scholar]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical Composition and Antibacterial Activities of Seven Eucalyptus Species Essential Oils Leaves. Biol Res 2015, 48, 7. [Google Scholar] [CrossRef]
- EMA. Committee on Herbal Medicinal Products (HMPC) Community Herbal Monograph on Eucalyptus Globulus Labill., Eucalyptus Polybractea R.T. Baker and/or Eucalyptus Smithii R.T. Baker, Aetheroleum. European Medicines Agenc 2014, 44, 2–11. [Google Scholar]
- Revision, D. European Union Herbal Monograph on Rosmarinus Officinalis L., Aetheroleum. EMEA European Medicines Agency 2022, 31, 1–6. [Google Scholar]
- Luo, K.; Zhao, P.; He, Y.; Kang, S.; Shen, C.; Wang, S.; Guo, M.; Wang, L.; Shi, C. Antibacterial Effect of Oregano Essential Oil against Vibrio Vulnificus and Its Mechanism. Foods 2022, 11, 403. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, J.; Song, Y.; Raka, R.N.; Xiang, J.; Wu, H.; Xiao, J.; Jin, J.; Hui, X.L. Antibacterial Activity of Oregano Essential Oils against Streptococcus Mutans in Vitro and Analysis of Active Components. BMC Complement Med Ther 2023, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. Journal of Essential Oil-Bearing Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial Mechanism of Oregano Essential Oil. Ind Crops Prod 2019, 139, 111498. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, D.; Xiang, H.; Feng, H.; Jiang, Y.; Xia, L.; Dong, J.; Lu, J.; Yu, L.; Deng, X. Subinhibitory Concentrations of Thymol Reduce Enterotoxins A and B and α-Hemolysin Production in Staphylococcus Aureus Isolates. PLoS One 2010, 5, 9736. [Google Scholar] [CrossRef]
- Kryvtsova, M. V.; Fedkiv, O.K.; Hrytsyna, M.R.; Salamon, I. Anty-Microbial, and Anty-Biofilm-Forming Properties of Origanum Vulgare L. Essential Oils on Staphylococcus Aureus and Its Antioxidant Action. Studia Biologica 2020, 14, 27–38. [Google Scholar] [CrossRef]
- Sipahi, N.; Kekeç, A.I.; Halaç, B. In Vitro Effect of Some Essential Oils against Multiple Antibiotic-Resistant Bacteria from Cats and Dogs. Pak Vet J 2022, 42, 561–565. [Google Scholar]
- Schillaci, D.; Napoli, E.M.; Cusimano, M.G.; Vitale, M.; Ruberto, G. Origanum Vulgare Subsp. Hirtum Essential Oil Prevented Biofilm Formation and Showed Antibacterial Activity against Planktonic and Sessile Bacterial Cells. J Food Prot 2013, 76, 1747–1752. [Google Scholar] [CrossRef]
- Lu, M.; Wong, K.I.; Li, X.; Wang, F.; Wei, L.; Wang, S.; Wu, M.X. Oregano Oil and Harmless Blue Light to Synergistically Inactivate Multidrug-Resistant Pseudomonas Aeruginosa. Front Microbiol 2022, 13, 810746. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M. del M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phytotherapy Research 2018, 32. [Google Scholar] [CrossRef] [PubMed]
- Lara, V.M.; Carregaro, A.B.; Santurio, D.F.; Sá, M.F. De; Santurio, J.M.; Alves, S.H. Antimicrobial Susceptibility of Escherichia Coli Strains Isolated from Alouatta Spp. Feces to Essential Oils. Evidence-based Complementary and Alternative Medicine 2016, 2016, 1643762. [Google Scholar] [CrossRef] [PubMed]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Ghalem, B.R.; Mohamed, B. Antibacterial Activity of Leaf Essential Oils of Eucalyptus Globulus and Eucalyptus Camaldulensis. Afr J Pharm Pharmacol 2008, 2, 211–215. [Google Scholar]
- Bachir, R.G.; Benali, M. Antibacterial Activity of the Essential Oils from the Leaves of Eucalyptus Globulus against Escherichia Coli and Staphylococcus Aureus. Asian Pac J Trop Biomed 2012, 2, 739–742. [Google Scholar] [CrossRef]
- Ameur, E.; Sarra, M.; Yosra, D.; Mariem, K.; Nabil, A.; Lynen, F.; Larbi, K.M. Chemical Composition of Essential Oils of Eight Tunisian Eucalyptus Species and Their Antibacterial Activity against Strains Responsible for Otitis. BMC Complement Med Ther 2021, 21, 209. [Google Scholar]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the Antibiofilm and Antiquorum Sensing Activities of Eucalyptus Globulus Essential Oil and Its Main Component 1,8-Cineole against Methicillin-Resistant Staphylococcus Aureus Strains. Microb Pathog 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Quatrin, P.M.; Verdi, C.M.; de Souza, M.E.; de Godoi, S.N.; Klein, B.; Gundel, A.; Wagner, R.; de Almeida Vaucher, R.; Ourique, A.F.; Santos, R.C.V. Antimicrobial and Antibiofilm Activities of Nanoemulsions Containing Eucalyptus Globulus Oil against Pseudomonas Aeruginosa and Candida Spp. Microb Pathog 2017, 112, 230–242. [Google Scholar] [CrossRef]
- Khedhri, S.; Polito, F.; Caputo, L.; Manna, F.; Khammassi, M.; Hamrouni, L.; Amri, I.; Nazzaro, F.; De Feo, V.; Fratianni, F. Chemical Composition, Phytotoxic and Antibiofilm Activity of Seven Eucalyptus Species from Tunisia. Molecules 2022, 27, 8227. [Google Scholar] [CrossRef] [PubMed]
- Azzam, N.F.A.E.M. Antibacterial Effect of Eucalyptus Essential Oil. Indian J Sci Technol 2020, 13, 799–804. [Google Scholar] [CrossRef]
- https://oshadhi.co.uk/kb/purity-adulteration-testing/ accessed on 20 May 2023.
- Elangovan, S.; Mudgil, P. Antibacterial Properties of Eucalyptus Globulus Essential Oil against MRSA: A Systematic Review. Antibiotics 2023, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial Activity of Essential Oils from Eucalyptus and of Selected Components against Multidrug-Resistant Bacterial Pathogens. Pharm Biol 2011, 49, 553625. [Google Scholar] [CrossRef] [PubMed]
- Van, L.T.; Hagiu, I.; Popovici, A.; Marinescu, F.; Gheorghe, I.; Curutiu, C.; Ditu, L.M.; Holban, A.M.; Sesan, T.E.; Lazar, V. Antimicrobial Efficiency of Some Essential Oils in Antibiotic-Resistant Pseudomonas Aeruginosa Isolates. Plants 2022, 11, 2003. [Google Scholar] [CrossRef]
- Jafari-Sales, A.; Pashazadeh, M. Study of Chemical Composition and Antimicrobial Properties of Rosemary (Rosmarinus Officinalis) Essential Oil on Staphylococcus Aureus and Escherichia Coli in Vitro. International Journal of Life Sciences and Biotechnology 2020, 3, 62–69. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Gong, X.; Wu, X.; Liu, L.; Chi, F. Rosemary and Tea Tree Essential Oils Exert Antibiofilm Activities in Vitro against Staphylococcus Aureus and Escherichia Coli. J Food Prot 2020, 83, 1261–1267. [Google Scholar] [CrossRef]
- Stojiljkovic, J. Antibacterial Activities of Rosemary Essential Oils and Their Components against Pathogenic Bacteria. Advances in Cytology & Pathology 2018, 3, 93–96. [Google Scholar]
- Santoyo, S.; Cavero, S.; Jaime, L.; Ibañez, E.; Señoráns, F.J.; Reglero, G. Chemical Composition and Antimicrobial Activity of Rosmarinus Officinalis L. Essential Oil Obtained via Supercritical Fluid Extraction. J Food Prot 2005, 68, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, O.; Uğur, A.; Saraç, N.; Ozcan, F.; Baygar, T. The in Vitro Antibiofilm Activity of Rosmarinus Officinalis L. Essential Oil against Multiple Antibiotic Resistant Pseudomonas Sp. and Staphylococcus Sp. J Food Agric Environ 2014, 12, 82–86. [Google Scholar]
- Bogavac, M.A.; Karaman, M.A.; Sudi, J.J.; Radovanović, B.B.; Janjušević, L.N.; Ćetković, N.B.; Tešanović, K.D. Antimicrobial Potential of Rosmarinus Officinalis Commercial Essential Oil in the Treatment of Vaginal Infections in Pregnant Women. Nat Prod Commun 2017, 12, 200136. [Google Scholar] [CrossRef]
- https://www.wildherbsofcrete.com/rosemary accessed on 20 May 2023.
- Xu, J.G.; Liu, T.; Hu, Q.P.; Cao, X.M. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus Aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial Activity and Mechanism of Clove Essential Oil against Foodborne Pathogens. LWT 2023, 173. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A Phyto-Compound Effective against Methicillin-Resistant and Methicillin-Sensitive Staphylococcus Aureus Clinical Strain Biofilms. PLoS One 2015, 10, 119564. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Reinders, R.D. Antibacterial Activity of Selected Plant Essential Oils against Escherichia Coil O157:H7. Lett Appl Microbiol 2003, 36, 162–167. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Gwon, G.; Kim, S. Il; Park, J.G.; Lee, J. Essential Oils and Eugenols Inhibit Biofilm Formation and the Virulence of Escherichia Coli O157:H7. Sci Rep 2016, 6, 36377. [Google Scholar] [CrossRef]
- Musthafa, K.S.; Voravuthikunchai, S.P. Anti-Virulence Potential of Eugenyl Acetate against Pathogenic Bacteria of Medical Importance. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology 2015, 107, 703–710. [Google Scholar] [CrossRef]
- Costa, L.V.; Moreira, J.M.A.R.; de Godoy Menezes, I.; Dutra, V.; do Bom Parto Ferreira de Almeida, A. Antibiotic Resistance Profiles and Activity of Clove Essential Oil (Syzygium Aromaticum) against Pseudomonas Aeruginosa Isolated of Canine Otitis. Vet World 2022, 15. [Google Scholar] [CrossRef]
- Purwasena, I.A.; Astuti, D.I.; Taufik, I.; Putri, F.Z. The Potential of Clove Essential Oil Microemulsion as an Alternative Biocide against Pseudomonas Aeruginosa Biofilm. J Pure Appl Microbiol 2020, 14, 261–269. [Google Scholar] [CrossRef]
- Muntean, D.; Licker, M.; Alexa, E.; Popescu, I.; Jianu, C.; Buda, V.; Dehelean, C.A.; Ghiulai, R.; Horhat, F.; Horhat, D.; et al. Evaluation of Essential Oil Obtained from Mentha×piperita L. against Multidrug-Resistant Strains. Infect Drug Resist 2019, 12, 2905–2914. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.; Qui, J.Z.; Wang, J.F.; Luo, M.J.; Li, H.E.; Leng, B.F.; Ren, W.Z.; Deng, X.M. Peppermint Oil Decreases the Production of Virulence- Associated Exoproteins by Staphylococcus Aureus. Molecules 2011, 16, 1642. [Google Scholar] [CrossRef]
- Horváth, P.; Koščová, J. In Vitro Antibacterial Activity of Mentha Essential Oils Against Staphylococcus Aureus. Folia Vet 2017, 61, 71–77. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and Anti-Biofilm Activities of Peppermint Essential Oil against Staphylococcus Aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Sarwar, W.; Ali, Q.; Ahmed, S. Microscopic Visualization of the Antibiofilm Potential of Essential Oils against Staphylococcus Aureus and Klebsiella Pneumoniae. Microsc Res Tech 2022, 85, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Pajohi Alamoti, M.; Bazargani-Gilani, B.; Mahmoudi, R.; Reale, A.; Pakbin, B.; Di Renzo, T.; Kaboudari, A. Essential Oils from Indigenous Iranian Plants: A Natural Weapon vs. Multidrug-Resistant Escherichia Coli. Microorganisms 2022, 10, 109. [Google Scholar] [CrossRef]
- Metin, S.; Didinen, B.I.; Telci, I.; Diler, O. Essential Oil of Mentha Suaveolens Ehrh., Composition and Antibacterial Activity against Bacterial Fish Pathogens. An Acad Bras Cienc 2021, 93, 20190478. [Google Scholar] [CrossRef]
- Pazarci, O.; Tutar, U.; Kilinc, S. Investigation of the Antibiofilm Effects of Mentha Longifolia Essential Oil on Titanium and Stainless Steel Orthopedic Implant Surfaces. Eurasian Journal of Medicine 2019, 51, 18432. [Google Scholar] [CrossRef]
- Iseppi, R.; Di Cerbo, A.; Aloisi, P.; Manelli, M.; Pellesi, V.; Provenzano, C.; Camellini, S.; Messi, P.; Sabia, C. In Vitro Activity of Essential Oils against Planktonic and Biofilm Cells of Extended-Spectrum β-Lactamase (ESBL)/Carbapenamase-Producing Gram-Negative Bacteria Involved in Human Nosocomial Infections. Antibiotics 2020, 9, 272. [Google Scholar] [CrossRef]
- https://www.perfumerflavorist.com/flavor/ingredients/article/21856932/authentication-of-natural-peppermint-oil accessed on 20 May 2023.
- Singh, N.S.; Singhal, N.; Kumar, M.; Virdi, J.S. Exploring the Genetic Mechanisms Underlying Amoxicillin-Clavulanate Resistance in Waterborne Escherichia Coli. Infection, Genetics and Evolution 2021, 90, 104767. [Google Scholar] [CrossRef] [PubMed]
- Sandulovici, R.C.; Carmen-Marinela, M.; Grigoroiu, A.; Moldovan, C.A.; Savin, M.; Ordeanu, V.; Voicu, S.N.; Cord, D.; Costache, G.M.; Galatanu, M.L.; et al. The Physicochemical and Antimicrobial Properties of Silver/Gold Nanoparticles Obtained by “Green Synthesis” from Willow Bark and Their Formulations as Potential Innovative Pharmaceutical Substances. Pharmaceuticals 2023, 16, 10048. [Google Scholar] [CrossRef] [PubMed]
- Stefan, D.S.; Popescu, M.; Luntraru, C.M.; Suciu, A.; Belcu, M.; Ionescu, L.E.; Popescu, M.; Iancu, P.; Stefan, M. Comparative Study of Useful Compounds Extracted from Lophanthus Anisatus by Green Extraction. Molecules 2022, 27, 7737. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Manrique-Moreno, M.; Klaiss-Luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of Essential Oils on Growth Inhibition, Biofilm Formation and Membrane Integrity of Escherichia Coli and Staphylococcus Aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sequeda, N.; Cáceres, M.; Stashenko, E.E.; Hidalgo, W.; Ortiz, C. Antimicrobial and Antibiofilm Activities of Essential Oils against Escherichia Coli O157:H7 and Methicillin-Resistant Staphylococcus Aureus (MRSA). Antibiotics 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Rambu, D.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Ungureanu-Iuga, M.; Oroian, M.; Mironeasa, S.; et al. Antioxidant, Cytotoxic, and Rheological Properties of Canola Oil Extract of Usnea Barbata ( L.) Weber Ex F. H. Wigg from Călimani Mountains, Romania. Plants 2022, 11, 854. [Google Scholar] [CrossRef]
- Guillín, Y.; Cáceres, M.; Torres, R.; Stashenko, E.; Ortiz, C. Effect of Essential Oils on the Inhibition of Biofilm and Quorum Sensing in Salmonella Enteritidis 13076 and Salmonella Typhimurium 14028. Antibiotics 2021, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Popescu, A.; Matei, E.; Caraiane, A.; Botnarciuc, M. Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea Barbata ( L.) Weber Ex F. H. Wigg from Călimani Mountains, Romania. Pharmaceuticals 2022, 15, 829. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Musuc, A.M.; Mitu, M.A.; Atkinson, I.; et al. In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea Barbata ( L. ) F. H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy. Antioxidants 2022, 11, 1934. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Sarbu, I.; Musuc, A.M.; Atkinson, I.; et al. Formulation and Development of Bioadhesive Oral Films Containing Usnea Barbata ( L.) F. H. Wigg Dry Ethanol Extract ( F-UBE-HPC ) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy. Pharmaceutics 2022, 14, 1808. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





