Submitted:
25 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
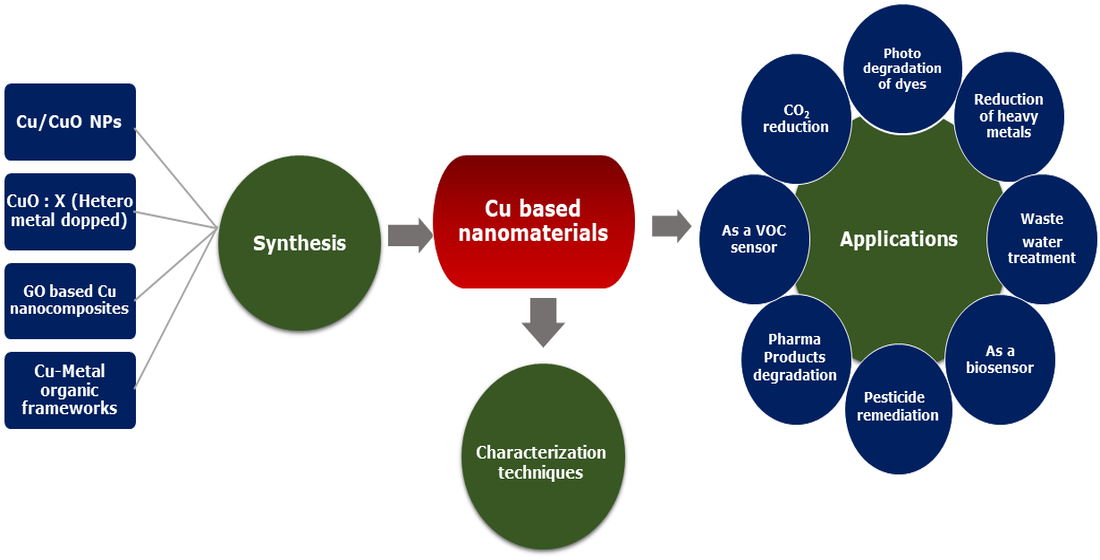
Keywords:
1. Introduction
2. Materials and Methodology
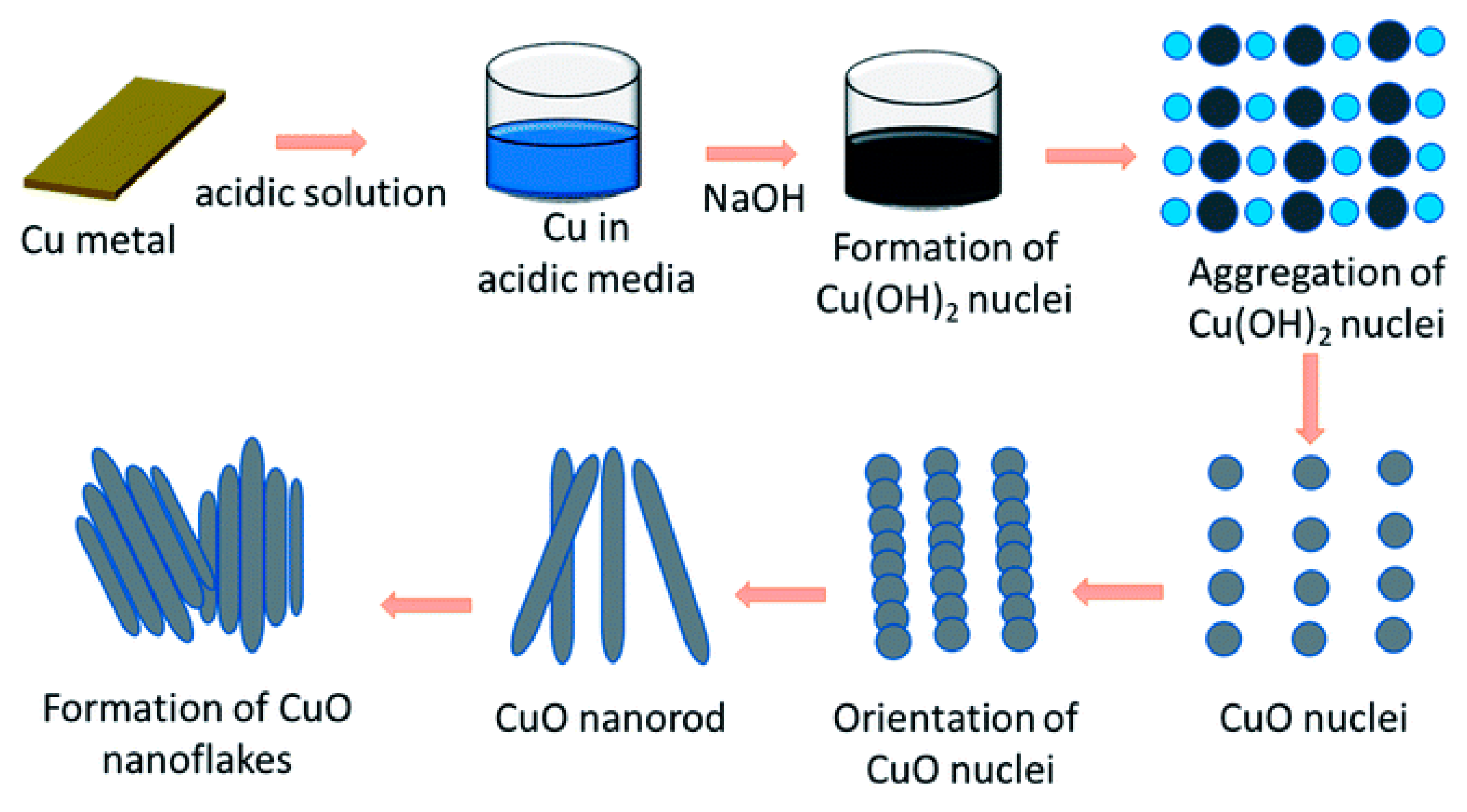
2.1. Synthesis of Cu Nanoparticles
2.2. Synthesis of Copper and Copper Oxide Nanoparticles
2.3. Synthesis of Heterometal Doped Copper-Based Nanocomposites
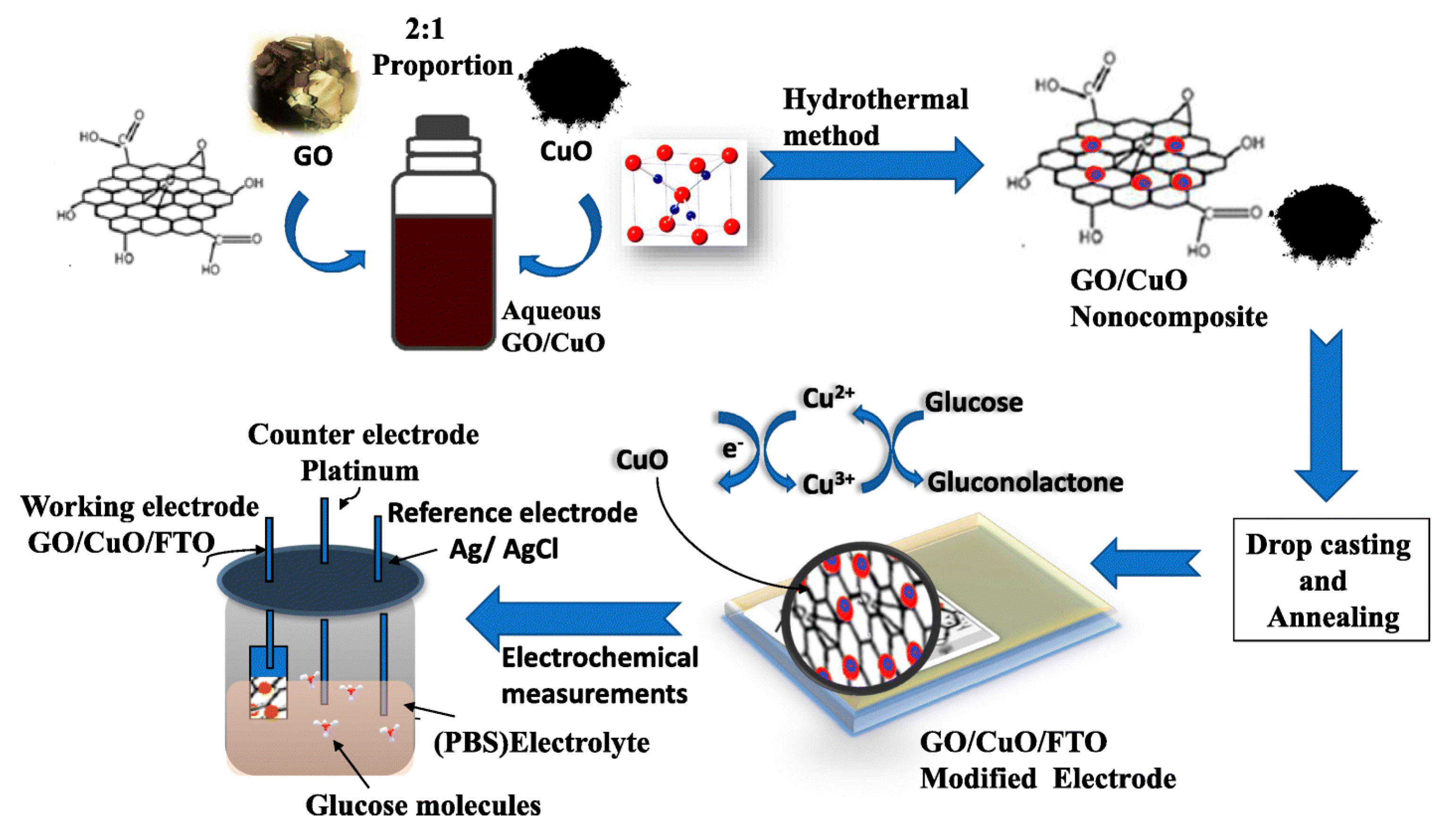
2.4. Synthesis of graphene-oxide (GO) based Copper nanocomposites
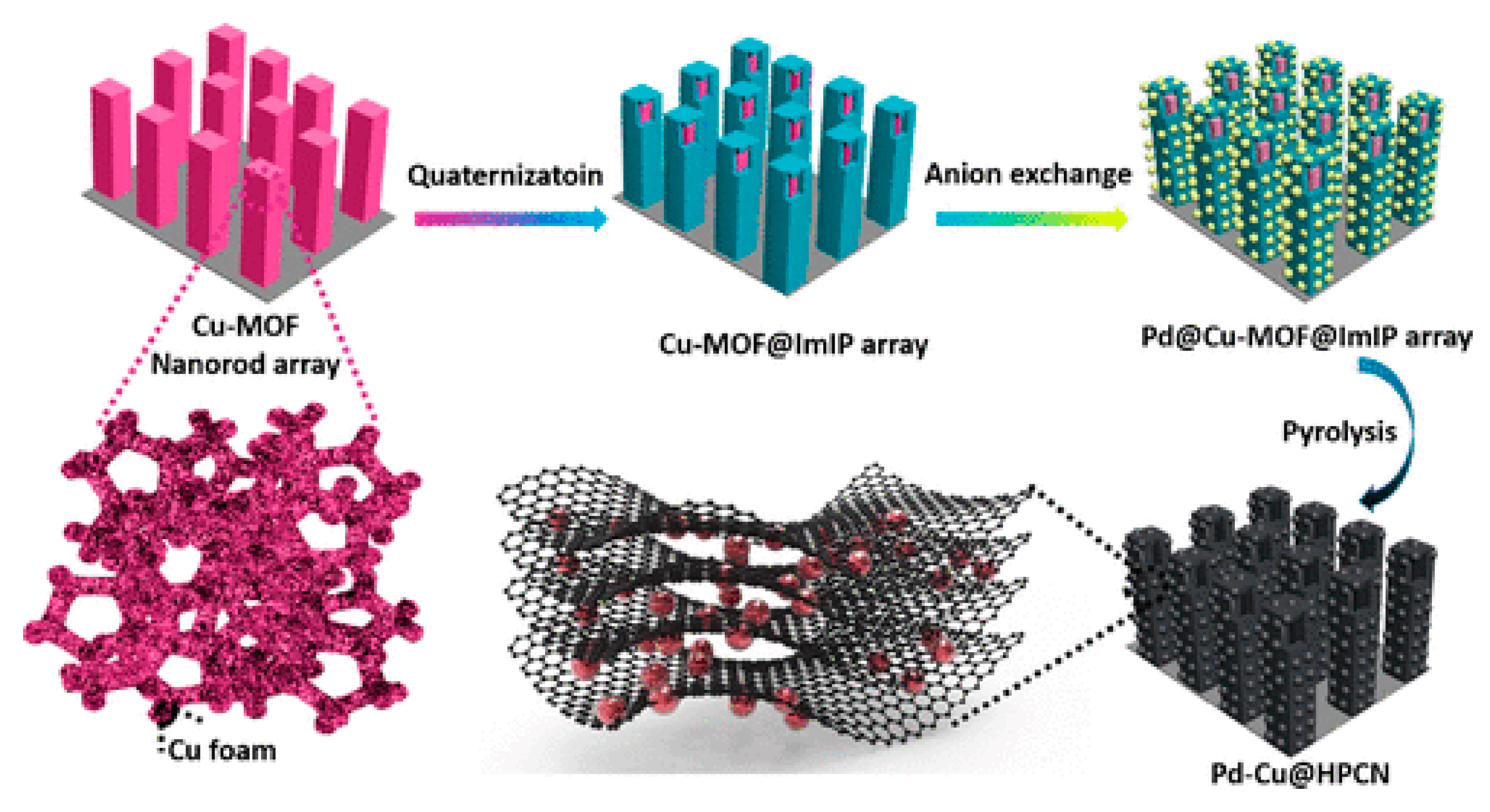
2.5. Synthesis of Copper-Based Organic and Metal-Organic Frameworks
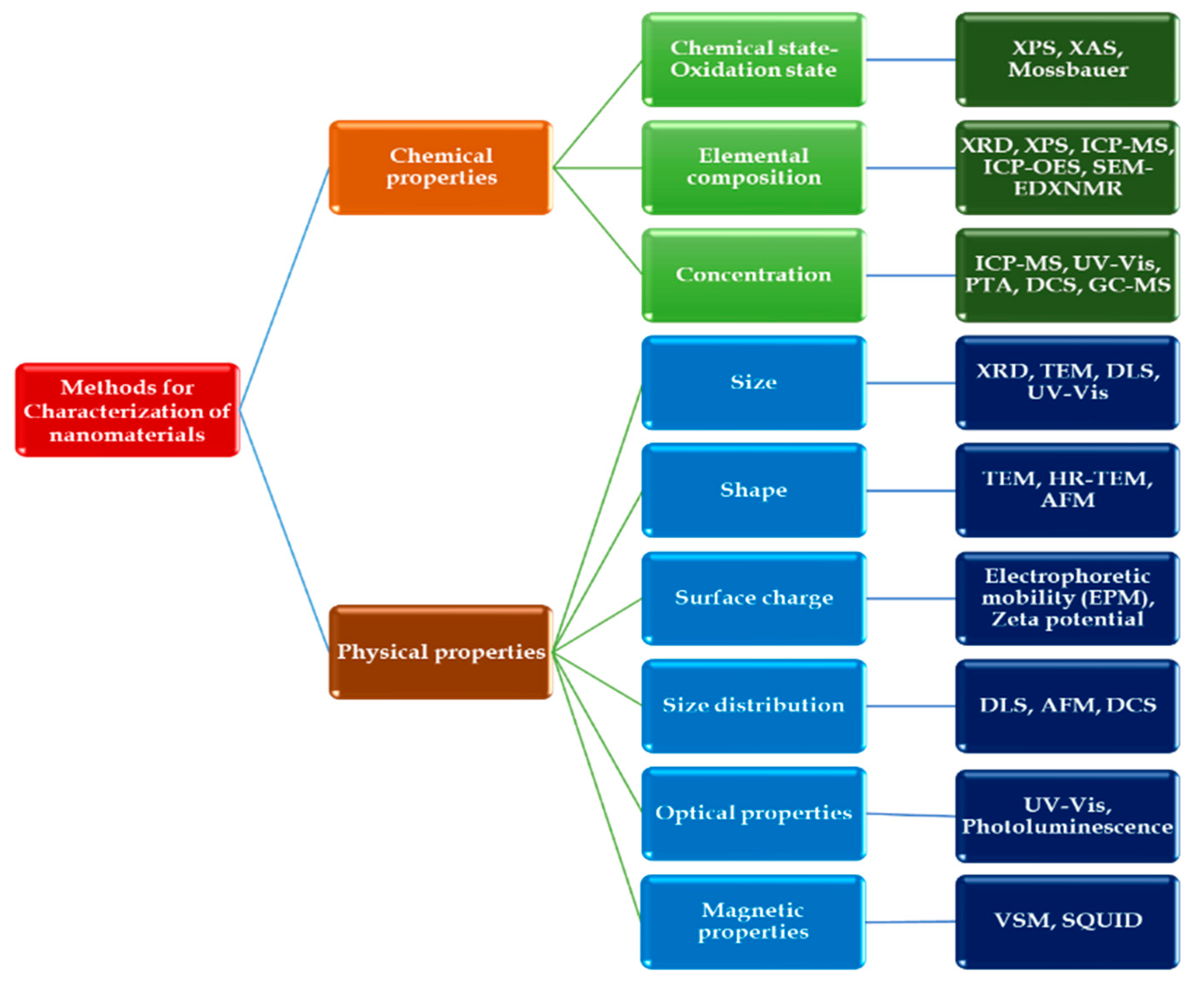
3. Characterization Methods for Copper-Based Nanomaterials
4. Copper-Based Nanomaterials for Environmental Pollution Management
4.1. Copper-Based Nanomaterials in Photodegradation of Industrial dyes/ Removal of Dyes
4.2. Copper in Reduction of other Heavy Metals Contamination
4.3. Copper-Based Nanomaterials in Wastewater Treatment
4.4. Copper-Based Materials as Biosensing Materials
4.5. Copper-Based Nanomaterials in Pesticides Remediation in Soil
4.6. Copper-Based Nanomaterials in the Degradation of Pharmaceutical Products
4.7. Copper-Based Nanomaterials as VOCs Sensor
4.8. Copper-Based Nanomaterials in Carbon Dioxide Reduction
5. Conclusions
Author Contributions
Funding
Ethics Approval and Consent to Participate
Data availability statement
Conflicts of Interest
Abbreviations
References
- Yang, C.; Bromma, K.; Sung, W.; Schuemann, J.; Chithrani, D. Determining the Radiation Enhancement Effects of Gold Nanoparticles in Cells in a Combined Treatment with Cisplatin and Radiation at Therapeutic Megavoltage Energies. Cancers 2018, 10, 150. [Google Scholar] [CrossRef]
- Fratoddi, I.; Cartoni, A.; Venditti, I.; Catone, D.; O'Keeffe, P.; Paladini, A.; Toschi, F.; Turchini, S.; Sciubba, F.; Testa, G.; et al. Gold nanoparticles functionalized by rhodamine B isothiocyanate: A new tool to control plasmonic effects. J. Colloid Interface Sci. 2018, 513, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Neuschmelting, V.; Harmsen, S.; Beziere, N.; Lockau, H.; Hsu, H.; Huang, R.; Razansky, D.; Ntziachristos, V.; Kircher, M.F. Dual-Modality Surface-Enhanced Resonance Raman Scattering and Multispectral Optoacoustic Tomography Nanoparticle Approach for Brain Tumor Delineation. Small 2018, 14, e1800740–e1800740. [Google Scholar] [CrossRef] [PubMed]
- Zaera, F. Nanostructured materials for applications in heterogeneous catalysis. Chem. Soc. Rev. 2012, 42, 2746–2762. [Google Scholar] [CrossRef]
- Nzilu, D.M.; Madivoli, E.S.; Makhanu, D.S.; Otenda, B.V.; Kareru, P.G.; Kairigo, P.K.; Tuhkanen, T. Environmental remediation using nanomaterial as adsorbents for emerging micropollutants. Environ. Nanotechnology, Monit. Manag. 2023, 20. [Google Scholar] [CrossRef]
- Khalaj, M.; Kamali, M.; Khodaparast, Z.; Jahanshahi, A. Copper-based nanomaterials for environmental decontamination – An overview on technical and toxicological aspects. Ecotoxicol. Environ. Saf. 2018, 148, 813–824. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2021, 12, 141. [Google Scholar] [CrossRef]
- Sandoval, S.S.; Silva, N. Review on Generation and Characterization of Copper Particles and Copper Composites Prepared by Mechanical Milling on a Lab-Scale. Int. J. Mol. Sci. 2023, 24, 7933. [Google Scholar] [CrossRef]
- Hong, X.; Zhu, H.; Du, D.; Zhang, Q.; Li, Y. Research Progress of Copper-Based Bimetallic Electrocatalytic Reduction of CO2. Catalysts 2023, 13, 376. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M.; Javed, R. Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Appl. Microbiol. Biotechnol. 2023, 107, 1039–1061. [Google Scholar] [CrossRef]
- Rubilar, O.; Rai, M.; Tortella, G.; Diez, M.C.; Seabra, A.B.; Durán, N. Biogenic nanoparticles: copper, copper oxides, copper sulphides, complex copper nanostructures and their applications. Biotechnol. Lett. 2013, 35, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lieber, C.M. Nanoelectronics from the bottom up. Nat. Mater. 2007, 6, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, D.; Eijkel, J.C.T.; Berg, A.v.D. Technologies for nanofluidic systems: top-down vs. bottom-up—a review. Lab a Chip 2005, 5, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Pothu, R.; Challa, P.; Rajesh, R.; Boddula, R.; Balaga, R.; Balla, P.; Perugopu, V.; Radwan, A.B.; Abdullah, A.M.; Al-Qahtani, N. Vapour-Phase Selective Hydrogenation of γ-Valerolactone to 2-Methyltetrahydrofuran Biofuel over Silica-Supported Copper Catalysts. Nanomaterials 2022, 12, 3414. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Hassan, K.; Sahajwalla, V. Utilising problematic waste to detect toxic gas release in the environment: fabricating a NiO doped CuO nanoflake based ammonia sensor from e-waste. Nanoscale Adv. 2022, 4, 4066–4079. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, X.; Liu, W.; Yang, S.; Huang, L.; Tang, S.; Zhang, Z.; Wang, Y.; Chen, F.; Qian, K. A Copper-Based Biosensor for Dual-Mode Glucose Detection. Front. Chem. 2022, 10, 861353. [Google Scholar] [CrossRef]
- Raul, P.K.; Das, B.; Umlong, I.M.; Devi, R.R.; Tiwari, G.; Kamboj, D.V. Toward a Feasible Solution for Removing Toxic Mercury and Chromium From Water Using Copper Oxide Nanoparticles. Front. Nanotechnol. 2022, 4. [Google Scholar] [CrossRef]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S. SELECTION OF A SUITABLE METHOD FOR THE SYNTHESIS OF COPPER NANOPARTICLES. Nano 2012, 7. [Google Scholar] [CrossRef]
- Espinosa-Lagunes, F.I.; Cruz, J.C.; Vega-Azamar, R.E.; Murillo-Borbonio, I.; Torres-González, J.; Escalona-Villalpando, R.A.; Gurrola, M.P.; Ledesma-García, J.; Arriaga, L.G. Copper nanoparticles suitable for bifunctional cholesterol oxidation reaction: harvesting energy and sensor. Mater. Renew. Sustain. Energy 2022, 11, 105–114. [Google Scholar] [CrossRef]
- Rao, M.P.C.; Kulandaivelu, K.; Ponnusamy, V.K.; Wu, J.J.; Sambandam, A. Surfactant-assisted synthesis of copper oxide nanorods for the enhanced photocatalytic degradation of Reactive Black 5 dye in wastewater. Environ. Sci. Pollut. Res. 2019, 27, 17438–17445. [Google Scholar] [CrossRef] [PubMed]
- Chandan, M.R.; Kumar, K.R.; Shaik, A.H. Two-dimensional Cu nanostructures for efficient photo-catalytic degradation of methylene blue. Environ. Sci. Adv. 2022, 1, 814–826. [Google Scholar] [CrossRef]
- Nahar, B.; Chaity, S.B.; Gafur, A.; Hossain, M.Z. Synthesis of Spherical Copper Oxide Nanoparticles by Chemical Precipitation Method and Investigation of Their Photocatalytic and Antibacterial Activities. J. Nanomater. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Wu, R.; Ai, J.; Ga, L. Synthesis of Fluorescent Copper Nanomaterials and Detection of Bi3+. Front. Chem. 2022, 10, 899672. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.; Ali, M.E.M.; Gomaa, E.; Mohsen, M. Copper sulfide and zinc oxide hybrid nanocomposite for wastewater decontamination of pharmaceuticals and pesticides. Sci. Rep. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Jardón-Maximino, N.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Lugo-Uribe, L.E.; Cabello-Alvarado, C.; Mata-Padilla, J.M.; Barriga-Castro, E.D. Synthesis of Copper Nanoparticles Stabilized with Organic Ligands and Their Antimicrobial Properties. Polymers 2021, 13, 2846. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, N.; Mnif, S.; Ben Nasr, F.; Fourati, N.; Zerrouki, C.; Chehimi, M.M.; Guermazi, H.; Aifa, S.; Guermazi, S. Non-doped and transition metal-doped CuO nano-powders: structure-physical properties and anti-adhesion activity relationship. RSC Adv. 2022, 12, 23527–23543. [Google Scholar] [CrossRef]
- Badawy, S.M.; El-Khashab, R.A.; Nayl, A.A. Synthesis, Characterization and Catalytic Activity of Cu/Cu2O Nanoparticles Prepared in Aqueous Medium. Bull. Chem. React. Eng. Catal. 2015, 10, 169–174. [Google Scholar] [CrossRef]
- Guzman, M.; Arcos, M.; Dille, J.; Godet, S.; Rousse, C. Effect of the Concentration of NaBH4 and N2H4 as Reductant Agent on the Synthesis of Copper Oxide Nanoparticles and its Potential Antimicrobial Applications. Nano Biomed. Eng. 2018, 10. [Google Scholar] [CrossRef]
- Guzman, M.; Arcos, M.; Dille, J.; Rousse, C.; Godet, S.; Malet, L. Effect of the Concentration and the Type of Dispersant on the Synthesis of Copper Oxide Nanoparticles and Their Potential Antimicrobial Applications. ACS Omega 2021, 6, 18576–18590. [Google Scholar] [CrossRef]
- Guzman, M.; Tian, W.; Walker, C.; Herrera, J.E. Copper oxide nanoparticles doped with lanthanum, magnesium and manganese: optical and structural characterization. R. Soc. Open Sci. 2022, 9, 220485. [Google Scholar] [CrossRef]
- Ponnar, M.; Thangamani, C.; Monisha, P.; Gomathi, S.; Pushpanathan, K. Influence of Ce doping on CuO nanoparticles synthesized by microwave irradiation method. Appl. Surf. Sci. 2018, 449, 132–143. [Google Scholar] [CrossRef]
- Mersian, H.; Alizadeh, M.; Hadi, N. Synthesis of zirconium doped copper oxide (CuO) nanoparticles by the Pechini route and investigation of their structural and antibacterial properties. Ceram. Int. 2018, 44, 20399–20408. [Google Scholar] [CrossRef]
- Ganesan, K.P.; Anandhan, N.; Gopu, G.; Amaliroselin, A.; Marimuthu, T.; Paneerselvam, R. An enhancement of ferromagnetic, structural, morphological, and optical properties of Mn-doped Cu2O thin films by an electrodeposition technique. J. Mater. Sci. Mater. Electron. 2019, 30, 19524–19535. [Google Scholar] [CrossRef]
- Rapp, R.A.; Ezis, A.; Yurek, G.J. Displacement reactions in the solid state. Met. Trans. 1973, 4, 1283–1292. [Google Scholar] [CrossRef]
- Luo, F.; Wei, J.; Liu, Q.; Wang, J. The study of room temperature ferromagnetism in Mn-doped Cu2O powders. Mater. Sci. Semicond. Process. 2021, 133, 105972. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Bharathi, D.; Saravanan, M.; Manikandan, E.; Kumar, S.S.; Pugazhendhi, A. Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of Gram negative and Gram positive bacteria. J. Photochem. Photobiol. B: Biol. 2018, 188, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Venugopal, K.; Bhat, A.H.; Kavitha, K.; Ramanan, A.; Rajagopal, K.; Srinivasan, R.; Manikandan, E. Enhanced Biosynthesis Synthesis of Copper Oxide Nanoparticles (CuO-NPs) for their Antifungal Activity Toxicity against Major Phyto-Pathogens of Apple Orchards. Pharm. Res. 2020, 37, 1–12. [Google Scholar] [CrossRef]
- Kardarian, K.; Nunes, D.; Sberna, P.M.; Ginsburg, A.; Keller, D.A.; Pinto, J.V.; Deuermeier, J.; Anderson, A.Y.; Zaban, A.; Martins, R.; et al. Effect of Mg doping on Cu 2 O thin films and their behavior on the TiO 2 /Cu 2 O heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 27–36. [Google Scholar] [CrossRef]
- Resende, J.; Jiménez, C.; Nguyen, N.D.; Deschanvres, J. Magnesium-doped cuprous oxide (Mg:Cu2O) thin films as a transparent p-type semiconductor. Phys. Status solidi (a) 2016, 213, 2296–2302. [Google Scholar] [CrossRef]
- Ivill, M.; Overberg, M.; Abernathy, C.; Norton, D.; Hebard, A.; Theodoropoulou, N.; Budai, J. Properties of Mn-doped Cu2O semiconducting thin films grown by pulsed-laser deposition. Solid-state Electron. 2003, 47, 2215–2220. [Google Scholar] [CrossRef]
- Das, K.; Sharma, S.N.; Kumar, M.; De, S.K. Luminescence properties of the solvothermally synthesized blue light emitting Mn doped Cu2O nanoparticles. J. Appl. Phys. 2010, 107, 024316. [Google Scholar] [CrossRef]
- Suganthi, A.; Vethanathan, S.J.K.; Perumal, S.; Koilpillai, D.P.; Karpagavalli, S. Optical and Electrical Properties of Solvothermally Synthesized Manganese Doped Cuprous Oxide Nanoparticles. IOSR J. Appl. Phys. 2017, 1, 43–48. [Google Scholar] [CrossRef]
- Jacob, S.S.K.; Kulandaisamy, I.; Valanarasu, S.; Arulanantham, A.M.S.; Ganesh, V.; AlFaify, S.; Kathalingam, A. Enhanced optoelectronic properties of Mg doped Cu2O thin films prepared by nebulizer pyrolysis technique. J. Mater. Sci. Mater. Electron. 2019, 30, 10532–10542. [Google Scholar] [CrossRef]
- Zhao, Z.-B.; Liu, J.-D.; Du, X.-Y.; Wang, Z.-Y.; Zhang, C.; Ming, S.-F. Fabrication of silver nanoparticles/copper nanoparticles jointly decorated nitride flakes to improve the thermal conductivity of polymer composites. Colloids Surfaces A: Physicochem. Eng. Asp. 2021, 635, 128104. [Google Scholar] [CrossRef]
- Hanh, T.T.; Chi, N.T.L.; Duy, N.N. Preparation of Copper Nanoparticles/Diatomite Nanocomposite for Improvement in Water Quality of Fishponds. J. Chem. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Habte, A.G.; Hone, F.G.; Dejene, F.B. Influence of Cu-Doping Concentration on the Structural and Optical Properties of SnO2 Nanoparticles by Coprecipitation Route. J. Nanomater. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Varma, K.S.; Shukla, A.D.; Tayade, R.J.; Joshi, P.A.; Das, A.K.; Modi, K.B.; Gandhi, V.G. Photocatalytic performance and interaction mechanism of reverse micelle synthesized Cu-TiO2 nanomaterials towards levofloxacin under visible LED light. Photochem. Photobiol. Sci. 2021, 21, 77–89. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Weber, I.C. A Strategy to Enhance Humidity Robustness of p-Type CuO Sensors for Breath Acetone Quantification. Small Sci. 2023, 3. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hussain, M.M.; Asiri, A.M.; Alamry, K.; Hasnat, M. An enzyme free detection of L-Glutamic acid using deposited CuO. GdO nanospikes on a flat glassy carbon electrode. Surf. Interfaces 2020, 20, 100617. [Google Scholar]
- Abebe, B.; Tsegaye, D.; Sori, C.; Prasad, R.C.K.R.; Murthy, H.C.A. Cu/CuO-Doped ZnO Nanocomposites via Solution Combustion Synthesis for Catalytic 4-Nitrophenol Reduction. ACS Omega 2023, 8, 9597–9606. [Google Scholar] [CrossRef] [PubMed]
- Thaysen, J.; Boisen, A.; Hansen, O.; Bouwstra, S. Atomic Force Microscopy Probe with Piezoresistive Read-out and a Highly Symmetrical Wheatstone Bridge Arrangement. Sens. Actuators A Phys. 2000, 83, 47–53. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Subash, M.; Logambal, S.; Udhayakumar, G.; Uthrakumar, R.; Inmozhi, C.; Al-Onazi, W.A.; Al-Mohaimeed, A.M.; Chen, T.-W.; Kanimozhi, K. Synthesis and characterization studies of pure and Ni doped CuO nanoparticles by hydrothermal method. J. King Saud Univ. - Sci. 2022, 34, 101831. [Google Scholar] [CrossRef]
- S.I. El-Hout, S.M. El-Sheikh, H.M. Hassan, F.A. Harraz, I.A. Ibrahim, E.A. El-Sharkawy, Appl. Catal. A Gen. 2015, 503, 176.
- Y. Cheng, Y. Fan, Y. Pei, M. Qiao, Catal. Sci. Technol. 2015, 5, 3903.
- B. Ma, Y. Wang, X. Tong, X. Guo, Z. Zheng, X. Guo, Catal. Sci. Technol. 2017, 7, 2805.
- Goswami, A.K. Rathi, C. Aparicio, O. Tomanec, M. Petr, R. Pocklanova, M.B. Gawande, R.S. Varma, R. Zboril, A.C.S. Appl, Mater. Interfaces. 2017, 9, 2815.
- Zhang, K.; Suh, J.M.; Lee, T.H.; Cha, J.H.; Choi, J.-W.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Copper oxide–graphene oxide nanocomposite: efficient catalyst for hydrogenation of nitroaromatics in water. Nano Converg. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Sedlackova, E.; Bytesnikova, Z.; Birgusova, E.; Svec, P.; Ashrafi, A.M.; Estrela, P.; Richtera, L. Label-Free DNA Biosensor Using Modified Reduced Graphene Oxide Platform as a DNA Methylation Assay. Materials 2020, 13, 4936. [Google Scholar] [CrossRef]
- Medha Gijare, Sharmila Chaudhari, Satish Ekar & Anil Garje, A facile synthesis of GO/CuO-blended nanofiber sensor electrode for efficient enzyme-free amperometric determination of glucose, Journal of Analytical Science and Technology 2021, vol. 12, 40.
- Zhang, T.; Zhang, J.; Yu, Y.; Li, J.; Zhou, Z.; Li, C. Synthesis of CuO/GO-DE Catalyst and Its Catalytic Properties and Mechanism on Ciprofloxacin Degradation. Nanomaterials 2022, 12, 4305 doiorg/103390/nano 12234305. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Arya, S. Highly selective and efficient electrochemical sensing of ascorbic acid via CuO/rGO nanocomposites deposited on conductive fabric. Appl. Phys. A 2022, 128, 1–12. [Google Scholar] [CrossRef]
- Katowah, D.F.; Saleh, S.M.; Alqarni, S.A.; Ali, R.; Mohammed, G.I.; Hussein, M.A. Network structure-based decorated CPA@CuO hybrid nanocomposite for methyl orange environmental remediation. Sci. Rep. 2021, 11, 1–21. [Google Scholar] [CrossRef]
- Malik, M.A.; Surepally, R.; Akula, N.; Cheedarala, R.K.; Alshehri, A.A.; Alzahrani, K.A. Oxidation of Alcohols into Carbonyl Compounds Using a CuO@GO Nano Catalyst in Oxygen Atmospheres. Catalysts 2023, 13, 55 doiorg/103390 /catal13010055. [Google Scholar] [CrossRef]
- Hajipour, P.; Bahrami, A.; Eslami, A.; Hosseini-Abari, A.; Hagh Ranjbar, H.R. Chemical bath synthesis of CuO-GO-Ag nanocomposites with enhanced antibacterial properties. J. Alloys Compd. 2020, 821, 153456. [Google Scholar] [CrossRef]
- Gijare, M.; Chaudhari, S.; Ekar, S.; Garje, A. A facile synthesis of GO/CuO-blended nanofiber sensor electrode for efficient enzyme-free amperometric determination of glucose. J. Anal. Sci. Technol. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Wu, Z.; Liu, Y.; Li, S. Facile hydrothermal synthesis of CuO–Cu2O/GO nanocomposites for the photocatalytic degradation of organic dye and tetracycline pollutants. New J. Chem. 2020, 44, 6420–6427. [Google Scholar] [CrossRef]
- Bekru, A.G.; Tufa, L.T.; Zelekew, O.A.; Gwak, J.; Lee, J.; Sabir, F.K. Microwave-Assisted Synthesis of rGO-ZnO/CuO Nanocomposites for Photocatalytic Degradation of Organic Pollutants. Crystals 2023, 13, 133 doiorg/103390/ cryst13010133. [Google Scholar] [CrossRef]
- Krishna, R.; Fernandes, D.M.; Ventura, J.; Freire, C.; Titus, E. Novel synthesis of highly catalytic active Cu@Ni/RGO nanocomposite for efficient hydrogenation of 4-nitrophenol organic pollutant. Int. J. Hydrogen Energy 2016, 41, 11608–11615. [Google Scholar] [CrossRef]
- Pérez-Poyatos, L.; Pastrana-Martínez, L.; Morales-Torres, S.; Sánchez-Moreno, P.; Bramini, M.; Maldonado-Hódar, F. Iron-copper oxide nanoparticles supported on reduced graphene oxide for the degradation of cyclophosphamide by photo-Fenton reaction. Catal. Today 2023, 423, 114010. [Google Scholar] [CrossRef]
- Haso, H.W.; Dubale, A.A.; Chimdesa, M.A.; Atlabachew, M. High Performance Copper Based Metal Organic Framework for Removal of Heavy Metals From Wastewater. Front. Mater. 2022, 9. [Google Scholar] [CrossRef]
- Hermawan, A. , Zhang, B. , Taufik, A., Asakura, Y., Hasegawa, T., Zhu, J., …Yin, S. (2020). CuO nanoparticles/Ti3C2Tx MXene hybrid Nanocomposites for Detection of Toluene Gas. ACS Applied Nano Materials. ACS Appl. Nano Mater. 2020, 3, 4755−4766. [Google Scholar] [CrossRef]
- Esokkiya, A.; Murugasenapathi, N.; Kumar, S.; Sudalaimani, S.; Santhosh, B.; Tamilarasan, P.; Sivakumar, C.; Giribabu, K. Electrochemically activated copper nitroprusside as a catalyst for sensing of carcinogenic acetaldehyde in red wine. Sensors Actuators B: Chem. 2022, 363. [Google Scholar] [CrossRef]
- Yelei Gong, Hao Li, Wenle Pei, Jincheng Fan, Ahmad Umar, M. S. Al-Assiri, Yao Wang, Nicolaas Frans de Rooij and Guofu Zhou, Assembly with copper (II) ions and D–p–A molecules on a graphene surface for ultra-fast acetic acid sensing at room temperature, RSC Adv., 2019, 9, 30432.
- Feng, X.; Sun, S.; Cheng, G.; Shi, L.; Yang, X.; Zhang, Y. Removal of Uranyl Ion from Wastewater by Magnetic Adsorption Material of Polyaniline Combined with CuFe2O4. Adsorpt. Sci. Technol. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Fan, W.; Wang, A.; Wang, L.; Jiang, X.; Xue, Z.; Li, J.; Wang, G. Hollow Carbon Nanopillar Arrays Encapsulated with Pd–Cu Alloy Nanoparticles for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2023, 15, 13600–13608. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. 3D-hierarchically structured MnO2 for catalytic oxidation of phenol solutions by activation of peroxymonosulfate: Structure dependence and mechanism. Appl. Catal. B: Environ. 2014, 164, 159–167. [Google Scholar] [CrossRef]
- Wang, L.; Ke, F.; Zhu, J. Metal–organic gel templated synthesis of magnetic porous carbon for highly efficient removal of organic dyes. Dalton Trans. 2016, 45, 4541–4547. [Google Scholar] [CrossRef]
- Dong, F.; Guo, W.; Park, S.-S.; Ha, C.-S. Uniform and monodisperse polysilsesquioxane hollow spheres: synthesis from aqueous solution and use in pollutant removal. J. Mater. Chem. 2011, 21, 10744–10749. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Library Cataloguing-in-Publication Data World Health Organization: Geneva, Switzerland, 2011; p. 518. ISBN 978-92-4-154815-1. [Google Scholar]
- Khan, S.A.; Khan, S.B.; Asiri, A.M. Toward the design of Zn–Al and Zn–Cr LDH wrapped in activated carbon for the solar assisted de-coloration of organic dyes. RSC Adv. 2016, 6, 83196–83208. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, S.B.; Asiri, A.M. Layered double hydroxide of Cd-Al/C for the mineralization and de-coloration of dyes in solar and visible light exposure. Sci. Rep. 2016, 6, 35107. [Google Scholar] [CrossRef]
- Wang, C.; Salmon, L.; Li, Q.; Igartua, M.E.; Moya, S.; Ciganda, R.; Ruiz, J.; Astruc, D. From Mono to Tris-1,2,3-triazole-Stabilized Gold Nanoparticles and Their Compared Catalytic Efficiency in 4-Nitrophenol Reduction. Inorg. Chem. 2016, 55, 6776–6780. [Google Scholar] [CrossRef]
- Mandal, S.; De, S. Catalytic and fluorescence studies with copper nanoparticles synthesized in polysorbates of varying hydrophobicity. Colloids Surfaces A: Physicochem. Eng. Asp. 2015, 467, 233–250. [Google Scholar] [CrossRef]
- Pakzad, K.; Alinezhad, H.; Nasrollahzadeh, M. Green synthesis of Ni@Fe3O4 and CuO nanoparticles using Euphorbia maculata extract as photocatalysts for the degradation of organic pollutants under UV-irradiation. Ceram. Int. 2019, 45, 17173–17182. [Google Scholar] [CrossRef]
- Bordbar Maryam, Sharifi-Zarchi Zeinab, Khodadadi Bahar. Green synthesis of copper oxide nanoparticles/clinoptilolite using Rheum palmatum L. root extract: high catalytic activity for reduction of 4-nitro phenol, rhodamine B, and methylene blue, Journal of Sol-Gel Science and Technology. 2016;81(3).
- Alshaikhi, H.A.; Asiri, A.M.; Alamry, K.A.; Marwani, H.M.; Alfifi, S.Y.; Khan, S.B. Copper Nanoparticles Decorated Alginate/Cobalt-Doped Cerium Oxide Composite Beads for Catalytic Reduction and Photodegradation of Organic Dyes. Polymers 2022, 14, 4458. [Google Scholar] [CrossRef] [PubMed]
- Cosma, D.; Urda, A.; Radu, T.; Rosu, M.C.; Mihet, M.; Socaci, C. Evaluation of the Photocatalytic Properties of Copper Oxides/ Graphene/TiO2 Nanoparticles Composites. Molecules 2022, 27, 5803 doiorg/103390/ molecules27185803. [Google Scholar] [CrossRef]
- Ahsan, H.; Shahid, M.; Imran, M.; Mahmood, F.; Siddique, M.H.; Ali, H.M.; Niazi, M.B.; Hussain, S.; Shahbaz, M.; Ayyub, M.; et al. Photocatalysis and adsorption kinetics of azo dyes by nanoparticles of nickel oxide and copper oxide and their nanocomposite in an aqueous medium. PeerJ 2022, 10, e14358. [Google Scholar] [CrossRef]
- Mohammed Rehaan Chandan, Kodi Rajesh Kumar and Aabid Hussain Shaik, Two-dimensional Cu nanostructures for efficient photo-catalytic degradation of methylene blue: Environ. Sci.: Adv., 2022, 1, 814.
- Borge, V.V.; Patil, R.M.; Dwivedi, P.R. Photocatalytic Decomposition of Rhodamine B Dye Using Copper Oxide Nanoparticles Prepared from Copper Chalcone Complexes. Int. J. Nanosci. 2022, 21. [Google Scholar] [CrossRef]
- Nazim, M.; Khan, A.A.P.; Asiri, A.M.; Kim, J.H. Exploring Rapid Photocatalytic Degradation of Organic Pollutants with Porous CuO Nanosheets: Synthesis, Dye Removal, and Kinetic Studies at Room Temperature. ACS Omega 2021, 6, 2601–2612. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Oluwalana, A.E. Enhanced Photocatalytic Degradation of Ternary Dyes by Copper Sulfide Nanoparticles. Nanomaterials 2021, 11, 2000. [Google Scholar] [CrossRef]
- Rasheed, S.; Batool, Z.; Intisar, A.; Riaz, S.; Shaheen, M.; Kousar, R. Enhanced photodegradation activity of cuprous oxide nanoparticles towards Congo red for water purification. Desalination Water Treat. 2021, 227, 330–337. [Google Scholar] [CrossRef]
- Nahar, B.; Chaity, S.B.; Gafur, A.; Hossain, M.Z. Synthesis of Spherical Copper Oxide Nanoparticles by Chemical Precipitation Method and Investigation of Their Photocatalytic and Antibacterial Activities. J. Nanomater. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Aksu, Z. Application of biosorption for the removal of organic pollutants: a review. Process. Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Library Cataloguing-in-Publication Data World Health Organization: Geneva, Switzerland, 2011; p. 518. ISBN 978-92-4-154815-1. [Google Scholar]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption Behavior of EDTA-Graphene Oxide for Pb (II) Removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. [Google Scholar] [CrossRef]
- Tesfaye, D. Removal of Lead from Wastewater Using Corncob Activated Carbon as an Adsorbent. 2016. M.Sc Thesis. Addis Ababa: Addis Ababa University.
- Haso, H.W.; Dubale, A.A.; Chimdesa, M.A.; Atlabachew, M. High Performance Copper Based Metal Organic Framework for Removal of Heavy Metals From Wastewater. Front. Mater. 2022, 9. [Google Scholar] [CrossRef]
- Fanta, F.T.; Dubale, A.A.; Bebizuh, D.F.; Atlabachew, M. Copper doped zeolite composite for antimicrobial activity and heavy metal removal from wastewater. BMC Chem. 2019, 13, 44. [Google Scholar] [CrossRef]
- Raul, P.K.; Das, B.; Umlong, I.M.; Devi, R.R.; Tiwari, G.; Kamboj, D.V. Toward a Feasible Solution for Removing Toxic Mercury and Chromium From Water Using Copper Oxide Nanoparticles. Front. Nanotechnol. 2022, 4. [Google Scholar] [CrossRef]
- Sikder, M.T.; Yoshihiro, M.; Islam, M.; Saito, T.; Tanaka, S.; Kurasaki, M. Preparation and characterization of chitosan–caboxymethyl-β-cyclodextrin entrapped nanozero-valent iron composite for Cu (II) and Cr (IV) removal from wastewater, Chem. Eng. J. 2014, 236, 378–387. [Google Scholar] [CrossRef]
- Mary, B.C.J.; Vijaya, J.J.; Bououdina, M.; Khezami, L.; Modwi, A.; Ismail, M.; Bellucci, S. Study of Barium Adsorption from Aqueous Solutions Using Copper Ferrite and Copper Ferrite/rGO Magnetic Adsorbents. Adsorpt. Sci. Technol. 2022, 2022, 1–24. [Google Scholar] [CrossRef]
- Xinrui Feng, Shaoshuai Sun, Ge Cheng, Lei Shi, Xiangshan Yang, and Yib Technology, Vol.l of Uranyl Ion from Wastewater by Magnetic Adsorption Material of Polyaniline Combined with CuFe2O4, Adsorption Science and Technology, 2021, 5584158. [CrossRef]
- Mahmoud, A.E.D.; Al-Qahtani, K.M.; Alflaij, S.O.; Al-Qahtani, S.F.; Alsamhan, F.A. Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Fouda, A.; Hassan, S.E.-D.; Hamza, M.F.; Alharbi, N.K.; Elkelish, A.; Alharthi, A.; Salem, W.M. Plant-Based Copper Oxide Nanoparticles; Biosynthesis, Characterization, Antibacterial Activity, Tanning Wastewater Treatment, and Heavy Metals Sorption. Catalysts 2023, 13, 348. [Google Scholar] [CrossRef]
- Wu, R.; Ai, J.; Ga, L. Synthesis of Fluorescent Copper Nanomaterials and Detection of Bi3+. Front. Chem. 2022, 10, 899672. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Sagboye, P.A.; Umenweke, G.; Ajala, O.J.; Omoarukhe, F.O.; Adeyanju, C.A.; Ogunniyi, S.; Adeniyi, A.G. CuO nanoparticles (CuO NPs) for water treatment: A review of recent advances. Environ. Nanotechnology, Monit. Manag. 2021, 15, 100443. [Google Scholar] [CrossRef]
- M. Fulekar, Bhawana Pathak, RHIZOFILTRATION: A Green Technology for Remediation of Heavy Metals, Engineering, Environmental Science, 2012, Corpus ID: 111110066.
- Panel Xiaolei Qu, Pedro J.J.Alvarez, QilinLi, Applications of nanotechnology in water and wastewater treatment, Water Research, 2014, Vol. 47, Issue 12, 1 Pages 3931-3946. [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Nitrophenols (Draft). Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA. 1990.
- U.S. Department of Health and Human Services. Hazardous Substances Data Bank (HSDB, online database). National Toxicology Information Program, National Library of Medicine, Bethesda, MD. 1993.
- Sarkar, C.; Dolui, S.K. Synthesis of copper oxide/reduced graphene oxide nanocomposite and its enhanced catalytic activity towards reduction of 4-nitrophenol. RSC Adv. 2015, 5, 60763–60769. [Google Scholar] [CrossRef]
- Sedlackova, E.; Bytesnikova, Z.; Birgusova, E.; Svec, P.; Ashrafi, A.M.; Estrela, P.; Richtera, L. Label-Free DNA Biosensor Using Modified Reduced Graphene Oxide Platform as a DNA Methylation Assay. Materials 2020, 13, 4936. [Google Scholar] [CrossRef]
- Nguyen, T. T. , & Wang, J. (). Recent advances in biosensors based on hydrogen peroxide. Biosensors & bioelectronics, 2012, 37(1), 1-11.
- Shukla, A. , & Gupta, V. K. Hydrogen peroxide-based biosensors: A review. Biosensors and Bioelectronics, 2008, 24(6), 1485-1493.
- Umar, A.; Haque, M.; Ansari, S.G.; Seo, H.-K.; Ibrahim, A.A.; Alhamami, M.A.M.; Algadi, H.; Ansari, Z.A. Label-Free Myoglobin Biosensor Based on Pure and Copper-Doped Titanium Dioxide Nanomaterials. Biosensors 2022, 12, 1151. [Google Scholar] [CrossRef]
- Haque, M.; Fouad, H.; Seo, H.-K.; Alothman, O.Y.; Ansari, Z.A. Cu-Doped ZnO Nanoparticles as an Electrochemical Sensing Electrode for Cardiac Biomarker Myoglobin Detection. IEEE Sensors J. 2020, 20, 8820–8832. [Google Scholar] [CrossRef]
- Ali, Y.; Knight, D.; Howlader, M.M.R. Nonenzymatic Electrochemical Glutamate Sensor Using Copper Oxide Nanomaterials and Multiwall Carbon Nanotubes. Biosensors 2023, 13, 237. [Google Scholar] [CrossRef]
- Arsalan, M.; Saddique, I.; Baoji, M.; Awais, A.; Khan, I.; Shamseldin, M.A.; Mehrez, S. Novel Synthesis of Sensitive Cu-ZnO Nanorod–Based Sensor for Hydrogen Peroxide Sensing. Front. Chem. 2022, 10, 932985. [Google Scholar] [CrossRef]
- Sajna, M.; Cabibihan, J.-J.; Malik, R.A.; Sadasivuni, K.K.; Geetha, M.; Alahmad, J.K.; Hijazi, D.A.; Alsaedi, F. Nonenzymatic electrochemical lactic acid sensor using CuO nanocomposite. Mater. Sci. Eng. B 2023, 288. [Google Scholar] [CrossRef]
- Güngör, S.; Taşaltın, C.; Gürol, I.; Baytemir, G.; Karakuş, S.; Taşaltın, N. Copper phthalocyanine-borophene nanocomposite-based non-enzymatic electrochemical urea biosensor. Appl. Phys. A 2022, 128, 1–8. [Google Scholar] [CrossRef]
- Saputra, F.; Uapipatanakul, B.; Lee, J.-S.; Hung, S.-M.; Huang, J.-C.; Pang, Y.-C.; Muñoz, J.E.R.; Macabeo, A.P.G.; Chen, K.H.-C.; Hsiao, C.-D. Co-Treatment of Copper Oxide Nanoparticle and Carbofuran Enhances Cardiotoxicity in Zebrafish Embryos. Int. J. Mol. Sci. 2021, 22, 8259. [Google Scholar] [CrossRef] [PubMed]
- Delepine, M. : Metallic salts of dithiocarbamic acids; preparation of isothiocyanates in aliphatic series. C. R. 144, 1125–1127.
- Malik, A.K. , Faubel, W.: Methods of analysis of dithiocarbamate pesticides: a review. Pestic. Sci., 1999, 55(10), 965–970.
- Al-Alam, J.; Bom, L.; Chbani, A.; Fajloun, Z.; Millet, M. Analysis of Dithiocarbamate Fungicides in Vegetable Matrices Using HPLC-UV Followed by Atomic Absorption Spectrometry. J. Chromatogr. Sci. 2016, 55, 429–435. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dithiocarbamate pesticides, ethylene thiourea and propylene thiourea: a general introduction. International Program on Chemical Safety. 2005. https://www.inchem.org/documents/ehc/ehc/ehc78.htm. WHO: The WHO Recommended Classification of Pesticides by Hazards and Guidelines to Classification, WHO/IPCS/IOMC, Geneva.
- Szolar, O. Environmental and pharmaceutical analysis of dithiocarbamates. Anal. Chim. Acta 2007, 582, 191–200. [Google Scholar] [CrossRef]
- Ghoto, S.A.; Khuhawar, M.Y.; Jahangir, T.M.; Mangi, J.U.D. Applications of copper nanoparticles for colorimetric detection of dithiocarbamate pesticides. J. Nanostructure Chem. 2019, 9, 77–93. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2. [Google Scholar] [CrossRef]
- Gworek, B.; Kijeńska, M.; Wrzosek, J.; Graniewska, M. Pharmaceuticals in the Soil and Plant Environment: a Review. Water, Air, Soil Pollut. 2021, 232, 1–17. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Lu, G.; Liu, X.; Wang, Y.; Wang, D.; Liu, W.; Yue, P.; Zhu, B.; Duan, X. Climate Transition fromWarm-Dry to Warm-Wet in Eastern Northwest China. Atmosphere 2021, 12, 548. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Efficient adsorptive removal of paracetamol and thiazolyl blue from polluted water onto biosynthesized copper oxide nanoparticles. Sci. Rep. 2023, 13, 1–15. [Google Scholar] [CrossRef]
- Ferrone, E. , Araneo, R. , Notargiacomo, A.; Pea, M., Rinaldi, A. ZnO Nanostructures and Electrospun ZnO-Polymeric Hybrid Nanomaterials in Biomedical, Health, and Sustainability Applications. Nanomaterials 2019, 9, 1449. [Google Scholar] [CrossRef]
- Shirzadi, A.; Nezamzadeh-Ejhieh, A. Enhanced photocatalytic activity of supported CuO–ZnO semiconductors towards the photodegradation of mefenamic acid aqueous solution as a semi real sample. J. Mol. Catal. A: Chem. 2016, 411, 222–229. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Moghaddam, N.S.M.; Rahimi, S.M.; Amarzadeh, M.; Nasseh, N. Efficient photocatalytic degradation of metronidazole in wastewater under simulated sunlight using surfactant- and CuS-activated zeolite nanoparticles. J. Environ. Manag. 2022, 319, 115697. [Google Scholar] [CrossRef]
- Dong, Q.; Dong, H.; Li, Y.; Xiao, J.; Xiang, S.; Hou, X.; Chu, D. Degradation of sulfamethazine in water by sulfite activated with zero-valent Fe-Cu bimetallic nanoparticles. J. Hazard. Mater. 2022, 431, 128601. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Nasr, R.A.; Vannier, R.-N.; Jamil, T.S. Improving of photocatalytic activity of barium ferrate via bismuth and copper co-doping for degradation of paracetamol under visible light irradiation. J. Environ. Sci. 2021, 112, 331–342. [Google Scholar] [CrossRef]
- Benhaddouch, T.E.; Pinzon, S.K.; Landi, D.M.C.; Marcial, J.; Mehta, P.; Romero, K.; Rockward, T.; Bhansali, S.; Dong, D. Review—Micro-Fuel Cell Principal Biosensors for Monitoring Transdermal Volatile Organic Compounds in Humans. ECS Sensors Plus 2022, 1, 041602. [Google Scholar] [CrossRef]
- Shuai, J.; Kim, S.; Ryu, H.; Park, J.; Lee, C.K.; Kim, G.-B.; Ultra, V.U., Jr.; Yang, W. Health risk assessment of volatile organic compounds exposure near Daegu dyeing industrial complex in South Korea. BMC Public Health 2018, 18, 528. [Google Scholar] [CrossRef] [PubMed]
- Dima, A.C.; Balaban, D.V.; Dima, A. Diagnostic Application of Volatile Organic Compounds as Potential Biomarkers for Detecting Digestive Neoplasia: A Systematic Review. Diagnostics 2021, 11, 2317. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Wang, B.; Pei, S. Hydrothermal synthesis of SnO2-CuO composite nanoparticles as a fast-response ethanol gas sensor. J. Alloy. Compd. 2021, 886. [Google Scholar] [CrossRef]
- Meng, F.; Yang, Z.; Yuan, Z.; Zhang, H.; Zhu, H. Hydrothermal synthesis of CuO/rGO nanosheets for enhanced gas sensing properties of ethanol. Ceram. Int. 2023, 49, 5595–5603. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z.; Xiao, Q.; Li, M.; Xu, Y.; Qiu, X. Sensitive detection of p-nitrotoluene based on a copper cluster modified carbon nitride nanosheets photoelectrochemical sensor. Appl. Catal. A: Gen. 2023, 649. [Google Scholar] [CrossRef]
- Lete, C.; Spinciu, A.-M.; Alexandru, M.-G.; Moreno, J.C.; Leau, S.-A.; Marin, M.; Visinescu, D. Copper(II) Oxide Nanoparticles Embedded within a PEDOT Matrix for Hydrogen Peroxide Electrochemical Sensing. Sensors 2022, 22, 8252. [Google Scholar] [CrossRef]
- Ma, P.; Ma, X. High-sensitivity and temperature-controlled switching methanol sensor prepared based on the dual catalysis of copper particles. Talanta 2021, 237, 122888. [Google Scholar] [CrossRef]
- Esokkiya, A.; Murugasenapathi, N.; Kumar, S.; Sudalaimani, S.; Santhosh, B.; Tamilarasan, P.; Sivakumar, C.; Giribabu, K. Electrochemically activated copper nitroprusside as a catalyst for sensing of carcinogenic acetaldehyde in red wine. Sensors Actuators B: Chem. 2022, 363. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Pei, W.; Fan, J.; Umar, A.; Al-Assiri, M.S.; Wang, Y.; de Rooij, N.F.; Zhou, G. Assembly with copper(ii) ions and D–π–A molecules on a graphene surface for ultra-fast acetic acid sensing at room temperature. RSC Adv. 2019, 9, 30432–30438. [Google Scholar] [CrossRef] [PubMed]
- Maake, P.J.; Mokoena, T.P.; Bolokang, A.S.; Hintsho-Mbita, N.; Tshilongo, J.; Cummings, F.R.; Swart, H.C.; Iwuoha, E.I.; Motaung, D.E. Fabrication of AgCu/TiO2 nanoparticle-based sensors for selective detection of xylene vapor. Mater. Adv. 2022, 3, 7302–7318. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Biswas, M.R.; Alzubaidi, M.S.; Shah, U.; Abd-Alrazaq, A.A.; Shah, Z. A Scoping Review to Find out Worldwide COVID-19 Vaccine Hesitancy and Its Underlying Determinants. Vaccines 2021, 9, 1243. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, S.B.; Asiri, A.M. Toward the design of Zn–Al and Zn–Cr LDH wrapped in activated carbon for the solar assisted de-coloration of organic dyes. RSC Adv. 2016, 6, 83196–83208. [Google Scholar] [CrossRef]
- Weng, Z.; Jiang, J.; Wu, Y.; Wu, Z.; Guo, X.; Materna, K.L.; Liu, W.; Batista, V.S.; Brudvig, G.W.; Wang, H. Electrochemical CO2 Reduction to Hydrocarbons on a Heterogeneous Molecular Cu Catalyst in Aqueous Solution. J. Am. Chem. Soc. 2016, 138, 8076–8079. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Lee, J.H.; Liu, Y.; Xie, Z.; Hwang, S.; Marinkovic, N.S.; Park, A.-H.A.; Kattel, S.; Chen, J.G. Electrochemical CO2 Reduction Reaction over Cu Nanoparticles with Tunable Activity and Selectivity Mediated by Functional Groups in Polymeric Binder. JACS Au 2021, 2, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, T.; Wang, R.; Sun, C.; Zhang, N.; Gao, R.; Song, Y. Heat-treated copper phthalocyanine on carbon toward electrochemical CO2 conversion into ethylene boosted by oxygen reduction. Chem. Commun. 2022, 58, 12192–12195. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, W.; Park, J.W.; Kim, C.; Kim, M.; Song, H. Branched Copper Oxide Nanoparticles Induce Highly Selective Ethylene Production by Electrochemical Carbon Dioxide Reduction. J. Am. Chem. Soc. 2019, 141, 6986–6994. [Google Scholar] [CrossRef]
- Suliman, M.H.; Yamani, Z.H.; Usman, M. Electrochemical Reduction of CO2 to C1 and C2 Liquid Products on Copper-Decorated Nitrogen-Doped Carbon Nanosheets. Nanomaterials 2022, 13, 47. [Google Scholar] [CrossRef]
- Amr, A.E.; Abo-Ghalia, M.H.; Moustafa, G.; Al-Omar, M.A.; Nossier, E.S.; Elsayed, E.A. Design, synthesis and docking studies of novel macrocyclic pentapeptides as anticancer multi-targeted kinase inhibitors. Molecules 2018, 23, 2416. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Burdyny, T.; Dinh, C.-T.; Kibria, M.G.; Fan, J.Z.; Liu, M.; Sargent, E.H.; Sinton, D. Joint tuning of nanostructured Cu-oxide morphology and local electrolyte programs high-rate CO2 reduction to C2H4. Green Chem. 2017, 19, 4023–4030. [Google Scholar] [CrossRef]
- Dutta, A.; Rahaman, M.; Mohos, M.; Zanetti, A.; Broekmann, P. Electrochemical CO2 Conversion Using Skeleton (Sponge) Type of Cu Catalysts. ACS Catal. 2017, 7, 5431–5437. [Google Scholar] [CrossRef]
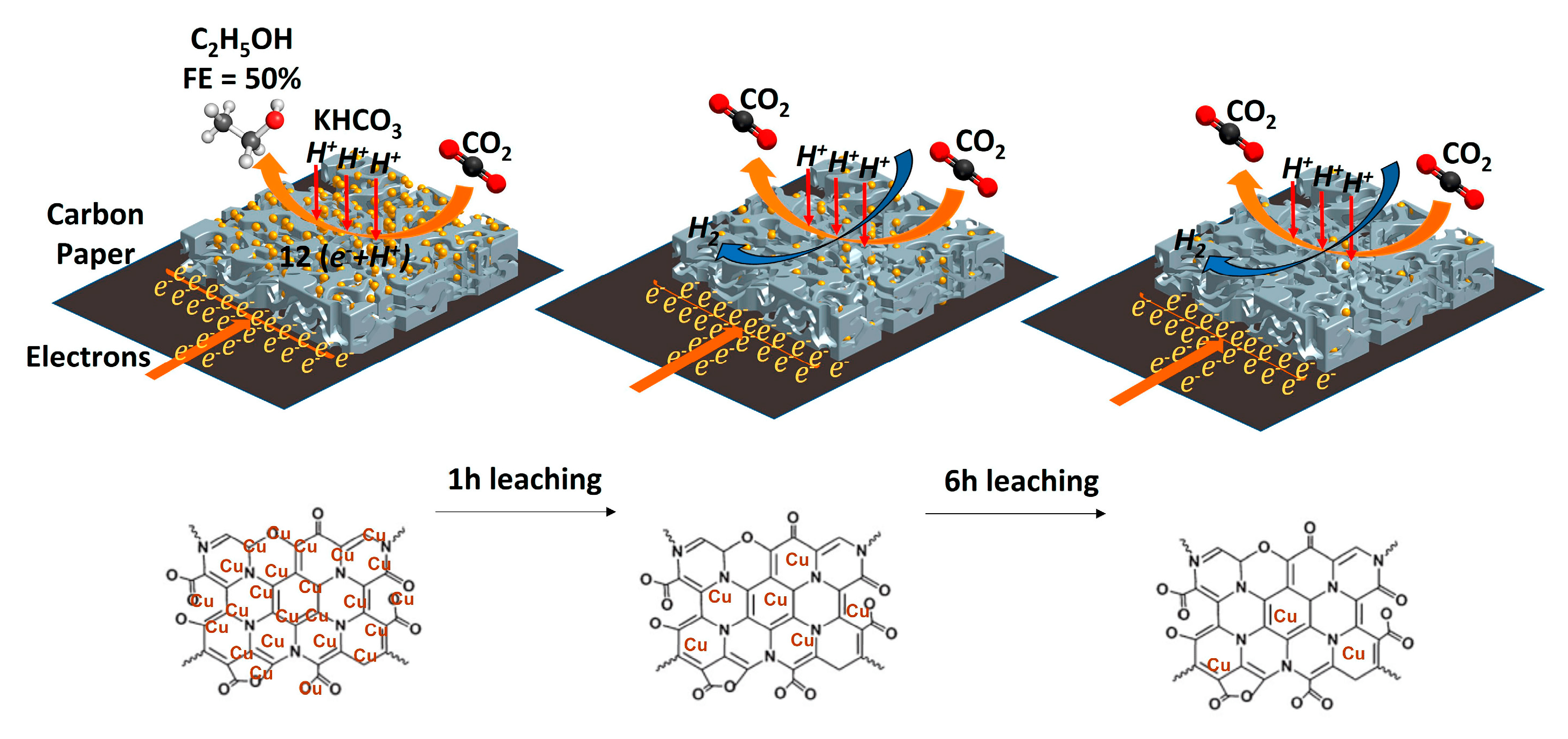
- Alkoshab, M.Q.; Thomou, E.; Abdulazeez, I.; Suliman, M.H.; Spyrou, K.; Iali, W.; Alhooshani, K.; Baroud, T.N. Low Overpotential Electrochemical Reduction of CO2 to Ethanol Enabled by Cu/CuxO Nanoparticles Embedded in Nitrogen-Doped Carbon Cuboids. Nanomaterials 2023, 13, 230. [Google Scholar] [CrossRef]
| Synthesized Nano material | Method | Solvent | Precursor | Reducing agent | Stabilizer/binding agent | Conditions | Product description | Ref. |
|---|---|---|---|---|---|---|---|---|
| CuO nanoflakes | Chemo-thermal | Water-Acidic medium | Recovered Cu foil from FPCBs, Cu (OH)2 | NaOH | - | Decomposed at 5200C | Width ∼10 to 50 nm; Length ∼30-80 nm | [16] |
| Cu2O | Reduction | Water | CuSO4 5H2O, NaOH, D-glucose, PVP, ethylene glycol | NaOH | Ethylene glycol | Heated at 80°C for 1 h, drying for 5 h in a vacuum oven at 55° C. | 150-200nm | [17] |
| Cupric oxide | Simple reduction | Ethanol | Cupric chloride (CuCl2), | NaOH | Polyethylene glycol | Heating for 16hrs, centrifuged, dried. | 20nm | [18] |
| CuNPs | Wet chemical | Octyl ether | Cu (II) | 1,2-hexa decanediol | Oleic acid+ Oleyl amine | 1050C, 10 min;150 to 2000C, 30min | 5-25nm | [19] |
| acetyl acetonate | ||||||||
| Cu/Cu2O | Chemical reduction | Ethylene glycol | Ethylene glycol, PVP, CuSO4, Ascorbic acid, Acetone, NaOH | Ascorbic acid | PVP | 800C at 350rpm for 36hrs. Dried at 6h at600C | 28/29nm | [20] |
| CuO nanorods | Chemical precipitation | Water | SDS or SLS, Copper nitrate | KOH, Ammonia | - | Stirring 700rpm, Centrifuged 3k rpm for 15min, Dried for 12h at600C, calcination 4000C,4h | 20-40nm | [21] |
| Cu nano | Chemical reduction | Water | Copper chloride, | Ascorbic acid | - | 10k rpm for 10 min. | 650nm | [22] |
| sheet | Ascorbic acid, CTAB, NaOH | length/ | ||||||
| 150nm diameter | ||||||||
| CuO NPs | Simple chemical reduction | Water | Copper acetate monohydrate, | NaOH | Stirred 900rpm, 4hr at 800C, centrifuged for 20min at 10krpm, dried at 1000C for 3hrs | 6.2nm | [23] | |
| Fluorescent CuO NPs | Aggregation induced luminescence | Water | Glutathione, Copper nitrate, NaOH | NaOH | Glutathione | pH 2.7, centrifuge 20min and stored at 40C | - | [24] |
| CuS | Microwave hydrothermal | Water | Copper acetate, Thiourea | Thiourea | 6 min, 2 min on and 30s off, repeat for 3 cycles. Filtered, dried 600C for 6h | 1.9nm | [25] | |
| Cu NPs | Agitation | Water | Copper sulphate, PEI, NaOH | NaOH | PEI | pH at 12.0, stirred continuously for 60min at 600C, centrifugation 13k rpm at RT, Dried 700C for 5h | 25nm | [26] |
| Nano material | Method | Solvent | Precursor | Reducing agent/ Stabilizer | Conditions | Product description | Ref. |
|---|---|---|---|---|---|---|---|
| h-BN (Ag/Cu) | Agitation followed by growing | Water | CuCl2, h-BN, Tri-methoxy silane, | Hydrazine hydrate | 6h stirring at room temperature, drying overnight at 600C | - | [45] |
| CuNPs/DA | Co-precapitation | Ethanol | CuSO4.5H2O,1% chitosan, ascorbic acid, | Sodium hyroxide | Stirring and followed by irradiated at 20kGy linear accelerator and dried at 800C | 20nm | [46] |
| SnO2/CuNPs | Co-precipitation | Water | tin dichloride dihydrate, Copper acetate monohydrate, | Ammonia | pH maintained at 9.8 and heated at 500C for 2 hours | 25nm-35nm | [47] |
| Cu-TiO2 | Reverse micelle sol-gel | Water | Copper nitrate trihydrate, triton X-114, TTIP, Toulene, Hexane | TTIP | Stir for 15hrs at 700 rpm. Centrifuging 10 min at 8krpm, 18hrs 1300C dry, calcination 4hrs at 4000C | 5.79 nm/0.0839 cm3/g | [48] |
| CeO2-CuO | Flame spray pyrolysis (FSP) | 1:2 xylene and 2-ethylhexanoic acid | Cerium (II) ethyl hexanoate, Soligen copper 8, Xylene, 2-ethylhexanoic acid | - | FSP followed by annealing at 5000C, 5h | 13.6nm | [49] |
| CuO-GdO | Hydrothermal | Water | Gadolinium chloride, ammonium hydroxide, copper chloride | Ammonium hydroxide | Autoclave 1500C, 16h; Calcined at 5000C | [50] | |
| CuS QDs@ZnO | Microwave assisted hydrothermal | Water | Zinc nitrate, HMT, Copper acetate monohydrate,Thiourea | Thiourea | Stirring, 6min irradiated and dried the sample in oven 700C for 10h | 36.5nm | [25] |
| Cu/CuO-ZnO | Solution combustion synthesis | Water | Copper nitrate trihydrate, Zinc nitrate, polyvinyl alcohol, Urea | Urea/ PVA | Dehydrated by heating to 1100C, powder obtained calcined 5000C for 3h. | 15-50nm | [51] |
| CuO@AgO/ZnO | Hydrothermalsynthesis | Water | Zinc acetate, sodium hydroxide, copper sulfate, silver nitrate | NaOH | Autoclave 1850C for 12h, desiccated at 700C for 8h, calcinated at 6000C for 5h. | 85nm | [52] |
| Ni/CuO | Hydrothermal synthesis | Water | Copper sulphate, NaOH, Nickel sulphate | NaOH | Hydrothermal 1800C for 12h, dried at 800C,12h | 19 to 28nm | [53] |
| Nano material | Method | Solvent | Precursor | Reducing agent /Stabilizer |
Conditions | Product description | Pollutant Degradation/ Sensing/Reduction |
Ref. |
|---|---|---|---|---|---|---|---|---|
| /GO-DE | Ultrasonic impregnation method | Water | Copper nitrate | NaOH | Ultrasonication 30min with reducing agent, filtered, dried 1100 for 2h | 0.52699 (µm) (Pore diameter) |
Ciprofloxacin | [61] |
| CuO-rGO | Simple liquid approach | Water | Copper acetate | Ammonia | Reflux 2h, agitated 1h. Centrifuged, dried 10h at 800C | 21.68nm | Ascorbic acid | [62] |
| CPA/N-SWCNTS-GO-CE/CuO nanocomposite | Chemical oxidative copoly-merization | 0.5M H2SO4/Water | CuO, Graphene, PPDA, TPA, Aniline | Ammonia | Ultra Sonication for 30min, stirring at 0-40C in N2 atm. 24hr, black powder dried at 600C for 24hr. | - | Methyl orange | [63] |
| CuO@GO | Reflux | Water/iso proponol | Copper acetate, graphite, Sodium nitrate, KMnO4, NH4OH, | NH4OH/ KMnO4 | Stirring at 820C for 2h, dried at 600C in hot air oven overnight | - | Synthesis of Alcohols to carbonyl compounds | [64] |
| CuO-GO-Ag | Chemical reduction | Copper sulphate, ammonia, SDS, GO nanosheets, Ag nanoparticles | Ammonia/SDS | The entire solution is Sonicated for 1h, pH set to 10.0, heated in oil bath, kept at 1120C for 30min, dried in hot air for 10h, annealed 4h at 4000C | 5-10 µm | Antibacterial properties | [65] | |
| GO/CUO | Simple chemical reduction | Water | Copper oxide, graphite powder, NaOH | NaOH | Stirring for half an hour to 1000C, | 70-200nm | Glucose | [66] |
| CuO-Cu2O/GO | Hydrothermal synthesis | Water | Copper acetate, CTAB | CTAB | Autoclave 1600C for 12h, dried at 600C for 24h | 0.21-0.24nm | Organic dyes and tetracycline pollutants | [67] |
| rGO-ZnO/CuO | Microwave irradiation | Water | Graphite powder, Zinc acetate, copper nitrate, NaOH, PEG | NaOH/PEG | Stirring for 20min at 700C with the successive addition of each precursor at pH 10, MW 10min, dried 800C for 6h and then 2000C for 2h | Length 230–780 nm; Diameter 30-96nm | 4-nitrophenol, methylene blue | [68] |
| Cu@Ni/rGO | Ultrasonication | Water | Graphite powder, NaNO3, potassium permanganate, nickel chloride hexahydrate, copper sulphate, NaOH, hydrazine hydrate | NaOH | ultrasonicated for 15min, N2H2.H2O with NaBH4 added under Nitrogen atm. For 30 min and filtered, washed and dried at RT. | - | 4-nitrophenol hydrogenation | [69] |
| rGO/bimetallic FexCuy | Reflux | Water | Iron acetate, copper acetate, graphite oxide, sodium borohydride, ethylene glycol, NaOH | Ethylene glycol | The contents refluxed 5h at 850C. Centrifuged. Freezed for later use. | 34.7 to 44.5nm | CycloPhophamide degradation | [70] |
| SI.No | Copper Based Nanoparticles | Synthesis Method | Pollutant | Degradation time | Rate constant (min-1) Degradation efficiency | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | Cu@Alg/Co–CeO2 | One-pot synthesis | ArO (0.07mM) | 300min | 75.26% | [88] | |
| CR (0.07mM) | 270min | 33.65% | |||||
| MB (0.07mM) | 180min | 49.70% | |||||
| MO (0.07mM) | 240min | 45.54% | |||||
| 2 | Cu/GO/TiO2 | Quartz boat sealed in a furnace and argon gas is flown. | MB/ Cu (1%)-TiO2-GO | 141.1min | 4.91 min-1 | [89] | |
| MB/ Cu (2%)-TiO2-GO | 112.7min | 6.15 min-1 | |||||
| MB/ Cu (3%)-TiO2-GO | 60.8min | 11.40 min-1 | |||||
| 3 | NiO/CuO | Co-Precipitation | RB-5 | 120min | 93% | [90] | |
| RR-2 | 92% | ||||||
| O-II | 96% | ||||||
| 4 | 2D Cu nanosheets | Oriented attachment mechanism | MB | 20min | 95% | [91] | |
| 5 | CuO | Thermal decomposition | RhB | 150min | 93% | [92] | |
| 6 | Porous CuO nanosheets | Precipitation | AR | 6min | 96.99% | [93] | |
| 7 | Copper sulfide NPs | Precipitation | CV | CuS1 | 120min | 56.9% (0.0066) | [94] |
| CuS2 | 72.8% (0.0104) | ||||||
| CuS3 | 84.6% (0.0145) | ||||||
| MB |
CuS1 | 180min | 31.8% (0.006) | ||||
| CuS2 | 60.1% (0.0078) | ||||||
| CuS3 | 100min | 99.2% (0.0481) | |||||
| RhB |
CuS1 | 120min | 26.5% (0.0025) | ||||
| CuS2 | 53% (0.0062) | ||||||
| CuS3 | 81.4% (0.0127) | ||||||
| 8 | Cu2O | Congo red | 180min | 90% | [95] | ||
| 9 | CuO | Simple chemical reduction | MB | 60min | 55.5% | [96] | |
| Nano material used |
Target ions |
Temperature | pH | Contact time | Ion Concentration |
The capacity of Adsorption and removal/ detection limit | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Copper doped zeolite | Cr3+ | Room temperature for 60min and kept in refrigerator prior to analysis | 7.5 to 2 before analysis | 60min | 0.658 mg/L | 100% | [103] | |
| Pb2+ | 0.696 mg/L | 100% | ||||||
| 0.795 mg/L | 99.37% | |||||||
| Cd2+ | ||||||||
| CuO NPs | Hg2+Cr6+ | Room temperature | 7.27 | 180min | 1 g/L | 82%85% | [104] | |
| Cu NPs | Cr6+ | 250C | 3 | 180min | 20 mg/ml | 13.1mg/g(65.6%) | [105] | |
| CuFe2O4 |
Ba2+ | 250C | 7 | 120min | 10mg in 25ml | 87mg/g | [106] | |
| CuFe2O4/ rGO |
Ba2+ | 250C | 7 | 120min | 10mg in 25ml | 162 mg/g | [106] | |
| CuFe2O4/PANI | UO22+ (Uranium ions) | 250C | 4 | 60min | 322.6 mg/g | [107] | ||
| CuO NPs | Pb (II) | Room temperature | 6 | 60min | 0.33g/L | 88.80mg/g | [108] | |
| Ni (II) | 54.90mg/g | |||||||
| Cd (II) | 15.60mg/g | |||||||
| CuO NPs | Co (II) Pb (II) Ni (II) Cd (II) Cr(VI) |
Sunlight | 6.6 | 200min | 2mg/ml | 73.2% 80.8% 72.4% 64.4% 91.4% |
[109] | |
| Fluorescent CuO NPs | Bi 3+ | Room temperature | 2.7 | 15min | 50 μ L | 10 mmol L−1 | [110] |
| Nano material |
Analyte | LOD/ Detection limit |
Linear Range(mM) |
Sensitivity (µAcm-2/ nM) /Response time |
Electro chemical method used |
Ref. |
|---|---|---|---|---|---|---|
| CuO/rGO | Ascorbic acid | 189.05 µM | 500-2000 µM | - | CV | [62] |
| CuO.GdO NSs/Nafion/GCE | Glutamate | 166 × 10−6 | 166×10−6 to 100×103 | 0.567 | I-V | [50] |
| Cu-TiO2 | Enzyme less myoglobin | 14pM | 3nM-15nM | 61.51 /10ms |
CV-EIS | [120] |
| Cu/Cu2O | Cholesterol oxidation | 2.6µM | 0.5 to 1mM | 850 | CV | [20] |
| Cu2O | Glucose | 1.37 µM | 0.28-2.8mM | LDI-MS | [17] | |
| Cu/ZnO | Enzyme less myoglobin | 0.46nM | 3nM-15nM | ~2.13-10.14 | CV-EIS | [121] |
| CuO-MWCNTs/ SPCE | Glutamate | 17.5 µM | 20-200 µM | 8500 | LSV | [122] |
| Cu-ZnO nanorods | Hydrogen peroxide | 0.16 µM | 0.001-11mM | 3415 | CV | [123] |
| CuO | Non enzymatic lactic acid | 0.04 mM | 0.05-40mM | 14.47 | CV | [124] |
| Copper phthalo cyanine-borophene nanocomposite | Non enzymatic Urea | 0.05 µM | 250-1000 µM | 10.43 | CV | [125] |
| Nanomaterial | Pharmaceutical drug | Concentration of drug | Catalyst loading | Temperature/pH | Degradation source | Degradation Efficiency |
Ref |
|---|---|---|---|---|---|---|---|
| CuO NPs | Thiazolyl blue | 100mg/L | 20mg/10ml | 300K/pH 8.0 | Sonication /120min |
84.1% | [136] |
| Paracetamol | 300K/pH 7.0 | 81.2% | |||||
| M Mn dopped Cu2O | Amoxicillin | 15mg/L | 1g/L | pH 9.0 | Sunlight | 92% | [137] |
| ZnO-CuO/ clinoptilolite |
Mefenamic acid |
0.1g/L | 0.1g/L | RT/ pH=5.6 | Hg Lamp 200 min |
70% | [138] |
| Zeolite/HDTMA-Br/CuS | Metronidazole | 10mg/L | 0.01g/L | pH 7.0 | Sunlight | 100% (200min) | [139] |
| CuO-GO-DE/H2O2 | Ciprofloxacin | 50mg/L | 2g/L | 500C/ pH 7.0 | Ultrasonic impregnation | 240min | [61] |
| Sulfite activated Fe-Cu | Sulfamethazine | 5mg/L | 80mg/L | 298K/pH 6.0 | Advanced oxidation process | 87% (60min) | [140] |
| Cu-TiO2 | Levofloxacin | 50mg/L | 1g/L | pH 7.0 | Visible LED | 75.5% (6h) | [48] |
| Ba/Bi/Fe/CuO | Paracetamol | 50mg/L | 0.75g/L | pH 9.0 | Metal halide lamp J(HQI-T250/OSRAM GmbH) | 98.1% (120min) | [141] |
| CuS QDs@ZnO | ceftriaxone | 0.2g/L | RT | Solar simulator | 100% (90min) | [25] |
| Materials | Fabrication Technique/Detection system | Response time/ Response (Rg/Ra) /Sensitivity |
Linear range | Analytes | Retention Time/recovery time /LOD | Ref |
|---|---|---|---|---|---|---|
| SnO2-CuO | Slurry coated on ceramic tube | 4s | 50 to 300ppm | Ethanol | 10s | [145] |
| CuO-rGO | Gas sensor | 10.54 | 100ppm | Ethanol | 25s | [146] |
| CuO/Ti3C2TxMXene | Drop casting on printed IDE | 11.4 | 2.3 to 50ppm | Toluene | 10s | [72] |
| CNNS-Cu | Deposited on glassy carbon electrode | Immediate detection | 0.1–100 μmol L−1 | p-nitro toluene | 0.13 μmol L−1 | [147] |
| NiO-CuO/NH3 sensor | Drop casting on printed IDE | 11.7s | 25ppm to 500ppm | NH3 | 21.5s | [16] |
| PEDOT-CuO | Drop casting on GCE | 2s | 40-10000ppm | H2O2 | 8.5µm | [148] |
| CeO2/CuO | Deposited on Al2O3sensor substrate on IDE | 90-457ppb | Acetone | 670s | [49] | |
| PNIPAM-Cu@CP | Electrodepositing Cu particles on carbon paper elctrode | 72.8 μA cm-2 mM-1 | 1-300mM | Methanol | 0.3mM | [149] |
| Copper nitro prusside | Deposited on the glassy carbon electrode | 15s | 2.5 × 10−8 to2.5 × 10−1 M | Acetaldehyde | 41 × 10−8 M | [150] |
| 4HQ-rGO/Cu2+ | Deposited on IDE | 5s | 1000ppm | Acetic acid | 24s | [151] |
| AgCu/TiO2 | Coated on Alumina substrate for KSGAS6S KENOSISTEC | 22/33 | 100ppm | Xylene | 33.2s | [152] |
| Nanomaterial | Experimental Condition |
Potential | Products | Faradic Efficiency | Ref. |
|---|---|---|---|---|---|
| Cu2-x-Sey | 41.5 mA/cm2 | -1.815V | Methanol | 77.6% | [153] |
| Por-Cu | 0.25 mg/cm2 49 mA/cm2 |
-0.976V vs RHE | Methane | 27% | [156] |
| Ethylene | 17% | ||||
| CO | 10% | ||||
| Cu-X X=Nafion, PVDF |
0.1M KHCO3 -0.6V |
-1.4V(vs RHE) | HCOOH, CH4 | 30% | [157] |
| CuPc/C | 0.5 M KHCO3 aq. | -0.4 V vs. RHE | Ethylene | 42.6% | [158] |
| Cubic Cu2O and branched CuO nps | 0.1M KHCO3 5mA@2KeV 3.0 V (vs Ag/AgCl) |
- | C2H4 | 64% | [159] |
| Cu/NC | -4.9 mA/cm2 | -0.8V vs RHE | Formate | 40.9% | [160] |
| Acetate | 16% | ||||
| Cu95Sn5 | 0.1 M KHCO3 6.58 mA/cm2 0 V to −1.1 V vs. RHE |
−0.9 V vs. RHE | CO | 93% | [161] |
| CuO | M KHCO3 50 mA/cm2 |
-1.1V | C2H4 | 41% | [162] |
| 3D Cu skeleton | -2V(vs. Ag/AgCl); -3.0 A/cm2; 0.5M NaHCO3 |
-1.0 V vsRHE | C2H4, C2H6 | - | [163] |
| Cu/CuxO PCC | 0.5 M KHCO3-0.1V to -1.1V vs RHE | -0.5V vs RHE | C2H5OH | 50% | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





