Submitted:
20 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
Keywords:
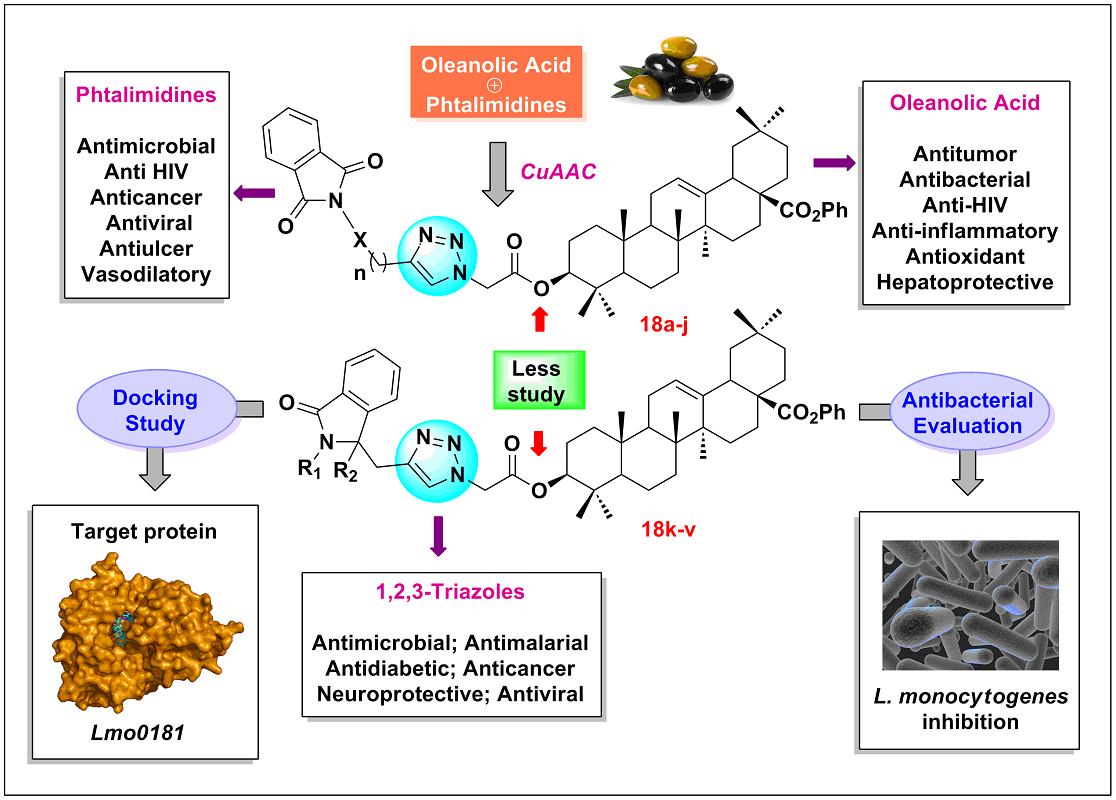
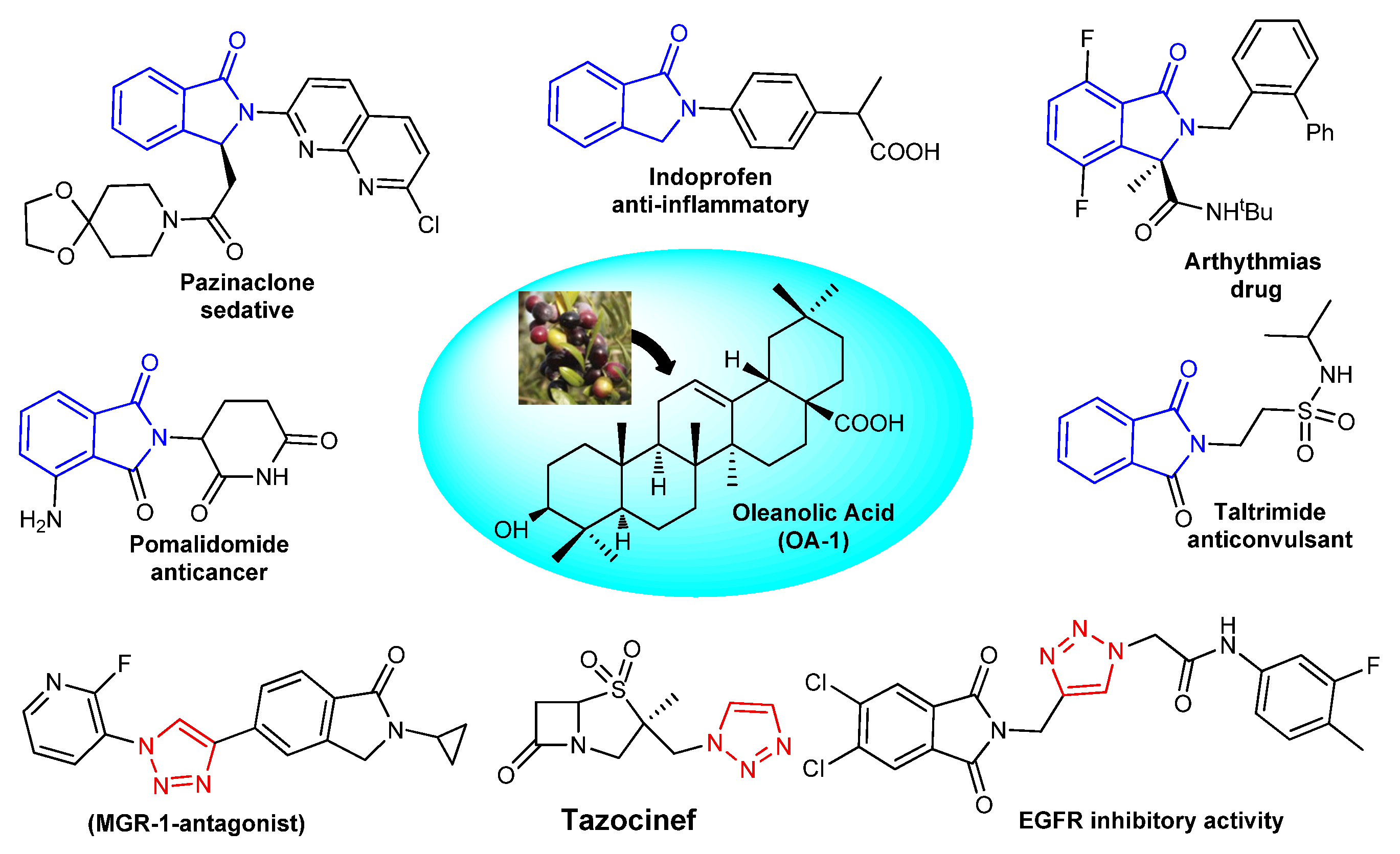
1. Introduction
2. Results and Discussion
2.1. Chemistry
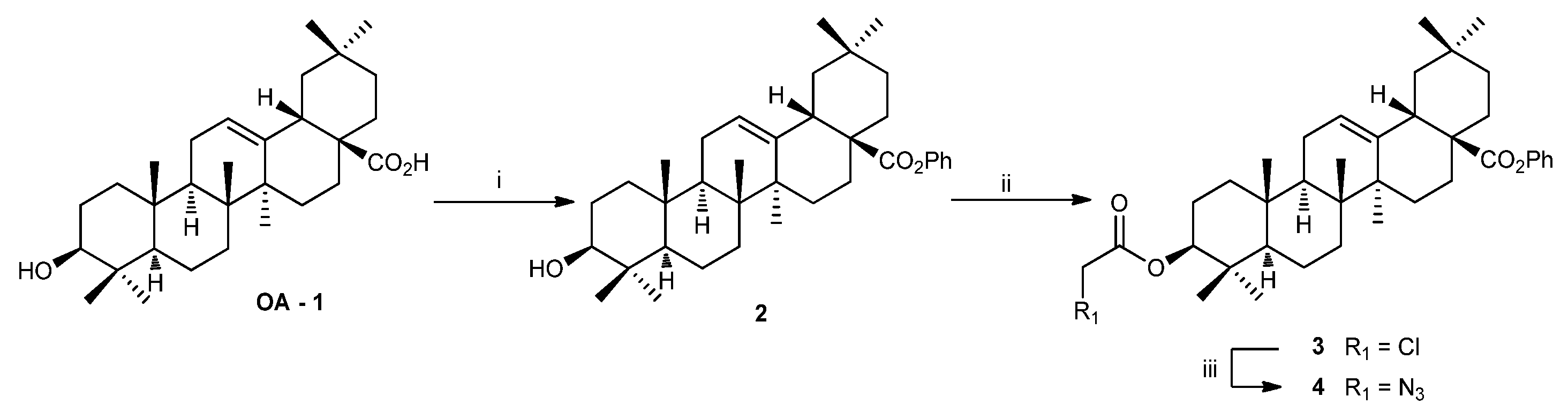
2.1.1. Isolation of oleanolic acid OA-1
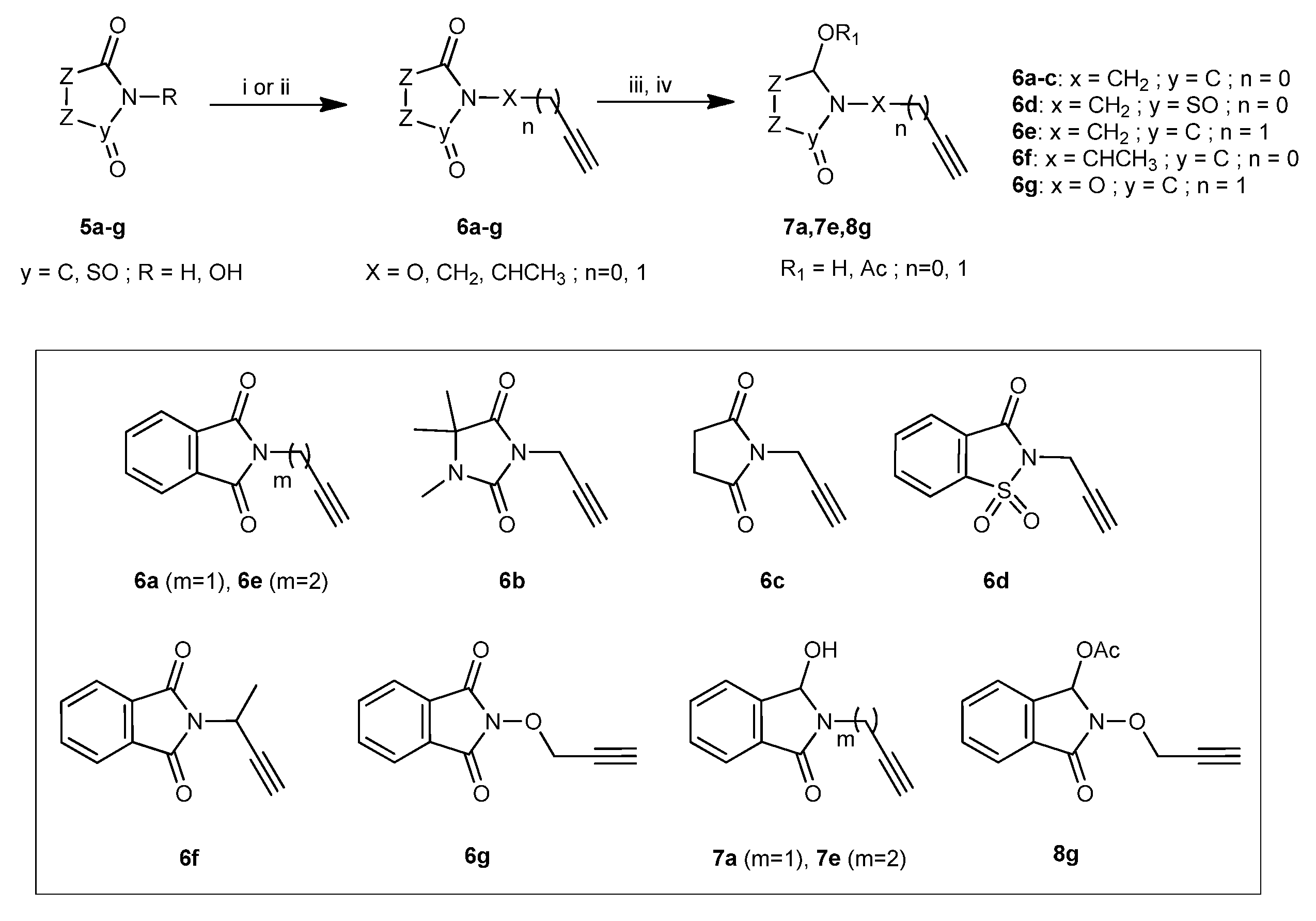
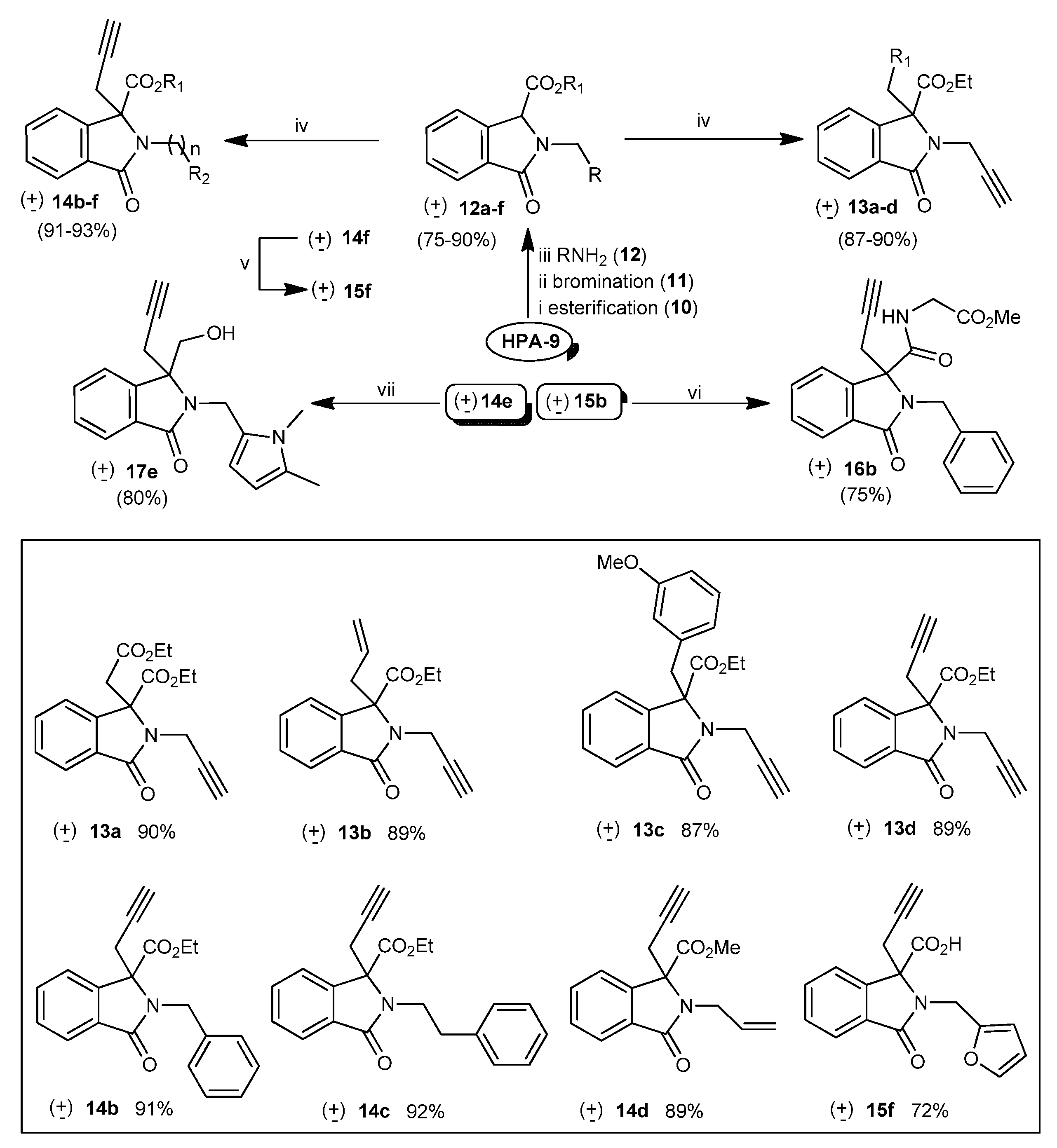
2.1.2. Synthesis
2.2. Antibacterial avtivity
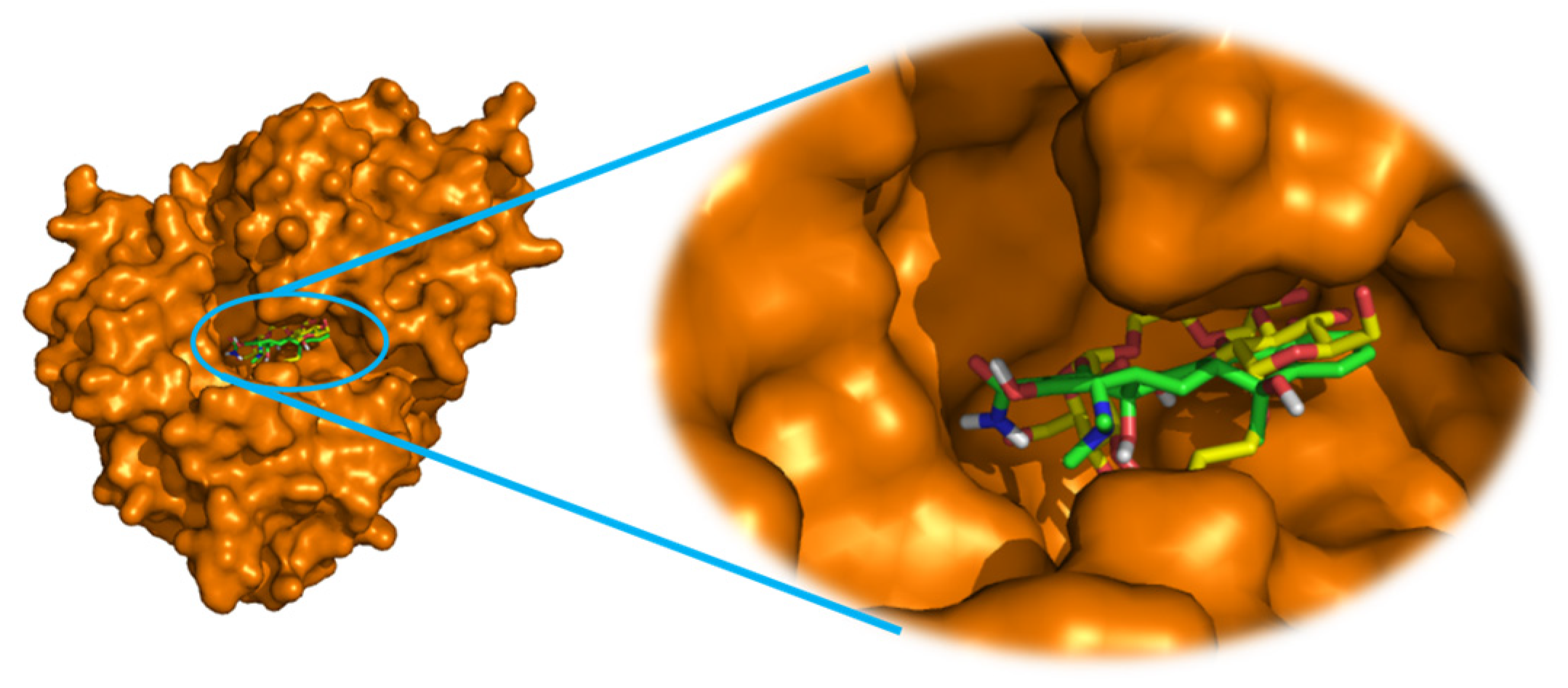
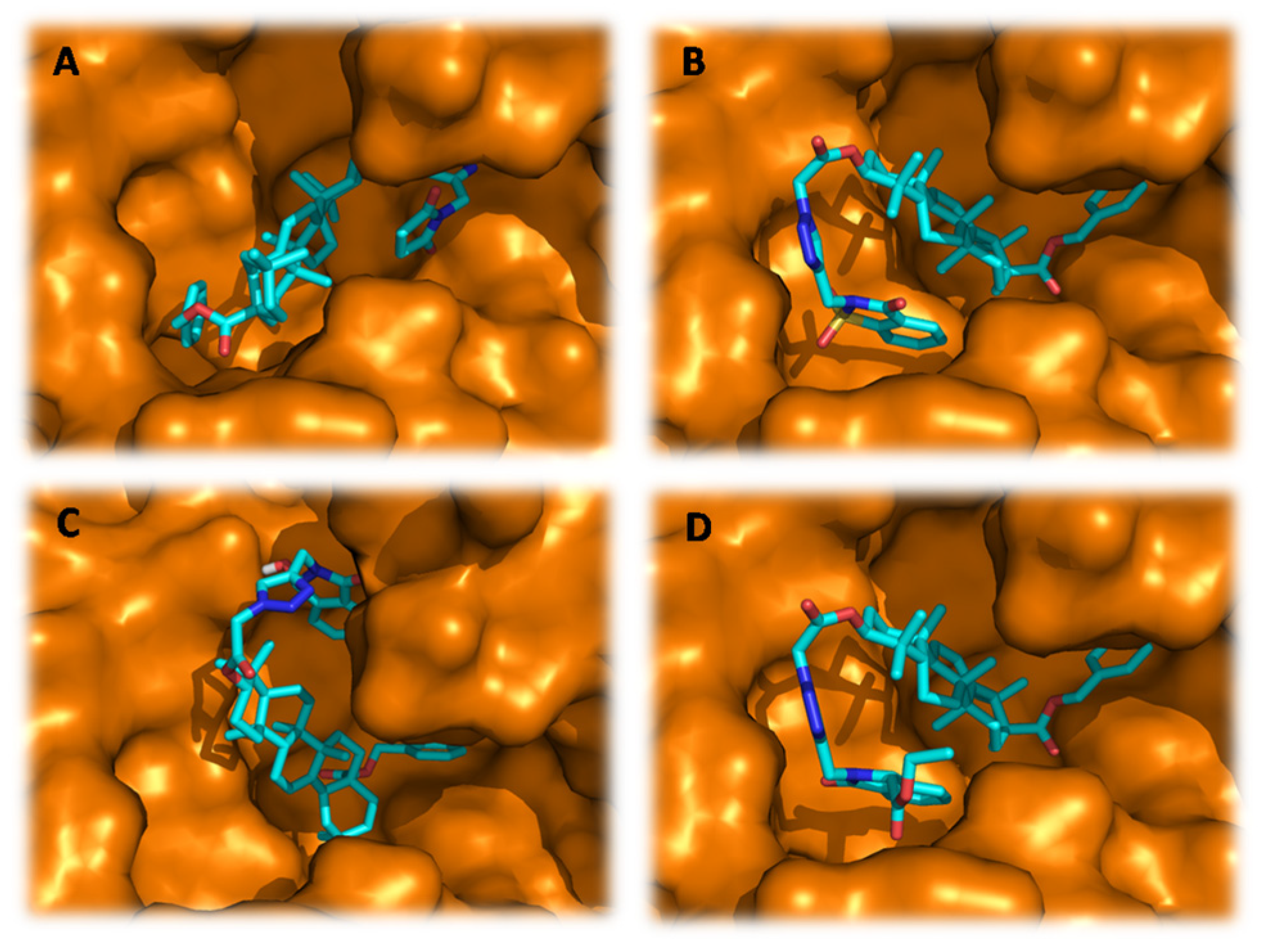
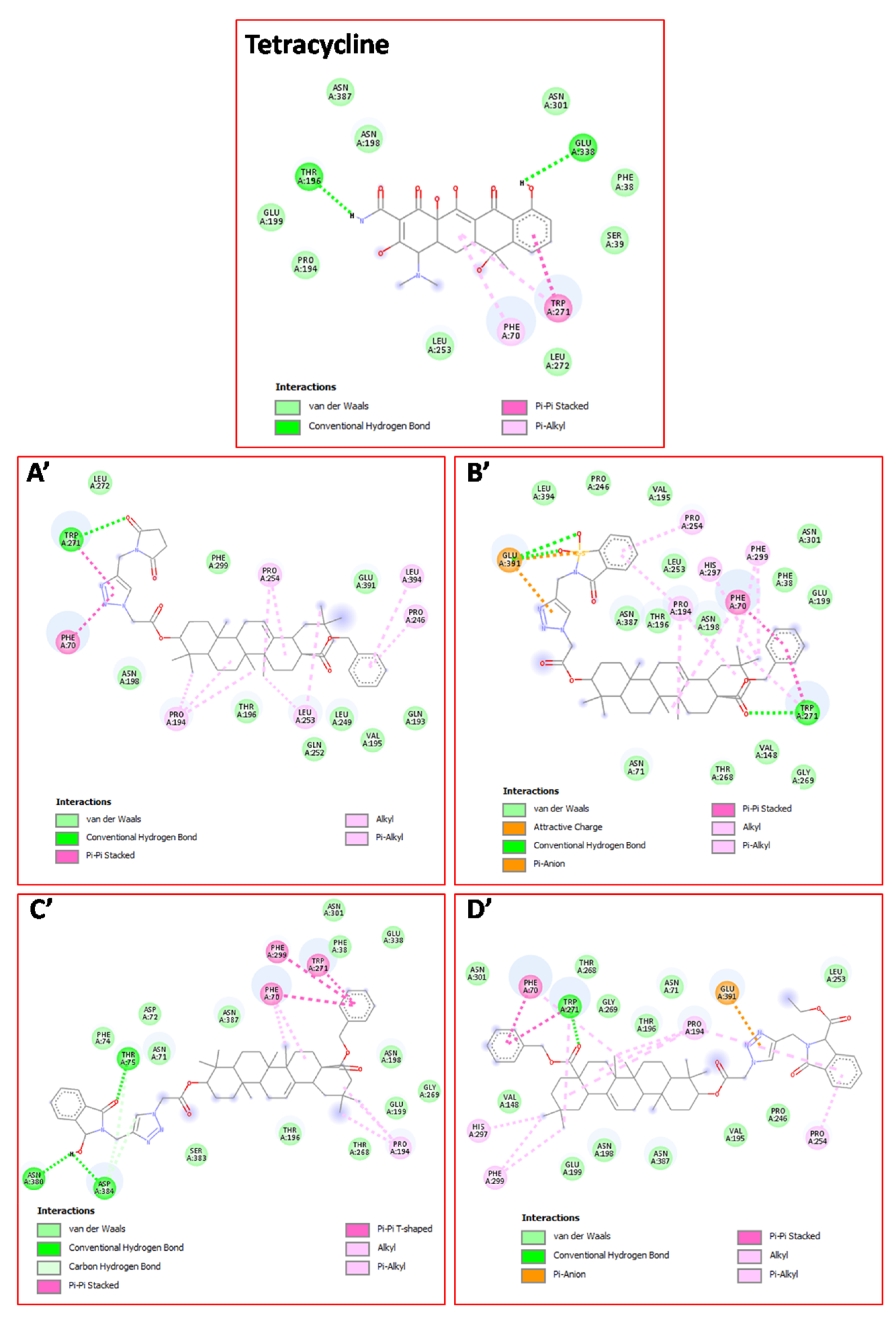
2.3. Molecular docking study
3. Materials and Methods
3.1. General experimental procedure
3.2. Chemistry
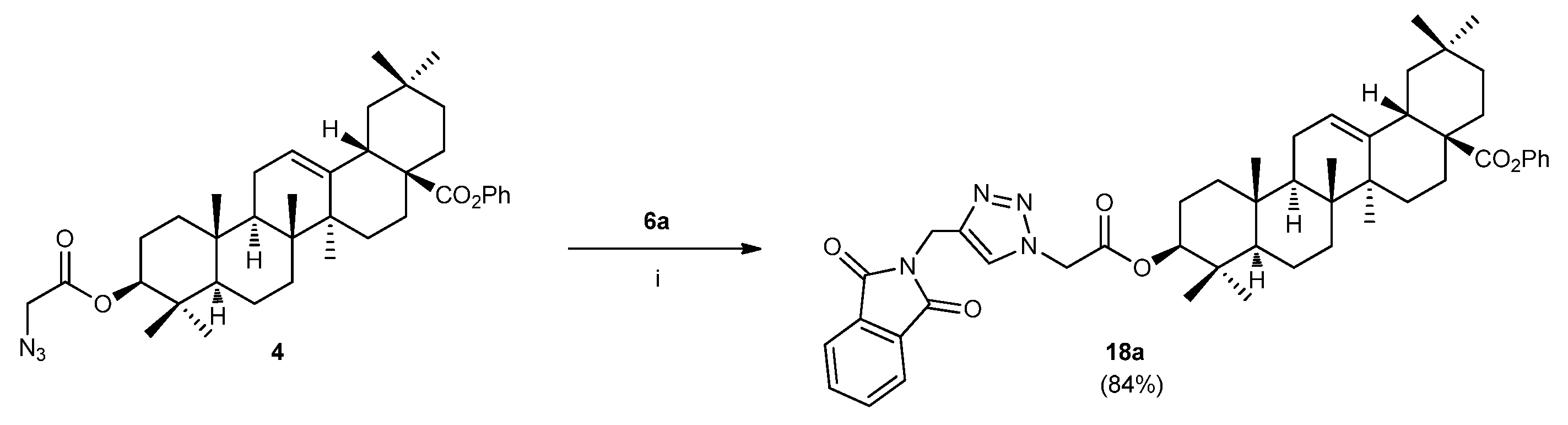
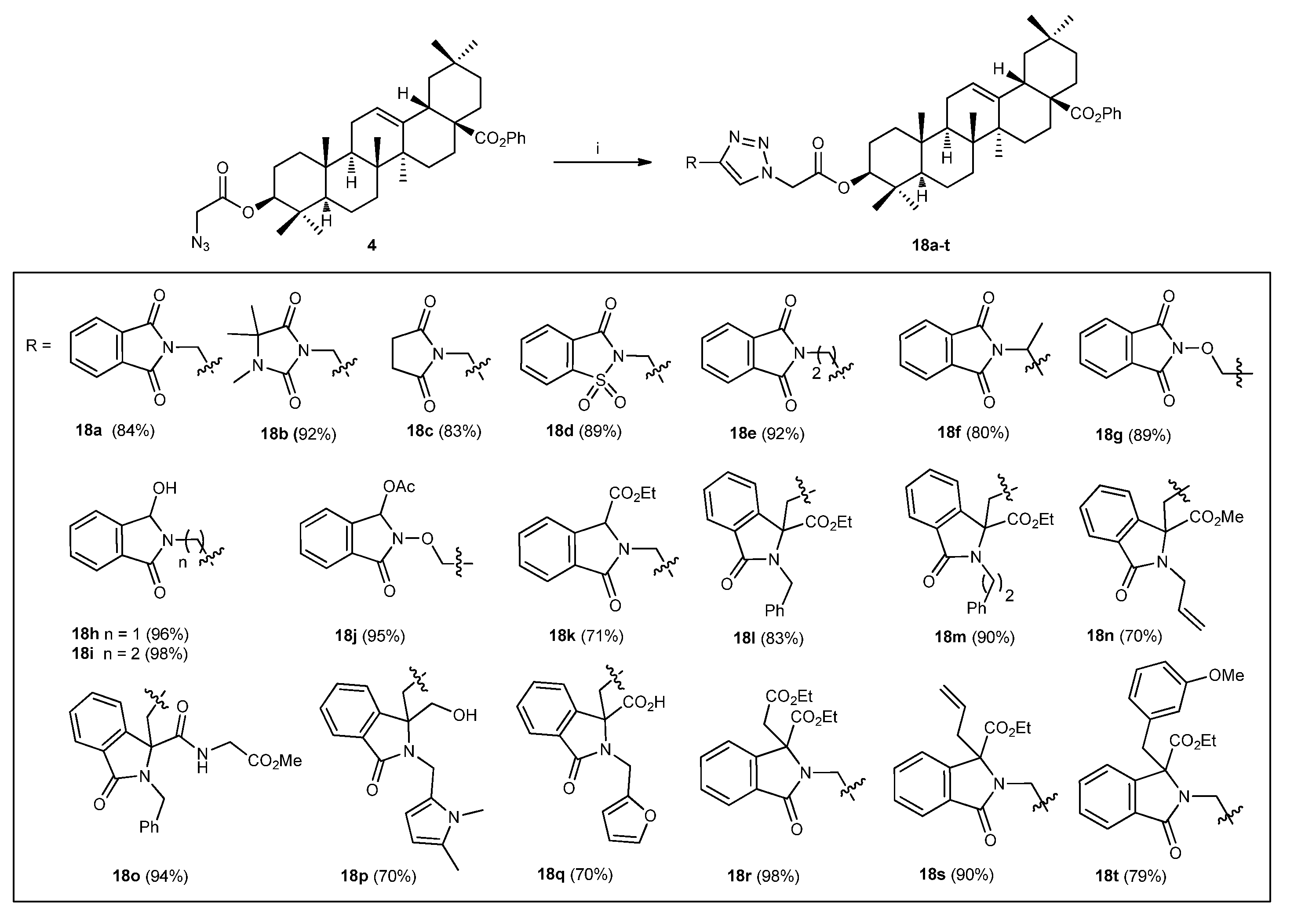
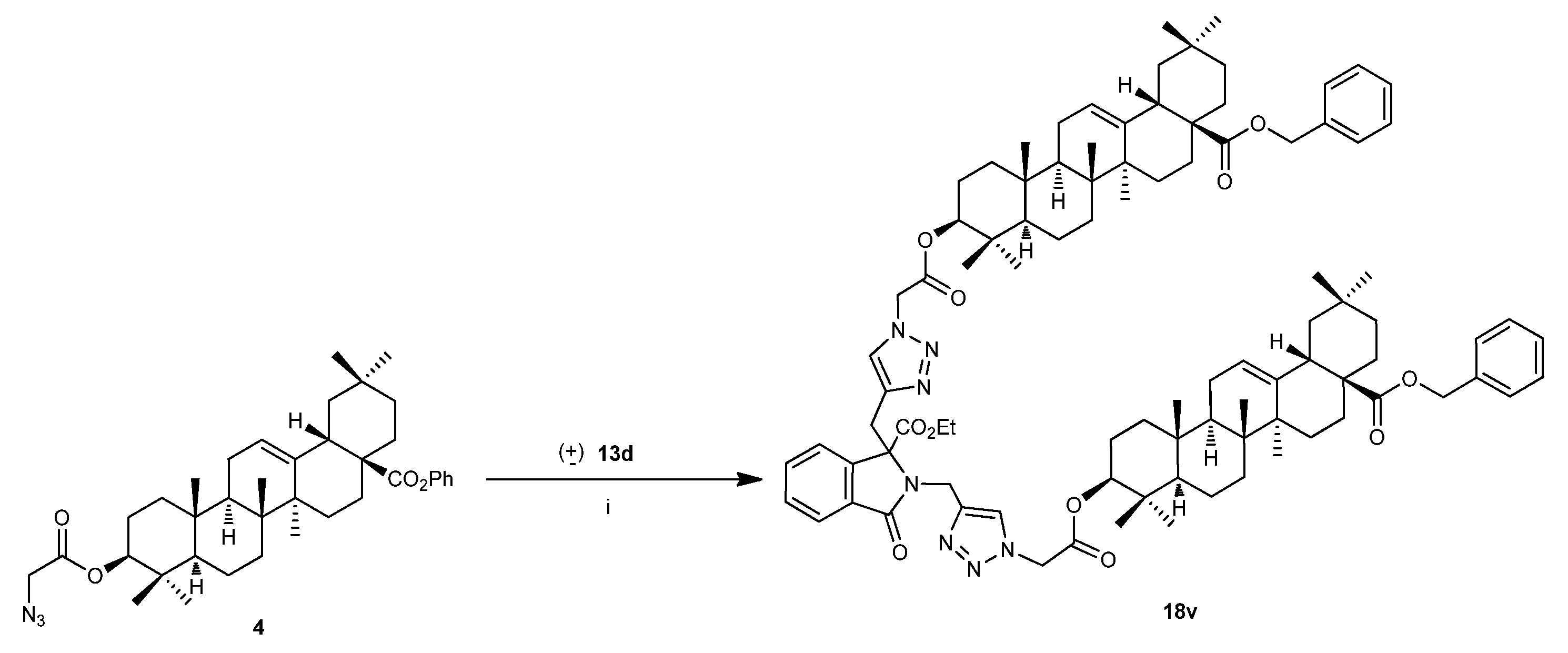
3.2.1. General procedure for the preparation of compounds 18
3.2.1.1. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((1,3-dioxoisoindolin-2-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18a)
3.2.1.2. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 2,2,6a,6b,9,9,12a-heptamethyl-10-(2-(4-((3,4,4-trimethyl-2,5-dioxoimidazolidin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18b)
3.2.1.3. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((2,5-dioxopyrrolidin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18c)
3.2.1.4. (4aS,6aS,6bR,10S,12aR)-benzyl 10-(2-(4-((1,1-dioxido-3-oxobenzo[d]isothiazol-2(3H)-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18d)
3.2.1.5. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-(2-(1,3-dioxoisoindolin-2-yl)ethyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18e)
3.2.1.6. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-(1-(1,3-dioxoisoindolin-2-yl)ethyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18f)
3.2.1.7. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-(((1,3-dioxoisoindolin-2-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18g)
3.2.1.8. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((1-hydroxy-3-oxoisoindolin-2-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18h)
3.2.1.9. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-(2-(1-hydroxy-3-oxoisoindolin-2-yl)ethyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18i)
3.2.1.10. methyl2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-oxoisoindoline-1-carboxylate (18j)
3.2.1.11. ethyl2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxoisoindoline-1-carboxylate (18k)
3.2.1.12. ethyl2-benzyl-1-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxoisoindoline-1-carboxylate (18l)
3.2.1.13. ethyl1-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxo-2-phenethylisoindoline-1-carboxylate (18m)
3.2.1.14. methyl2-allyl-1-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxoisoindoline-1-carboxylate (18n)
3.2.1.15. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((2-benzyl-1-((2-methoxy-2-oxoethyl)carbamoyl)-3-oxoisoindolin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18o)
3.2.1.16. (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((2-((1,5-dimethyl-1H-pyrrol-2-yl)methyl)-1-(hydroxymethyl)-3-oxoisoindolin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18p)
3.2.1.17. 1-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(furan-2-ylmethyl)-3-oxoisoindoline-1-carboxylic acid (18q)
3.2.1.18. ethyl2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-1-(2-ethoxy-2-oxoethyl)-3-oxoisoindoline-1-carboxylate (18r)
3.2.1.19. ethyl 1-allyl-2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxoisoindoline-1-carboxylate (18s)
3.2.1.20. ethyl2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-1-(3-methoxybenzyl)-3-oxoisoindoline-1-carboxylate (18t)
3.2.1.21. ethyl1,2-bis((1-(2-(((3S,4aR,6aR,6bS,8aS,12aR,14aS,14bS)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxo-2,3-dihydro-1H-isoindole-1-carboxylate (18v)
3.3. Antibacterial activity
3.4. Molecular docking procedure
3.5. Statistical analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural Waste: Review of the evolution,approaches and perspectives on alternative uses. Global. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic acid: Extraction, characterization and biological activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Lee, W.; Yang, E.-J.; Ku, S.-K.; Song, K.-S.; Bae, J.-S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 2013, 36, 94–102. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, Q.; Bo, S.; Zeng, X.; Wei, Y.; Lei, S.; Xiao, X.; Xiao, J.; Wang, Z. Advances in research on hepatoprotective activity and synthesis of oleanolic acid derivatives. J. Appl. Biopharm. Pharmacokinet. 2015, 3, 27–33. [Google Scholar] [CrossRef]
- Ghafoor, K. Antioxidant properties of oleanolic acid from grape peel. Agro-food Ind. Hi Tech. 2014, 25, 54–57. [Google Scholar]
- Juan, M.E.; Wenzel, U.; Ruiz-Gutierrez, V.; Daniel, H.; Planas, J.M. Olive fruit extracts inhibit proliferation and induce apoptosisin HT-29 human colon cancer cells. J. Nutr. 2006, 136, 2553–2557. [Google Scholar] [CrossRef]
- Yamai, H.; Sawada, N.; Yoshida, T.; Seike, J.; Takizawa, H.; Kenzaki, K.; Miyoshi, T.; Kondo, K.; Bando, Y.; Ohnishi, Y.; Tangoku, A. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int. J. Cancer 2009, 125, 952–960. [Google Scholar] [CrossRef]
- Kuo, R.-Y.; Qian, K.; Morris-Natschke, S.L.; Lee, K.-H. Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat. Prod. Rep. 2009, 26, 1321–1344. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, X.; Dong, J.; Chen, D.; Liu, J.; Zhang, L.; Wen, X. Synthesis and evaluation of novel oleanolic acid derivatives as potential antidiabetic agents. Chem. Biol. Drug. Des. 2014, 83, 297–305. [Google Scholar] [CrossRef]
- Melo, T.S.; Gattass, C.R.; Soares, D.C.; Cunha, M.R.; Ferreira, C.; Tavares, M.T.; Saraiva, E.; Parise-Filho, R.; Braden, H.; Delorenzi, J.C. Oleanolic acid (OA) as an antileishmanial agent: Biological evaluation and in silico mechanistic insights. Parasitol. Int. 2016, 65, 227–237. [Google Scholar] [CrossRef]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef]
- Hichri, F.; Ben Jannet, H.; Cheriaa, J.; Jegham, S.; Mighri, Z. Antibacterial activities of a few prepared derivatives of oleanolic acid and of other natural triterpenic compounds. C. R. Chim. 2003, 6, 473–483. [Google Scholar] [CrossRef]
- Chouaïb, K.; Hichri, F.; Nguir, A.; Daami-Remadi, M.; Elie, N.; Touboul, D.; Ben Jannet, H. Semi-synthesis of new antimicrobial esters from the natural oleanolic and maslinic acids. Food chem. 2015, 183, 8–17. [Google Scholar] [CrossRef]
- Park, S.N.; Lim, Y.K.; Choi, M.H.; Cho, E.; Bang, I.S.; Kim, J.M. , Ahn, S.J.; Kook, J.K. Antimicrobial mechanism of oleanolic and ursolic acids on Streptococcus mutans UA159. Curr. Microbiol. 2018, 75, 11–19. [Google Scholar] [CrossRef]
- Savela, R.; Méndez-Gálvez, C. Isoindolinone synthesis via one-pot type transition metal catalyzed C-C bond forming reactions. Chem. Eur. J. 2021, 27, 5344–5378. [Google Scholar] [CrossRef]
- Upadhyay, S.P.; Thapa, P.; Sharma, R.; Sharma, M. 1-Isoindolinone scaffold-based natural products with a promising diverse bioactivity. Fitoterapia 2020, 146, 104722. [Google Scholar] [CrossRef]
- Thakur, K.; Singh, G. A Comprehensive Review on SAR and Activities of Isoindolinone. Eur. J. Mol. Clin. Med. 2020, 7, 3658–3668. [Google Scholar]
- Othman, I.M.; Gad-Elkareem, M.A.; El-Naggar, M.; Nossier, E.S.; Amr, A.E.G.E. Novel phthalimide based analogues: Design, synthesis, biological evaluation, and molecular docking studies. J. Enzyme Inhib. Med. Chem. 2019, 34, 1259–1270. [Google Scholar] [CrossRef]
- Ferland, J.-M.; Demerson, C.A.; Humber, L.G. Synthesis of derivatives of isoindole and of pyrazino[2,1-a]isoindole. Can. J. Chem. 1985, 63, 361–365. [Google Scholar] [CrossRef]
- Zhuang, Z.-P.; Kung, M.-P.; Mu, M.; Kung, H.F. Isoindol-1-one analogues of 4-(2‘-methoxyphenyl)-1-[2‘-[N-(2‘‘-pyridyl)-p-iodobenzamido]ethyl]piperazine (p-MPPI) as 5-HT1A receptor ligands. J. Med. Chem. 1998, 41, 157–166. [Google Scholar] [CrossRef]
- Linden, M.; Hadler, D.; Hofmann, S. Randomized, double-blind, placebo-controlled trial of the efficacy and tolerability of a new isoindoline derivative (DN-2327) in generalized anxiety. Hum. Psychopharmacol. 1997, 12, 445–452. [Google Scholar] [CrossRef]
- Norman, M.H.; Minick, D.J.; Rigdon, G.C. Effect of linking bridge modifications on the antipsychotic profile of some phthalimide and isoindolinone derivatives. J. Med. Chem. 1996, 39, 149–157. [Google Scholar] [CrossRef]
- Meng, X.B.; Han, D.; Zhang, S.N.; Guo, W., Cui, J.R.; Li, Z.J. Synthesis and anti-inflammatory activity of N-phthalimidomethyl 2,3-dideoxy- and 2,3-unsaturated glycosides. Carbohydr. Res. 2007, 342, 1169–1174. [CrossRef]
- Laboratori Baldacci, S. P. A. Japanese Patent 5,946,268,1984; Chem. Abstr., 1984, 101, 54922. [Google Scholar]
- Achinami, K.; Ashizawa, N.; Kobayasui, F. Japanese Patent 03,133,955, 1991; Chem. Abstr. 1991, 115, 255977j. [Google Scholar]
- De Clercq, E. Toward Improved Anti-HIV Chemotherapy: Therapeutic Strategies for Intervention with HIV Infections. J. Med. Chem. 1995, 38, 2491–2517. [Google Scholar] [CrossRef]
- Pendrak, I.; Barney, S.; Wittrock, R.; Lambert, D.M.; Kingsbury, W.D. Synthesis and Anti-HSV Activity of A-Ring-Deleted Mappicine Ketone Analog. J. Org. Chem. 1994, 59, 2623–2625. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Ravindranath, K.R.; Viswanathan, N. Mappicine, a minor alkaloid from Mappia foetida miers. J. Chem. Soc. Perkin Trans. 1. 1974, 1, 1215–1217. [Google Scholar] [CrossRef]
- Mertens, A.; Zilch, J.H.; Konig, B.; Schafer, W.; Poll, T.; Kampe, W.; Seidel, S.; Leser, U.; Leinert, H. Selective non-nucleoside HIV-1 reverse transcriptase inhibitors. New 2,3-dihydrothiazolo[2,3-a]isoindol-5(9bH)-ones and related compounds with anti-HIV-1 activity. J. Med. Chem. 1993, 36, 2526–2535. [CrossRef]
- Rezaei, Z.; Moghimi, S.; Javaheri, R.; Asadi, M.; Mahdavi, M.; Shabani, S.; Edraki, N.; Firuzi, O.; Safavi, M.; Amini, M.; Asadipour, A. Synthesis and biological evaluation of 1, 3, 4-thiadiazole linked phthalimide derivatives as anticancer agents. Lett. Drug Des. Discov. 2017, 14, 1138–1144. [Google Scholar] [CrossRef]
- Taylor, E.C.; Zhou, P.; Jenning, L.D.; Mao, Z.; Hu, B.; Jun, J.-G. Novel synthesis of a conformationally-constrained analog of DDATHF. Tetrahedron Lett. 1997, 38, 521–524. [Google Scholar] [CrossRef]
- Armoiry, X.; Aulagner, G.; Facon, T. Lenalidomide in the treatment of multiple myeloma: a review. J. Clin. Pharm.Ther. 2008, 33, 219–226. [Google Scholar] [CrossRef]
- Kim, K.H.; Noh, H.J.; Choi, S.U.; Lee, K.R. Isohericenone, a new cytotoxic isoindolinone alkaloid from Hericium erinaceum. J. Antibiot. 2012, 65, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.M.; El-masry, A.H.; Mohamed, N.A.; Awad, G.E.; Habib, B.S. Synthesis, characterization and antimicrobial activity of some novel isoindole-1,3-dione derivatives. Der Pharm. Chem. 2013, 5, 97–108. [Google Scholar]
- Dabholkar, V.V.; Udawant, D.; Jaiswar, R.; Gopinathan, A. Novel 2-(5-(substituted-benzyl)-1,3,4-thiadiazol-2-yl)-substituted-isoindoline-1,3-dione derivatives—Their one pot synthesis and Antimicrobial evaluation. J. Chem. Sci. 2019, 9, 274–280. [Google Scholar]
- Takahashi, I.; Kawakami, T.; Hirano, V.; Yokota, H.; Kitajima, H. Novel phthalimidine synthesis. Mannich condensation of o-phthalaldehyde with primary amines using 1,2,3-1H-benzotriazole and 2-mercaptoethanol as dual synthetic auxiliaries. Synlett 1996, 4, 353–355. [CrossRef]
- Klaus, S.; Thomas, M. The chemistry of isoindole natural products. Beilstein. J. Org. Chem. 2013, 9, 2048–2078. [Google Scholar] [CrossRef]
- Kovtunenko, V.A.; Voitenko, Z.V. The chemistry of isoindoles. Russ. Chem. Rev. 1994, 63, 997–1018. [Google Scholar] [CrossRef]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S.; Bérubé, G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discov. 2013, 8, 1029–1047. [Google Scholar] [CrossRef]
- Sflakidou, E.; Leonidis, G.; Foroglou, E.; Siokatas, C.; Sarli, V. Recent Advances in Natural Product-Based Hybrids as Anti-Cancer Agents. Molecules 2022, 27, 6632. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.H.; Yadav, J.P. Concept of hybrid drugs and recent advancements in anticancer hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef]
- Marinho, J.A.; Guimarães, D.S.M.; Glanzmann, N.; de Almeida Pimentel, G.; da Costa Nunes, I.K.; Pereira, H.M.G.; Navarro, M.; de Pilla Varotti, F.; da Silva, A.D.; Abramo, C. In vitro and in vivo antiplasmodial activity of novel quinoline derivative compounds by molecular hybridization. Eur. J. Med. Chem. 2021, 215, 113271. [Google Scholar] [CrossRef]
- Hosseini-Zare, M.S.; Sarhadi, M.; Zarei, M.; Thilagavathi, R.; Selvam, C. Synergistic effects of curcumin and its analogs with other bioactive compounds: A comprehensive review. Eur. J. Med. Chem. 2021, 210, 113072. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigbe, B.A. Pentacyclic triterpenoids with nitrogen-containing heterocyclic moiety, privileged hybrids in anticancer drug discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef] [PubMed]
- Cars, O.; Nordberg, P. Antibiotic resistance–The faceless threat. Int. J. Risk Saf. Med. 2005, 17, 103–110. [Google Scholar]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- Mashayekh, K.; Shiri, P. An overview of recent advances in the applications of click chemistry in the synthesis of bioconjugates with anticancer activities. Chemistry Select. 2019, 4, 13459–13478. [Google Scholar] [CrossRef]
- Brennan, J.L.; Hatzakis, N.S.; Tshikhudo, T.R.; Dirvianskyte, N.; Razumas, V.; Patkar, S.; Vind, J.; Svendsen, A.; Nolte, R.J.; Rowan, A.E.; Brust, M. Bionanoconjugation via click chemistry: The creation of functional hybrids of lipases and gold nanoparticles. Bioconjug. Chem. 2006, 17, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Zaia, J. The 2022 Nobel Prize in Chemistry for the development of click chemistry and bioorthogonal chemistry. Anal. Bioanal. Chem. 2023, 415, 527–532. [Google Scholar] [CrossRef]
- Sun, L.; Huang, T.; Dick, A.; Meuser, M.E.; Zalloum, W.A.; Chen, C. H.; Ding, X.; Gao, P.; Cockin, S.; Lee, K-H. ; Zhan, P.; Liu, X. Design, synthesis andstructure-activity relationships of 4-phenyl-1H-1, 2, 3-triazole phenylalaninederivatives as novel HIV-1 capsid inhibitors with promising antiviral activities. Eur. J. Med. Chem. 2020, 190, 112085–112103. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Sangwan, J. Regioselective synthesis, antibacterial, and antioxidant activities of ester-linked 1, 4-disubstituted 1, 2, 3-triazoles. Monatsh.Chem. 2020, 151, 807–819. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Chahal, M. Synthesis, antimalarial and antioxidant activity of coumarin appended 1, 4-disubstituted 1, 2, 3-triazoles. Monatsh. Chem. 2021, 152, 1001–1012. [Google Scholar] [CrossRef]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, S.J.; Lv, Z.S.; Gao, F.; Wang, Y.L.; Zhang, F.; Bai, L.Y.; Deng, J.L.; Wang, Q.; Fan, Y.L. Design, synthesis, and evaluation of tetraethylene glycol-tetheredisatin–1, 2, 3-triazole–coumarin hybrids as novel anticancer agents. J. Heterocycl. Chem. 2019, 56, 1127–1132. [Google Scholar] [CrossRef]
- Endoori, S.; Gulipalli, K.C.; Bodige, S.; Ravula, P.; Seelam, N. Design, synthesis, anticancer activity, and in silico studies of novel imidazo [1, 2-a] pyridinebased 1 H-1, 2, 3-triazole derivatives. J. Heterocycl. Chem. 2021, 58, 1311–1320. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, L.; Huang, X.; Wang, X.; Gong, M. Chalcone hybrids and theirantimalarial activity. Arch. Pharm. 2020, 353, 1900350–1900360. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.M.D.; Gonzaga, D.T.; da Silva, F.C.; Ferreira, V.F.; Garcia, C.R. Plasmodium falciparum knockout for the GPCR-like PfSR25 receptor displays greatersusceptibility to 1, 2, 3-triazole compounds that block malaria parasite development. Biomolecules 2020, 10, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Chouaïb, K.; Romdhane, A.; Delemasure, S.; Dutartre, P.; Elie, N.; Touboul, D. Regiospecific synthesis, anti-inflammatory and anticancer evaluation of novel 3, 5-disubstituted isoxazoles from the natural maslinic and oleanolic acids. Ind. Crops Prod. 2016, 85, 287–299. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, L.; Li, L.; Liu, J.; Li, H.; Zhang, L.; Chen, L.; Cheng, K.; Zheng, M.; Wen, X.; Zhang, P.; Hao, J.; Gong, Y.; Zhang, X.; Zhu, X.; Chen, J.; Liu, H.; Jiang, H.; Luo, C.; Sun, H. Identification of pentacyclic triterpenes derivatives as potent inhibitors against glycogen phosphorylase based on 3D-QSAR studies. Eur. J. Med. Chem. 2011, 46, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, X.; Dong, J.; Chen, D.; Liu, J.; Zhang, L.; Wen, X. Synthesis and evaluation of novel oleanolic acid derivatives as potential antidiabetic agents. Chem. Biol. Drug Des. 2014, 83, 297–305. [Google Scholar] [CrossRef]
- Ihmaid, S.K.; Alraqa, S.Y.; Aouad, M.R.; Aljuhani, A.; Elbadawy, H.M.; Salama, S.A.; Rezki, N.; Ahmed, H.E. Design of molecular hybrids of phthalimide-triazole agents with potent selective MCF-7/HepG2 cytotoxicity: Synthesis, EGFR inhibitory effect, and metabolic stability. Bioorg. Chem. 2021, 111, 104835. [Google Scholar] [CrossRef]
- Gutekunst, W.R.; Hawker, C.J. ; A general approach to sequence-controlled polymers using macrocyclic ring opening metathesis polymerization. J. Am. Chem. Soc. 2015, 137, 8038–8041. [Google Scholar] [CrossRef]
- Rubio-Ruiz, B.; Perez-Lopez, A.M.; Sebastián, V.; Unciti-Broceta, A. A minimally-masked inactive prodrug of panobinostat that is bioorthogonally activated by gold chemistry. Bioorg. Med. Chem. 2021, 41, 116217. [Google Scholar] [CrossRef] [PubMed]
- Rateb, H.S.; Ahmed, H.E.; Ahmed, S.; Ihmaid, S.; Afifi, T.H. Discovery of novel phthalimide analogs: Synthesis, antimicrobial and antitubercular screening with molecular docking studies. EXCLI journal 2016, 15, 781. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Hadley, M.; Ustinova, K.; Pavlicek, J.; Knox, T.; Noonepalle, S.; Tavares, M.T.; Zimprich, C.A.; Zhang, G.; Robers, M.B.; Bařinka, C. Discovery of a new isoxazole-3-hydroxamate-based histone deacetylase 6 inhibitor SS-208 with antitumor activity in syngeneic melanoma mouse models. J. Med. Chem. 2019, 62, 8557–8577. [Google Scholar] [CrossRef] [PubMed]
- Anwar, U.; Casaschi, A.; Grigg, R.; Sansano, J.M. Palladium catalysed queuing processes. Part 2: Termolecular cyclization–anion capture employing carbon monoxide as a relay switch with in situ generated vinylstannanes. Tetrahedron 2001, 57, 1361–1367. [CrossRef]
- Padula, D.; Mazzeo, G.; Santoro, E.; Scafato, P.; Belviso, S.; Superchi, S. Amplification of the chiroptical response of UV-transparent amines and alcohols by N-phthalimide derivatization enabling absolute configuration determination through ECD computational analysis. Org. Biomol. Chem. 2020, 18, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Kumara Swamy, K.C.; Bhuvan Kumar, N.N.; Balaraman, E.; Pavan Kumar, K.V.P. Mitsunobu and Related Reactions: Advances and Applications. Chem. Rev. 2009, 109, 2551–2651. [Google Scholar] [CrossRef]
- Kumari Sahu, A.; Unnava, R.; Shit, S.; Saikia, A.K. In(OTf)3-catalyzed one-pot tandem mannich and conia-ene cyclization reaction of N-propargyl amido alcohols with 1,3-dicarbonyl compounds: An approach to construct tetrahydro-1H-pyrrolo[2,1-a]isoindolone-1,1-dicarboxylate and its application. J. Org. Chem. 2020, 85, 1961−1971. [CrossRef]
- Szemes, F.; Fousse, A.; Bousquet, T.; Ben Othman, R.; Othman, M.; Dalla, V. Towards simplifying the chemistry of N-acyliminium ions: A one-pot protocol for the preparation of 5-acetoxy pyrrolidin-2-ones and 2-acetoxy N-alkoxycarbonyl pyrrolidines from imides. Synthesis 2006, 5, 875–879. [Google Scholar] [CrossRef]
- Ben Othman, R.; Othman, M.; Coste, S.; Decroix, B. One-pot enyne metathesis/diels–alder reaction for the construction of highly functionalized novel polycyclic aza-compounds. Tetrahedron 2008, 64, 559–567. [Google Scholar] [CrossRef]
- Rammah, M.M.; Othman, M.; Ciamala, K.; Strohmann, C.; Rammah, M.B. Silver-catalyzed spirolactonization: First synthesis of spiroisoindole-γ-methylene-γ-butyrolactones. Tetrahedron 2008, 64, 3505–3516. [Google Scholar] [CrossRef]
- Chortani, S.; Othman, M.; Lawson, A.M.; Romdhane, A.; Ben Jannet, H.; Knorr, M.; Brieger, L.; Strohmann, C.; Daïch, A. Aza-heterocyclic frameworks through intramolecular π-system trapping of spiro-N-acyliminiums generated from isoindolinone. New J. Chem. 2021, 45, 2393–2403. [Google Scholar] [CrossRef]
- Pesquet, A.; Marzag, H.; Knorr, M.; Strohmann, C.; Lawson, A.M.; Ghinet, A.; Dubois, J.; Amaury, F.; Daïch, A.; Othman, M. Access to 3-spiroindolizines containing an isoindole ring through intra-molecular arylation of spiro-N-acyliminium species: a new family of potent farnesyltransferase inhibitors. Org. Biomol. Chem. 2019, 17, 2798–2808. [Google Scholar] [CrossRef]
- Yadava, P.; Kaushik, C.P.; Mukesh Kumar, M.; Kumar, A. Phthalimide/Naphthalimide containing 1,2,3-triazole hybrids: Synthesis and antimicrobial evaluation. J. Mol. Struct. 2023, 1276, 134688. [Google Scholar] [CrossRef]
- Horchani, M.; Hajlaoui, A.; Harrath, A.H.; Mansour, L.; Ben Jannet, H.; Romdhane, A. New pyrazolo-triazolo-pyrimidine derivatives as antibacterial agents: Design and synthesis, molecular docking and DFT studies. J. Mol. Struct. 2020, 1199, 127007. [Google Scholar] [CrossRef]
- Horchani, M.; Edziri, H.; Harrath, A.H.; Ben Jannet, H.; Romdhane, A. Access to new schiff bases tethered with pyrazolopyrimidinone as antibacterial agents: Design and synthesis, molecular docking and DFT analysis. J. Mol. Struct. 2022, 1248, 131523. [Google Scholar] [CrossRef]
- Dbeibia, A.; Taheur, F.B.; Altammar, K.A.; Haddaji, N.; Mahdhi, A.; Amri, Z.; Mzoughi, R.; Jabeur, C. Control of Staphylococcus aureus methicillin resistant isolated from auricular infections using aqueous and methanolic extracts of Ephedra alata. Saudi J. Biol. Sci. 2022, 29, 1021–1028. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Light, S.H.; Cahoon, L.A.; Halavaty, A.S.; Freitag, N.E.; Anderson, W.F. Structure to function of an alpha-glucan metabolic pathway that promotes Listeria monocytogenes pathogenesis. Nat. Microbiol. 2016, 2, 16202–16202. [Google Scholar] [CrossRef]
- The PyMOL molecular graphics system, Version 1.5.0.4 Schrö.
| Compound |
S. aureus ATCC 25923 |
L. monocytogenes ATCC19115 |
S. thyphimurium ATCC14080 |
P. aeruginosa ATCC27853 |
|---|---|---|---|---|
| OA-1 | 12.2 ± 1.7a | na | 12.8 ± 1.1c,d | 14.0 ± 1.0c |
| 18a | na | 12.0 ± 1.4b | na | na |
| 18b | na | 15.0 ± 0.0e | na | na |
| 18c | na | 9.5 ± 2.1a | na | na |
| 18d | na | 12.0 ± 0.0b | na | na |
| 18e | na | 14.0 ± 0.0d | na | na |
| 18f | na | 9.0 ± 0.0a | 12.0 ± 0.7c | na |
| 18g | na | 15.0 ± 0.0e | na | na |
| 18h | na | 12.0 ± 0.0b | na | 16.0 ± 0.0d |
| 18i | na | na | na | na |
| 18j | na | na | na | na |
| 18k | na | 14.0 ± 0.7d | 10.0 ± 1.4a | na |
| 18l | na | na | na | na |
| 18m | na | 13.0 ± 0.0c | na | 13.0 ± 0.0b |
| 18n | na | na | na | na |
| 18o | na | na | na | na |
| 18p | na | na | na | na |
| 18q | na | 12.0 ± 0.7b | 11.0 ± 0.0b | na |
| 18r | na | na | na | na |
| 18s | na | na | na | na |
| 18t | na | na | na | na |
| 18v | na | na | na | na |
| TET | 18.0 ± 0.0b | 12.5 ± 0.0b,c | 12.0 ± 0.1c | 11.0 ± 1.4a |
| Compound | S. aureus | L. monocytogenes | S. thyphimurium | P. aeruginosa | ||||
|---|---|---|---|---|---|---|---|---|
| ATCC 25923 | ATCC19115 | ATCC14080 | ATCC 27853 | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| OA-1 | 78.02 | 171.18 | 147.9 | 1353.18 | 59.97 | 633.66 | 21.39 | 85.59 |
| 18a | na | na | 12.4 | 1588.22 | na | na | na | na |
| 18b | na | na | 59.97 | 1917.99 | na | na | na | na |
| 18c | na | na | 21.13 | 1353.18 | na | na | na | na |
| 18d | na | na | 9.56 | 151.86 | na | na | na | na |
| 18e | na | na | 39.01 | 2496.67 | na | na | na | na |
| 18f | na | na | 39.01 | 624.16 | 78.02 | 312.08 | na | na |
| 18g | na | na | 9.48 | 155.65 | na | na | na | na |
| 18h | na | na | 9.89 | 633.66 | na | na | 158.41 | 633.66 |
| 18i | na | na | na | na | na | na | na | na |
| 18j | na | na | na | na | na | na | na | na |
| 18k | na | na | 18.48 | 591.63 | 147.9 | 591.63 | na | na |
| 18l | na | na | na | na | na | na | na | na |
| 18m | na | na | 21.39 | 85.59 | na | na | 42.79 | 171.18 |
| 18n | na | na | na | na | na | na | na | na |
| 18o | na | na | na | na | na | na | na | na |
| 18p | na | na | na | na | na | na | na | na |
| 18q | na | na | 16.88 | 540.44 | 135.11 | 540.44 | na | na |
| 18r | na | na | na | na | na | na | na | na |
| 18s | na | na | na | na | na | na | na | na |
| 18t | na | na | na | na | na | na | na | na |
| 18v | na | na | na | na | na | na | na | na |
| TET | 4.5 | 288 | 576.01 | 1152.02 | 9 | 576.01 | 576.01 | 1152.02 |
| Compound | Binding Energy (kcal/mol) |
|---|---|
| 18a | -10.7 |
| 18b | -10.8 |
| 18c | -11.7 |
| 18d | -12.0 |
| 18e | -10.7 |
| 18f | -10.9 |
| 18g | -10.9 |
| 18h | -12.3 |
| 18k | -11.6 |
| 18m | -10.6 |
| 18q | -11.0 |
| Tetracycline | -11.1 |
| Docked Compounds | Interacting Residues | Binding Energy (kcal/mol) |
|---|---|---|
| 18c | van der Waals: Gln193, Val195, Thr196, Asn198, Leu249, Gln252, Leu272, Phe299, Glu391; H bond: Trp271*(3.13); Alkyl/Pi–Alkyl:Pro194(3.92)(4.90)(5.06), Pro246(5.14), Leu253(4.11)(5.26), Pro254(4.85)(5.21), Leu394(5.37) ; Pi-Pi: Phe70(4.10), Trp271(4.33). | -11.7 |
| 18d | van der Waals: Phe38, Asn71, Val148, Val195, Thr196, Asn198, Glu199, Pro246, Leu253, Thr268, Gly269, Asn301, Asn387, Leu394; H bond: Trp271*(3.15), Glu391**(3.27)(3.38); Alkyl/Pi–Alkyl: Phe70(5.14), Pro194(4.86), Pro254(4.77), Trp271(4.89), His297(4.07), Phe299 (4.43) (4.76); Pi-Pi: Phe70(3.87), Trp271(4.66) ; Pi-Anion: Glu391(3.81). | -12.0 |
| 18h | van der Waals: Phe38, Asn71, Asp72, Phe74, Thr196, Asn198, Glu199, Thr268, Gly269, Asn301, Glu338, Ser383, Asn387 ; H-bond: Thr75*(3.13), Asn*380(2.55), Asp384*(2.12); C-H bond: Thr75(3.28), Asp384(3.71)(3.76); Alkyl/Pi–Alkyl: Phe70(5.05)(5.26), Pro194(4.27)(5.19); Pi-Pi: Phe70(5.06), Trp271(4.47), Phe299(5.16). | -12.3 |
| 18k | van der Waals: Phe38, Asn71, Val148, Val195, Thr196, Asn198, Glu199, Pro246, Leu249, Gln252, Leu253, Thr268, Gly269, Asn301, Asn387 ; H-bond: Trp271*(3.25); Alkyl/Pi–Alkyl: Phe70(5.28), Trp271(4.89), Pro194(4.69)(4.89)(5.34) (5.36), Pro254(4.58), His297(3.99), Phe299(4.40)(4.72); Pi-Pi: Phe70(3.77), Trp271(4.45) ; Pi-Anion: Glu391(3.68). | -11.6 |
| Tetracycline | van der Waals: Phe38, Ser39, Pro194, Asn198, Glu199, Leu253, Leu272, Asn301, Asn387; H-bond: Thr196*(2.12), Glu338*(2.82); Alkyl/Pi-Alkyl: Phe70(4.42), Trp271(5.47);Pi-Pi: Trp271(4.09) | -11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





