Submitted:
25 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Resonance-like Responses to Low-Frequency Magnetic Fields and Biophysical Models
2.1. Ions as Primary Targets
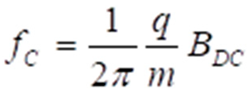
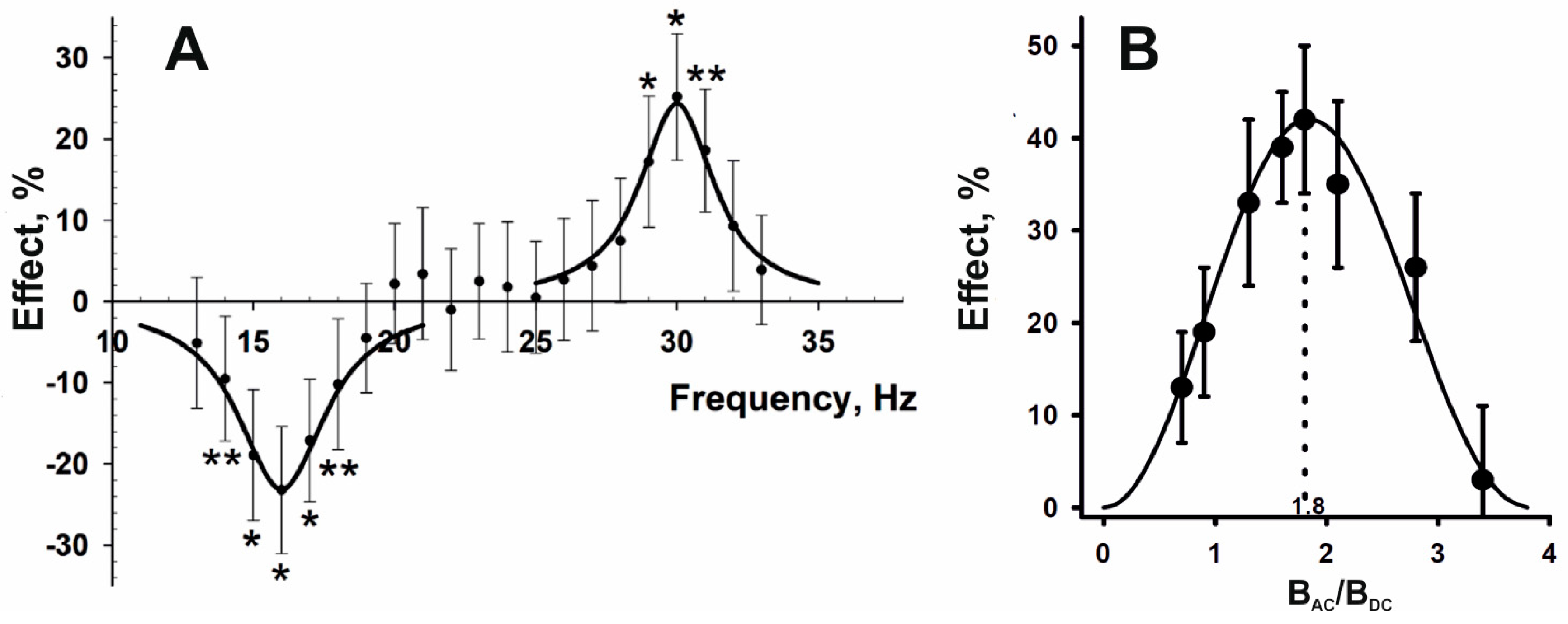
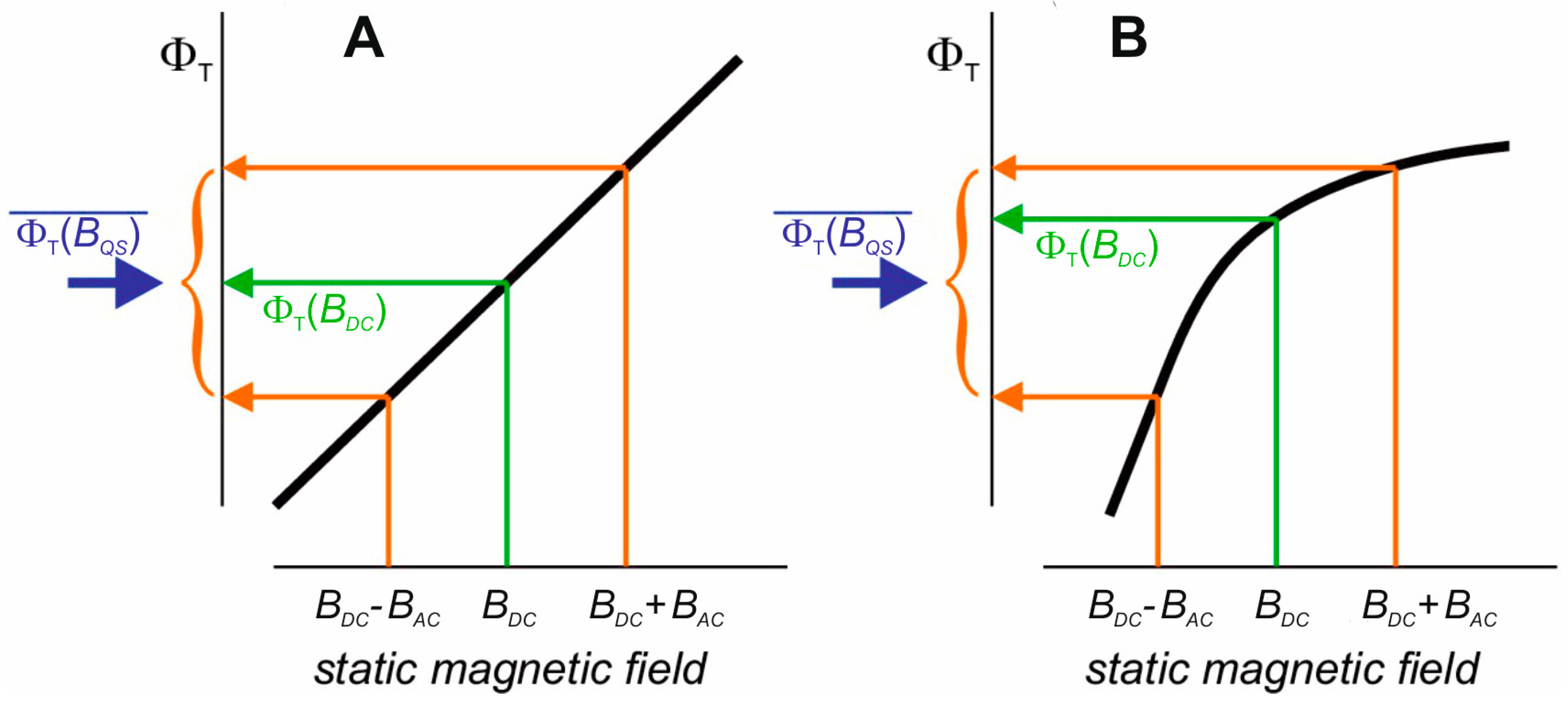
2.2. Magnetic Moments as Primary Targets
3. Radical Pair Magnetoreception and Its Application to the Effects of Low-Frequency Magnetic Fields
4. Some Inconsistencies
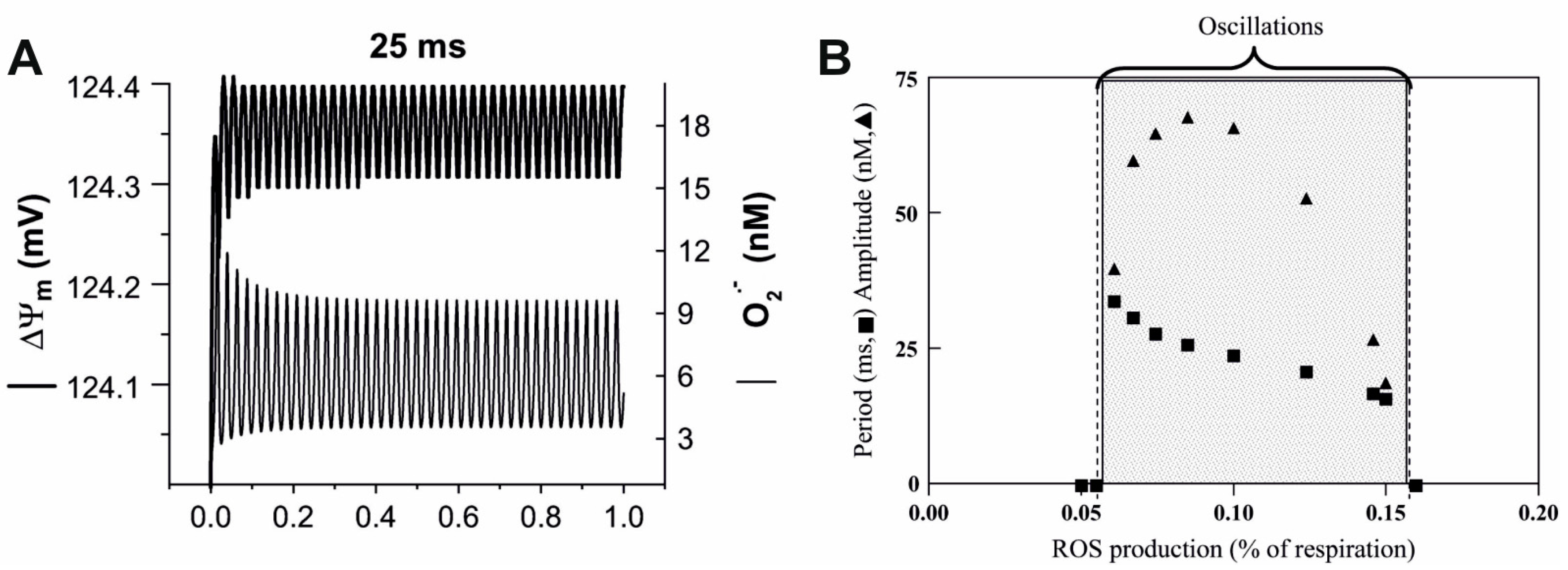
5. Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Akasofu, S. I.; Chapman, S. Solar-terrestrial Physics; Clarendon Press: Oxford, UK, 1972. [Google Scholar]
- Sarimov, R.; Binhi, V. Low-frequency magnetic fields in cars and office premises and the geomagnetic field variations. Bioelectromagnetics 2020, 41, 360–368. [Google Scholar] [CrossRef]
- Binhi, V.N.; Rubin, A.B. Magnetobiology: The kT paradox and possible solutions. Electromagn. Biol. Med. 2007, 26, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N. Magnetobiology: Underlying Physical Problems; Academic Press: London, UK, 2002. [Google Scholar]
- Blackman, C.F.; Benane, S.G.; Rabinowitz, J.R.; House, D.E.; Joines, W.T. A role for the magnetic field in the radiation-induced efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics 1985, 6, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Schrot, J.; Liboff, A.R. Low-intensity magnetic fields alter operant behavior in rats. Bioelectromagnetics 1986, 7, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Liboff, A.R.; Rozek, R.J.; Sherman, M.L.; McLeod, B.R.; Smith, S.D. Ca2+-45 cyclotron resonance in human lymphocytes. J. Bioelectricity 1987, 6, 13–22. [Google Scholar]
- Smith, S.D.; McLeod, B.R.; Liboff, A.R.; Cooksey, K. Calcium cyclotron resonance and diatom mobility. Bioelectromagnetics 1987, 8, 215–227. [Google Scholar] [CrossRef]
- Liboff, A.R. Geomagnetic cyclotron resonance in living cells. J. Biol. Phys. 1985, 13, 99–102. [Google Scholar] [CrossRef]
- Sandweiss, J. On the cyclotron resonance model of ion transport. Bioelectromagnetics 1990, 11, 203–205. [Google Scholar] [CrossRef]
- Halle, B. On the cyclotron resonance mechanism for magnetic field effects on transmembrane ion conductivity. Bioelectromagnetics 1988, 9, 381–385. [Google Scholar] [CrossRef]
- Lednev, V.V.; Srebnitskaya, L.K.; Il’Yasova, Y.N.; Rozhdestvenskaya, S.Y.; Klimov, A.A.; Belova, N.A.; Tiras, K.P. Magnetic parametric resonance in biosystems: Experimental verification of the theoretical predictions with the use of regenerating planarians Dugesia tigrina as a test-system. Biophysics 1996, 41, 815–825. [Google Scholar]
- Prato, F.S.; Carson, J.J.L.; Ossenkopp, K.P.; Kavaliers, M. Possible mechanisms by which extremely low frequency magnetic fields affect opioid function. FASEB J. 1995, 9, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E. Empirical test of an ion parametric resonance model for magnetic field interactions with PC-12 cells. Bioelectromagnetics 1994, 15, 239–260. [Google Scholar] [CrossRef]
- Baureus Koch, C.L.M.; Sommarin, M.; Persson, B.R.R.; Salford, L.G.; Eberhardt, J.L. Interaction between weak low frequency magnetic fields and cell membranes. Bioelectromagnetics 2003, 24, 395–402. [Google Scholar] [CrossRef]
- Sarimov, R.; Markova, E.; Johansson, F.; Jenssen, D.; Belyaev, I. Exposure to ELF magnetic field tuned to Zn inhibit growth of cancer cells. Bioelectromagnetics 2005, 26, 631–638. [Google Scholar] [CrossRef]
- Belova, N.A.; Potselueva, M.M.; Srebnitskaya, L.K.; Znobishcheva, A.V.; Lednev, V.V. The influence of weak magnetic fields on the production of the reactive oxygen species in peritoneal neutrophils of mice. Biophysics 2010, 55, 586–591. [Google Scholar] [CrossRef]
- Kantserova, N.P.; Ushakova, N.V.; Krylov, V.V.; Lysenko, L.A.; Nemova, N.N. Modulation of Ca2+-dependent protease activity in fish and invertebrates by weak low-frequency magnetic fields. Russ. J. Bioorg. Chem. 2013, 39, 373–377. [Google Scholar] [CrossRef]
- Kuz’mina, V.V.; Ushakova, N.V.; Krylov, V.V. The effect of magnetic fields on the activity of proteinases and glycosidases in the intestine of the crucian carp Carassius carassius. Biol. Bull. 2015, 42, 61–66. [Google Scholar] [CrossRef]
- Tiras, H.P.; Petrova, O.N.; Myakisheva, S.N.; Popova, S.S.; Aslanidi, K.B. Effects of weak magnetic fields on different phases of planarian regeneration. Biophysics 2015, 60, 126–130. [Google Scholar] [CrossRef]
- Khokhlova, G.; Abashina, T.; Belova, N.; Panchelyuga, V.; Petrov, A.; Abreu, F.; Vainshtein, M. Effects of combined magnetic fields on bacteria Rhodospirillum rubrum VKM B-1621. Bioelectromagnetics 2018, 39, 485–490. [Google Scholar] [CrossRef]
- Krylov, V.V.; Papchenkova, G.A.; Golovanova, I.L. Influence of calcium resonance-tuned low-frequency magnetic fields on Daphnia magna. Int. J. Mol. Sci. 2022, 23, 15727. [Google Scholar] [CrossRef]
- Prato, F.S.; Kavaliers, M.; Thomas, A.W. Extremely low frequency magnetic fields can either increase or decrease analgaesia in the land snail depending on field and light conditions. Bioelectromagnetics 2000, 21, 287–301. [Google Scholar] [CrossRef]
- Ermakov, A.; Afanasyeva, V.; Ermakova, O.; Blagodatski, A.; Popov, A. Effect of weak alternating magnetic fields on planarian regeneration. Biochem. Biophys. Res. Commun. 2022, 592, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.P.; Blackman, C.F. Clarification and application of an ion parametric resonance model for magnetic field interactions with biological systems. Bioelectromagnetics 1994, 15, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Lednev, V.V. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics 1991, 12, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Lednev, V.V. Bioeffects of weak static and alternating magnetic fields. Biofizika 1996, 41, 224–232. [Google Scholar] [PubMed]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E. The ion parametric resonance model predicts magnetic field parameters that affect nerve cells. FASEB J 1995, 9, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Trillo, M.A.; Ubeda, A.; Blanchard, J.P.; House, D.E.; Blackman, C.F. Magnetic fields at resonant conditions for the hydrogen ion affect neurite outgrowth in PC-12 cells: A test of the ion parametric resonance model. Bioelectromagnetics 1996, 17, 10–20. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. A physical mechanism of magnetoreception: Extension and analysis. Bioelectromagnetics 2017, 38, 41–52. [Google Scholar] [CrossRef]
- Ponomarev, V.O.; Novikov, V.V. Effect of low-frequency alternating magnetic fields on the rate of biochemical reactions proceeding with formation of reactive oxygen species. Biophysics 2009, 54, 163–168. [Google Scholar] [CrossRef]
- Lednev, V.V. Biological Effects of the Extremely Weak Alternating Magnetic Fields: The Identification of Primary Targets. In Modelling of Geophysical Processes; Sidorin, A., Ed.; Schmidt Institute of the Physics of the Earth: Moscow, Russia, 2003; pp. 130–136. [Google Scholar]
- Belova, N.A.; Panchelyuga, V.A. Lednev’s model: Theory and experiment. Biophysics 2010, 55, 661–674. [Google Scholar] [CrossRef]
- Belova, N.A.; Ermakov, A.M.; Znobishcheva, A.V.; Srebnitskaya, L.K.; Lednev, V.V. The influence of extremely weak alternating magnetic fields on the regeneration of planarians and the gravitropic response of plants. Biophysics 2010, 55, 623–627. [Google Scholar] [CrossRef]
- Belova, N.A.; Ermakova, O.N.; Ermakov, A.M.; Rojdestvenskaya, Z.Ye.; Lednev, V.V. The bioeffects of extremely weak power-frequency alternating magnetic fields. Environmentalist 2007, 27, 411–416. [Google Scholar] [CrossRef]
- Lednev, V.V.; Belova, N.A.; Ermakov, A.M.; Akimov, E.B.; Tonevitsky, A.G. Modulation of cardiac rhythm in the humans exposed to extremely weak alternating magnetic fields. Biophysics 2008, 53, 648–654. [Google Scholar] [CrossRef]
- Binhi, V.N. Primary physical mechanism of the biological effects of weak magnetic fields. Biophysics 2016, 61, 170–176. [Google Scholar] [CrossRef]
- Binhi, V.N. Do naturally occurring magnetic nanoparticles in the human body mediate increased risk of childhood leukaemia with EMF exposure? Int. J. Radiat. Biol. 2008, 84, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Savin, A.V. Molecular gyroscopes and biological effects of weak extremely low-frequency magnetic fields. Phys. Rev. E Stat. Nonlin. Soft. Matter. Phys. 2002, 65, 051912. [Google Scholar] [CrossRef] [PubMed]
- McLeod, B.R.; Smith, S.D.; Liboff, A.R. Calcium and potassium cyclotron resonance curves and harmonics in diatoms (A. coffeaeformis) J. Bioelectricity 1987, 6, 153–168. [Google Scholar] [CrossRef]
- Haiech, J.; Klee, C.B.; Demaille, J.G. Effects of cations on affinity of calmodulin for calcium: Ordered binding of calcium ions allows the specific activation of calmodulin-stimulated enzymes. Biochemistry 1981, 20, 3890–3897. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Disorientation of inexperienced young pigeons after transportation in total darkness. Nature 1981, 291, 433–434. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetic compass orientation in birds and its physiological basis. Naturwissenschaften 2002, 89, 445–452. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. The effect of yellow and blue light on magnetic compass orientation in European robins Erithacus rubecula. J. Comp. Physiol. A 1999, 184, 295–299. [Google Scholar] [CrossRef]
- Muheim, R.; Backman, J.; Akesson, S. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 2002, 205, 3845–3856. [Google Scholar] [CrossRef] [PubMed]
- Ritz, T.; Adem, S.; Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Buchachenko, A.L.; Sagdeev, R.Z.; Salikhov, K.M. Magnetic and Spin Effects in Chemical Reactions; Nauka: Novosibirsk, 1978. [Google Scholar]
- Buchachenko, A.L.; Molin, Y.N.; Sagdeev, R.Z.; Salikhov, K.M.; Frankevich, E.L. Magneto-spin effects in chemical reactions. Sov. Phys. Usp. 1987, 30, 79–80. [Google Scholar] [CrossRef]
- Cashmore, A.; Jarillo, J.; Wu, Y-J. ; Liu, D. Cryptochromes: blue light receptors for plants and animals. Science 1999, 284, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Brautigam, C.A.; Smith, B.S.; Ma, Z.; Palnitkar, M.; Tomchick, D.R.; Machius, M.; Deisenhofer, J. Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. PNAS 2004, 101, 12142–12147. [Google Scholar] [CrossRef]
- Sancar, A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 2003, 103, 2203–2237. [Google Scholar] [CrossRef]
- Maeda, K.; Robinson, A.J.; Henbest, K.B.; Hogben, H.J.; Biskup, T.; Ahmad, M.; Schleicher, E.; Weber, S.; Timmel, C.R.; Hore, P.J. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. PNAS 2012, 109, 4774–4779. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Solov'yov, I.A.; Chandler, D.E.; Schulten, K. Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophys. J. 2007, 92, 2711–2726. [Google Scholar] [CrossRef]
- Ahmad, M.; Galland, P.; Ritz, T.; Wiltschko, R.; Wiltschko, W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 2006, 225, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.M.; Li, J.; Henbest, K.B.; Neil, S.R.; Maeda, K.; Storey, J.; Schleicher, E.; Biskup, T.; Rodriguez, R.; Weber, S.; et al. Millitesla magnetic field effects on the photocycle of an animal cryptochrome. Sci. Rep. 2017, 7, 42228. [Google Scholar] [CrossRef]
- Bradlaugh, A.A.; Fedele, G.; Munro, A.L.; Hansen, C.N.; Hares, J.M.; Patel, S.; Kyriacou, C.P.; Jones, A.R.; Rosato, E.; Baines, R.A. Essential elements of radical pair magnetosensitivity in Drosophila. Nature 2023, 615, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Dejean, V.; Sowood, D.J.C.; et al. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Marley, R.; Giachello, C.N.G.; Scrutton, N.S.; Baines, R.A.; Jones, A.R. Cryptochrome-dependent magnetic field effect on seizure response in Drosophila larvae. Sci. Rep. 2014, 4, 5799. [Google Scholar] [CrossRef] [PubMed]
- Pooam, M.; Arthaut, L.D.; Burdick, D.; Link, J.; Martino, C.F.; Ahmad, M. Magnetic sensitivity mediated by the arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 2018, 249, 319–332. [Google Scholar] [CrossRef]
- Hammad, M.; Albaqami, M.; Pooam, M.; Kernevez, E.; Witczak, J.; Ritz, T.; Martino, C.; Ahmad, M. Cryptochrome mediated magnetic sensitivity in arabidopsis occurs independently of light-induced electron transfer to the flavin. Photochem. Photobiol. Sci. 2020, 19, 341–352. [Google Scholar] [CrossRef]
- Wan, G.; Hayden, A.N.; Iiams, S.E.; Merlin, C. Cryptochrome-1 mediates light-dependent inclination magnetosensing in monarch butterflies. Nat. Commun. 2021, 12, 771. [Google Scholar] [CrossRef]
- Gegear, R.J.; Casselman, A.; Waddell, S.; Reppert, S.M. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 2008, 454, 1014–1018. [Google Scholar] [CrossRef]
- Foley, L.E.; Gegear, R.J.; Reppert, S.M. Human cryptochrome exhibits light-dependent magnetosensitivity. Nat. Commun. 2011, 2, 356. [Google Scholar] [CrossRef]
- Kerpal, C.; Richert, S.; Storey, J.G.; Pillai, S.; Liddell, P.A.; Gust, D.; Mackenzie, S.R.; Hore, P.J.; Timmel, C.R. Chemical compass behaviour at microtesla magnetic fields strengthens the radical pair hypothesis of avian magnetoreception. Nat. Commun. 2019, 10, 3707. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.A.; Lau, J.C.; Hogben, H.J.; Biskup, T.; Kattnig, D.R.; Hore, P.J. Alternative radical pairs for cryptochrome-based magnetoreception. J. R. Soc. Interface 2014, 11, 20131063. [Google Scholar] [CrossRef]
- Müller, P.; Ahmad, M. Light-activated cryptochrome reacts with molecular oxygen to form a flavin–superoxide radical pair consistent with magnetoreception. J. Biol. Chem. 2011, 286, 21033–21040. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Schulten, K. Magnetoreception through cryptochrome may involve superoxide. Biophys. J. 2009, 96, 4804–4813. [Google Scholar] [CrossRef] [PubMed]
- Hogben, H.J.; Efimova, O.; Wagner-Rundell, N.; Timmel, C.R.; Hore, P. Possible involvement of superoxide and dioxygen with cryptochrome in avian magnetoreception: origin of Zeeman resonances observed by in vivo EPR spectroscopy. Chem. Phys. Lett. 2009, 480, 118–122. [Google Scholar] [CrossRef]
- Evans, E.W.; Kattnig, D.R.; Henbest, K.B.; Hore, P.J.; Mackenzie, S.R.; Timmel, C.R. Sub-millitesla magnetic field effects on the recombination reaction of flavin and ascorbic acid radicals. J. Chem. Phys. 2016, 145, 085101. [Google Scholar] [CrossRef]
- Moser, C.C.; Page, C.C.; Farid, R.; Dutton, P.L. Biological electron transfer. J. Bioenerg. Biomembr. 1995, 27, 263–274. [Google Scholar] [CrossRef]
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 2022, 19, 20220325. [Google Scholar] [CrossRef]
- McLendon, G.; Hake, R. Interprotein electron transfer. Chem. Rev. 1992, 92, 481–490. [Google Scholar] [CrossRef]
- Hore, P. Upper bound on the biological effects of 50/60 Hz magnetic fields mediated by radical pairs. eLife 2019, 8, e44179. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Mohtat, N.; Cozens, F.L.; McLean, J.; Thansandote, A. Application of the radical pair mechanism to free radicals in organized systems: Can the effects of 60 Hz be predicted from studies under static fields? Bioelectromagnetics 1994, 15, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Brocklehurst, B. Spin correlation in the geminate recombination of radical ions in hydrocarbons. Part 1. Theory of the magnetic field effect. J. Chem. Soc., Faraday Trans. 2 1976, 72, 1869–1884. [Google Scholar] [CrossRef]
- Eveson, R.W.; Timmel, C.R.; Brocklehurst, B.; Hore, P.J.; McLauchlan, K.A. The effects of weak magnetic fields on radical recombination reactions in micelles. Int. J. Radiat. Biol. 2000, 76, 1509–1522. [Google Scholar] [PubMed]
- Timmel, C.R.; Till, U.; Brocklehurst, B.; Mclauchlan, K.A.; Hore, P.J. Effects of weak magnetic fields on free radical recombination reactions. Mol. Phys. 1998, 95, 71–89. [Google Scholar] [CrossRef]
- Kattnig, D.R.; Evans, E.W.; Dejean, V.; Dodson, C.A.; Wallace, M.I.; Mackenzie, S.R.; Timmel, C.R.; Hore, P.J. Chemical amplification of magnetic field effects relevant to avian magnetoreception. Nat. Chem. 2016, 8, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.M.; Fay, T.P.; Manolopoulos, D.E.; Kerpal, C.; Richert, S.; Timmel, C.R. On the low magnetic field effect in radical pair reactions. J. Chem. Phys. 2018, 149, 034103. [Google Scholar] [CrossRef]
- Blackman, C.F.; Benane, S.G.; House, D.E. Frequency-dependent interference by magnetic fields of nerve growth factor-induced neurite outgrowth in PC-12 cells. Bioelectromagnetics 1995, 16, 387–395. [Google Scholar] [CrossRef]
- Blackman, C.F.; Benane, S.G.; House, D.E.; Elliott, D.J. Importance of alignment between local DC magnetic field and an oscillating magnetic field in responses of brain tissue in vitro and in vivo. Bioelectromagnetics 1990, 11, 159–167. [Google Scholar] [CrossRef]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E. Effect of ac and dc magnetic field orientation on nerve cells. Biochem. Biophys. Res. Commun. 1996, 220, 807–811. [Google Scholar] [CrossRef]
- García-Sancho, J.; Montero, M.; Alvarez, J.; Fonteriz, R.I.; Sanchez, A. Effects of extremely-law-frequency electromagnetic fields on ion transport in several mammalian cells. Bioelectromagnetics 1994, 15, 579–588. [Google Scholar] [CrossRef]
- Picazo, M.L.; Vallejo, D.; Bardasano, J.L. An Introduction to the study of ELF magnetic field effects on white blood cells in mice. Electro- Magnetobiol. 1994, 13, 77–84. [Google Scholar] [CrossRef]
- Qin, S.; Yin, H.; Yang, C.; Dou, Y.; Liu, Z.; Zhang, P.; Yu, H.; Huang, Y.; Feng, J.; Hao, J.; et al. A magnetic protein biocompass. Nature Mater. 2016, 15, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Belova, N.A.; Lednev, V.V. Dependence of gravitotropic reaction in segments of flax stems on frequency and amplitude of variable components of a weak combined magnetic field. Biofizika 2000, 45, 1108–1111. [Google Scholar]
- Cortassa, S.; Aon, M.A.; Winslow, R.L.; O'Rourke, B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys. J. 2004, 87, 2060–2073. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; O'Rourke, B. The fundamental organization of cardiac mitochondria as a network of coupled oscillators. Biophys. J. 2006, 91, 4317–4327. [Google Scholar] [CrossRef]
- Cortassa, S.; Aon, M.A.; Marbán, E.; Winslow, R.L.; O'Rourke, B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys. J. 2003, 84, 2734–2755. [Google Scholar] [CrossRef]
- Casani-Galdon, P.; Garcia-Ojalvo, J. Signaling oscillations: Molecular mechanisms and functional roles. Curr. Opin. Cell Biol. 2022, 78, 102130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



