1. Introduction
Nowadays, the development of biodegradable polymers that are derived from renewable natural resources has gained increasing interest. Starch is an attracting raw material to produce biodegradable materials but it still have a limited ability to process [
1]. This drawback can be solved by changing the molecular order within the granules. Plasticization could change the structure of starch molecules in the presence of a plasticizer under high temperature and shear, resulting in a homogeneous, amorphous substance known as “thermoplastic starch” (TPS) or “plasticized starch” in the preferential conditions [
2]. TPS is a flexible and renewable material that can be easily used in a variety of thermoplastification processes using standard equipments which is common in the production of synthetic polymers [
3]. Plasticizer plays an important role in TPS production and processing. It can penetrate into starch molecules and destroy the intra- and inter- molecular hydrogen bonds between starch molecules. Glycerol is one of the most widely used plasticizers to prepare thermoplastic starch [
4]. In addition, native starches lack the characteristics needed for industrial use; therefore, their molecular structure needs to be modified. Oxidation is considered one of the most effective methods to modify the molecular structure of starches, and the preferred oxidant is sodium hypochlorite (NaClO) [
5,
6]. After treating with NaClO, the molecular structure of starch was transformed and resulted in a higher content of carbonyl and carboxyl groups, which can potentially form intra-molecule hydrogen bonds between starch molecules as well as contribute to enhance the interaction between oxidized starch molecules and plasticizer. The use of oxidized starch was expected that could improve some properties of thermoplastic starch [
7].
In recent years, starch from under-exploited sources have received a great deal of attention from researchers. Among these sources, Canna (
Canna Edulis Ker), which also known as “
achira”[
8], could be considered as promising green materials in the future, especially for food packaging application due to its high amylose content [
9]. Andrade-Mahecha
et al. [
10] developed biodegradable films based on achira flour and glycerol. The results showed that, achira flour is considered as a promising material for the development of biodegradable films with good mechanical properties and low vapor permeability. Canales
et al. [
11] prepared and studied on characterization of thermoplastics achira starch and the results suggested that all thermoplastic achira starches developed was a viable alternative for application in the industry dedicated to the processing of bioplastics. Study of Carolina Caicedo [
12] on characteristics of thermoplastic canna starch modified with oleic acid and lactic acid also provided promising results for the development of new biodegradable polymers which can use in manufacturing packaging materials with nature origins in the future.
Although there are some studies on the preparation of thermoplastic from CS, the reports on the effects of oxidized canna starch (OCS) on the physicochemical characteristics of respective thermoplastic starch are few. In this study, CS was oxidized and the obtained oxidized starches were used to prepare thermoplastic samples. The characteristics of TPS samples were examined and the effect of different degrees of oxidation of starch on the properties of OTPS samples was also evaluated.
2. Materials and Methods
2.1. Materials
Canna (
Canna Edulis Ker.) starch was isolated from fresh canna according to the method of Hung
et al. [
13] The obtained CS has 33% amylose; 60% amylopectin; 0.22% ash and 0.31% crude fiber.
Sodium hypochlorite was supplied by Viet Tri Chemical Joint Stock Company, with density of 1.112 g/ml, active chlorine content of 8%.
Hydrochloric acid, sodium hydroxide and glycerol were purchased from Xilong Chemical Co., Ltd. (China).
2.2. Preparation of oxidized starches
Starch oxidation was performed according to the method described by our previous article [
14]. Oxidation reaction was carried out in a three-neck, round-bottom flask with a glass condenser and a heating magnetic stirrer. Canna starch was transfered into a NaClO solution (1, 2 and 3%, w/w) which was adjusted to pH = 9 and stirred at room temperature for two hours. The pH of the mixture was maintained throughout the procedure by using HCl and NaOH solution. After the oxidation period, the mixture was neutralized with 0.5 M HCl solution and filtered by using a Buchner filter funnel then washed five times with distilled water. Oxidized starch was precipitated in ethanol and then dried in the oven at 50°C under vacuum to a constant weight. Obtained OCS samples were labeled as: OCS1, OCS3 and OCS5, respectively.
2.3. Preparation of thermoplastic starch
Different TPS samples were prepared by using an internal mixer (Brabender Plastograph®EC Plus). Native canna starch and oxidized canna starch (dried at 70 oC for 12 hours) and glycerol (30wt%) was blended uniformly and stored for 16-18 h at room temperature. This mixture then was manually fed into Brabender internal mixer (Germany) with screw speed of 60 rpm at 160 oC for 10 min. The extruded thermoplastic starch samples were then compression molded into 2 mm-thick plates by using a hot press at 160 oC and 20 MPa for 15 min. Obtained TPS samples were labeled as: TPS-CS, TPS-OCS1, TPS-OCS3, TPS-OCS5, respectively.
2.4. Characterization
2.4.1. Carboxyl, carbonyl content and total oxidation degree
The carboxyl, carbonyl content and total oxidation degree (TOD) was determined according to the titrimetric method described by Y. Wang [
15].
2.4.2. Mechanical properties
Tensile and elongation at break properties of samples were determined according to ASTM D638 using Instron 5980 Testing Machine. All the test samples were conditioned at 25 oC within 24 h before testing. An average value of five tests was reported.
2.4.3. Fourier transform infrared spectroscopy (FTIR)
Reflectance IR spectra of samples were obtained in a wavenumber range of 400-4000 cm-1 on a Fourier transform infrared (FTIR) spectrometer (Nicolet iS10). The sample was placed in the position of the diamond crystal and was in full contact with it. Infrared light passes through the diamond crystal and interacts with the surface of the sample, then it is reflected off, thereby the spectrometer can collect the reflectance IR spectra of the sample. The data was collected and processed by using the OMNIC 5a software.
2.4.4. Morphology
The morphology of the fracture surface of TPS samples was observed by Scanning Electron Microscopy (SEM) (JEOL SM-6510 LV) at 5kV. After being immersed in liquid nitrogen, the TPS samples were fractured and the surface was coated with a thin platinium coating before observation.
2.4.5. X-ray diffraction (XRD)
X-ray diffraction (XRD) patterns and crystallinity of samples were obtained by using an X-ray diffractometer (Bruker, Model D8-ADVANCE) with the measuring parameters of CuKα cell (λ = 1,5406 Å), U = 40 kV, I = 30 mA. The 2θ scan range was from 5 to 40 °.
2.4.6. Differential Scanning Calorimetry (DSC)
The glass transition temperature and transition enthalpy of samples were analyzed and calculated by differential scanning calorimetry (Rigaku DSC 8231). About 5.0 mg of samples were hermetically sealed in aluminum pans and heated from 20 to 100 °C at heating rate of 10 °C min−1. To keep an inert atmosphere for the DSC cell, it was flushed with nitrogen at a flow rate of 30 mL.min−1.
2.4.7. Thermogravimetry Analysis (TGA)
The thermal stability of samples was determined by using a TGA209F1 thermogravimetry analysis system. A nitrogen atmosphere was used for all measurements, at a constant heating and cooling rate of 10 oC/min from room temperature to 450 oC.
3. Results and Discussions
3.1. Carboxyl, carbonyl content and total oxidation degree (TOD) of oxidized canna starch
The carbonyl, carboxyl contents and TOD of oxidized canna starch (OCS) samples were shown in
Table 1.
As can be seen in
Table 1, oxidation with sodium hypochlorite clearly increased both the carbonyl and carboxyl content of canna starch. After treating with oxidant, content of carboxyl groups increase significantly, whereas content of carbonyl groups lightly increase. The hydroxyl groups in starch molecules are oxidized to carbonyl and carboxyl groups, which results in the presence of these groups in oxidized starch.
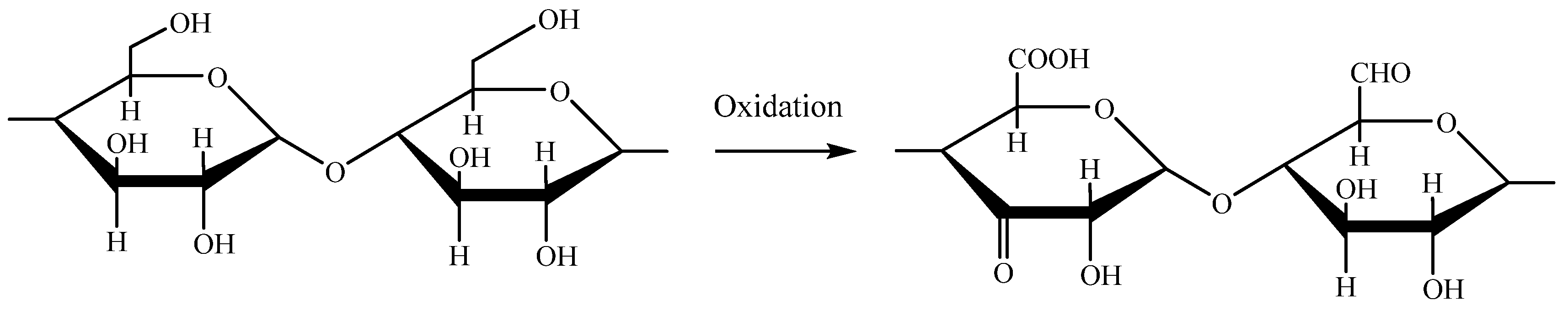
Figure 1.
Chemical reaction sodium hypochlorite (NaOCl) oxidation of starch.
Figure 1.
Chemical reaction sodium hypochlorite (NaOCl) oxidation of starch.
Numerous studies on the oxidation of various starch sources with different concentrations of sodium hypochlorite were reported [
14,
16,
17,
18,
19] and these studies also showed that the carbonyl and carboxyl contents of oxidized starch gradually increased with the increasing the concentration of hypochlorite agent. It was also reported that the content of carboxyl groups was always higher than that of the carboxyl groups at the same concentration of oxidation agent [
16]. The amount of carbonyl and/or carboxyl groups in oxidized starches directly affects the physicochemical characteristics of the starches, possibly making them suitable for a variety of industrial purposes.
3.2. IR spectra
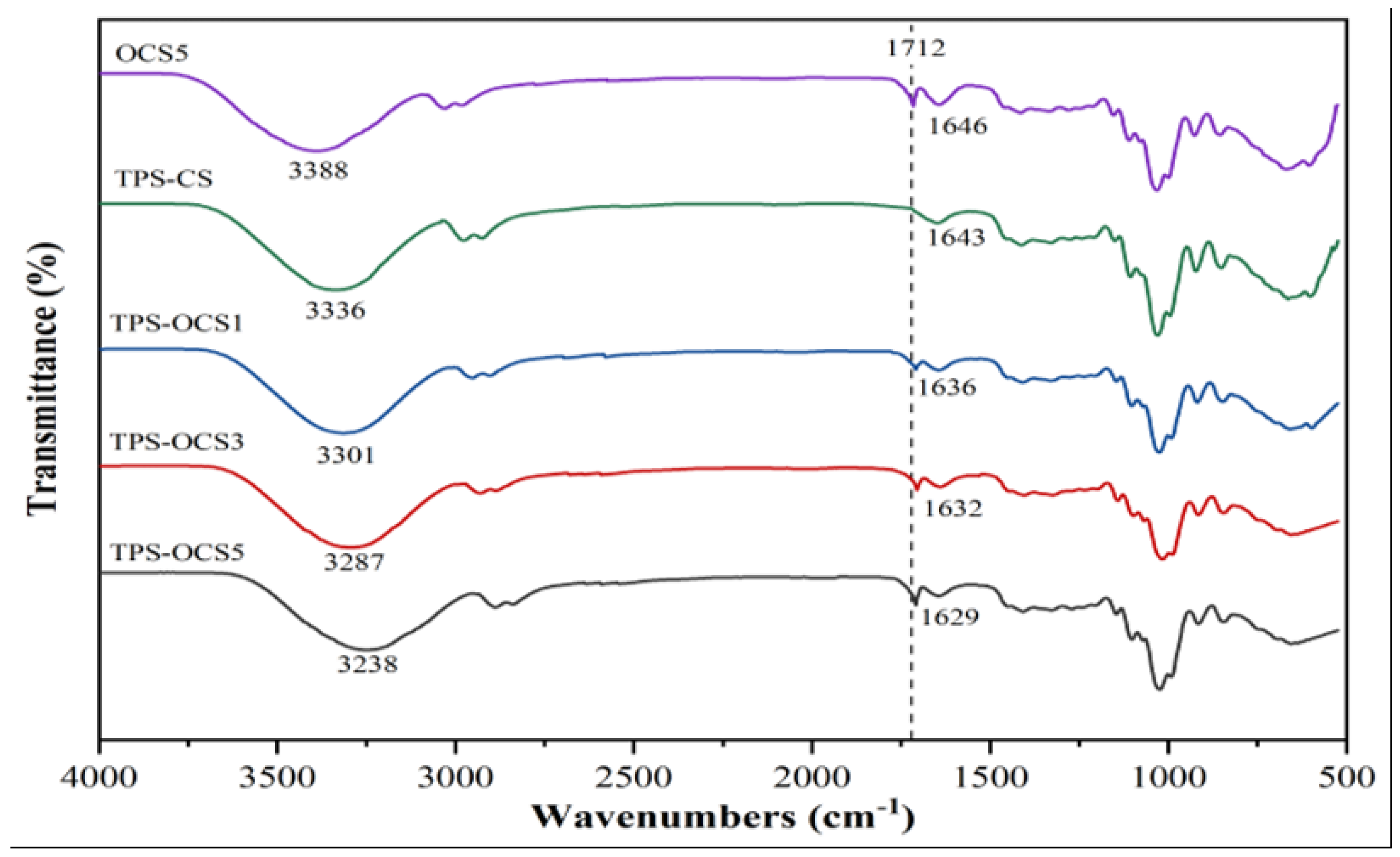
The IR spectra of OCS5, TPS-CS and TPS-OCS samples were presented in
Figure 2.
From the
Figure 2, it is observed that the infrared spectra of both OCS5 and TPS-OCS samples showed an absorption peak near 1710 cm
−1, which was assigned to the characteristic absorption peak of C=O-linkage in carbonyl and the intensity of this peak was enhanced with the increase of concentration of hypochlorite.
Hydrogen bond played an important role in the properties of TPS. As can be seen in
Figure 3, the peak of hydroxyl group stretching in OCS5 is at 3388 cm
-1, whereas, the visible broad absorption band at 3336, 3301, 3287 and 3238 cm
-1 in TPS-CS and TPS-OCS samples, respectively, is assigned to the hydrogen bonds formed by the interaction between O-H groups in starch chains and in the plasticizer. The formation of hydrogen bonds between starch and plasticizer leads to the decrease of the wavenumber value of stretching vibrations of O–H groups in starch molecules [
20]. This suggests that the hydrogen bonds that have newly formed between glycerol and starch are stronger than the intermolecular and intramolecular hydrogen bonds between the hydroxyl groups of the starch.
In addition, in TPS, it was testified that lower wavenumber indicate stronger hydrogen bond interaction [
20]. Therefore, by comparing the wavenumber values of stretching vibrations of O–H groups in TPS – CS and TPS-OCS samples, it can be suggested that the higher oxidation degree of CS lead to stronger hydrogen bonds between starch molecules and glycerol.
The characteristic peak around 1640 cm-1 can be assigned to the bending vibration of -OH groups of water molecules absorbed by amorphous region of starch granules. However, this peak on the IR spectra of TPS samples was clearly smaller than that on the IR spectra of OCS5. This may be due to the partial evaporation of water during the plasticization.
3.3. XRD
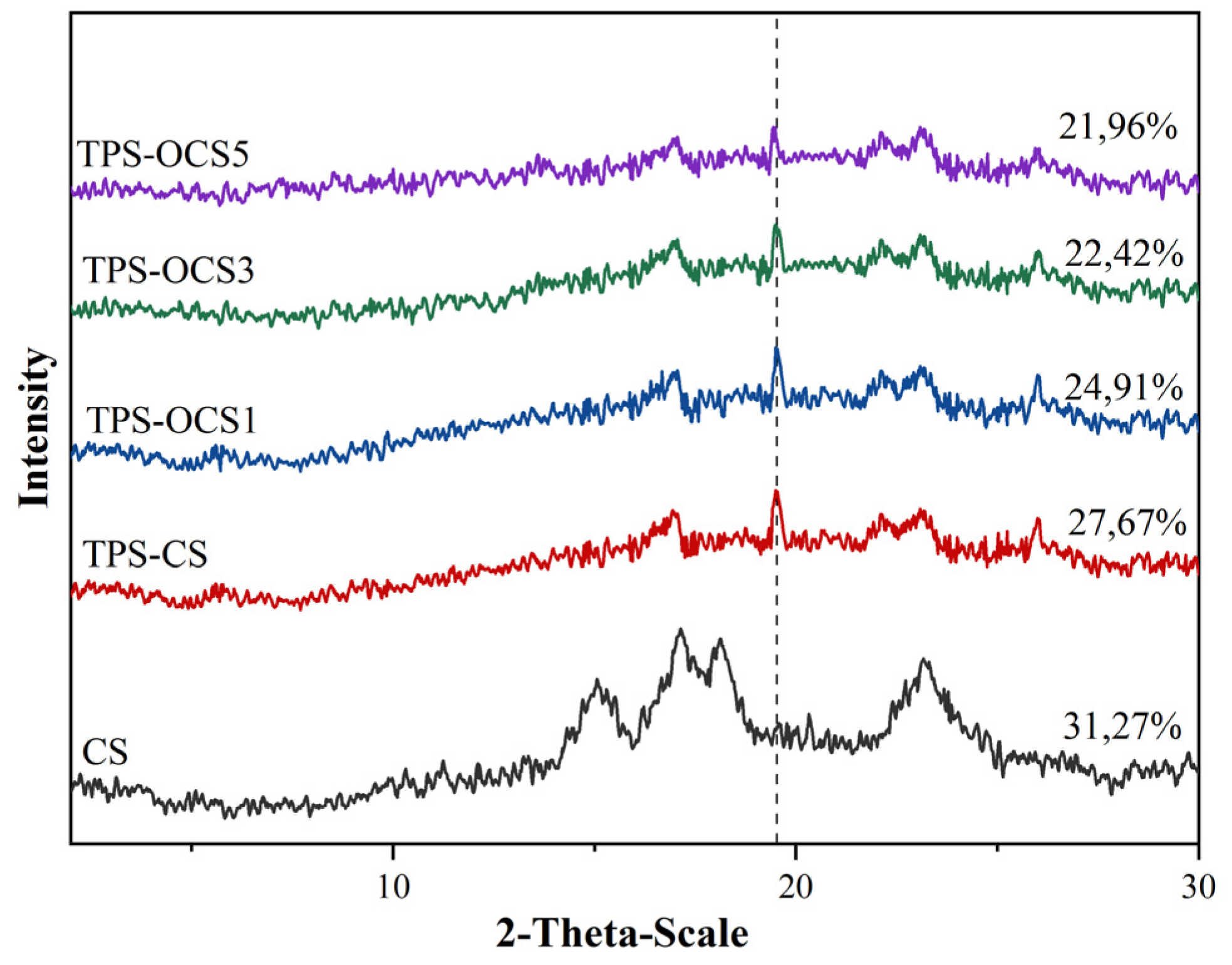
XRD pattern of native CS, TPS-CS and TPS-OCS samples and respective crystallinity were shown in
Figure 4.
As shown in
Figure 4, XRD peaks of native canna starch changed after extrusion process with the presence of glycerol. The native CS clearly showed the typical A-type crystalline structure with a single peak at 2θ near 15°, a doublet peak at 2θ = 17 and 18°, and a peak near 2θ = 23°. After plasticization process, XRD pattern of TPS-CS and TPS-OCS samples showed the characteristics of B-type of crystallization with a single peak at 2θ = ~17° and doublet peak at 22 and 23°. This is due to the starch destructuration in the thermoplasticization causes the TPS to recrystallize in a new structure.
However, it clearly orbserved that XRD pattern of all TPS samples showed the appearance of V
h-type crystallization peaks at 2θ = 19,5°. The existence of this crystalline structure was closely related to the interactions between amylose component in starch and plasticizer during plasticization. In addition, the XRD peaks of OTPS were smaller, and the intensity was lower than for the NTPS. This suggested that the crystallinity degree of the OTPS was lower than that of the NTPS. This result was confirmed by the crystallinity of all samples which was also shown in
Figure 4. The crystallinity decreased with the increase of TOD. The decrease in crystallinity of TPS-OCS samples can be explained by the effects of both oxidation and plasticization. The formation of carbonyl and carboxyl groups had a significant increase as compared to the native starch, lead to the decrease in the relative crystallinity of oxidized starches. This decrease can be attributed to the partial degradation of the crystalline region [
5]. In addition, in plasticization, the mechanical breakdown of molecular bonds caused by the strong shear forces also contributed to the decrease in crystallinity of TPS-OCS samples [
22].
3.4. Morphology
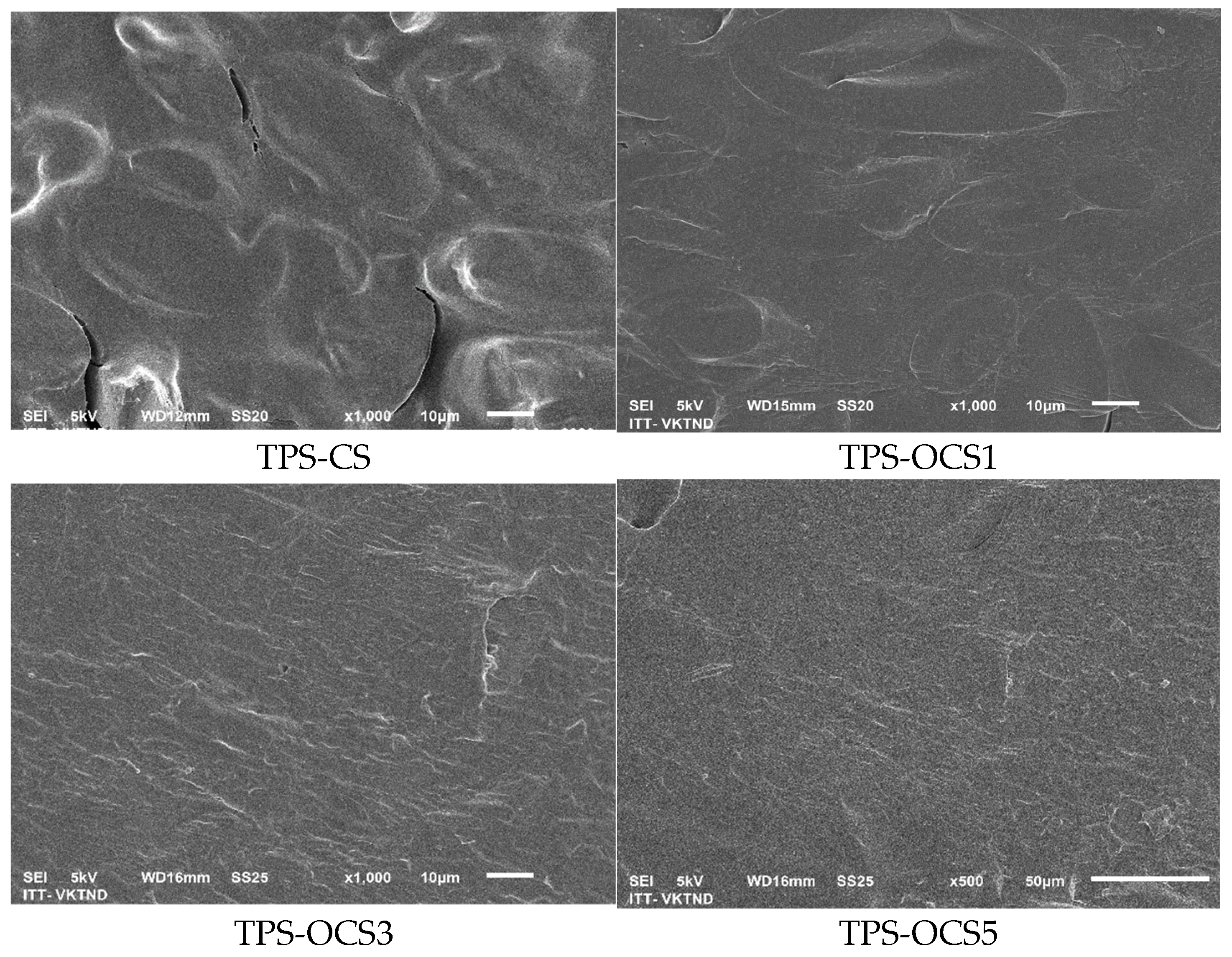
SEM images of the fracture surfaces of OCS-based TPS samples were showed in
Figure 5.
As can be seen in
Figure 5, it was also observed that the morphology of TPS samples are clearly changed after the extrusion process. Extrusion with the presence of glycerol at a high temperature resulted in complete destruction of starch structure with no native starch granules. The morphology of OTPS samples was smoother significantly when compare to the NTPS, respectively. This can be explained by the better plasticizing effect leads to the less the starch crystallinity, therefore, results in the smoother of the surface. However, there’s no significantly difference between morphology of TPS-OCS samples at different levels of oxidation. In other words, in this study, under the same experimental conditions, the degree of oxidation has no significant effect on the surface morphology of the OTPS samples.
3.5. DSC
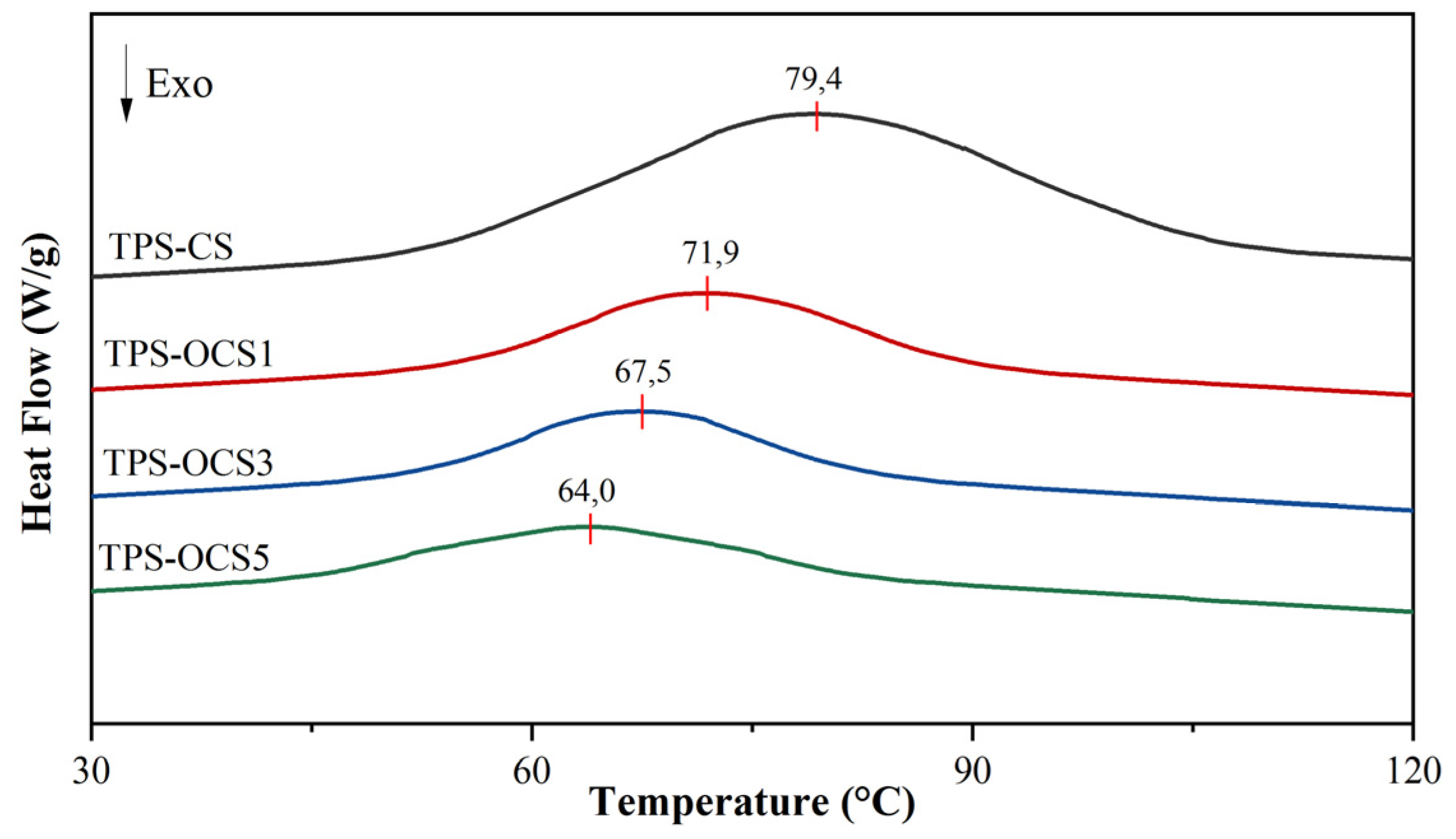
DSC curves of NTPS and OTPS were shown in
Figure 6 and the glass transition temperature (T
g) determined from DSC curves were showed in
Table 2.
As shown in
Table 2, it could clearly observed that T
g of OTPS decreased with increasing the concentration of oxidized agent. In the oxidation, the hydroxyl groups of starch was transformed to carboxyl and carbonyl groups, therefore, the hydrogen bonds between starch molecules weakened, leading to lower T
g.
During plasticization, glycerol molecules replace starch–starch interactions with starch–plasticizer interactions, results in the increase of polymeric chain molibility. The effectiveness of starch plasticization can be evaluated by comparing the phase transition enthalpy (ΔH
g). The less degree of plasticization, the higher is this enthalpy [
23]. Thit is due to it is less able to disrupt intermolecular hydrogen bonds of starch molecules in order to form hydrogen bonds between starch and plasticizer molecules. The ΔH
g values indicated that oxidized starches plasticized more easily than native starch. This suggested that the higher content of carboxyl and carbonyl groups in oxidized starches could help the plasticization becomes easier.
3.6. TGA
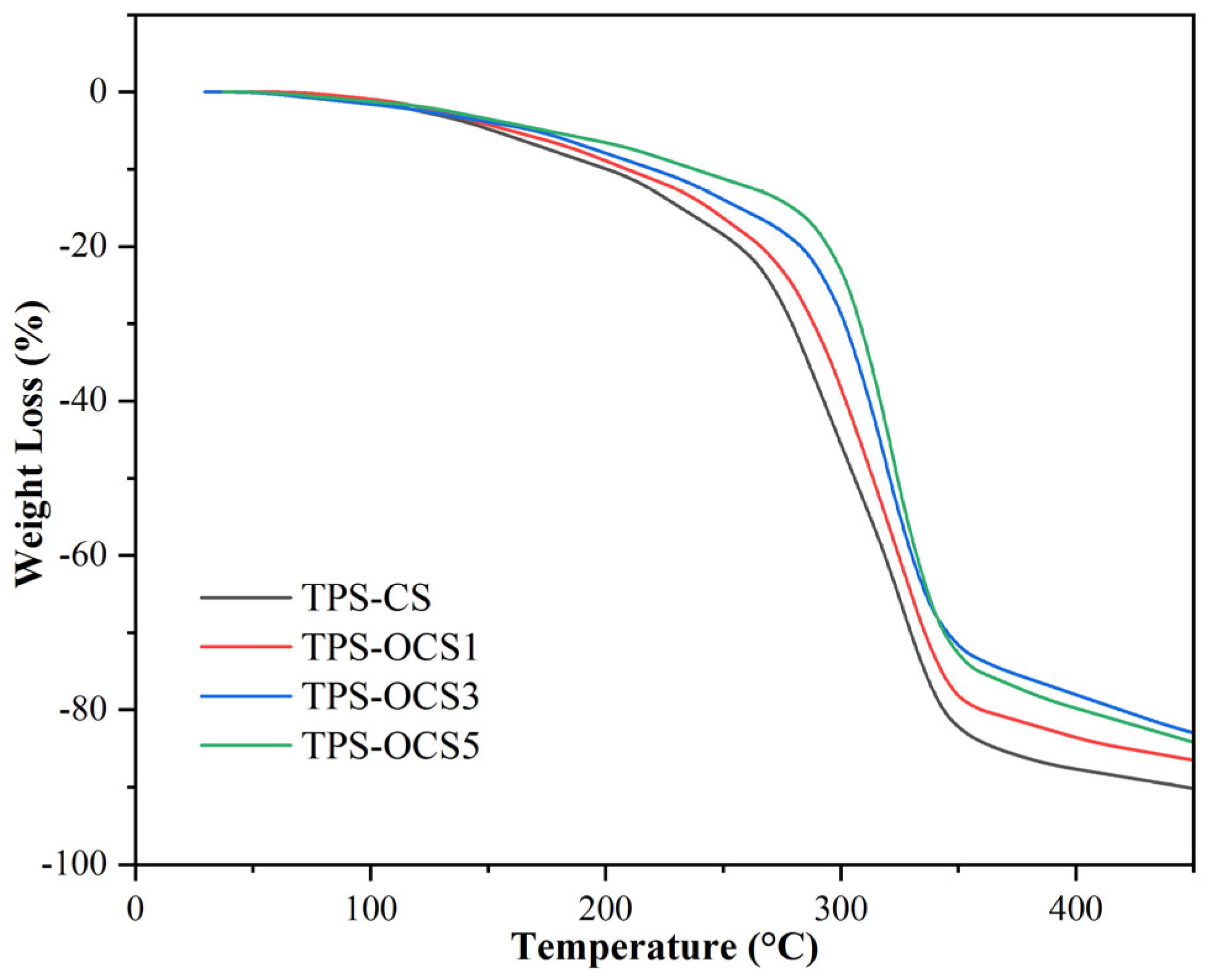
Thermogravimetry curves of NTPS and OTPS samples were shown in
Figure 7.
The TGA curves are similar for both NTPS and OTPS. The weight loss at the temperature below 100
o C is mainly ascribed to moisture evaporation, whereas the weight loss in range 100
oC to 300
oC can be ascribed to the decomposition of the glycerol rich phase in TPS [
7,
24]. Followed by the weight loss occurred around 300 °C and this corresponds to the decomposition of starch.
T
5%, T
10% and T
max, which represents the temperature of 5%, 10% and maximum weight loss, respectively, are determined from TGA curves and listed in the
Table 3.
As can be seen in
Table 3, T
5% and T
10% of TPS-OCS samples increase with the increase of the TOD and higher than that of respective NTPS samples. The cause may be due to the presence of oxidized starches enhances the binding of water and glycerol to starch, therefor, it was more difficult for them to evaporate during processing. The values of T
max of TPS-CS, TPS-OCS1 and TPS-OCS3 are almost the same, whereas T
max of TPS-OCS5 is the highest. This indicated that oxidized starch can improve the thermal stability of thermoplastic starches at lower temperature. This also means that at the practical processing temperature, the degradation of starch-based materials can be prevented by using oxidized starch. This results was also reported by Zhang [
7].
3.7. Mechanical properties
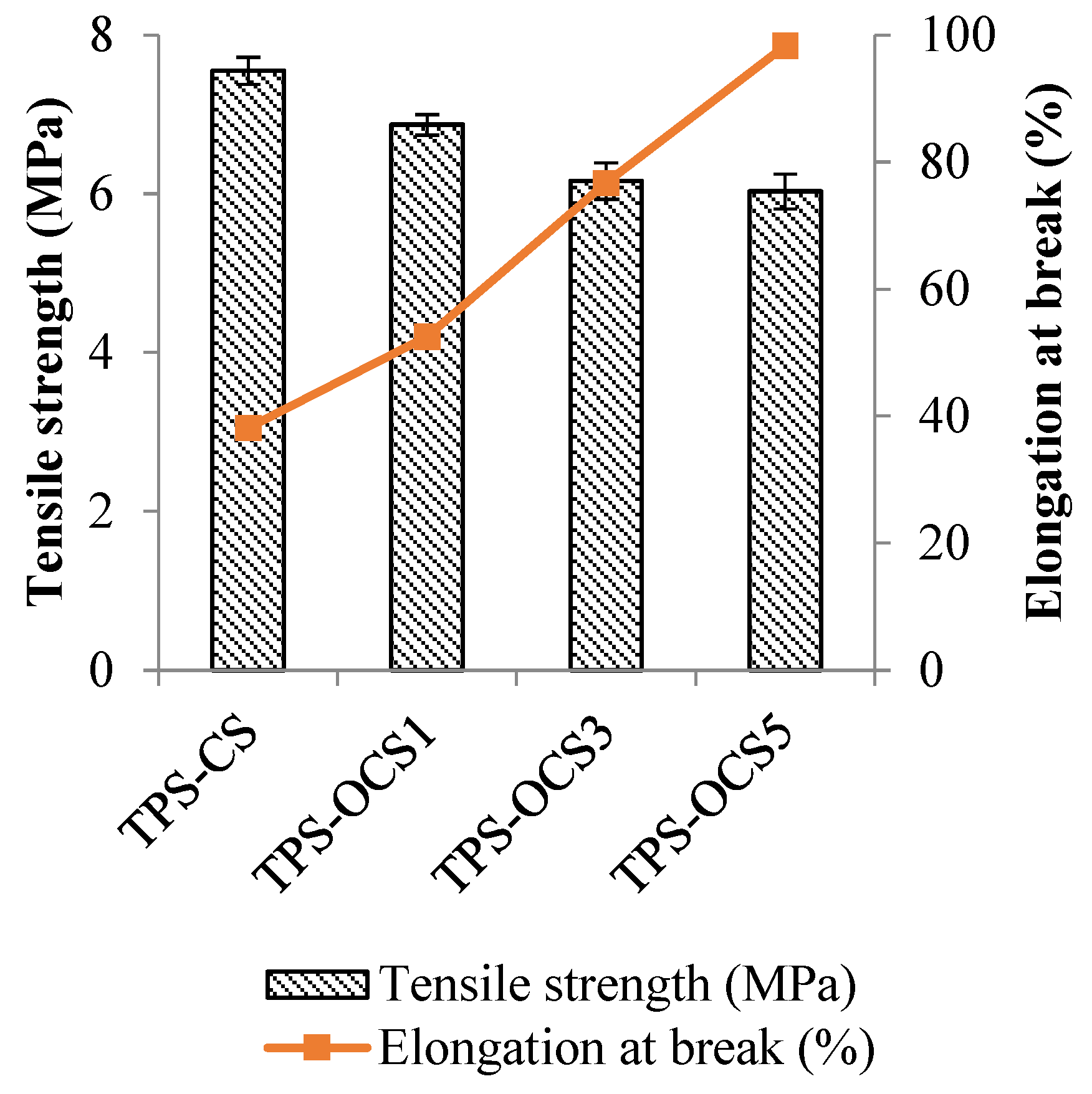
The tensile strength and elongation at break of TPS samples are shown in
Figure 8.
As can be seen in
Figure 8, tensile strength of TPS samples decreased with the increase of oxidation degree of CS, whereas the elongation at break was significantly improved. These results indicated that the flexibility of TPS was improved with the increase of oxidation degree of CS, which due to the oxidized starch disrupts the hydrogen bonds between starch chains. A higher oxidation degree of oxidized starch resulted in a stronger interaction with plasticizer molercules, leading to an improvement in the mobility of starch chains.
4. Conclusions
Herein, effect of oxidation on physicochemical characteristics of thermoplastic canna starchwere studied. The results showed that oxidized canna starch was successfully plasticized to form thermoplastic starch and the higher content of carboxyl and carbonyl groups in oxidized starches led to the better plasticization in TPS-OCS samples. SEM images confirmed that the better plasticizing effect in OTPS samples led to the clearly smoother fractured surface. DSC data indicated that both glass transition temperature Tg and phase transition enthalpy ΔHg of OTPS was lower than that of NTPS and suggested that the higher TOD of OCS could help the plasticization becomes easier. The tensile strength slightly decreased and the elongation at break significantly increased with the increase of the degree of oxidation. Oxidized starch also improved the thermal stability of thermoplastic starch. The obtained results of this study suggested that canna-based OTPS can be considered a potential material that can replace traditional starch sources in developing biodegradable films.
Author Contributions
All authors of this manuscript contributed to the development of this work. Conceptualization: T.T.N. and T.T.H.P.; data curation: T.D.N and V.K.N.; formal analysis: T.T.N. and T.T.P.; investigation: T.H.N. and N.A.P.; methodology: T.T.N. and T.T.H.P.; project administration: T.T.H.P.; software: T.D.N. and N.A.P.; writing – original draft: T.T.N. and T.H.N.; writing – review and editing: V.K.N. and T.T.P.
Funding
This research was funded by Ministry of Science and Technology of Vietnam (Grant No. ĐTĐL.CN.07/21)
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bangar, S. P; Whiteside, W. S.; Ashogbon, A. O.; Kumar, M. Recent advances in thermoplastic starches for food packaging: A review. Food Packag. and Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Ismail, S.; Mansor, N.; Majeed, Z.; Man, Z. Effect of Water and [Emim][OAc] as Plasticizer on Gelatinization of Starch. Procedia Eng 2016, 148, 524–529. [Google Scholar] [CrossRef]
- Nafchi, A. M. et al. Thermoplastic starches: Properties, challenges, and prospects. Starch – Starke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Tyagi, V.; Bhattacharya, B. Role of plasticizers in bioplastics. MOJ Food Process. Technol. 2019, 7, 128–130. [Google Scholar] [CrossRef]
- Kuakpetoon, D.; Wang, Y. J. Characterization of Different Starches Oxidized by Hypochlorite. Starch – Starke 2001, 53, 211–218. [Google Scholar] [CrossRef]
- Vanier, N. L.; El Halal, S. L. M.; Dias, A. R. G.; and Zavareze, E. R. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Zhao, G.; Wang, Y. Influence of oxidized starch on the properties of thermoplastic starch. Carbohydr. Polym. 2013, 96, 358–364. [Google Scholar] [CrossRef]
- Parvatha Reddy, P. Plant Protection in Tropical Root and Tuber Crops, Springer, 2015th edition, 281–291.
- Guilbert, S.; Cuq, B.; Contard, N. Recent Innovations in Edible and/or Biodegradable Packaging Materials. Food. Addit. Contam. 1997, 14, 741–751. [Google Scholar] [CrossRef]
- Andrade-Mahecha, M. M.; Tapia-Blacido, D. R.; Menegalli, F. C. Development and optimization of biodegradable films based on achira flour. Carbohydr. Polym. 2012, 88, 449–458. [Google Scholar] [CrossRef]
- Canales, Z. E. C.; Velazquez, G.; Gomez-Aldap, C. A.; Fonseca-Florido, H. Preparation and Characterization of Thermoplastics Achira (Canna indica L.) Starch by Three Succination Methods. Starch – Starke 2021, 74, 2100040. [Google Scholar] [CrossRef]
- Caicedo, C.; Garcia, A. F. Rheological, Thermal, Superficial, and Morphological Properties of Thermoplastic Achira Starch Modified with Lactic Acid and Oleic Acid. Molecules 2019, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hung, P. V.; Morita, N. Physicochemical properties and enzymatic digestibility of starch from edible canna (Canna edulis) grown in Vietnam. Carbohydr. Polym. 2005, 61, 314–321. [Google Scholar] [CrossRef]
- Tung, N. T.; Khoi, N. V.; Huy, N. Q.; Hung, N. K.; Hoang, T. Characterization of tapioca starch oxidized by sodium hypochlorite. Vietnam J. Chem. 2009, 47, 389–392. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Ding, W.; Zhou, J.; Shi, B. Preparation of highly-oxidized starch using hydrogen peroxide and its application as a novel ligand for zirconium tanning of leather. Carbohydr. Polym. 2017, 174, 823–829. [Google Scholar] [CrossRef]
- Termvejsayanona, N.; Sangseethong, K.; Sriroth, K. Characterization of physicochemical properties of hypochlorite and peroxide-oxidized cassava starches. Carbohydr. Polym. 2010, 82, 446–53. [Google Scholar] [CrossRef]
- García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C. G.; Castaño, J.; Guadarrama-Lezama, A. Y.; Rodríguez Llamazares, S. Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides 2022, 3, 136–177. [Google Scholar] [CrossRef]
- Gonçalves, J. R.; Fonseca, L. M.; El Halal, S. L.; Pinto, V. Z.; Dias, A. R. G.; Jacques, A. C.; Zavareze, E. R. Oxidation of potato starch with different sodium hypochlorite concentrations and its effect on biodegradable films. LWT - Food Sci. Technol. 2015, 60, 1–7. [Google Scholar] [CrossRef]
- Sánchez-Rivera, M.M.; García-Suárez, F.J.L.; Velázquez del Valle, M.; Gutierrez-Meraz, F.; Bello-Pérez, L.A. Partial characterization of banana starches oxidized by different levels of sodium hypochlorite. Carbohydr. Polym. 2005, 62, 50–56. [Google Scholar] [CrossRef]
- Kahar, A. W. M.; Ismail, H.; Othman, N. Morphology and Tensile Properties of High-Density Polyethylene/Natural Rubber/Thermoplastic Tapioca Starch Blends: The Effect of Citric Acid-Modified Tapioca Starch. J. Appl. Polym. Sci. 2012, 125, 768–775. [Google Scholar] [CrossRef]
- Altayan, M. M.; Al Darouich, T.; Karabet, F. Thermoplastic starch from corn and wheat: a comparative study based on amylose content. Polym. Bull. 2021, 78, 3131–3147. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yua, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Zuo, Y.; Gu, J.; Tan, H.; Zhang, Y. Thermoplastic starch prepared with different plasticizers: relation between degree of plasticization and properties. J Wuhan Univ Technol-Mater Sci 2015, 30, 423–428. [Google Scholar] [CrossRef]
- García, N. L.; Famá, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. A comparison between the physico-chemical properties of tuber and cereal starches. Food Res. Int. 2009, 42, 976–982. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).