Submitted:
25 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The role of EBUS-TBNA in the molecular profiling of lung cancer
3. Elastography
4. “Unusual” applications of EBUS
Pulmonary embolism
Pleural lesions
Pleural and pericardial effusion
Thyroid lesions
Intratumoral therapy
5. EUS-B Fine Needle Aspiration
6. Novel devices improving EBUS TBNA performance and future perspectives
7. Conclusion
Funding
Conflicts of Interest
References
- Schieppati, E. La puncion mediastinal a traves del espolon traqueal [Mediastinal puncture through the tracheal spur]. Rev As Med Argent 1949, 663, 497–499. [Google Scholar]
- Wang, K.P.; Terry, P.; Marsh, B. Bronchoscopic needle aspiration biopsy of paratracheal tumors. Am Rev Respir Dis 1978, 118, 17–21. [Google Scholar] [PubMed]
- Oho, K.; Kato, H.; Ogawa, I.; Hayashi, N.; Hayata, Y. A new needle for transfiberoptic bronchoscope use. Chest 1979, 76, 492. [Google Scholar] [CrossRef]
- Wang, K.P.; Terry, P. Transbronchial needle aspiration in the diagnosis and staging of bronchogenic carcinoma. Am Rev Respir Dis 1983, 127, 344–347. [Google Scholar]
- Yarmus, L.; Feller-Kopman, D.; Browning, R.; Wang, K. TBNA: should EBUS be used on all lymph node aspirations? J Bronchology Interv Pulmonol 2011, 18, 115–117. [Google Scholar] [CrossRef]
- Yasufuku, K.; Chiyo, M.; Sekine, Y.; Chhajed, P.N.; Shibuya, K.; Iizasa, T.; Fujisawa, T. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004, 126, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, H.; Garfield, D.H.; Teng, J.; Han, B.; Sun, J. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of non-lymph node thoracic lesions. Ann Thorac Med 2013, 8, 14–21. [Google Scholar]
- Annema, J.T.; van Meerbeeck, J.P.; Rintoul, R.C.; Dooms, C.; Deschepper, E.; Dekkers, O.M.; De Leyn, P.; Braun, J.; Carroll, N.R.; Praet, M.; et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010, 304, 2245–2252. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians' evidence-based clinical practice guidelines. Chest 2013, 143, e211S–e250S. [Google Scholar] [CrossRef]
- Vilmann, P.; Clementsen, P.F.; Colella, S.; Siemsen, M.; De Leyn, P.; Dumonceau, J.M.; Herth, F.J.; Larghi, A.; Vazquez-Sequeiros, E.; Hassan, C.; et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015, 47, 545–559. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.E.B.; Barnes, D.J.; Troy, L.K. Diagnosing Lung Cancer: The Complexities of Obtaining a Tissue Diagnosis in the Era of Minimally Invasive and Personalised Medicine. J Clin Med 2018, 7, 163. [Google Scholar] [CrossRef]
- Um, S.W.; Kim, H.K.; Jung, S.H.; Han, J.; Lee, K.J.; Park, H.Y.; Choi, Y.S.; Shim, Y.M.; Ahn, M.J.; Park, K.; et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015, 10, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Yasufuku, K.; Pierre, A.; Darling, G.; de Perrot, M.; Waddell, T.; Johnston, M.; da Cunha Santos, G.; Geddie, W.; Boerner, S.; Le, L.W; et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011, 142, 1393–1400.e1. [Google Scholar] [CrossRef]
- Ernst, A.; Anantham, D.; Eberhardt, R.; Krasnik, M.; Herth, F.J. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008, 3, 577–582. [Google Scholar] [CrossRef]
- Navani, N.; Brown, J.M.; Nankivell, M.; Woolhouse, I.; Harrison, R.N.; Jeebun, V.; Munavvar, M.; Ng, B.J.; Rassi, D.M.; Falzon, M; et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: A multicenter study of 774 patients. Am J Respir Crit Care Med 2012, 185, 1316–1322. [Google Scholar] [CrossRef]
- Rooper, L.M.; Nikolskaia, O.; Carter, J.; Ning, Y.; Lin, M.T.; Maleki, Z. A single EBUS-TBNA procedure can support a large panel of immunohistochemical stains, specific diagnostic subtyping, and multiple gene analyses in the majority of non-small cell lung cancer cases. Hum Pathol 2016, 51, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Esterbrook, G.; Anathhanam, S.; Plant, P.K. Adequacy of endobronchial ultrasound transbronchial needle aspiration samples in the subtyping of non-small cell lung cancer. Lung Cancer 2013, 80, 30–34. [Google Scholar] [CrossRef]
- Labarca, G.; Folch, E.; Jantz, M.; Mehta, H.J.; Majid, A.; Fernandez-Bussy, S. Adequacy of samples obtained by endobronchial ultrasound with transbronchial needle aspiration for molecular analysis in patients with non-small cell lung cancer: systematic review and meta-analysis. Ann Am Thorac Soc 2018, 15, 1205–1216. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, G.K.; Lee, H.S.; Kim, M.S.; Lee, J.M.; Kim, H.Y.; Nam, B.H.; Zo, J.I.; Hwangbo, B. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008, 134, 368–374. [Google Scholar] [CrossRef]
- Wahidi, M.M.; Herth, F.; Yasufuku, K.; Shepherd, R.W.; Yarmus, L.; Chawla, M.; Lamb, C.; Casey, K.R.; Patel, S.; Silvestri, G.A.; Feller-Kopman, D.J. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 816–835. [Google Scholar] [CrossRef]
- Yarmus, L.; Akulian, J.; Gilbert, C.; Feller-Kopman, D.; Lee, H.J.; Zarogoulidis, P.; Lechtzin, N.; Ali, S.Z.; Sathiyamoorthy, V. Optimizing endobronchial ultrasound for molecular analysis: how many passes are needed? Ann Am Thorac Soc 2013, 10, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Wang, H.; Zerbo, A.; Beaudoin, S.; Ofiara, L.; Fiset, P.O.; Benedetti, A.; Gonzalez, A.V. Programmed Death Ligand 1 Testing of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Samples Acquired For the Diagnosis and Staging of Non-Small Cell Lung Cancer. J Bronchology Interv Pulmonol 2020, 27, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Schuurmans, M.M.; Theron, J.; Louw, M.; Wright, C.A.; Brundyn, K.; Bolliger, C.T. Utility of rapid on-site evaluation of transbronchial needle aspirates. Respiration 2005, 72, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Trisolini, R.; Cancellieri, A.; Tinelli, C.; Paioli, D.; Scudeller, L.; Casadei, G.P.; Forti Parri, S.; Livi, V.; Bondi, A.; Boaron, M.; Patelli, M. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest 2011, 139, 395–401. [Google Scholar] [CrossRef]
- Yarmus, L.; Van der Kloot, T.; Lechtzin, N.; Napier, M.; Dressel, D.; Feller-Kopman, D. A randomized prospective trial of the utility of rapid on-site evaluation of transbronchial needle aspirate specimens. J Bronchol Int Pulmonol 2011, 18, 121–127. [Google Scholar] [CrossRef]
- Marcoux, M.; Ost, D.E. What's new in endobronchial ultrasound for mediastinal staging? Curr Opin Pulm Med 2020, 26, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Oki, M.; Saka, H.; Kitagawa, C.; Kogure, Y.; Murata, N.; Adachi, T.; Ando, M. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration 2013, 85, 486–492. [Google Scholar] [CrossRef]
- Trisolini, R.; Cancellieri, A.; Tinelli, C.; et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration with and without rapid on-site evaluation for lung cancer genotyping. Chest 2015, 148, 1430–1437. [Google Scholar] [CrossRef]
- Tsaknis, G.; Naeem, M.; Rathinam, S.; Caswell, A.; Haycock, J.; McKenna, J.; Reddy, R.V. Utilization of high-pressure suction for EBUS-TBNA sampling in suspected lung Cancer. J Bronchology Interv Pulmonol 2022, 29, 115–124. [Google Scholar] [CrossRef]
- Fernandez-Bussy, S.; Biswas, A.; Labarca, G.; et al. Comparison of endobronchial ultrasound-guided transbronchial needle aspiration with stylet retracted partially versus completely for molecular testing. J Bronchol Int Pulmonol 2019, 26, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, F.; Mao, X.; Zheng, X.; Li, Y.; Zhu, L.; Sun, J. Determining factors of endobronchial ultrasound-guided transbronchial needle aspiration specimens for lung cancer subtyping and molecular testing. Endosc Ultrasound 2019, 8, 404–411. [Google Scholar] [PubMed]
- Leighl, N.B.; Rekhtman, N.; Biermann, W.A.; Huang, J.; Mino-Kenudson, M.; Ramalingam, S.S.; West, H.; Whitlock, S.; Somerfield, M.R. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol 2014, 32, 3673–3679. [Google Scholar] [PubMed]
- Krogerus, L.; Kholov√°, I. Cell Block in Cytological Diagnostics: Review of Preparatory Techniques. Acta Cytol 2018, 62, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Santos, J.; Serra, P.; Andreo, F.; Llatjós, M.; Castellà, E.; Monsó, E. Contribution of cell blocks obtained through endobronchial ultrasound-guided transbronchial needle aspiration to the diagnosis of lung cancer. BMC Cancer 2012, 12, 34. [Google Scholar] [CrossRef]
- Martin-Deleon, R.; Teixido, C.; Lucena, C.M.; Martinez, D.; Fontana, A.; Reyes, R.; García, M.; Viñolas, N.; Vollmer, I.; Sanchez, M.; et al. EBUS-TBNA Cytological Samples for Comprehensive Molecular Testing in Non-Small Cell Lung Cancer. Cancers 2021, 13, 2084. [Google Scholar] [CrossRef]
- Jug, R.; Giovacchini, C.X.; Liu, B.; Green, C.L.; Clarke, J.M.; Mahmood, K.; Pavlisko, E.N. EBUS-FNA cytologic-histologic correlation of PD-L1 immunohistochemistry in non-small cell lung cancer. J Am Soc Cytopathol 2020, 9, 485–493. [Google Scholar] [CrossRef]
- Yoshimura, K.; Inoue, Y.; Karayama, M.; Tsuchiya, K.; Mori, K.; Suzuki, Y.; Iwashita, Y.; Kahyo, T.; Kawase, A.; Tanahashi, M.; et al. Heterogeneity analysis of PD-L1 expression and copy number status in EBUS-TBNA biopsy specimens of non-small cell lung cancer: Comparative assessment of primary and metastatic sites. Lung Cancer 2019, 134, 202–209. [Google Scholar] [CrossRef]
- Sakata, K.K.; Midthun, D.E.; Mullon, J.J.; Kern, R.M.; Nelson, D.R.; Edell, E.S.; Schiavo, D.N.; Jett, J.R.; Aubry, M.C. Comparison of Programmed Death Ligand-1 Immunohistochemical Staining Between Endobronchial Ultrasound Transbronchial Needle Aspiration and Resected Lung Cancer Specimens. Chest 2018, 154, 827–837. [Google Scholar] [CrossRef]
- 40 Ben Dori, S.; Aizic, A.; Sabo, E.; Hershkovitz, D. Spatial heterogeneity of PD-L1 expression and the risk for misclassification of PD-L1 immunohistochemistry in non-small cell lung cancer. Lung Cancer 2020, 147, 91–98. [Google Scholar] [CrossRef]
- Tajarernmuang, P.; Ofiara, L.; Beaudoin, S.; Wang, H.; Benedetti, A.; Gonzalez, A.V. Real-World Outcomes of Patients With Advanced Non-small Cell Lung Cancer Treated With Anti-PD1 Therapy on the Basis of PD-L1 Results in EBUS-TBNA vs Histological Specimens. Chest 2021, 160, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Chan, H.P.; Soon, Y.Y.; Huang, Y.; Soo, R.A.; Kee, A.C.L. A systematic review and meta-analysis of the adequacy of endobronchial ultrasound transbronchial needle aspiration for next-generation sequencing in patients with non-small cell lung cancer. Lung Cancer 2022, 166, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhuri, S.; Mehrotra, M.; Bolivar, A.M.; Kanagal-Shamanna, R.; Barkoh, B.A.; Hannigan, B.; Zalles, S.; Ye, W.; Duose, D.; Broaddus, R. Salvaging the supernatant: next generation cytopathology for solid tumor mutation profiling. Mod Pathol 2018, 31, 1036. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Tsukada, H.; Hwang, D.H.; Chambers, E.; Cibas, E.S.; Bale, T.; Supplee, J.; Ulrich, B.; Sholl, L.M. Liquid biopsy of fine-needle aspiration supernatant for lung cancer genotyping. Lung Cancer 2018, 122, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, B.; Ye, W.; Mehrotra, M.; Lam, V.; Bolivar, A.; Zalles, S.; Barkoh, B.A.; Duose, D.; Hu, P.C.; Broaddus, R. Liquid biopsy assay for lung carcinoma using centrifuged supernatants from fine-needle aspiration specimens. Ann Oncol 2019, 30, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, M.; Ost, D.E. What's new in endobronchial ultrasound for mediastinal staging? Curr Opin Pulm Med 2020, 26, 346–358. [Google Scholar] [CrossRef]
- Chaft, J.E.; Rimner, A.; Weder, W.; Azzoli, C.G.; Kris, M.G.; Cascone, T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol 2021, 18, 547–557. [Google Scholar] [CrossRef]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Shukla, N.; Hanna, N. Neoadjuvant and Adjuvant Immunotherapy in Early-Stage Non-Small Cell Lung Cancer. Lung Cancer 2021, 12, 51–60. [Google Scholar] [CrossRef]
- Tsuboi, M.; Weder, W.; Escriu, C.; Blakely, C.; He, J.; Dacic, S.; Yatabe, Y.; Zeng, L.; Walding, A.; Chaft, J.E. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol 2021, 17, 4045–4055. [Google Scholar] [CrossRef]
- Cao, C.; Guo, A.; Chen, C.; Chakos, A.; Bott, M.; Yang, C.J.; Zielinski, R.; Melfi, F. Systematic Review of Neoadjuvant Immunotherapy for Patients With Non-Small Cell Lung Cancer. Semin Thorac Cardiovasc Surg 2021, 33, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P. The challenges of evaluating predictive biomarkers using small biopsy tissue samples and liquid biopsies from non-small cell lung cancer patients. J Thorac Dis 2019, 11, S57–S64. [Google Scholar] [CrossRef]
- Hofman, P. EGFR Status Assessment for Better Care of Early Stage Non-Small Cell Lung Carcinoma: What Is Changing in the Daily Practice of Pathologists? Cells 2021, 10, 2157. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Bubendorf, L.; Cooper, W.A.; Illei, P.; Borralho Nunes, P.; Ong, B.H.; Tsao, M.S.; Yatabe, Y.; Kerr, K.M. Molecular testing in stage I-III non-small cell lung cancer: Approaches and challenges. Lung Cancer 2021, 162, 42–53. [Google Scholar] [CrossRef]
- Agrawal, S.; Goel, A.D.; Gupta, N.; Lohiya, A.; Gonuguntla, H.K. Diagnostic utility of endobronchial ultrasound (EBUS) features in differentiating malignant and benign lymph nodes-a systematic review and meta-analysis. Respir Med 2020, 171, 106097. [Google Scholar] [CrossRef] [PubMed]
- Izumo, T.; Sasada, S.; Chavez, C.; Matsumoto, Y.; Tsuchida, T. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014, 44, 956–962. [Google Scholar] [CrossRef]
- Fournier, C.; Dhalluin, X.; Wallyn, F.; Machuron, F.; Bouchindhomme, B.; Copin, M.C.; Valentin, V. Performance of Endobronchial Ultrasound Elastography in the Differentiation of Malignant and Benign Mediastinal Lymph Nodes: Results in Real-life Practice. J Bronchology Interv Pulmonol 2019, 26, 193–198. [Google Scholar] [CrossRef]
- Hern√°ndez Roca, M.; P√©rez Pallar√©s, J.; Prieto Merino, D.; Del Mar Valdivia Salas, M.; Solano, J.G.; Fern√°ndez √Ålvarez, J.; Lozano Vicente, D.; Wasniewski, S.; Mart√≠nez D√≠az, J.J.; Torregrosa, C.E.; et al. Diagnostic Value of Elastography and Endobronchial Ultrasound in the Study of Hilar and Mediastinal Lymph Nodes. J Bronchology Interv Pulmonol 2019, 26, 184–192. [Google Scholar] [CrossRef]
- Verhoeven, R.L.J.; de Korte, C.L.; van der Heijden, E. Optimal endobronchial ultrasound strain elastography assessment strategy: an explorative study. Respiration 2019, 97, 337–347. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nakajima, T.; Inage, T.; Sata, Y.; Sakairi, Y.; Tamura, H.; Wada, H.; Suzuki, H.; Chiyo, M.; Yoshino, I. The combination of endobronchial elastography and sonographic findings during endobronchial ultrasound-guided transbronchial needle aspiration for predicting nodal metastasis. Thorac Cancer 2019, 10, 2000–2005. [Google Scholar] [CrossRef]
- Trisolini, R.; Verhoeven, R.L.J.; Cancellieri, A.; De Silvestri, A.; Natali, F.; Van der Heijden, E. Role of endobronchial ultrasound strain elastography in the identification of fibrotic lymph nodes in sarcoidosis: a pilot study. Respirology 2020, 25, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Razavi, F.; Farahani, M.; Hashemi, M.; Emami, H.; Mohammadi, F.; Kiani, A. The utility of elastography during EBUS-TBNA in a population with a high prevalence of anthracosis. Clin Respir J 2020, 14, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Casoni, G.L.; Gurioli, C.; Romagnoli, M.; Poletti, V. Diagnosis of pulmonary thromboembolism with endobronchial ultrasound. Eur Respir J 2008, 32, 1416–1417. [Google Scholar] [CrossRef]
- Aumiller, J.; Herth, F.J.; Krasnik, M.; Eberhardt, R. Endobronchial ultrasound for detecting central pulmonary emboli: a pilot study. Respiration 2009, 77, 298–302. [Google Scholar] [CrossRef]
- Hunsaker, A.R.; Lu, M.T.; Goldhaber, S.Z.; Rybicki, F.J. Imaging in acute pulmonary embolism with special clinical scenarios. Circ Cardiovasc Imaging 2010, 3, 491–500. [Google Scholar] [CrossRef]
- Bertini, P.; Ribechini, A.; Guarracino, F. Improved diagnosis of pulmonary embolism causing cardiac arrest by combined endobronchial ultrasound and echocardiography. Cardiovasc Ultrasound 2020, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.; Corcoran, J.P.; Maldonado, F.; Feller-Kopman, D.; Janssen, J.; Astoul, P. Advanced medical interventions in pleural disease. Eur Respir Rev 2016, 25, 199–213. [Google Scholar] [CrossRef]
- Kassier, M.; et al. Sampling pleural nodules with an EBUS scope: A novel application. Respiratory Medicine Case Reports 2018, 25, 36–38. [Google Scholar] [CrossRef]
- Lococo, F.; Rossi, G.; Agostini, L.; Filice, A.; Paci, M.; Rapicetta, C. "Dry" pleural mesothelioma successfully diagnosed on endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA). Intern Med 2014, 53, 467–469. [Google Scholar] [CrossRef]
- DeWitt, J.; Kongkam, P.; Attasaranya, S.; LeBlanc, J.K.; Sherman, S.; Sheski, F.D. Endoscopic ultrasound-guided transesophageal thoracentesis. Endoscopy 2008, 40, E118–E119. [Google Scholar] [CrossRef]
- Cocciardi, S.; Borah, A.; Terrigno, R.; Abouzgheib, W.; Boujaoude, Z. A case report of an expensive yet necessary thoracentesis. Expanding the boundaries of endoscopic ultrasound transbronchial needle aspiration. Medicine (Baltimore) 2019, 98, e17555. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Khanna, A.; Talwar, D. Endobronchial ultrasound: A new technique of pericardiocentesis in posterior loculated pericardial effusion. Chest 2016, 150, e121–e123. [Google Scholar] [CrossRef] [PubMed]
- Ceron, L.; Manzato, M.; Mazzaro, F.; Bellavere, F. A new diagnostic and therapeutic approach to pericardial effusion: transbronchial needle aspiration. Chest 2003, 123, 1753–1758. [Google Scholar] [CrossRef]
- Madan, K.; Mittal, S.; Hadda, V.; Jain, D.; Mohan, A.; Guleria, R. Endobronchial ultrasound-guided transbronchial needle aspiration of thyroid: Report of two cases and systematic review of literature. Lung India 2016, 33, 682–687. [Google Scholar] [CrossRef]
- Kennedy, M.P.; Breen, M.; O'Regan, K.; McCarthy, J.; Horgan, M.; Henry, M.T. Endobronchial ultrasound-guided transbronchial needle aspiration of thyroid nodules: Pushing the boundary too far? Chest 2012, 142, 1690–1691. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Deng, Y.; Tin, M.S.; Lok, V.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; Wong, M.C.S. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest 2022, 161, 1101–1111. [Google Scholar] [CrossRef]
- DeMaio, A.; Sterman, D. Bronchoscopic intratumoural therapies for non-small cell lung cancer. Eur Respir Rev 2020, 29, 200028. [Google Scholar] [CrossRef]
- Celikoglu, F. Intratumoural chemotherapy of lung cancer for diagnosis and treatment of draining lymph node metastasis. J Pharm Pharmacol 2010, 62, 287–295. [Google Scholar] [CrossRef]
- Marabelle, A.; Tselikas, L.; de Baere, T.; et al. Intratumoural immunotherapy: using the tumour as the remedy. Ann Oncol 2017, 28, xii33–xii43. [Google Scholar] [CrossRef]
- Hohenforst-Schmidt, W.; Zarogoulidis, P.; Darwiche, K.; Vogl, T.; Goldberg, E.P.; Huang, H.; Simoff, M.; Li, Q.; Browning, R.; Turner, F.J.; Le Pivert, P.; et al. Intratumoural chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des Devel Ther 2013, 7, 571–583. [Google Scholar]
- Mori, V.; Roy, G.S.; Bates, J.H.T.; Kinsey, C.M. Cisplatin pharmacodynamics following endobronchial ultrasound-guided transbronchial needle injection into lung tumours. Sci Rep 2019, 9, 6819. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Anker, C.J.; Garrison, G.; Kinsey, C.M. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc 2015, 12, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Sun, L.; Yan, X.; Ai, Z.; Xu, J. Intratumoral chemotherapy with paclitaxel liposome combined with systemic chemotherapy: a new method of neoadjuvant chemotherapy for stage III unresectable non-small cell lung cancer. Med Oncol 2015, 32, 345. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; et al. Intratumoral Treatment with Chemotherapy and Immunotherapy for NSCLC with EBUS-TBNA 19G. J Cancer 2021, 12, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Mori, V.; Bates, J.H.T.; Jantz, M.; Mehta, H.J.; Kinsey, C.M. A computational modeling approach for dosing endoscopic intratumoral chemotherapy for advanced non-small cell lung cancer. Sci Rep 2022, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, B.; Lee, H.S.; Lee, G.K.; Lim, K.Y.; Lee, S.H.; Kim, H.Y.; Lee, J.; Zo, J.I. Transoesophageal needle aspiration using a convex probe ultrasonic bronchoscope. Respirology 2009, 14, 843–849. [Google Scholar] [CrossRef]
- Herth, F.J.; Krasnik, M.; Kahn, N.; Eberhardt, R.; Ernst, A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. 2010, 138, 790–794. [Google Scholar] [CrossRef]
- Oki, M.; Saka, H.; Ando, M.; Tsuboi, R.; Nakahata, M.; Oka, S.; Kogure, Y.; Kitagawa, C. Transbronchial vs transesophageal needle aspiration using an ultrasound bronchoscope for the diagnosis of mediastinal lesions: a randomized study. Chest 2015, 147, 1259–1266. [Google Scholar] [CrossRef]
- Chapman, G.S.; Kumar, D.; Redmond, J.; Munderloh, S.H.; Gandara, D.R. Upper abdominal computerized tomography scanning in staging non-small cell lung carcinoma. Cancer 1984, 54, 1541–1543. [Google Scholar] [CrossRef]
- Ettinghausen, S.E.; Burt, M.E. Prospective evaluation of unilateral adrenal masses in patients with operable non-small-cell lung cancer. J Clin Oncol 1991, 9, 1462–1466. [Google Scholar] [CrossRef]
- Christiansen, I.S.; Ahmad, K.; Bodtger, U.; Naur, T.M.H.; Sidhu, J.S.; Nessar, R.; Salih, G.N.; H√∏egholm, A.; Annema, J.T.; Clementsen, P.F. EUS-B for suspected left adrenal metastasis in lung cancer. J Thorac Dis 2020, 12, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Crombag, L.M.; Annema, J.T. Left Adrenal Gland Analysis in Lung Cancer Patients Using the Endobronchial Ultrasound Scope: A Feasibility Trial. Respiration 2016, 91, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, I.S.; Bodtger, U.; Naur, T.M.H.; Ahmad, K.; Singh Sidhu, J.; Nessar, R.; Salih, G.N.; H√∏egholm, A.; Annema, J.T.; Clementsen, P.F. EUS-B-FNA for Diagnosing Liver and Celiac Metastases in Lung Cancer Patients. Respiration 2019, 98, 428–433. [Google Scholar] [CrossRef] [PubMed]
- El, H.I.; Wu, H.; Reuss, S.; Randolph, M.; Harris, A.; Gromski, M.A.; Al-Haddad, M. Prospective Assessment of the Performance of a New Fine Needle Biopsy Device for EUS-Guided Sampling of Solid Lesions. Clin. Endosc 2018, 51, 576–583. [Google Scholar]
- Balwan, A.; Bixby, B.; Grotepas, C.; Witt, B.L.; Iravani, A.; Ansari, S.; Reddy, C.B. Core needle biopsy with endobronchial ultrasonography: single center experience with 100 cases. J Am Soc Cytopathol 2020, 9, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gu, Y.; Wang, H.; Yu, D.; Zhang, H.; Wang, H. Novel ProCore 25-gauge needle for endobronchial ultrasound-guided transbronchial needle aspiration reduces the puncture time and frequency, with comparable diagnostic rate for mediastinal and hilar lymphadenopathy. Thorac Cancer 2020, 11, 748–753. [Google Scholar] [CrossRef]
- Dhooria, S.; Sehgal, I.S.; Prasad, K.T.; Muthu, V.; Gupta, N.; Bal, A.; Ram, B.; Aggarwal, A.N.; Agarwal, R. Diagnostic yield and safety of the ProCore versus the standard EBUS-TBNA needle in subjects with suspected sarcoidosis. Expert Rev Med Devices 2021, 18, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ghori, U.; Chaddha, U.; Murgu, S. Combined EBUS-IFB and EBUS-TBNA vs EBUS-TBNA Alone for Intrathoracic Adenopathy: A Meta-Analysis. Ann Thorac Surg 2021. [Google Scholar] [CrossRef]
- Gonuguntla, H.K.; Shah, M.; Gupta, N.; Agrawal, S.; Poletti, V.; Nacheli, G.C. Endobronchial ultrasound-guided transbronchial cryo-nodal biopsy: a novel approach for mediastinal lymph node sampling. Respirol Case Rep 2021, 9, e00808. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.R.; Huang, Z.S.; Fu, W.L.; Wu, X.L.; Wu, N.; Kuebler, W.M.; Herth, F.J.F.; Fan, Y. Transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions: a randomised trial. Eur Respir J 2021, 58, 2100055. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Panchabhai, T.S.; Mehta, A.C. EBUS-TBNA for the diagnosis of lymphoma. Still an Achilles heel. Ann Am Thorac Soc 2015, 12, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliv√©, I.; Mons√≥, E.; Andreo, F.; Sanz-Santos, J.; Taron, M.; Molina-Vila, M.A.; Llatj√≥s, M.; Castell√†, E.; Moran, T.; Bertran-Alamillo, J.; et al. Endobronchial ultrasound-guided transbronchial needle aspiration for identifying EGFR mutations. Eur Respir J 2010, 35, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; Nikolskaia, O.; Carter, J.; Ning, Y.; Lin, M.; Maleki, Z. A single EBUS-TBNA procedure can support a large panel of immunohistochemical stains, specific diagnostic subtyping, and multiple gene analyses in the majority of non-small cell lung cancer cases. Hum Pathol 2016, 51, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, A.; Wu, X.; Huang, Z.; Kontogianni, K.; Sun, K.; Fu, W.; Wu, N.; Kuebler, W.M.; Herth, F.G.F. Transbronchial needle aspiration combined with cryobiopsy in the diagnosis of mediastinal diseases: a multicentre, open-label, randomised trial. Lancet Respir Med 2023, 11, 256–264. [Google Scholar] [CrossRef]
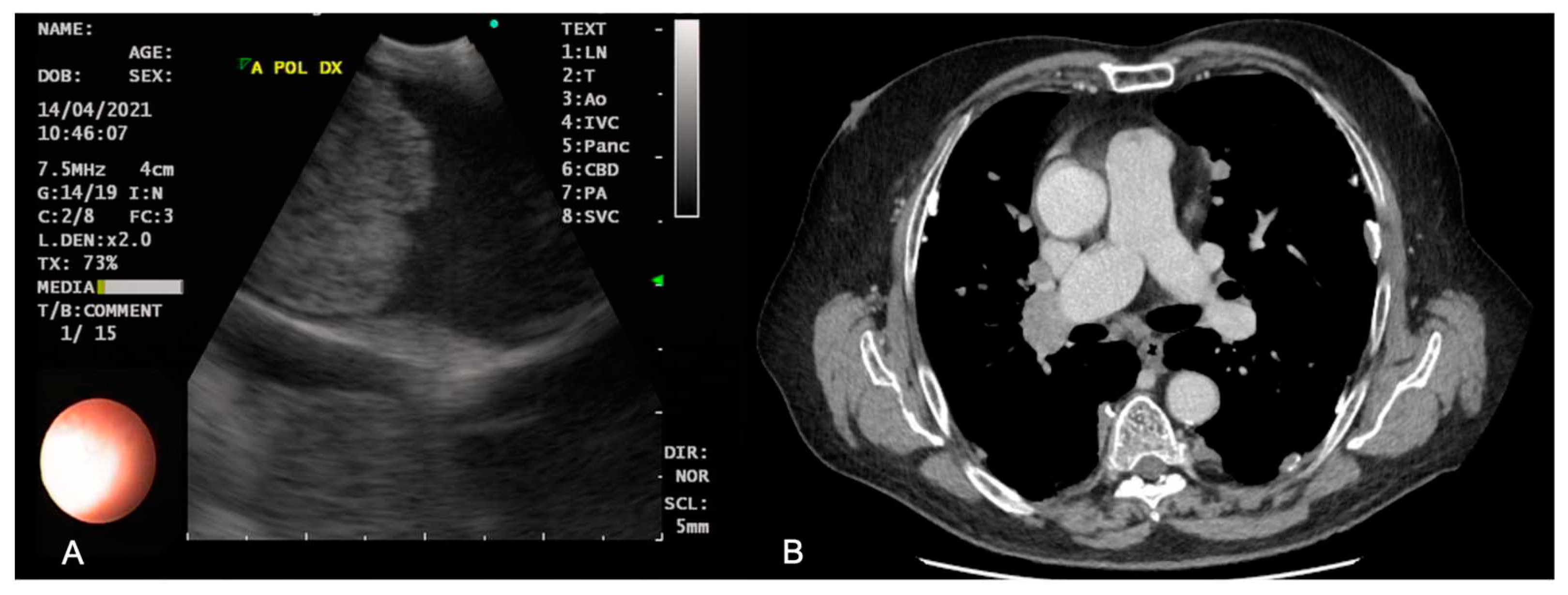
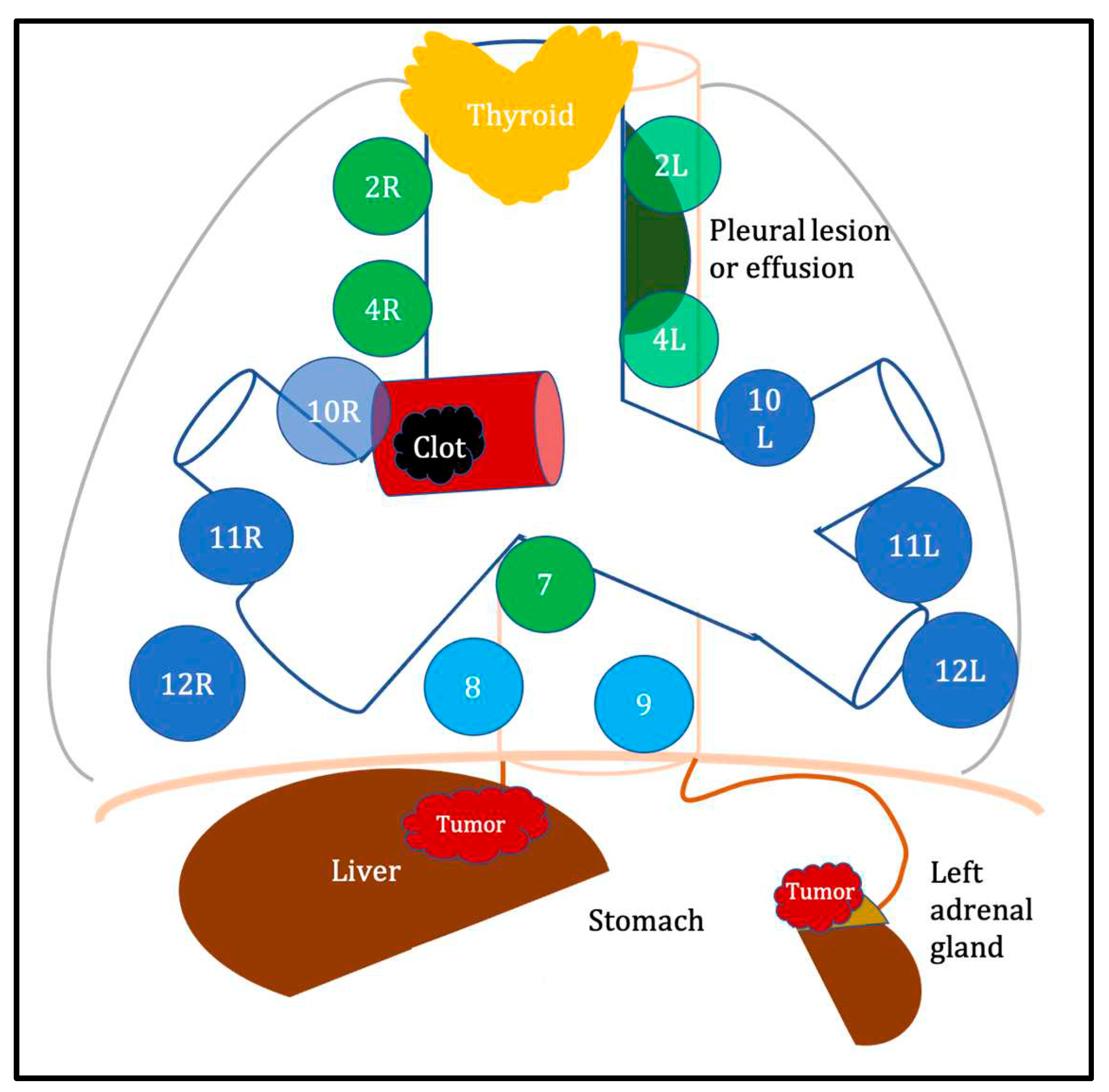
| Indication | Specific complementary tools | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Pulmonary vessel assessment | Color Doppler; echocardiography | Bedside feasibility; not contraindicated in kidney failure, pregnancy and allergy to contrast medium | Poor sensibility in peripheral pulmonary embolism | [63,64,65,66] |
| Pleural lesion sampling | - | Minimally invasive compared to surgery | Limited to lesions located next to airways or esophagus | [67,68] |
| Pleural/pericardial effusion drainage/sampling | - | Valuable option in loculated effusions | Potential infection | [70,71,72,73] |
| Thyroid lesion sampling | - | Valuable option in intrathoracic goiter/thyroid lesions | Potential infection | [74,75] |
| Intratumoral therapy | Computational modeling to design delivery strategy | Higher concentration at tumor site with lower systemic side effects | Risk of extravasation and airway irritation | [77,78,79,80,81,82,83,84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





