1. Introduction
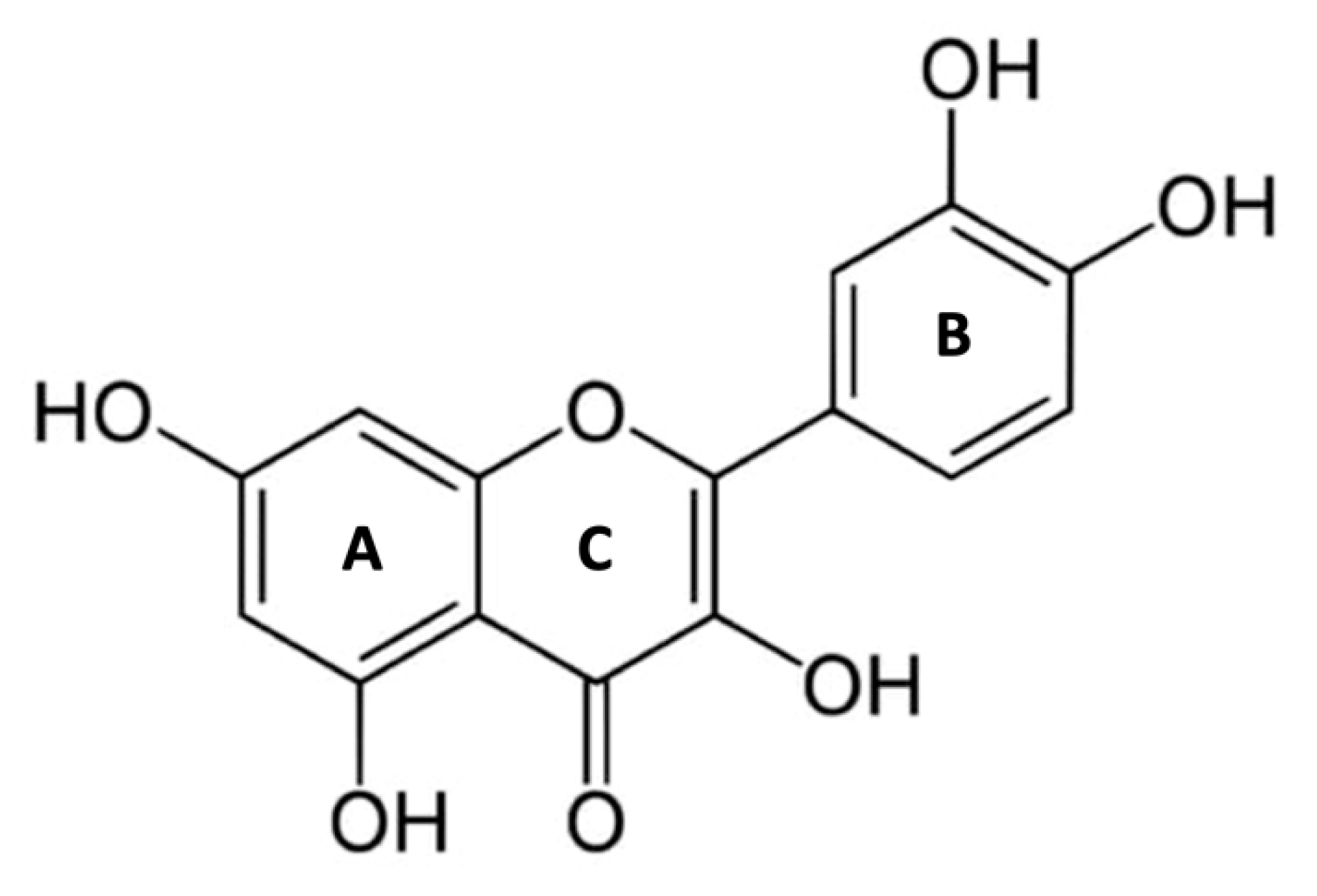
Quercetin (Que) is a 3,30,40,5,7-pentahydroxyflavone with the chemical formula C15H10O7. The presence of five hydroxyl groups in the structure and the pyrocatechol-kind of benzene ring makes Que a strong antioxidant and a good scavenger of free radicals [
1]. The structure of Que (
Figure 1) contains a ketocarbonyl group, and the oxygen atom present on the first carbon, being basic, can form salts with acids. Furthermore, a dihydroxy group between the A ring, o-dihydroxy group B, C ring C2, C3 double bond, and 4-carbonyl are the active groups in Que. Que’s biological activities have been largely attributed to these active phenolic hydroxyl groups and double bonds [
2].
Que is highly present in daily human foods, such as onions, apples, red wine, and tea, exerting various biological effects including antioxidant, anticancer, anti-inflammatory, anti-viral, and anti-aging effects, but also antiaggregant and vasodilator effects, making it protective against cardiovascular diseases (CVD) [
3,
4,
5]. In humans consuming flavonoid-rich foods, the concentration range of Que plasma levels is 0.3-7.6 μM, mainly in the form of glucuronidated and sulfated metabolites [
6].
The production of free radicals and reactive oxygen species (ROS) occurs in cells due to common metabolic processes or external sources [
7]. Such stress can be limited by antioxidant systems, followed by a rapid return to a physiological redox state. However, excessive oxidative stress, characterized by the disruption of the balance between oxidation and antioxidation systems, causes non-specific and irreversible damage to biological molecules, such as lipids, proteins, and DNA, leading to a loss of function [
8].
In several chronic diseases, such as cancer, neurodegenerative diseases, and metabolic diseases, oxidative stress is often the original triggering factor [
9].
Potential endogen ROS sources include the nicotinamide adenine dinucleotide phosphate oxidase (NOX), xanthine oxidase (XO), lipoxygenase (LOX), myeloperoxidase (MPO), and monoamine-oxidase (MAO)[
10], the increased activation of these enzymes has been involved in oxidative stress and the initiation and progression of inflammatory diseases. Que has shown a promising protective capacity to attenuate the expression and the enzymatic activity of several endogenous oxidative enzymes [
11,
12].
This review collects the most recent data from MEDLINE (PubMed), Google Scholar, ScienceDirect, Scopus, Cochrane, SID, and SciFinder, on the inhibitory activity of Que against oxidative enzymes. Particular attention was directed at the possible binding modes of Que the interactions involved and, to confirm the data already present, further docking analyses were carried out using Glide software [
13].
2. Quercetin antioxidant activity: in silico approach
Que is a potent antioxidant in the flavonoid family due to the presence of a phenolic hydroxyl group and double bonds [
14]. In recent years, several in silico studies have been conducted to elucidate the molecular basis of its antioxidant activity. For instance, Zheng et al. [
15] used the Density Functional Theory (DFT) approach to calculate various properties, such as electronegativity, hardness, softness, electrophilic index, and ionization potential in gas, ethanol, and water phases of Que to explain its antioxidant activity [
16]. The study was performed using Gaussian 09 and the M062X/6-311+g** level of theory and confirmed that Que acts as an electron donor, indicating its antioxidant activity (
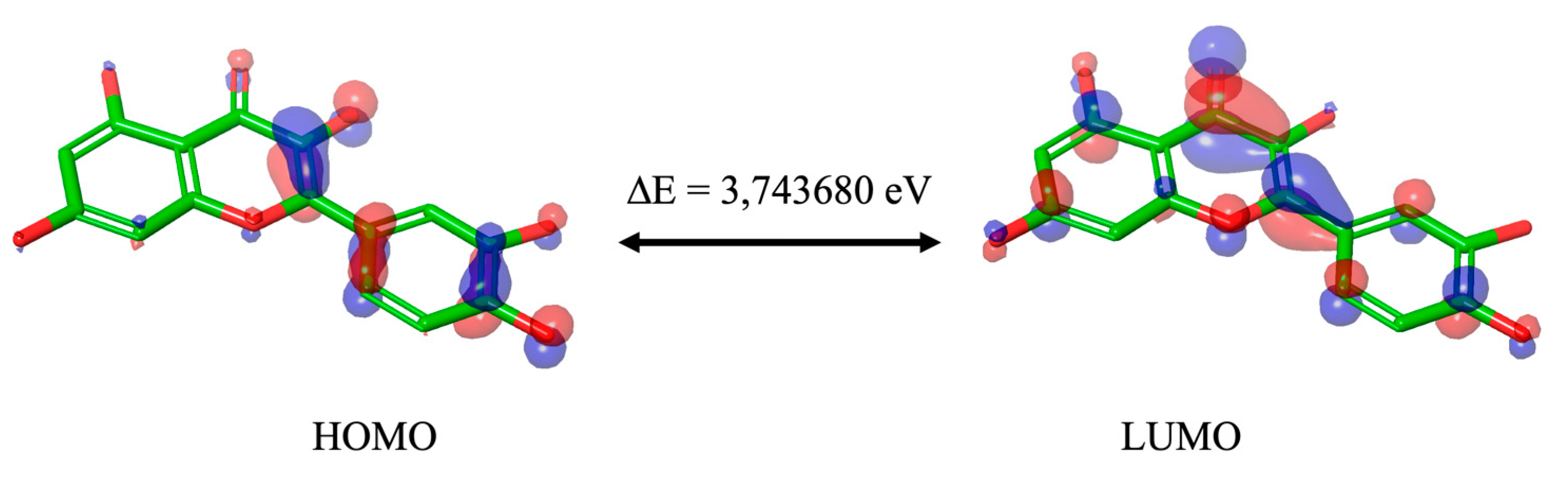
Table 1). The energies for the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) were -6.85 eV and -0.95 eV, respectively. HOMO is predominantly located on the B-ring and C-ring, while LUMO is distributed over the entire molecule [
15].
Similarly, Thao et al. [
17] calculated the Electrostatic Potential (ESP), HOMO, and LUMO in the water phase at the M052X/6-311++G(d,p) level of theory using Gaussian 09. The study found that the negative changes in Que are mainly located in the phenolic and ketone group, with HOMO localized in the conjugated systems C21-C22-C20, C17-C11-C19, C10-C12-C13, and C18-C14-C8-C9-O1. Meanwhile, LUMO is distributed in the conjugated system C15-C9-C8-C14, C16-C18, C12-C13, C17-C20 π bonds, and the phenolic groups.
Villar and colleagues [
18] also used Gaussian 9 to calculate HOMO and LUMO of Que, employing the Hartree-Fock method in the 6-31G** basis set. The study reported the values of HOMO and LUMO as -7,04 eV and -1,62 eV, respectively.
We optimized the Que using B3LYP/6-311++G** method with Jaguar software, and calculated ESP, Average Local Ionization Energy (ALIE), Electron Density (ED), HOMO, and LUMO (
Table 2). ESP is an effective tool for understanding and interpreting the chemical and reactivity properties of molecular systems [
19], ALIE is the energy required to remove an electron from a specific point in a system and the lowest values reveal the locations of the least tightly held electrons [
20], ED measures the probability of finding an electron in the volume surrounding a molecule [
21]. HOMO and LUMO orbitals were presented in color-coded surfaces, which give the location of positive (blue) and negative (red) electrostatic potentials (
Figure 2). According to Koopman’s theorem (1934), during molecular interactions, the LUMO accepts electrons, and its energy corresponds to the Electron Affinity (EA=-ELUMO), while the HOMO represents electron donors, and its energy is associated with the Ionization Potential (IP=-EHOMO) [
22]. The energy gap (ΔE) is the difference between HOMO-LUMO orbitals, which specifies the molecule’s capacity in the free radical optimization mechanism and small values indicate that the intramolecular charge transfer is easy, and the molecule participates effectively in radical scavenging reaction [
23].
Table 2 contains the values in eV of the calculated properties in silico and
Figure S1 shows ALIE, ESP, and ED calculated in silico for Que.
Although the different DFT studies mentioned cannot be directly compared due to the use of different software and levels of theory, HOMO LUMO orbitals carried out by us and Zheng et colleagues [
15] presented similar results, with the HOMO orbitals predominantly located in the B and C ring and the LUMO orbitals distributed throughout the molecule. Meanwhile, Thao et al. [
17] found both orbitals distributed throughout the molecule. Reported values are good indicators of chemical reactivity, especially for aromatic systems [
20,
24] and the results suggest that Que is a highly effective free radical scavenger.
3. Molecular mechanisms shared
3.1. NAD(P)H Oxidase
NOX (EC 1.6.3.1.) is a transmembrane glycoprotein that catalyzes the production of superoxide (O2−) from oxygen and NADPH. O2− reacts quickly to produce a burst of additional oxidants, including hydrogen peroxide (H2O2), which is typically further converted by MPO into hypochlorous acid (HOCl) and hydroxyl radical (• OH), for this reason, NOX is usually considered a major source of ROS and oxidative stress in eukaryotic cells [
25].
NOX consists of membranous subunits (gp91-phox, p22-phox), which form a heterodimeric flavoprotein, and cytosolic subunits (p40-phox, p47-phox, and p67-phox), which exist in unstimulated cells. There are six human homologs of the catalytic subunit of the phagocyte NOX: NOX1, NOX2, NOX3, NOX4, NOX5, Dual Oxidase (DUOX)-1, and DUOX-2.
NOX2 is the primary phagocytic oxidase; NOX1 and NOX2 are expressed in neurons, vascular smooth muscle cells, and microglia; NOX3 and NOX5 are expressed in myocardial cells; NOX4, which does not require any cytosolic components and is constitutively active, is expressed highly in the kidney and vascular smooth muscle cells; NOX5 is expressed in fetal tissues and spleen; finally, DUOX1 and 2 are highly expressed in the thyroid where they produce H2O2 [
25].
NOX inhibition has potential therapeutic significance, and several compounds have been registered as NOX inhibitors in the patent literature. [
26].
Que showed inhibitory activity against NOX in different cells and systems. In lung epithelial A549 cells, Que prevented lipopolysaccharide (LPS)-induced oxidative stress by downregulating the NOX gene. In fact, Que treatment almost completely abolished the mRNA and protein levels of NOX2 induced by LPS stimulation [
27].
In RAW264.7 macrophages, Que significantly enhances Heme oxygenase-1 (HO-1) protein expression, likely through the reduction of Kelch Like ECH Associated Protein 1 (Keap1), a negative of nuclear factor erythroid 2-related factor 2 (Nrf2), which regulates the expression of genes encoding many cytoprotective and stress-responsive enzymes such as HO-1. [
12]. As an antioxidant enzyme, HO-1 induction could be a physiological compensatory mechanism that may protect cells and tissues from exaggerated oxidative injury during inflammation [
28]. Interestingly, modifications in the Que structure resulted in different inhibitory effects on NOX activity; quercetin-3-glucuronide (Q3G) did not induce HO-1 expression in macrophages. It appears that the 3-hydroxyl group is a key structural requirement for HO-1 induction in macrophages, and the glycosylation of the 3- hydroxyl group in Que removed its ability [
12]. These results were confirmed in Human Umbilical Vein Endothelial Cells (HUVECs) and Apo-E Knockout (KO) mice [
29].
In vascular smooth muscle cells (VSMCs) from spontaneously hypertensive rats (SHR), Que and its most abundant metabolite in plasma, Q3G, inhibited NOX in a non-competitive manner. Que seems to be locally regenerated in vivo by glucuronidase to protect VSMCs from oxidative stress since it is about 10-fold more potent than Q3G, indicating that the glycosylation of the 3-hydroxyl group affected the ability to inhibit NOX [
30].
Villar and colleagues conducted molecular docking studies using AutoDock 4.2.6/Vina to investigate the activity of Que in the binding pocket of NOX[
18]. They retrieved the crystal structure of the enzyme (code ID 2CDU) from the Protein Data Bank [
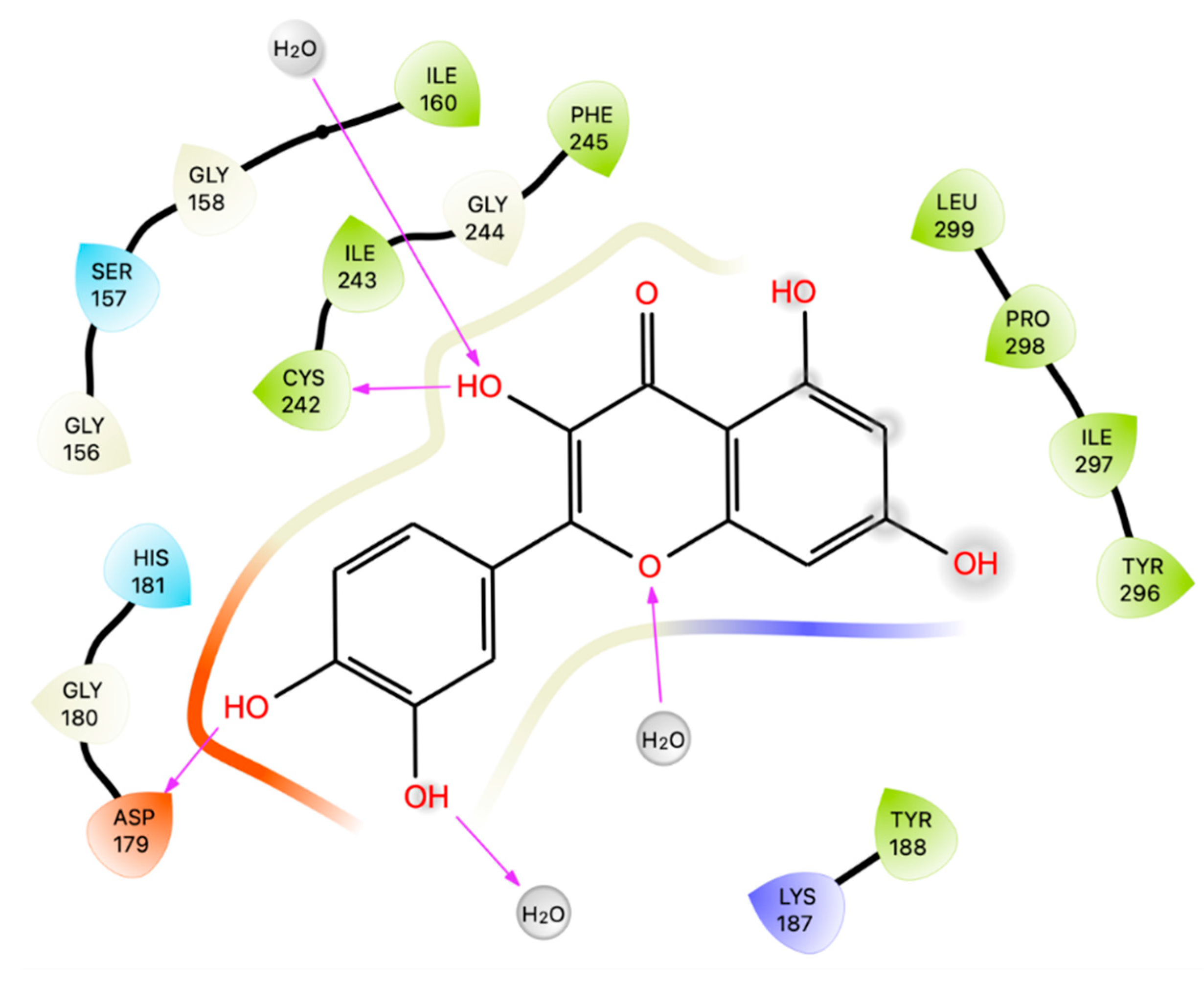
31]. Que and dextromethorphan, the reference compound, showed binding energy of -8.3 kcal/mol and -8.0 kcal/mol, respectively. Que established π-cation interactions with Pro 120, Asp 179 and Lys 213, as well ashydrogen bonds with Asp 179, Gly 180 and Val 214. Que also established hydrophobic interactions with Gly 158 and π-sigma interactions with Ile 243.
In our docking study using Glide software [
13] and the same crystal structure used by Villar and colleagues [
18], we found, as expected, that Que establishes H-bonds with Asp 179 and Cys 242 (Figure3). The results from docking simulations were submitted to Prime MM-GBSA calculations to calculate the ligand-receptor binding energy. Que showed a comparable Glide Gscore (-5.35 Kcal/mol) to the reference compound dextromethorphan (-5.62 Kcal/mol) at the ATP-site, but, its MM-GBSA dG bind value was much lower (-4.82 kcal/mol) than that of dextromethorphan (-15.14 kcal/mol). These results suggest that Que may not be a good NOX ligand at the ATP-site.
3.2. Xanthine Oxidase
XO (EC 1.17.3.2) is an iron-molybdenum flavoprotein found in mammalian tissues that play a crucial role in purine catabolism [
32]. It catalyzes the oxidation of hypoxanthine to xanthine and xanthine to uric acid (UA) with the production of superoxide anion. The increase in ROS could lead to oxidative stress damage, metabolic syndrome, and cardiovascular diseases such as hypertension and ischemia reperfusion [
33]. Furthermore, the accumulation of UA has been shown to initiate the inflammatory process through NLRP3 inflammasome and ROS production, contributing to inflammation-related tissue damage [
34]. The deposition of crystals of UA or its monosodium salt in human joints with accompanying joint inflammation is the primary cause of gout [
35]. Two FDA-approved drugs, allopurinol, and febuxostat, inhibit XO and are used to treat gout [
36]. However, their side effects, such as bone marrow depression, allergic reactions, and renal and gastrointestinal toxicities, limit their use [
37].
Que inhibits XO activity in a concentration-dependent manner both in vitro and in vivo. In vitro, Que inhibits XO showing an IC50 value of 2.74 ± 0.04 × 10−6 mol L−1 [
38], instead, in vivo, Que elicited a hypouricemic effect when orally administered to hyperuricemic mice at a dose of 100 mg/kg body weight [
39].
XO has been shown to play a role in atrial remodeling induced by oxidative stress in alloxan-induced diabetic rabbits [
40]. Que ameliorates oxidative stress injury and myocardial remodeling after myocardial ischemia [
41].
Moreover, XO has a central role in the metabolism of the antitumor drug 6-mercaptopurine (6-MP), yielding pharmacologically inactive 6-thiouric acid (6-TU) [
42]. The simultaneous administration of XO inhibitors, allopurinol, and 6-MP shows a slow elimination of the latter compound [
43].
Since Que and its metabolites showed an inhibitory effect on UA production, it was shown the same effect on the formation of 6-TU, with an IC50 of 1.4 μM, five-fold stronger than allopurinol (IC50 = 7.0 μM) [
44].
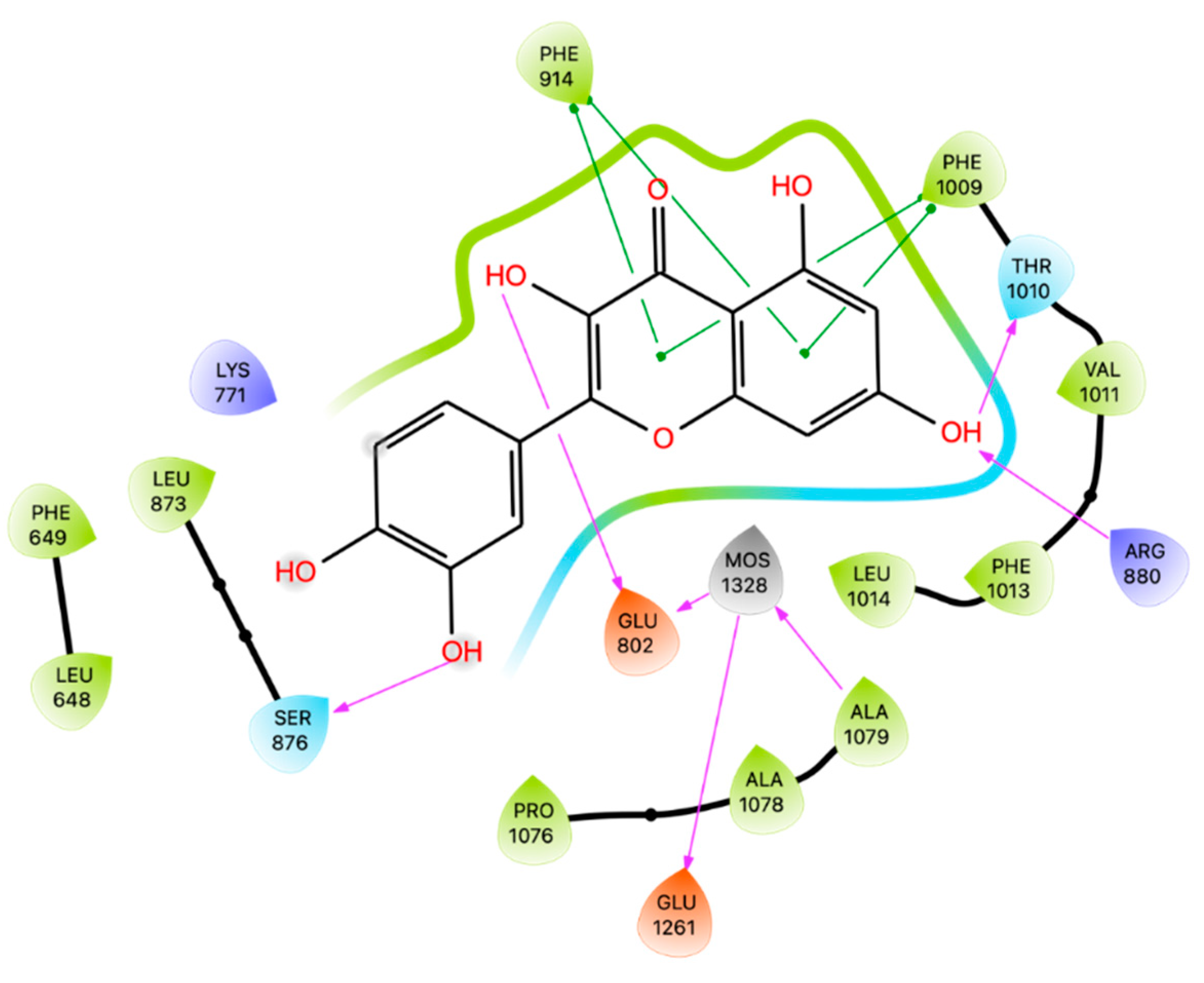
Different studies have employed docking techniques to investigate the binding of Que to XO. In the X-ray [
45], Que adopts an orientation with its benzopyran part sandwiched between Phe 914 and Phe 1009 and the B ring pointing toward the solvent channel leading to the active molybdenum center. Que binds with hydrogen bonds the catalytically relevant residues Arg 880 and Glu 802.
Zhang and colleagues [
46] employed AutoDock 4.2 software to dock Que with XO using 1FIQ as X-ray structure from bovine milk. The active cavity identified is a long, narrow channel to the flavin adenine dinucleotide (FAD) reaction site, where reduction of the substrate O2 takes place in the presence of XO. The best cluster identified has an energy of -5.67 kcal/mol with the calculated binding free energy of -6.33 kcal/mol, and it forms two hydrogen bonds between the hydroxyl groups of Que and the hydrogen atoms on the amino acid residues of XO. Specifically, the hydroxyl group at positions 5 and 7 in the A-ring of Que was connected with Glu263 and Arg394 [
38].
Mohos and colleagues perform docking using Autodock 4.2 program package and the structure of XO (PDB code 3EUB) without the native ligand. Que binds at the XO pocket and interacts with Glu 802, Ser 876, Phe 914, Phe 1009 and Thr 1010 [
44].
Employing the crystal of XO bound to Que code 3NVY [
45], we docked the ligand on the binding site of XO using Glide software. Confirming the results obtained by Mohos et al., Que establishes H-bonds with Glu 802, Ser 876, and Thr 1010 Arg 880 and π-π stackings with Phe 914 and Phe 1009 (
Figure 4). The best pose resulting from the docking simulation was then superimposed upon the native Que, showing a RMSD of 0,111 Å. As before, docking results were submitted to Prime MM-GBSA calculations giving energy of -22,18 kcal/mol.
These studies confirm the potential of Que as an inhibitor of XO, offering a natural alternative to traditional drugs with fewer side effects.
3.3. Myeloperoxidase
MPO (EC 1.11.2.2) is an enzyme found in the azurophilic/primary granules of neutrophils and, to a lesser extent, in monocytes [
47]. MPO catalyzes the oxidation of chloride and other halide ions in H2O2 to generate HOCl and other highly reactive products that mediate efficient antimicrobial action [
48]
Although MPO is safeguarded in the azurophilic/primary granules, which require stronger stimulation and are the last to be released during the hierarchical release of neutrophil granules, plenty of evidence suggests that it spills into the surrounding tissue during inflammation [
49]. As such, elevated plasma and tissue levels of MPO have been linked to many inflammatory disorders, including cardiovascular diseases, such as coronary artery disease, arterial hypertension, pulmonary arterial hypertension, peripheral arterial disease, myocardial ischemia/reperfusion-related injury, stroke, and venous thrombosis [
50]. These findings have implicated MPO as an important therapeutic target in treating inflammatory conditions.
Que has been shown to have potent inhibitory activity against MPO. In human neutrophils treated with an inflammatory and oxidant agent, phorbol 12-myristate 13-acetate (PMA), Que reduced MPO activity by 83,7% at 25 µM. In plasma rats, Que co-treatment with cadmium reduced MPO activity after cadmium treatment by 53% at 50 mg/Kg [
51,
52]. The inhibitory activity of Que against MPO also appears to improve damage caused by reperfusion of the ischemic brain in rats. Reperfusion injury is associated with an imbalance of oxidative stress and antioxidant defense system, Que ameliorates disease progression by reducing MPO-associated ROS [
53].
MPO is generally regarded as a risk factor for vascular complications in diabetic individuals [
54]. In in vitro study, MPO exacerbated high glucose-mediated endothelial dysfunction through its reaction with H2O2 to generate HOCl in HUVECs, Que treatment attenuated MPO activity and, subsequently its endothelial cytotoxicity. In murine models, these studies are confirmed in vivo, where expressions and activities of MPO and NADPH oxidase in aortas from diabetic animals were significantly higher than those in control mice. Que supplementation (3.5 mg daily for each animal) significantly reduced NADPH oxidase and MPO expressions and improved endothelial function [
55].
MPO is also associated with the formation of neutrophil extracellular traps (NETs), which can modulate the inflammatory process and induce tissue damage [
56]. In human neutrophils, prior incubation with Que reduced NET release after PMA stimulation [
57]. Moreover, NETs have a cytotoxic effect on the A549 cell line, and Que was able to reduce the cytotoxic effects of NETs, probably acting by reducing MPO and elastase activity [
57].
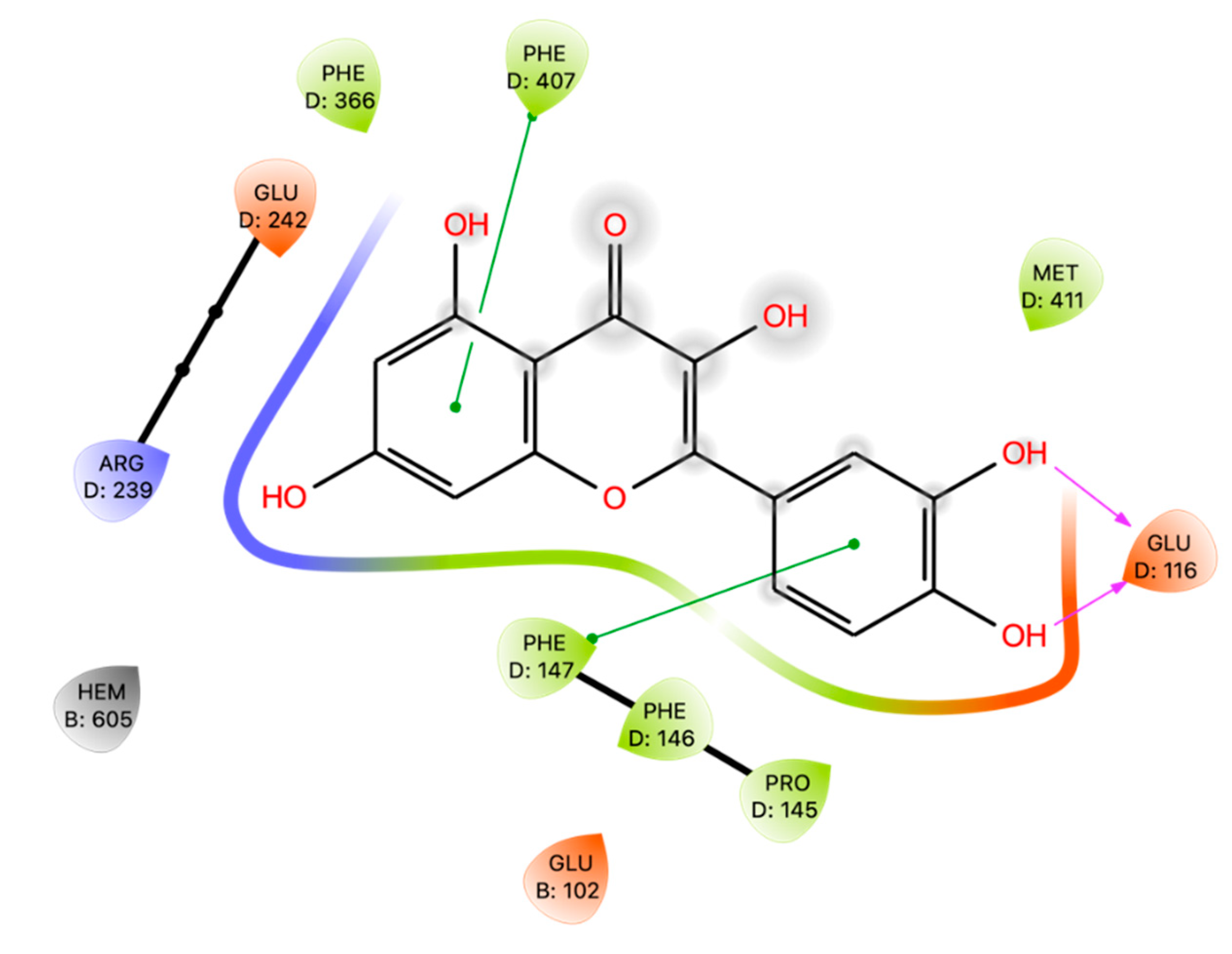
Docking studies have shown that Que interacts with MPO by orienting with ring A and ring C above the iron-heme located in the active site of MPO [
11]. It also established noncovalent interactions with Arg 239 and Phe 407, which are near the catalytic heme site, giving a possible explanation for its inhibition of MPO activity.
Pereira and colleagues [
57] investigated the interactions between Que and MPO and elastase, which are involved in the neutrophil extracellular traps. Focusing on MPO, (PDB code 5FIW) the best pose obtained a Vina Score of -8.1kcal/mol and the complexes are thermodynamically stable. Que establishes 71 Van der Waals interactions within the residues located near the heme group and in the catalytic region.
We reproduced docking studies on MPO (PDB code 5FIW) of Que using Glide software and calculating the Prime MM-GBSA energy.
Que interacts with Phe 147 and Phe 407 by means of π-π interactions and establishes hydrogen-bonds with Glu 116 (
Figure 5). The binding energies of the results derived from docking are -4.91 kcal/mol for GlideScore, greater than that identified by Pereira but with a dG Bind of -28.81kcal/mol.
3.4. Lipoxygenase
LOXs (EC 1.13.11.12) are a large monomeric protein family with non-heme, non-sulfur, iron cofactor-containing dioxygenases that catalyze the oxidation of polyunsaturated fatty acids (PUFA) in lipids containing a cis,cis-1,4-pentadiene into cell signaling agents [
58]. The nomenclature of these enzymes is based on the specific position of the carbon oxygenated, and some examples are 5-LOX, 12-LOX, and 15-LOX, widely distributed in mammals [
59].
The main products of LOXs are leukotrienes and lipoxins, which play important roles in several inflammation-related diseases, such as arthritis, asthma, cancer, and allergies. Therefore, inhibition of the LOX pathway and targeting LOX with inhibitors is a promising therapeutic approach for treating a wide spectrum of human diseases [
60].
Que has been extensively studied for its inhibitory effects on rabbit 15-LOX-1, 5-LOX, and 12-LOX and has shown high efficiency as LOXs inhibitor [
61,
62]. The inhibitory effect of Que has also been confirmed in mammalian 15-LOX showing an IC50 of 0.35 mM [
63]. In addition, Que has been found to inhibit low-density lipoprotein peroxidation catalyzed by mammalian 15-LOX [
64].
Pinto and colleagues investigated the mechanism of Que inhibition on 5-LOX. They found a characteristic competitive mechanism with a value of Ki of 3.73 μM, demonstrating a high efficiency of this polyphenol as a lipoxygenase inhibitor. The competitive inhibition produced by Que on LOX activity suggests that the substrate, such as linoleic acid, is displaced from the active site and that, consequently, the binding site should be located near or at LOX catalytic site [
65].
Que modulates the time course of the lipoxygenase reaction in a complex manner by exerting three distinct effects: (i) prolongation of the kinetic lag period, (ii) instant decrease in the initial rate after the lag phase being overcome, (iii) time-dependent inactivation of the enzyme during the reaction, but not in the absence of substrate [
62].
Vyshnevska et al. [
66] have performed molecular docking investigations on herbal compounds, including Que, as potential 5-LOX and COX-2 inhibitors. Concerning 5-LOX, (PDB code 6NCF) [
67] was chosen, and the simulations were carried out with AutoDock Vina. The obtained results were compared with the reference compound 3-acetyl-11-keto-beta-boswellic acid (AKBA), a non-competitive inhibitor of 5-LOX which was the co-crystallized ligand of 6NCF. AKBA showed a binding energy of -9.1 kcal/mol, whereas Que reached -8.2 kcal/mol and established hydrophobic interactions with Val 107, Val 110 and Thr 137, H-bonds with His 130, Glu 134, Arg 138 and Asp 166, and π-cation interactions with Arg 101.
We have reproduced the docking studies performed by Vyshnevska [
66] and found the interactions with the same amino acids with a Glide score of -5,94 Kcal/mol and dGbind of -37,42 Kcal/mol (
Figure 6 and
Table 3). Que displays a better docking score and MMGBSA dG Bind in comparison with those of AKBA, -3,37 kcal/mol and -28,21 kcal/mol, respectively.
These results demonstrated the potential of Que as an inhibitor of 5-LOX.
3.5. Monoamine oxidase
MAO (EC 1.4.3.4) is a riboflavin protein distributed on the outer mitochondrial membrane that catalyzes the oxidative deamination of biogenic and xenobiotic amines, producing the corresponding aldehydes, hydrogen peroxide, and ammonia [
68]. There are two isoforms of MAO, MAO-A and MAO-B, which differ in their substrate and tissue distributions. MAO-A preferentially deaminates serotonin and norepinephrine, while MAO-B metabolizes dopamine [
69]. The generation of H2O2 via MAOs is reported to be a cytotoxic factor involved in oxidative stress and neurodegenerative disorders such as Parkinson’s disease [
70,
71].
Selective inhibition of MAO-A leads to increased levels of neurotransmitters within noradrenergic and serotoninergic neurons of the Central Nervous System (CNS) with clinical antidepressant action, while inhibition of MAO-B leads to increased levels of dopamine in the Parkinsonian brain with partial depletion of dopaminergic neurons in Substantia Nigra pars compacta with anti-Parkinsonian action [
69].
Food-derived compounds such as flavonoids, alkaloids, and phenylpropanoids have been reported to have an MAO-A and MAO-B inhibitory effect. Among them, the flavonoid Que has been found to be as most active MAO-A inhibitor, with an IC50 range value of 1-4 μM compared to MAO-B (IC50 value ≥ 90 μM) [
72,
73].
Although Que is present mainly as glucuronidated and sulfated metabolites in plasma [
74], it is possible to generate Que aglycone from its conjugated derivatives due to β-glucuronidase activity during inflammation [
75]. Que is hydrophobic, and it seems to be able to cross mitochondrial membranes and act as an MAO-A inhibitor [
76].
MAO-A, located in the digestive system, is crucial in detoxifying exogenous amines such as tyramine in fermented dairy products. Some MAO-A inhibitors increase the risk of poisoning by exogenous amines. Interestingly, in murine model, the inhibitory effect on MAO-A from the small intestine was not observed in the range of 0.1−100 μM [
77,
78]. These studies suggest that Que can selectively inhibit MAO-A from the brain.
Therefore, Que and its derivatives, both synthetic and from natural extracts, are widely studied in order to find more selective and more powerful MAO-A inhibitors [
79,
80]
Larit et al. [
80] performed a docking simulation of Que and other flavonoids on hMAO-A using Glide software. Crystal structures 2BXR [
81], 2BXS [
81], 2Z5X [
82], and 2Z5Y [
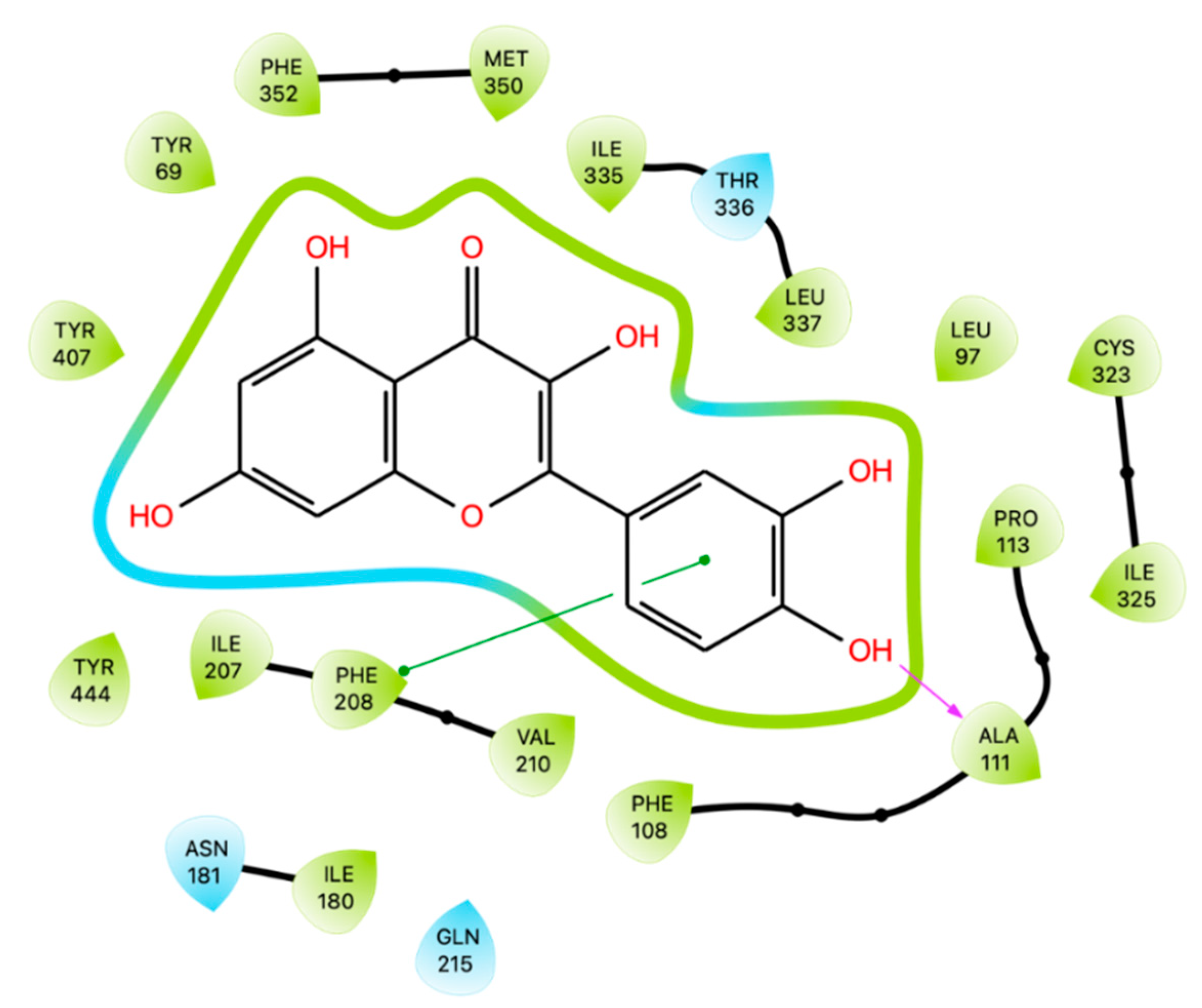
82] were selected to generate the grids. The best docking score for Que was -11.3 kcal/mol and interacted with Ala 111, Ile 180, Asn 181, Phe 208, Gln 215, Thr 336 and Tyr 444.
Based on the study performed by Larit [
80], we replicated the docking simulation of Que on the active site of hMAO-A (PDB code 2Z5Y) finding only an hydrogen-bond with Ala 111 and a π-π interaction with Phe 208 (
Figure 7). After conducting docking simulations, Prime MM-GBSA calculations were carried out, and the resulting energy was reported in
Table 3 along with the values for all the other enzymes. Among the enzymes studied, MAO-A exhibited the highest docking score following XO. These findings suggest that Que has the potential to inhibit MAO-A and could therefore have therapeutic applications for neurodegenerative and psychiatric disorders.
4. Conclusion
Que, a flavonoid widely distributed in fruits and vegetables, is known for its antimicrobial, antiviral, antioxidative, and anti-inflammatory properties. Its antioxidant activity is mainly attributed to its intrinsic ability to neutralize free radicals due to the hydroxyl substitutions and the catechol-type B-ring. Additionally, it can inhibit the expression of pro-inflammatory and oxidant genes and endogenous oxidizing enzymes. Docking studies have demonstrated the potential of Que to interact with oxidative enzymes, making it a promising lead compound for developing new anti-inflammatory agents. The studies have shown that Que has the best results in molecular docking simulations with XO, followed by MAO-A, 5-LOX, NOX, and MPO, which are implicated in several inflammatory pathologies. However, further research is needed to fully understand the mechanisms of action and to determine the efficacy in clinical trials of Que.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: Calculated
in silico properties: (A) ALIE, (B) ED, and (C) ESP.
Author Contributions
A.D.P., S.O., and C.S. wrote the article and created tables and figures. M.C.F. and B.E. reviewed the article. A.D.P and A.F. critically reviewed the article and supervised the project. All authors contributed and approved the final version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable here.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Y.M.; Zhang, Z.Y.; Wang, R.X. Protective Mechanisms of Quercetin Against Myocardial Ischemia Reperfusion Injury. Front Physiol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Yao, L.; Gu, W.; Zhao, S.; Shen, Z.; Lin, Z.; Liu, W.; Yan, T. Pharmacological Activity of Quercetin: An Updated Review. Evidence-based Complementary and Alternative Medicine 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.U. Quercetin and Its Role in Biological Functions: An Updated Review. EXCLI J 2018, 17, 856. [Google Scholar] [CrossRef] [PubMed]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytother Res 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Dagher, O.; Mury, P.; Thorin-Trescases, N.; Noly, P.E.; Thorin, E.; Carrier, M. Therapeutic Potential of Quercetin to Alleviate Endothelial Dysfunction in Age-Related Cardiovascular Diseases. Front Cardiovasc Med 2021, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Frei, B. Consumption of Flavonoid-Rich Foods and Increased Plasma Antioxidant Capacity in Humans: Cause, Consequence, or Epiphenomenon? Free Radic Biol Med 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian Journal of Clinical Biochemistry 2015, 30, 11. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin Interv Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol 2020, 11. [Google Scholar] [CrossRef]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous Mechanisms of Reactive Oxygen Species (ROS) Generation. Postepy Hig Med Dosw (Online) 2016, 70, 1150–1165. [Google Scholar] [CrossRef]
- Lu, N.; Sui, Y.; Tian, R.; Peng, Y.Y. Inhibitive Effects of Quercetin on Myeloperoxidase-Dependent Hypochlorous Acid Formation and Vascular Endothelial Injury. J Agric Food Chem 2018, 66, 4933–4940. [Google Scholar] [CrossRef]
- Luo, M.; Tian, R.; Yang, Z.; Peng, Y.Y.; Lu, N. Quercetin Suppressed NADPH Oxidase-Derived Oxidative Stress via Heme Oxygenase-1 Induction in Macrophages. Arch Biochem Biophys 2019, 671, 69–76. [Google Scholar] [CrossRef]
- Schrödinger Software Release 2022-1 | Macs in Chemistry Available online:. Available online: https://www.macinchem.org/blog/files/279f5ca3fa90d6bf7db20adc4b0afed0-2841.php (accessed on 17 May 2023).
- Vo, Q.V.; Nam, P.C.; Thong, N.M.; Trung, N.T.; Phan, C.T.D.; Mechler, A. Antioxidant Motifs in Flavonoids: O−H versus C−H Bond Dissociation. ACS Omega 2019, 4, 8935–8942. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Sci Rep 2017, 7. [Google Scholar] [CrossRef]
- Boulebd, H. DFT Study of the Antiradical Properties of Some Aromatic Compounds Derived from Antioxidant Essential Oils: C–H Bond vs. O–H Bond. Free Radic Res 2019, 53, 1125–1134. [Google Scholar] [CrossRef]
- Thao, T.H.D.; Dung, V.T.N.; Dao, D.Q. Antioxidant vs. pro-Oxidant Activities of Quercetin in Aqueous Phase: A Density Functional Theory Study. Vietnam Journal of Chemistry 2019, 57, 696–701. [Google Scholar] [CrossRef]
- Villar, L.; Da, P.; Pantoja, S.; Samantha, S.; Trindade, A.; Paulo, J.; Silva, B.; Fernanda, C.; Romeiro, R.; Carla, A.; et al. Computational Study of the Main Flavonoids from Chrysobalanus Icaco L. against NADPH-Oxidase and in Vitro Antioxidant Activity. Research, Society and Development 2022, 11, e5011628542–e5011628542. [Google Scholar] [CrossRef]
- Gadre, S.R.; Suresh, C.H.; Mohan, N. Electrostatic Potential Topology for Probing Molecular Structure, Bonding and Reactivity. Molecules 2021, Vol. 26, Page 3289 2021, 26, 3289. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Bulat, F.A. Average Local Ionization Energy: A Review. J Mol Model 2010, 16, 1731–1742. [Google Scholar] [CrossRef]
- Cukrowski, I.; Dhimba, G.; Riley, D.L. A Molecular-Wide and Electron Density-Based Approach in Exploring Chemical Reactivity and Explicit Dimethyl Sulfoxide (DMSO) Solvent Molecule Effects in the Proline Catalyzed Aldol Reaction. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Amati, M.; Stoia, S.; Baerends, E.J. The Electron Affinity as the Highest Occupied Anion Energy with a Sufficiently Accurate Approximation of the Exact–Sham Potential. J Chem Theory Comput 2020, 16, 443. [Google Scholar] [CrossRef]
- Islam, N. Investigation of Comparative Shielding of Morin against Oxidative Damage by Radicals: A DFT Study. http://www.editorialmanager.com/cogentchem 2015, 1, 1078272. [Google Scholar] [CrossRef]
- Sjoberg, P.; Murray, J.S.; Brinck, T.; Politzer’, P.; Politzer, P. Average Local Ionization Energies on the Molecular Surfaces of Aromatic Systems as Guides to Chemical Reactivity. 2011, 68, 1440–1443. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH Oxidases: An Overview from Structure to Innate Immunity-Associated Pathologies. Cellular & Molecular Immunology 2014 12:1 2014, 12, 5–23. [Google Scholar] [CrossRef]
- Kim, J.A.; Neupane, G.P.; Lee, E.S.; Jeong, B.S.; Park, B.C.; Thapa, P. NADPH Oxidase Inhibitors: A Patent Review. Expert Opin Ther Pat 2011, 21, 1147–1158. [Google Scholar] [CrossRef]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-KB in Lung Epithelial Cells. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Grosser, N.; Abate, A.; Oberle, S.; Vreman, H.J.; Dennery, P.A.; Becker, J.C.; Pohle, T.; Seidman, D.S.; Schröder, H. Heme Oxygenase-1 Induction May Explain the Antioxidant Profile of Aspirin. Biochem Biophys Res Commun 2003, 308, 956–960. [Google Scholar] [CrossRef]
- Luo, M.; Tian, R.; Lu, N. Quercetin Inhibited Endothelial Dysfunction and Atherosclerosis in Apolipoprotein E-deficient Mice: Critical Roles for NADPH Oxidase and Heme Oxygenase-1. J Agric Food Chem 2020, 68, 10875–10883. [Google Scholar] [CrossRef]
- Jimenez, R.; Lopez-Sepulveda, R.; Romero, M.; Toral, M.; Cogolludo, A.; Perez-Vizcaino, F.; Duarte, J. Quercetin and Its Metabolites Inhibit the Membrane NADPH Oxidase Activity in Vascular Smooth Muscle Cells from Normotensive and Spontaneously Hypertensive Rats. Food Funct 2015, 6, 409–414. [Google Scholar] [CrossRef]
- Lountos, G.T.; Jiang, R.; Wellborn, W.B.; Thaler, T.L.; Bommarius, A.S.; Orville, A.M. The Crystal Structure of NAD(P)H Oxidase from Lactobacillus Sanfranciscensis: Insights into the Conversion of O2 into Two Water Molecules by the Flavoenzyme. Biochemistry 2006, 45, 9648–9659. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine Oxidoreductase: One Enzyme for Multiple Physiological Tasks. Redox Biol 2021, 41, 101882. [Google Scholar] [CrossRef]
- Wang, C.H.; Zhang, C.; Xing, X.H. Xanthine Dehydrogenase: An Old Enzyme with New Knowledge and Prospects. Bioengineered 2016, 7, 395–405. [Google Scholar] [CrossRef]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci Rep 2017, 7. [Google Scholar] [CrossRef]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The Crystallization of Monosodium Urate. Curr Rheumatol Rep 2014, 16, 400. [Google Scholar] [CrossRef]
- Avena-Woods, C.; Hilas, O. Febuxostat (Uloric), A New Treatment Option for Gout. Pharmacy and Therapeutics 2010, 35, 82. [Google Scholar]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An Old Disease in New Perspective – A Review. J Adv Res 2017, 8, 495. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Zhang, G.; Gong, D. Mechanistic Insights into the Inhibition of Quercetin on Xanthine Oxidase. Int J Biol Macromol 2018, 112, 405–412. [Google Scholar] [CrossRef]
- Zhu, J.X.; Wang, Y.; Kong, L.D.; Yang, C.; Zhang, X. Effects of Biota Orientalis Extract and Its Flavonoid Constituents, Quercetin and Rutin on Serum Uric Acid Levels in Oxonate-Induced Mice and Xanthine Dehydrogenase and Xanthine Oxidase Activities in Mouse Liver. J Ethnopharmacol 2004, 93, 133–140. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Qiu, J.; Li, J.; Liang, X.; Zhang, Z.; Zhang, X.; Fu, H.; Korantzopoulos, P.; Letsas, K.P.; et al. Xanthine Oxidase Inhibitor Allopurinol Prevents Oxidative Stress-Mediated Atrial Remodeling in Alloxan-Induced Diabetes Mellitus Rabbits. J Am Heart Assoc 2018, 7. [Google Scholar] [CrossRef]
- Barteková, M.; Šimončíková, P.; Fogarassyová, M.; Ivanová, M.; Okruhlicová, L.; Tribulová, N.; Dovinová, I.; Barančík, M. Quercetin Improves Postischemic Recovery of Heart Function in Doxorubicin-Treated Rats and Prevents Doxorubicin-Induced Matrix Metalloproteinase-2 Activation and Apoptosis Induction. Int J Mol Sci 2015, 16, 8168–8185. [Google Scholar] [CrossRef]
- Wong, D.R.; Derijks, L.J.J.; Den Dulk, M.O.; Gemmeke, E.H.K.M.; Hooymans, P.M. The Role of Xanthine Oxidase in Thiopurine Metabolism: A Case Report. Ther Drug Monit 2007, 29, 845–848. [Google Scholar] [CrossRef]
- Pacher, P.; Nivorozhkin, A.; Szabó, C. Therapeutic Effects of Xanthine Oxidase Inhibitors: Renaissance Half a Century after the Discovery of Allopurinol. Pharmacol Rev 2006, 58, 87. [Google Scholar] [CrossRef]
- Mohos, V.; Pánovics, A.; Fliszár-Nyúl, E.; Schilli, G.; Hetényi, C.; Mladěnka, P.; Needs, P.W.; Kroon, P.A.; Pethő, G.; Poór, M. Inhibitory Effects of Quercetin and Its Human and Microbial Metabolites on Xanthine Oxidase Enzyme. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Cao, H.; Pauff, J.M.; Hille, R. X-Ray Crystal Structure of a Xanthine Oxidase Complex with the Flavonoid Inhibitor Quercetin. J Nat Prod 2014, 77, 1693–1699. [Google Scholar] [CrossRef]
- Enroth, C.; Eger, B.T.; Okamoto, K.; Nishino, T.; Nishino, T.; Pai, E.F. Crystal Structures of Bovinemilk Xanthine Dehydrogenase and Xanthine Oxidase: Structure-Based Mechanism of Conversion. Proc Natl Acad Sci U S A 2000, 97, 10723–10728. [Google Scholar] [CrossRef]
- Blair-Johnson, M.; Fiedler, T.; Fenna, R. Human Myeloperoxidase: Structure of a Cyanide Complex and Its Interaction with Bromide and Thiocyanate Substrates at 1.9 Å Resolution†. Biochemistry 2001, 40, 13990–13997. [Google Scholar] [CrossRef]
- Koeth, R.A.; Haselden, V.; Tang, W.H.W. Myeloperoxidase in Cardiovascular Disease. Adv Clin Chem 2013, 62, 1–32. [Google Scholar] [CrossRef]
- Rehring, J.F.; Bui, T.M.; Galán-Enríquez, C.S.; Urbanczyk, J.M.; Ren, X.; Wiesolek, H.L.; Sullivan, D.P.; Sumagin, R. Released Myeloperoxidase Attenuates Neutrophil Migration and Accumulation in Inflamed Tissue. Front Immunol 2021, 12. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase - A Bridge Linking Inflammation and Oxidative Stress with Cardiovascular Disease. Clin Chim Acta 2019, 493, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, F.H.; Cardoso, A.M.; Schmatz, R.; Gonçalves, J.F.; Baldissarelli, J.; Martins, C.C.; Zanini, D.; de Oliveira, L.S.; da Costa, P.; Pimentel, V.C.; et al. Protective Effect of Quercetin in Ecto-Enzymes, Cholinesterases, and Myeloperoxidase Activities in the Lymphocytes of Rats Exposed to Cadmium. Mol Cell Biochem 2014, 396, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, B.A.; Hajiali, F.; Adineh, M.; Nassiri-Asl, M. Anti-Inflammatory Effects of Quercetin and Vitexin on Activated Human Peripheral Blood Neutrophils. J Pharmacopuncture 2017, 20, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Annapurna, A.; Ansari, M.A.; Manjunath, P.M. Partial Role of Multiple Pathways in Infarct Size Limiting Effect of Quercetin and Rutin against Cerebral Ischemia-Reperfusion Injury in Rats. Eur Rev Med Pharmacol Sci 2013, 17, 491–500. [Google Scholar] [PubMed]
- Qaddoumi, M.G.; Alanbaei, M.; Hammad, M.M.; Al Khairi, I.; Cherian, P.; Channanath, A.; Thanaraj, T.A.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Investigating the Role of Myeloperoxidase and Angiopoietin-like Protein 6 in Obesity and Diabetes. Sci Rep 2020, 10. [Google Scholar] [CrossRef]
- Tian, R.; Jin, Z.; Zhou, L.; Zeng, X.P.; Lu, N. Quercetin Attenuated Myeloperoxidase-Dependent HOCl Generation and Endothelial Dysfunction in Diabetic Vasculature. J Agric Food Chem 2021, 69, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps (NETs): Double-Edged Swords of Innate Immunity. J Immunol 2012, 189, 2689. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.S.; Percebom, I.; Mendes, S.; Souza, P.S.S.; Diniz, L.F.A.; Costa, M.F.; Lopes, B.R.P.; Toledo, K.A. Quercetin Inhibits Neutrophil Extracellular Traps Release and Their Cytotoxic Effects on A549 Cells, as Well the Release and Enzymatic Activity of Elastase and Myeloperoxidase. Brazilian Journal of Biology 2022, 84, e252936. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific Roles of Lipoxygenases in Development and Responses to Stress in Plants. Plants 2022, 11. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Laparra-Llopis, J.M.; Schneider, C.; Espín, J.C. Targeting Mammalian 5-Lipoxygenase by Dietary Phenolics as an Anti-Inflammatory Mechanism: A Systematic Review. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Wisastra, R.; Dekker, F.J. Inflammation, Cancer and Oxidative Lipoxygenase Activity Are Intimately Linked. Cancers (Basel) 2014, 6, 1500. [Google Scholar] [CrossRef]
- Bhaskar, S.; Kumar, K.S.; Krishnan, K.; Antony, H. Quercetin Alleviates Hypercholesterolemic Diet Induced Inflammation during Progression and Regression of Atherosclerosis in Rabbits. Nutrition 2013, 29, 219–229. [Google Scholar] [CrossRef]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-Lipoxygenases by Flavonoids: Structure-Activity Relations and Mode of Action. Biochem Pharmacol 2003, 65, 773–781. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.L.; Tsushida, T.; Terao, J. Inhibition of Mammalian 15-Lipoxygenase-Dependent Lipid Peroxidation in Low-Density Lipoprotein by Quercetin and Quercetin Monoglucosides. Arch Biochem Biophys 1998, 349, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.L.; Abdalla, D.S.P.; Terao, J. Inhibitory Effect of Flavonoids on Low-Density Lipoprotein Peroxidation Catalyzed by Mammalian 15-Lipoxygenase. IUBMB Life 2000, 49, 289–295. [Google Scholar] [CrossRef] [PubMed]
- de Carmen Pinto, M.; Duque, A.L.; Macías, P. Fluorescence Quenching Study on the Interaction between Quercetin and Lipoxygenase. J Fluoresc 2011, 21, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Vyshnevska, L.; Severina, H.I.; Prokopenko, Y.; Shmalko, A. Molecular Docking Investigation of Anti-Inflammatory Herbal Compounds as Potential LOX-5 and COX-2 Inhibitors. Pharmacia 69(3): 733-744 2022, 69, 733–744. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and Mechanistic Insights into 5-Lipoxygenase Inhibition by Natural Products. Nature Chemical Biology 2020 16:7 2020, 16, 783–790. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Sobarzo-Sánchez, E.; Uriarte, E.; Khatkar, A. Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders. Molecules 2019, Vol. 24, Page 418 2019, 24, 418. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P.M.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front Pharmacol 2016, 7, 340. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr Neuropharmacol 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Gaweska, H.; Fitzpatrick, P.F. Structures and Mechanism of the Monoamine Oxidase Family. Biomol Concepts 2011, 2, 365–377. [Google Scholar] [CrossRef]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S.; et al. A New Series of Flavones, Thioflavones, and Flavanones as Selective Monoamine Oxidase-B Inhibitors. Bioorg Med Chem 2010, 18, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Malik, N.; Sobarzo-Sánchez, E.; Uriarte, E.; Khatkar, A. Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Lodi, F.; Jimenez, R.; Moreno, L.; Kroon, P.A.; Needs, P.W.; Hughes, D.A.; Santos-Buelga, C.; Gonzalez-Paramas, A.; Cogolludo, A.; Lopez-Sepulveda, R.; et al. Glucuronidated and Sulfated Metabolites of the Flavonoid Quercetin Prevent Endothelial Dysfunction but Lack Direct Vasorelaxant Effects in Rat Aorta. Atherosclerosis 2009, 204, 34–39. [Google Scholar] [CrossRef]
- Kawai, Y. β-Glucuronidase Activity and Mitochondrial Dysfunction: The Sites Where Flavonoid Glucuronides Act as Anti-Inflammatory Agents. J Clin Biochem Nutr 2014, 54, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Oyeleye, S.I.; Ogunsuyi, O.B.; Adebayo, A.A. Phytochemicals and Hormonal Effects. Encyclopedia of Food Chemistry 2019, 550–560. [Google Scholar] [CrossRef]
- Yoshino, S.; Hara, A.; Sakakibara, H.; Kawabata, K.; Tokumura, A.; Ishisaka, A.; Kawai, Y.; Terao, J. Effect of Quercetin and Glucuronide Metabolites on the Monoamine Oxidase-A Reaction in Mouse Brain Mitochondria. Nutrition 2011, 27, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Bandaruk, Y.; Mukai, R.; Kawamura, T.; Nemoto, H.; Terao, J. Evaluation of the Inhibitory Effects of Quercetin-Related Flavonoids and Tea Catechins on the Monoamine Oxidase-A Reaction in Mouse Brain Mitochondria. J Agric Food Chem 2012, 60, 10270–10277. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Khatkar, A. In Silico Design, Synthesis of Hybrid Combinations: Quercetin Based MAO Inhibitors with Antioxidant Potential. Curr Top Med Chem 2019, 19, 156–170. [Google Scholar] [CrossRef]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; León, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.H.; Belouahem-Abed, D.; et al. Inhibition of Human Monoamine Oxidase A and B by Flavonoids Isolated from Two Algerian Medicinal Plants. Phytomedicine 2018, 40, 27–36. [Google Scholar] [CrossRef]
- De Colibus, L.; Li, M.; Binda, C.; Lustig, A.; Edmondson, D.E.; Mattevi, A. Three-Dimensional Structure of Human Monoamine Oxidase A (MAO A): Relation to the Structures of Rat MAO A and Human MAO B. Proc Natl Acad Sci U S A 2005, 102, 12684–12689. [Google Scholar] [CrossRef]
- Son, S.Y.; Ma, J.; Kondou, Y.; Yoshimura, M.; Yamashita, E.; Tsukihara, T. Structure of Human Monoamine Oxidase A at 2.2-Å Resolution: The Control of Opening the Entry for Substrates/Inhibitors. Proc Natl Acad Sci U S A 2008, 105, 5739–5744. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any in-jury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).