Submitted:
13 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
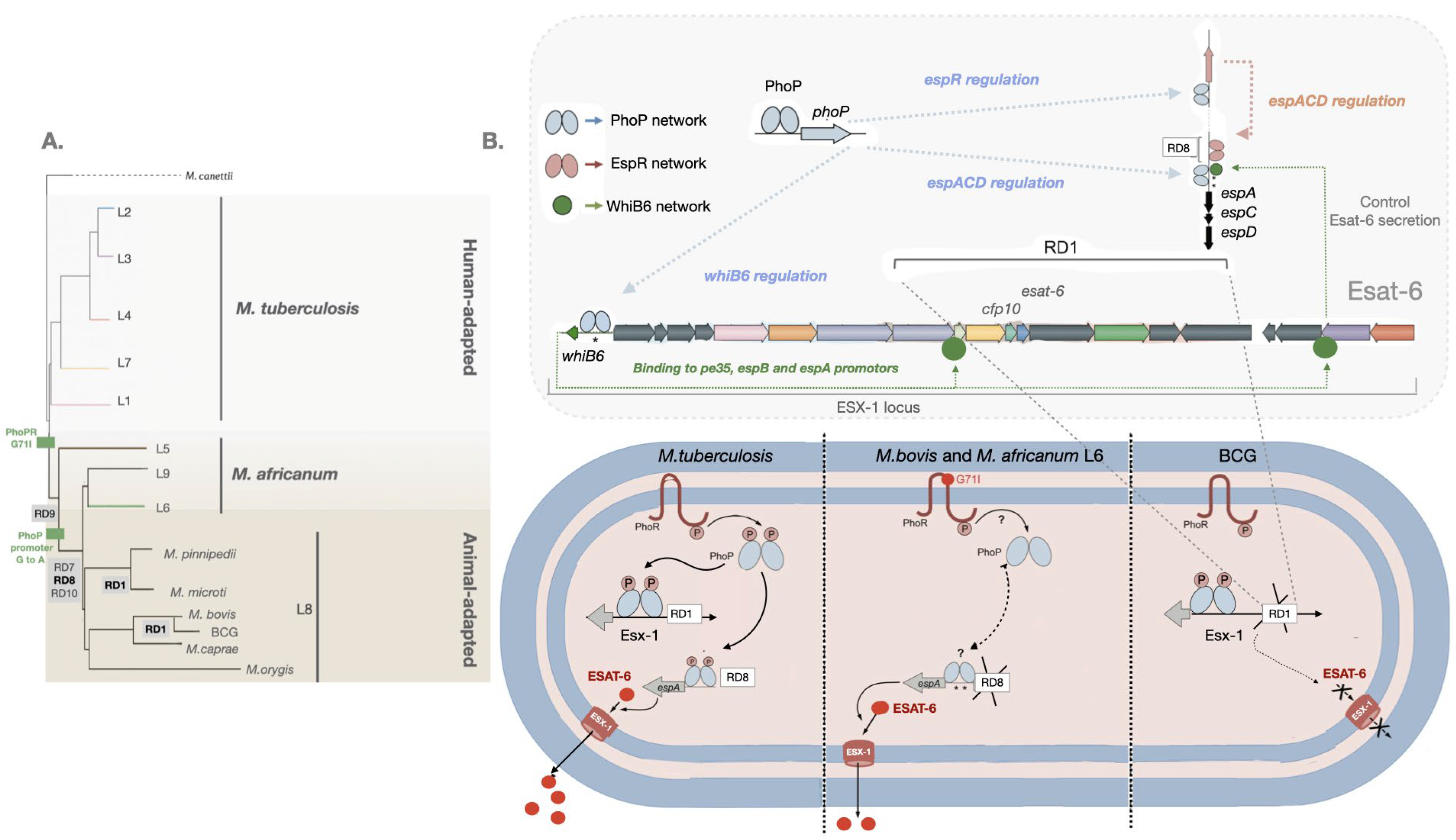
2. Virulence evolution among MTBC
3. ESAT-6 is required for virulence of Mtb
3. ESAT-6 in Mtb pathogenesis
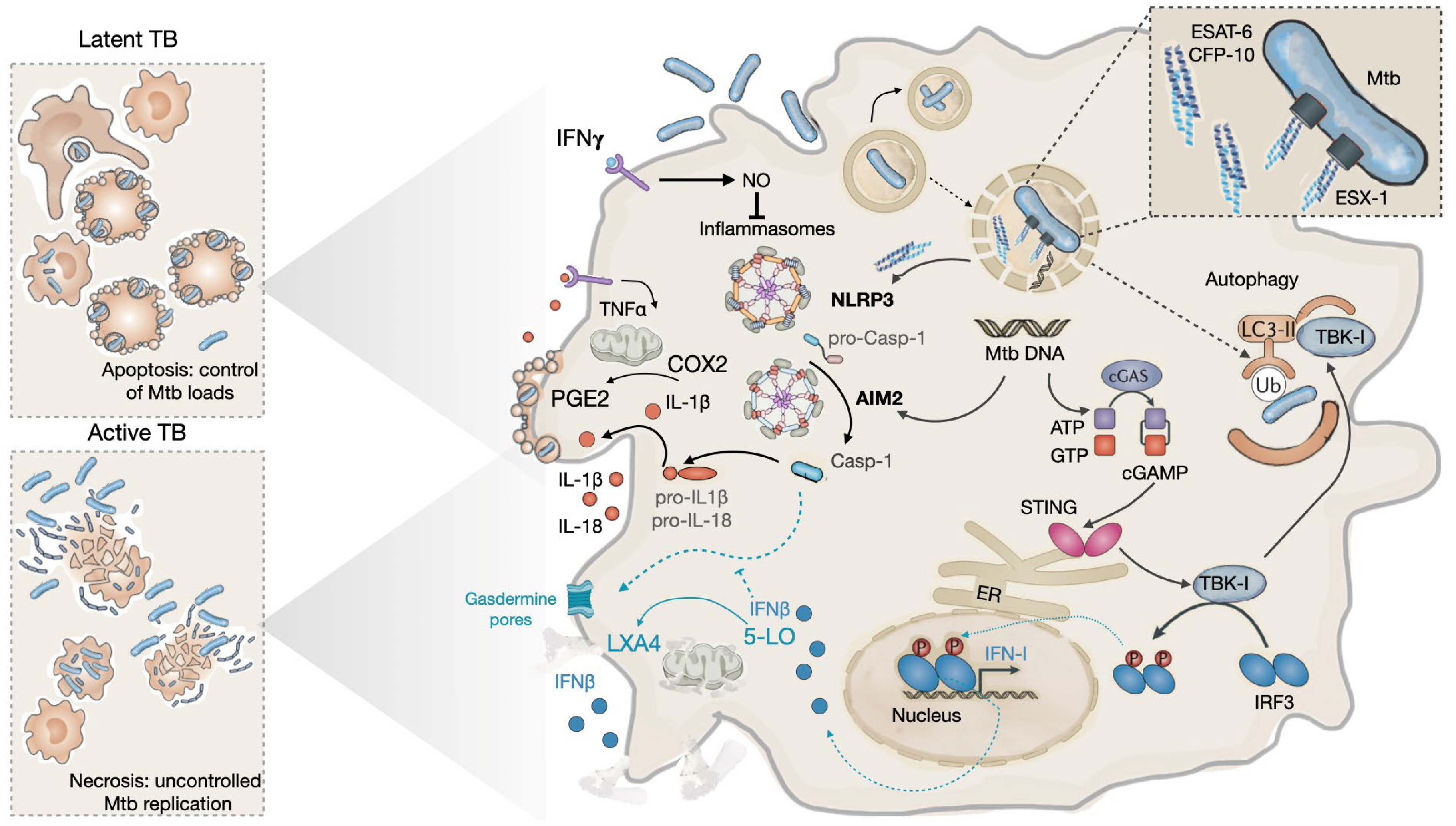
3.1. During the early phases of infection: the innate phase
3.2. During latency
3.2. During progression to disease
4. ESAT-6 from a virulence factor to diagnostic tools and vaccines for TB
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perrin, P. Human and tuberculosis co-evolution: An integrative view. Tuberculosis 2015, 95, S112–S116. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.G. Commentary: Medicine, population, and tuberculosis. Int. J. Epidemiol. 2005, 34, 521–524. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Report 2022 Factsheet. Available online: https://www.who.int/publications/m/item/global-tuberculosis-report-2022-factsheet (accessed on 20 April 2023).
- Chen, X.; Hu, T.Y. Strategies for advanced personalized tuberculosis diagnosis: Current technologies and clinical approaches. Precision Clinical Medicine 2021, 4, 35–44. [Google Scholar] [CrossRef]
- Behr, M.A.; Kaufmann, E.; Duffin, J.; Edelstein, P.H.; Ramakrishnan, L. Latent Tuberculosis: Two Centuries of Confusion. American Journal of Respiratory and Critical Care Medicine 2021, 204, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Carranza, C.; Pedraza-Sanchez, S.; de Oyarzabal-Mendez, E.; Torres, M. Diagnosis for Latent Tuberculosis Infection: New Alternatives. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Barry, C.E., 3rd; Maartens, G. Tuberculosis. The Lancet 2016, 387, 1211–1226. [Google Scholar] [CrossRef]
- Dheda, K.; Gumbo, T.; Maartens, G.; Dooley, K.E.; McNerney, R.; Murray, M.; Furin, J.; Nardell, E.A.; London, L.; Lessem, E.; et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. The Lancet Respiratory Medicine 2017, 5, 291–360. [Google Scholar] [CrossRef]
- Cambier, C.J.; Falkow, S.; Ramakrishnan, L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell 2014, 159, 1497–1509. [Google Scholar] [CrossRef]
- Azevedo-Pereira, J.M.; Pires, D.; Calado, M.; Mandal, M.; Santos-Costa, Q.; Anes, E. HIV/Mtb Co-Infection: From the Amplification of Disease Pathogenesis to an “Emerging Syndemic”. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Barry, C.E.; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, R.J.; Young, D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nature Reviews Microbiology 2009, 7, 845–855. [Google Scholar] [CrossRef]
- Blaser, M.J.; Kirschner, D. The equilibria that allow bacterial persistence in human hosts. Nature 2007, 449, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.T.; Smith, E.G.; Banerjee, A.; Smith, R.M.M.; Dale, J.; Innes, J.A.; Hunt, D.; Tweddell, A.; Wood, A.; Anderson, C.; et al. Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to-person transmission in the UK. The Lancet 2007, 369, 1270–1276. [Google Scholar] [CrossRef]
- Grange, J.M. Mycobacterium bovis infection in human beings. Tuberculosis 2001, 81, 71–77. [Google Scholar] [CrossRef]
- Thoen, C.O.; LoBue, P.A. Mycobacterium bovis tuberculosis: forgotten, but not gone. The Lancet 2007, 369, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gonzalez, P.; Cervera-Hernandez, M.E.; Martinez-Gamboa, A.; Garcia-Garcia, L.; Cruz-Hervert, L.P.; Bobadilla-del Valle, M.; Ponce-de Leon, A.; Sifuentes-Osornio, J. Human tuberculosis caused by Mycobacterium bovis: a retrospective comparison with Mycobacterium tuberculosis in a Mexican tertiary care centre, 2000–2015. BMC Infect. Dis. 2016, 16, 657. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, E.G.; Griffiths, R.J.; Roberts, C. Pulmonary tuberculosis due to Mycobacterium bovis. Thorax 1986, 41, 685. [Google Scholar] [CrossRef] [PubMed]
- de Jong, B.C.; Hill, P.C.; Aiken, A.; Awine, T.; Martin, A.; Adetifa, I.M.; Jackson-Sillah, D.J.; Fox, A.; Kathryn, D.; Gagneux, S.; et al. Progression to Active Tuberculosis, but Not Transmission, Varies by Mycobacterium tuberculosis Lineage in The Gambia. The Journal of Infectious Diseases 2008, 198, 1037–1043. [Google Scholar] [CrossRef]
- Mostowy, S.; Onipede, A.; Gagneux, S.; Niemann, S.; Kremer, K.; Desmond Edward, P.; Kato-Maeda, M.; Behr, M. Genomic Analysis Distinguishes Mycobacterium africanum. J. Clin. Microbiol. 2004, 42, 3594–3599. [Google Scholar] [CrossRef]
- Silva, M.L.; Cá, B.; Osório, N.S.; Rodrigues, P.N.S.; Maceiras, A.R.; Saraiva, M. Tuberculosis caused by Mycobacterium africanum: Knowns and unknowns. PLOS Pathogens 2022, 18, e1010490. [Google Scholar] [CrossRef]
- de Jong, B.C.; Antonio, M.; Gagneux, S. Mycobacterium africanum—Review of an Important Cause of Human Tuberculosis in West Africa. PLoS Negl. Trop. Dis. 2010, 4, e744. [Google Scholar] [CrossRef]
- Coscolla, M.; Gagneux, S.; Menardo, F.; Loiseau, C.; Ruiz-Rodriguez, P.; Borrell, S.; Otchere, I.D.; Asante-Poku, A.; Asare, P.; Sánchez-Busó, L.; et al. Phylogenomics of Mycobacterium africanum reveals a new lineage and a complex evolutionary history. Microbial Genomics 2021, 7. [Google Scholar] [CrossRef]
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nature Reviews Microbiology 2018, 16, 202–213. [Google Scholar] [CrossRef]
- Müller, B.; Dürr, S.; Alonso, S.; Hattendorf, J.; Laisse, C.J.M.; Parsons, S.D.C.; van Helden, P.; Zinsstag, J. Zoonotic Mycobacterium bovis–induced Tuberculosis in Humans. Emerging Infectious Disease journal 2013, 19, 899. [Google Scholar] [CrossRef]
- Borham, M.; Oreiby, A.; El-Gedawy, A.; Hegazy, Y.; Khalifa, H.O.; Al-Gaabary, M.; Matsumoto, T. Review on Bovine Tuberculosis: An Emerging Disease Associated with Multidrug-Resistant Mycobacterium Species. Pathogens 2022, 11. [Google Scholar] [CrossRef]
- Prodinger, W.M.; Indra, A.; Koksalan, O.K.; Kilicaslan, Z.; Richter, E. Mycobacterium caprae infection in humans. Expert Rev. Anti Infect. Ther. 2014, 12, 1501–1513. [Google Scholar] [CrossRef]
- Tagliapietra, V.; Boniotti, M.B.; Mangeli, A.; Karaman, I.; Alborali, G.; Chiari, M.; D’Incau, M.; Zanoni, M.; Rizzoli, A.; Pacciarini, M.L. Mycobacterium microti at the Environment and Wildlife Interface. Microorganisms 2021, 9, 2084. [Google Scholar] [CrossRef] [PubMed]
- Fabre, M.; Hauck, Y.; Soler, C.; Koeck, J.-L.; van Ingen, J.; van Soolingen, D.; Vergnaud, G.; Pourcel, C. Molecular characteristics of “Mycobacterium canettii” the smooth Mycobacterium tuberculosis bacilli. Infection, Genetics and Evolution 2010, 10, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Asensio, J.; Malaga, W.; Pawlik, A.; Astarie-Dequeker, C.; Passemar, C.; Moreau, F.; Laval, F.; Daffé, M.; Martin, C.; Brosch, R.; et al. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proceedings of the National Academy of Sciences 2014, 111, 11491–11496. [Google Scholar] [CrossRef] [PubMed]
- Riojas, M.A.; McGough, K.J.; Rider-Riojas, C.J.; Rastogi, N.; Hazbón, M.H. Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis. International Journal of Systematic and Evolutionary Microbiology 2018, 68, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.E.; Patton, E.A.; Gibbons-Burgener, S.N.; Klos, R.F.; Tans-Kersten, J.L.; Carlson, B.W.; Keller, S.J.; Pritschet, D.J.; Rollo, S.; Dutcher, T.V.; et al. Human-to-Cattle Mycobacterium tuberculosis Complex Transmission in the United States. Frontiers in Veterinary Science 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- WHO. Roadmap for zoonotic tuberculosis. Available online: https://www.who.int/publications/i/item/9789241513043 (accessed on 29 May 2023).
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of major human infectious diseases. Nature 2007, 447, 279–283. [Google Scholar] [CrossRef]
- Comas, I.; Coscolla, M.; Luo, T.; Borrell, S.; Holt, K.E.; Kato-Maeda, M.; Parkhill, J.; Malla, B.; Berg, S.; Thwaites, G.; et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat. Genet. 2013, 45, 1176–1182. [Google Scholar] [CrossRef]
- Urbanowski, M.E.; Ordonez, A.A.; Ruiz-Bedoya, C.A.; Jain, S.K.; Bishai, W.R. Cavitary tuberculosis: the gateway of disease transmission. The Lancet Infectious Diseases 2020, 20, e117–e128. [Google Scholar] [CrossRef] [PubMed]
- Brites, D.; Gagneux, S. Old and new selective pressures on Mycobacterium tuberculosis. Infection, Genetics and Evolution 2012, 12, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Alteri, C.J.; Rios-Sarabia, N.; De la Cruz, M.A.; González-y-Merchand, J.A.; Soria-Bustos, J.; Maldonado-Bernal, C.; Cedillo, M.L.; Yáñez-Santos, J.A.; Martínez-Laguna, Y.; Torres, J.; et al. The Flp type IV pilus operon of Mycobacterium tuberculosis is expressed upon interaction with macrophages and alveolar epithelial cells. Frontiers in Cellular and Infection Microbiology 2022, 12. [Google Scholar] [CrossRef]
- Ramsugit, S.; Pillay, M. Pili of Mycobacterium tuberculosis: current knowledge and future prospects. Arch. Microbiol. 2015, 197, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Kalscheuer, R.; Palacios, A.; Anso, I.; Cifuente, J.; Anguita, J.; Jacobs, W.R., Jr.; Guerin, M.E.; Prados-Rosales, R. The Mycobacterium tuberculosis capsule: a cell structure with key implications in pathogenesis. Biochem. J. 2019, 476, 1995–2016. [Google Scholar] [CrossRef] [PubMed]
- Anes, E.; Pires, D.; Mandal, M.; Azevedo-Pereira, J.M. Spatial localization of cathepsins: Implications in immune activation and resolution during infections. Front. Immunol. 2022, 13, 955407. [Google Scholar] [CrossRef]
- Armstrong, J.A.; Hart, P.D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 1975, 142, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Bernard, E.M.; Pombo, J.P.; Carmo, N.; Fialho, C.; Gutierrez, M.G.; Bettencourt, P.; Anes, E. Mycobacterium tuberculosis Modulates miR-106b-5p to Control Cathepsin S Expression Resulting in Higher Pathogen Survival and Poor T-Cell Activation. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Pires, D.; Calado, M.; Velez, T.; Mandal, M.; Catalão, M.J.; Neyrolles, O.; Lugo-Villarino, G.; Vérollet, C.; Azevedo-Pereira, J.M.; Anes, E. Modulation of Cystatin C in Human Macrophages Improves Anti-Mycobacterial Immune Responses to Mycobacterium tuberculosis Infection and Coinfection With HIV. Front. Immunol. 2021, 12, 4693. [Google Scholar] [CrossRef]
- Pires, D.; Mandal, M.; Pinho, J.; Catalão, M.J.; Almeida, A.J.; Azevedo-Pereira, J.M.; Gaspar, M.M.; Anes, E. Liposomal Delivery of Saquinavir to Macrophages Overcomes Cathepsin Blockade by Mycobacterium tuberculosis and Helps Control the Phagosomal Replicative Niches. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Pires, D.; Marques, J.; Pombo, J.P.; Carmo, N.; Bettencourt, P.; Neyrolles, O.; Lugo-Villarino, G.; Anes, E. Role of Cathepsins in Mycobacterium tuberculosis Survival in Human Macrophages. Sci. Rep. 2016, 6, 32247. [Google Scholar] [CrossRef]
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Sherman, D.R.; Gerner, M.Y.; Urdahl, K.B. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host & Microbe 2018, 24, 439–446.e434. [Google Scholar] [CrossRef]
- Mishra, B.B.; Moura-Alves, P.; Sonawane, A.; Hacohen, N.; Griffiths, G.; Moita, L.F.; Anes, E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 2010, 12, 1046–1063. [Google Scholar] [CrossRef] [PubMed]
- Aguilo, J.I.; Alonso, H.; Uranga, S.; Marinova, D.; Arbués, A.; de Martino, A.; Anel, A.; Monzon, M.; Badiola, J.; Pardo, J.; et al. ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cell. Microbiol. 2013, 15, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Dallenga, T.; Repnik, U.; Corleis, B.; Eich, J.; Reimer, R.; Griffiths, G.W.; Schaible, U.E. M. tuberculosis-Induced Necrosis of Infected Neutrophils Promotes Bacterial Growth Following Phagocytosis by Macrophages. Cell Host & Microbe 2017, 22, 519–530.e513. [Google Scholar] [CrossRef]
- Derrick, S.C.; Morris, S.L. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell. Microbiol. 2007, 9, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Anes, E.; Azevedo-Pereira, J.M.; Pires, D. Cathepsins and Their Endogenous Inhibitors in Host Defense During Mycobacterium tuberculosis and HIV Infection. Front. Immunol. 2021, 12, 726984. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Ramakrishnan, L. The Role of the Granuloma in Expansion and Dissemination of Early Tuberculous Infection. Cell 2009, 136, 37–49. [Google Scholar] [CrossRef]
- Pagán, A.J.; Ramakrishnan, L. Immunity and Immunopathology in the Tuberculous Granuloma. Cold Spring Harb. Perspect. Med. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.J.; Linas, B.; Trevejo-Nuñez, G.J.; Kincaid, E.; Tamura, T.; Takatsu, K.; Ernst, J.D. Mycobacterium tuberculosis Infects Dendritic Cells with High Frequency and Impairs Their Function In Vivo1. The Journal of Immunology 2007, 179, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Rathinam, V.A.K.; Martens, G.W.; Martinot, A.J.; Kornfeld, H.; Fitzgerald, K.A.; Sassetti, C.M. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome–dependent processing of IL-1β. Nat. Immunol. 2013, 14, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-W.; Jacobs Jr, W.R. Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell. Microbiol. 2011, 13, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Brosch, R.; Gordon, S.V.; Marmiesse, M.; Brodin, P.; Buchrieser, C.; Eiglmeier, K.; Garnier, T.; Gutierrez, C.; Hewinson, G.; Kremer, K.; et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proceedings of the National Academy of Sciences 2002, 99, 3684–3689. [Google Scholar] [CrossRef] [PubMed]
- Brosch, R.; Gordon, S.V.; Garnier, T.; Eiglmeier, K.; Frigui, W.; Valenti, P.; Dos Santos, S.; Duthoy, S.; Lacroix, C.; Garcia-Pelayo, C.; et al. Genome plasticity of BCG and impact on vaccine efficacy. Proceedings of the National Academy of Sciences 2007, 104, 5596–5601. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Marinova, D.; Gonzalo-Asensio, J.; Aguilo, N.; Martin, C. MTBVAC from discovery to clinical trials in tuberculosis-endemic countries. Expert Review of Vaccines 2017, 16, 565–576. [Google Scholar] [CrossRef]
- Smith, N.H.; Hewinson, R.G.; Kremer, K.; Brosch, R.; Gordon, S.V. Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis. Nature Reviews Microbiology 2009, 7, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Broset, E.; Martín, C.; Gonzalo-Asensio, J. Evolutionary Landscape of the Mycobacterium tuberculosis Complex from the Viewpoint of PhoPR: Implications for Virulence Regulation and Application to Vaccine Development. mBio 2015, 6, e01289–e01215. [Google Scholar] [CrossRef] [PubMed]
- Frigui, W.; Bottai, D.; Majlessi, L.; Monot, M.; Josselin, E.; Brodin, P.; Garnier, T.; Gicquel, B.; Martin, C.; Leclerc, C.; et al. Control of M. tuberculosis ESAT-6 Secretion and Specific T Cell Recognition by PhoP. PLOS Pathogens 2008, 4, e33. [Google Scholar] [CrossRef]
- Pérez, E.; Samper, S.; Bordas, Y.; Guilhot, C.; Gicquel, B.; Martín, C. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol. Microbiol. 2001, 41, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Mahairas, G.G.; Sabo, P.J.; Hickey, M.J.; Singh, D.C.; Stover, C.K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 1996, 178, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Pym, A.S.; Brodin, P.; Brosch, R.; Huerre, M.; Cole, S.T. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 2002, 46, 709–717. [Google Scholar] [CrossRef]
- de Jong, B.C.; Hill, P.C.; Brookes, R.H.; Gagneux, S.; Jeffries, D.J.; Otu, J.K.; Donkor, S.A.; Fox, A.; McAdam, K.P.W.J.; Small, P.M.; et al. Mycobacterium africanum Elicits an Attenuated T Cell Response to Early Secreted Antigenic Target, 6 kDa, in Patients with Tuberculosis and Their Household Contacts. The Journal of Infectious Diseases 2006, 193, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Asensio, J.; Pérez, I.; Aguiló, N.; Uranga, S.; Picó, A.; Lampreave, C.; Cebollada, A.; Otal, I.; Samper, S.; Martín, C. New insights into the transposition mechanisms of IS6110 and its dynamic distribution between Mycobacterium tuberculosis Complex lineages. PLoS Genet. 2018, 14, e1007282. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.Y.; Menendez, M.C.; Perez, E.; Samper, S.; Gomez, A.B.; Garcia, M.J.; Martin, C. IS6110 Mediates Increased Transcription of the phoP Virulence Gene in a Multidrug-Resistant Clinical Isolate Responsible for Tuberculosis Outbreaks. J. Clin. Microbiol. 2004, 42, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.M.; Gey van Pittius, N.C.; DiGiuseppe Champion, P.A.; Cox, J.; Luirink, J.; Vandenbroucke-Grauls, C.M.J.E.; Appelmelk, B.J.; Bitter, W. Type VII secretion — mycobacteria show the way. Nature Reviews Microbiology 2007, 5, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Gröschel, M.I.; Sayes, F.; Simeone, R.; Majlessi, L.; Brosch, R. ESX secretion systems: mycobacterial evolution to counter host immunity. Nature Reviews Microbiology 2016, 14, 677–691. [Google Scholar] [CrossRef]
- Andersen, P.; Andersen, A.B.; Sørensen, A.L.; Nagai, S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. The Journal of Immunology 1995, 154, 3359–3372. [Google Scholar] [CrossRef]
- Sørensen, A.L.; Nagai, S.; Houen, G.; Andersen, P.; Andersen, A.B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infection and Immunity 1995, 63, 1710–1717. [Google Scholar] [CrossRef]
- Brandt, L.; Elhay, M.; Rosenkrands, I.; Lindblad Erik, B.; Andersen, P. ESAT-6 Subunit Vaccination against Mycobacterium tuberculosis. Infection and Immunity 2000, 68, 791–795. [Google Scholar] [CrossRef]
- Ruhwald, M.; de Thurah, L.; Kuchaka, D.; Zaher, M.R.; Salman, A.M.; Abdel-Ghaffar, A.-R.; Shoukry, F.A.; Michelsen, S.W.; Soborg, B.; Blauenfeldt, T.; et al. Introducing the ESAT-6 free IGRA, a companion diagnostic for TB vaccines based on ESAT-6. Sci. Rep. 2017, 7, 45969. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; Rosenkrands, I.; Andersen, P.; Cole, S.T.; Brosch, R. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol. 2004, 12, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; McIntosh, F.; Radomski, N.; Dewar, K.; Simeone, R.; Enninga, J.; Brosch, R.; Rocha, E.P.; Veyrier, F.J.; Behr, M.A. Insights on the Emergence of Mycobacterium tuberculosis from the Analysis of Mycobacterium kansasii. Genome Biol. Evol. 2015, 7, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Wirth, S.E.; Krywy, J.A.; Aldridge, B.B.; Fortune, S.M.; Fernandez-Suarez, M.; Gray, T.A.; Derbyshire, K.M. Polar assembly and scaffolding proteins of the virulence-associated ESX-1 secretory apparatus in mycobacteria. Mol. Microbiol. 2012, 83, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Anes, E.; Peyron, P.; Staali, L.; Jordao, L.; Gutierrez, M.G.; Kress, H.; Hagedorn, M.; Maridonneau-Parini, I.; Skinner, M.A.; Wildeman, A.G.; et al. Dynamic life and death interactions between Mycobacterium smegmatis and J774 macrophages. Cell. Microbiol. 2006, 8, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Coros, A.; Callahan, B.; Battaglioli, E.; Derbyshire, K.M. The specialized secretory apparatus ESX-1 is essential for DNA transfer in Mycobacterium smegmatis. Mol. Microbiol. 2008, 69, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Flint, J.L.; Kowalski, J.C.; Karnati, P.K.; Derbyshire, K.M. The RD1 virulence locus of Mycobacterium tuberculosis regulates DNA transfer in Mycobacterium smegmatis. Proceedings of the National Academy of Sciences 2004, 101, 12598–12603. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Becq, J.; Gicquel, B.; Deschavanne, P.; Neyrolles, O. Horizontally acquired genomic islands in the tubercle bacilli. Trends Microbiol. 2008, 16, 303–308. [Google Scholar] [CrossRef]
- Ates, L.S.; Brosch, R. Discovery of the type VII ESX-1 secretion needle? Mol. Microbiol. 2017, 103, 7–12. [Google Scholar] [CrossRef]
- Boritsch, E.C.; Supply, P.; Honoré, N.; Seeman, T.; Stinear, T.P.; Brosch, R. A glimpse into the past and predictions for the future: the molecular evolution of the tuberculosis agent. Mol. Microbiol. 2014, 93, 835–852. [Google Scholar] [CrossRef] [PubMed]
- MacGurn, J.A.; Raghavan, S.; Stanley, S.A.; Cox, J.S. A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol. Microbiol. 2005, 57, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, F.; Brosch, R. ESX/Type VII Secretion Systems—An Important Way Out for Mycobacterial Proteins. Microbiology Spectrum 2019, 7, 7–4. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-Y.; Guo, S.; McLaughlin, B.; Morisaki, H.; Engel, J.N.; Brown, E.J. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol. Microbiol. 2004, 53, 1677–1693. [Google Scholar] [CrossRef]
- Guinn, K.M.; Hickey, M.J.; Mathur, S.K.; Zakel, K.L.; Grotzke, J.E.; Lewinsohn, D.M.; Smith, S.; Sherman, D.R. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2004, 51, 359–370. [Google Scholar] [CrossRef]
- Hsu, T.; Hingley-Wilson, S.M.; Chen, B.; Chen, M.; Dai, A.Z.; Morin, P.M.; Marks, C.B.; Padiyar, J.; Goulding, C.; Gingery, M.; et al. The primary mechanism of attenuation of bacillus Calmette–Guérin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proceedings of the National Academy of Sciences 2003, 100, 12420–12425. [Google Scholar] [CrossRef]
- Lewis, K.N.; Liao, R.; Guinn, K.M.; Hickey, M.J.; Smith, S.; Behr, M.A.; Sherman, D.R. Deletion of RD1 from Mycobacterium tuberculosis Mimics Bacille Calmette-Guérin Attenuation. The Journal of Infectious Diseases 2003, 187, 117–123. [Google Scholar] [CrossRef]
- Sassetti, C.M.; Rubin, E.J. Genetic requirements for mycobacterial survival during infection. Proceedings of the National Academy of Sciences 2003, 100, 12989–12994. [Google Scholar] [CrossRef]
- Stanley, S.A.; Raghavan, S.; Hwang, W.W.; Cox, J.S. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proceedings of the National Academy of Sciences 2003, 100, 13001–13006. [Google Scholar] [CrossRef]
- Simeone, R.; Bottai, D.; Frigui, W.; Majlessi, L.; Brosch, R. ESX/type VII secretion systems of mycobacteria: Insights into evolution, pathogenicity and protection. Tuberculosis 2015, 95, S150–S154. [Google Scholar] [CrossRef]
- Steenken, W., Jr.; Oatway, W.H., Jr.; Petroff, S.A. Biological Studies of the Tubercle Bacillus : Iii. Dissociation and Pathogenicity of the R and S Variants of the Human Tubercle Bacillus (H(37)). J. Exp. Med. 1934, 60, 515–540. [Google Scholar] [CrossRef]
- Solans, L.; Aguiló, N.; Samper, S.; Pawlik, A.; Frigui, W.; Martín, C.; Brosch, R.; Gonzalo-Asensio, J. A Specific Polymorphism in Mycobacterium tuberculosis H37Rv Causes Differential ESAT-6 Expression and Identifies WhiB6 as a Novel ESX-1 Component. Infection and Immunity 2014, 82, 3446–3456. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Mandal, M.; Matos, A.I.; Peres, C.; Catalão, M.J.; Azevedo-Pereira, J.M.; Satchi-Fainaro, R.; Florindo, H.F.; Anes, E. Development of Chitosan Particles Loaded with siRNA for Cystatin C to Control Intracellular Drug-Resistant Mycobacterium tuberculosis. Antibiotics 2023, 12. [Google Scholar] [CrossRef]
- Pires, D.; Valente, S.; Calado, M.; Mandal, M.; Azevedo-Pereira, J.M.; Anes, E. Repurposing Saquinavir for Host-Directed Therapy to Control Mycobacterium tuberculosis Infection. Front. Immunol. 2021, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Behura, A.; Das, M.; Kumar, A.; Naik, L.; Mishra, A.; Manna, D.; Patel, S.; Mishra, A.; Singh, R.; Dhiman, R. ESAT-6 impedes IL-18 mediated phagosome lysosome fusion via microRNA-30a upon Calcimycin treatment in mycobacteria infected macrophages. Int. Immunopharmacol. 2021, 101, 108319. [Google Scholar] [CrossRef]
- Bettencourt, P.; Pires, D.; Anes, E. Immunomodulating microRNAs of mycobacterial infections. Tuberculosis (Edinburgh, Scotland) 2016, 97, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Lee, W.L.; Alexander, D.C.; Grinstein, S.; Liu, J. The ESAT-6/CFP-10 secretion system of Mycobacterium marinum modulates phagosome maturation. Cell. Microbiol. 2006, 8, 1417–1429. [Google Scholar] [CrossRef]
- Simeone, R.; Bobard, A.; Lippmann, J.; Bitter, W.; Majlessi, L.; Brosch, R.; Enninga, J. Phagosomal Rupture by Mycobacterium tuberculosis Results in Toxicity and Host Cell Death. PLOS Pathogens 2012, 8, e1002507. [Google Scholar] [CrossRef]
- van der Wel, N.; Hava, D.; Houben, D.; Fluitsma, D.; van Zon, M.; Pierson, J.; Brenner, M.; Peters, P.J. M. tuberculosis and M. leprae Translocate from the Phagolysosome to the Cytosol in Myeloid Cells. Cell 2007, 129, 1287–1298. [Google Scholar] [CrossRef]
- Osman, M.M.; Pagán, A.J.; Shanahan, J.K.; Ramakrishnan, L. Mycobacterium marinum phthiocerol dimycocerosates enhance macrophage phagosomal permeabilization and membrane damage. PLoS One 2020, 15, e0233252. [Google Scholar] [CrossRef]
- Osman, M.M.; Shanahan, J.K.; Chu, F.; Takaki, K.K.; Pinckert, M.L.; Pagán, A.J.; Brosch, R.; Conrad, W.H.; Ramakrishnan, L. The C terminus of the mycobacterium ESX-1 secretion system substrate ESAT-6 is required for phagosomal membrane damage and virulence. Proceedings of the National Academy of Sciences 2022, 119, e2122161119. [Google Scholar] [CrossRef]
- Quigley, J.; Hughitt, V.K.; Velikovsky Carlos, A.; Mariuzza Roy, A.; El-Sayed Najib, M.; Briken, V. The Cell Wall Lipid PDIM Contributes to Phagosomal Escape and Host Cell Exit of Mycobacterium tuberculosis. mBio 2017, 8, e00148–00117. [Google Scholar] [CrossRef]
- Mishra, B.B.; Lovewell, R.R.; Olive, A.J.; Zhang, G.; Wang, W.; Eugenin, E.; Smith, C.M.; Phuah, J.Y.; Long, J.E.; Dubuke, M.L.; et al. Nitric oxide prevents a pathogen-permissive granulocytic inflammation during tuberculosis. Nature Microbiology 2017, 2, 17072. [Google Scholar] [CrossRef]
- Elkington, P.T.; Green, J.A.; Emerson, J.E.; Lopez-Pascua, L.D.; Boyle, J.J.; O'Kane, C.M.; Friedland, J.S. Synergistic up-regulation of epithelial cell matrix metalloproteinase-9 secretion in tuberculosis. Am. J. Respir. Cell Mol. Biol. 2007, 37, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Volkman, H.E.; Pozos, T.C.; Zheng, J.; Davis, J.M.; Rawls, J.F.; Ramakrishnan, L. Tuberculous Granuloma Induction via Interaction of a Bacterial Secreted Protein with Host Epithelium. Science 2010, 327, 466–469. [Google Scholar] [CrossRef]
- Ha, S.-H.; Choi, H.; Park, J.-Y.; Abekura, F.; Lee, Y.-C.; Kim, J.-R.; Kim, C.-H. Mycobacterium tuberculosis–Secreted Protein, ESAT-6, Inhibits Lipopolysaccharide-Induced MMP-9 Expression and Inflammation Through NF-κB and MAPK Signaling in RAW 264.7 Macrophage Cells. Inflammation 2020, 43, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Augenstreich, J.; Arbues, A.; Simeone, R.; Haanappel, E.; Wegener, A.; Sayes, F.; Le Chevalier, F.; Chalut, C.; Malaga, W.; Guilhot, C.; et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell. Microbiol. 2017, 19, e12726. [Google Scholar] [CrossRef] [PubMed]
- Refai, A.; Gritli, S.; Barbouche, M.-R.; Essafi, M. Mycobacterium tuberculosis Virulent Factor ESAT-6 Drives Macrophage Differentiation Toward the Pro-inflammatory M1 Phenotype and Subsequently Switches It to the Anti-inflammatory M2 Phenotype. Frontiers in Cellular and Infection Microbiology 2018, 8. [Google Scholar] [CrossRef]
- Li, F.; Luo, J.; Xu, H.; Wang, Y.; Jiang, W.; Chang, K.; Deng, S.; Chen, M. Early secreted antigenic target 6-kDa from Mycobacterium tuberculosis enhanced the protective innate immunity of macrophages partially via HIF1α. Biochemical and Biophysical Research Communications 2020, 522, 26–32. [Google Scholar] [CrossRef]
- Watson, Robert O. ; Bell, Samantha L.; MacDuff, Donna A.; Kimmey, Jacqueline M.; Diner, Elie J.; Olivas, J.; Vance, Russell E.; Stallings, Christina L.; Virgin, Herbert W.; Cox, Jeffery S. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host & Microbe 2015, 17, 811–819. [Google Scholar] [CrossRef]
- Grover, A.; Izzo, A.A. BAT3 Regulates Mycobacterium tuberculosis Protein ESAT-6-Mediated Apoptosis of Macrophages. PLoS One 2012, 7, e40836. [Google Scholar] [CrossRef] [PubMed]
- Welin, A.; Eklund, D.; Stendahl, O.; Lerm, M. Human Macrophages Infected with a High Burden of ESAT-6-Expressing M. tuberculosis Undergo Caspase-1- and Cathepsin B-Independent Necrosis. PLoS One 2011, 6, e20302. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, R.; Gulen Muhammet, F.; Sala, C.; Perin, Sonia G.; Lou, Y.; Rybniker, J.; Schmid-Burgk, Jonathan L.; Schmidt, T.; Hornung, V.; Cole, Stewart T.; et al. Mycobacterium tuberculosis Differentially Activates cGAS- and Inflammasome-Dependent Intracellular Immune Responses through ESX-1. Cell Host & Microbe 2015, 17, 799–810. [Google Scholar] [CrossRef]
- Yabaji, S.M.; Dhamija, E.; Mishra, A.K.; Srivastava, K.K. ESAT-6 regulates autophagous response through SOD-2 and as a result induces intracellular survival of Mycobacterium bovis BCG. Biochim. Biophys. Acta 2020, 1868, 140470. [Google Scholar] [CrossRef]
- Majlessi, L.; Brosch, R. Mycobacterium tuberculosis Meets the Cytosol: The Role of cGAS in Anti-mycobacterial Immunity. Cell Host & Microbe 2015, 17, 733–735. [Google Scholar] [CrossRef]
- Shafiani, S.; Dinh, C.; Ertelt James, M.; Moguche, Albanus O.; Siddiqui, I.; Smigiel, Kate S.; Sharma, P.; Campbell, Daniel J.; Way, Sing S.; Urdahl, Kevin B. Pathogen-Specific Treg Cells Expand Early during Mycobacterium tuberculosis Infection but Are Later Eliminated in Response to Interleukin-12. Immunity 2013, 38, 1261–1270. [Google Scholar] [CrossRef]
- Banaiee, N.; Kincaid, E.Z.; Buchwald, U.; Jacobs, W.R., Jr.; Ernst, J.D. Potent Inhibition of Macrophage Responses to IFN-γ by Live Virulent Mycobacterium tuberculosis Is Independent of Mature Mycobacterial Lipoproteins but Dependent on TLR21. The Journal of Immunology 2006, 176, 3019–3027. [Google Scholar] [CrossRef]
- Sreejit, G.; Ahmed, A.; Parveen, N.; Jha, V.; Valluri, V.L.; Ghosh, S.; Mukhopadhyay, S. The ESAT-6 Protein of Mycobacterium tuberculosis Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage. PLOS Pathogens 2014, 10, e1004446. [Google Scholar] [CrossRef]
- Jha, V.; Rameshwaram, N.R.; Janardhan, S.; Raman, R.; Sastry, G.N.; Sharma, V.; Subba Rao, J.; Kumar, D.; Mukhopadhyay, S. Uncovering Structural and Molecular Dynamics of ESAT-6:β2M Interaction: Asp53 of Human β2-Microglobulin Is Critical for the ESAT-6:β2M Complexation. The Journal of Immunology 2019, 203, 1918–1929. [Google Scholar] [CrossRef]
- Bretl, D.J.; Demetriadou, C.; Zahrt, T.C. Adaptation to Environmental Stimuli within the Host: Two-Component Signal Transduction Systems of Mycobacterium tuberculosis. Microbiology and Molecular Biology Reviews 2011, 75, 566–582. [Google Scholar] [CrossRef]
- Polena, H.; Boudou, F.; Tilleul, S.; Dubois-Colas, N.; Lecointe, C.; Rakotosamimanana, N.; Pelizzola, M.; Andriamandimby, S.F.; Raharimanga, V.; Charles, P.; et al. Mycobacterium tuberculosis exploits the formation of new blood vessels for its dissemination. Sci. Rep. 2016, 6, 33162. [Google Scholar] [CrossRef] [PubMed]
- Lerner, T.R.; Borel, S.; Greenwood, D.J.; Repnik, U.; Russell, M.R.G.; Herbst, S.; Jones, M.L.; Collinson, L.M.; Griffiths, G.; Gutierrez, M.G. Mycobacterium tuberculosis replicates within necrotic human macrophages. J. Cell Biol. 2017, 216, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Kumar, N.P.; Wei, W.; et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014, 511, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mourik, B.C.; Lubberts, E.; de Steenwinkel, J.E.M.; Ottenhoff, T.H.M.; Leenen, P.J.M. Interactions between Type 1 Interferons and the Th17 Response in Tuberculosis: Lessons Learned from Autoimmune Diseases. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O'Garra, A. Type I interferons in infectious disease. Nature Reviews Immunology 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Behar, S.M.; Martin, C.J.; Booty, M.G.; Nishimura, T.; Zhao, X.; Gan, H.X.; Divangahi, M.; Remold, H.G. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011, 4, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Roca Francisco, J.; Ramakrishnan, L. TNF Dually Mediates Resistance and Susceptibility to Mycobacteria via Mitochondrial Reactive Oxygen Species. Cell 2013, 153, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; Cardona, P.-J.; Kim, M.-J.; Allain, S.; Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009, 10, 943–948. [Google Scholar] [CrossRef]
- Griffin, G.K.; Newton, G.; Tarrio, M.L.; Bu, D.-x.; Maganto-Garcia, E.; Azcutia, V.; Alcaide, P.; Grabie, N.; Luscinskas, F.W.; Croce, K.J.; et al. IL-17 and TNF-α Sustain Neutrophil Recruitment during Inflammation through Synergistic Effects on Endothelial Activation. The Journal of Immunology 2012, 188, 6287–6299. [Google Scholar] [CrossRef] [PubMed]
- Poh, X.Y.; Loh, F.K.; Friedland, J.S.; Ong, C.W.M. Neutrophil-Mediated Immunopathology and Matrix Metalloproteinases in Central Nervous System – Tuberculosis. Front. Immunol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Dallenga, T.; Schaible, U.E. Neutrophils in tuberculosis--first line of defence or booster of disease and targets for host-directed therapy? Pathogens and Disease 2016, 74, ftw012. [Google Scholar] [CrossRef] [PubMed]
- Mahghani, G.A.; Kargar, M.; Ghaemi, E.A.; Kafilzadeh, F.; Davoodi, H. Role of ESAT-6 in pathogenicity of Beijing and non-Beijing Mycobacterium tuberculosis isolates. Microb. Pathog. 2022, 162, 105366. [Google Scholar] [CrossRef] [PubMed]
- StopTBPartnership. MTBVAC. Available online: https://newtbvaccines.org/vaccine/mtbvac/ (accessed on 29 May 2023).
- Martín, C.; Marinova, D.; Aguiló, N.; Gonzalo-Asensio, J. MTBVAC, a live TB vaccine poised to initiate efficacy trials 100 years after BCG. Vaccine 2021, 39, 7277–7285. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.R.; Constant, P.; Raynaud, C.; Lanéelle, M.-A.; Triccas, J.A.; Gicquel, B.; Daffé, M.; Guilhot, C. Analysis of the Phthiocerol Dimycocerosate Locus of Mycobacterium tuberculosis: EVIDENCE THAT THIS LIPID IS INVOLVED IN THE CELL WALL PERMEABILITY BARRIER *. J. Biol. Chem. 2001, 276, 19845–19854. [Google Scholar] [CrossRef]
- Bouzeyen, R.; Javid, B. Therapeutic Vaccines for Tuberculosis: An Overview. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Bellini, C.; Horváti, K. Recent Advances in the Development of Protein- and Peptide-Based Subunit Vaccines against Tuberculosis. Cells 2020, 9, 2673. [Google Scholar] [CrossRef]
- Sable, S.B.; Posey, J.E.; Scriba, T.J. Tuberculosis Vaccine Development: Progress in Clinical Evaluation. Clin. Microbiol. Rev. 2019, 33, e00100–00119. [Google Scholar] [CrossRef]
- Dockrell, H.M.; McShane, H. Tuberculosis vaccines in the era of Covid-19 - what is taking us so long? eBioMedicine 2022, 79. [Google Scholar] [CrossRef]
- Tkachuk, A.P.; Gushchin, V.A.; Potapov, V.D.; Demidenko, A.V.; Lunin, V.G.; Gintsburg, A.L. Multi-subunit BCG booster vaccine GamTBvac: Assessment of immunogenicity and protective efficacy in murine and guinea pig TB models. PLoS One 2017, 12, e0176784. [Google Scholar] [CrossRef]
- Tkachuk, A.P.; Bykonia, E.N.; Popova, L.I.; Kleymenov, D.A.; Semashko, M.A.; Chulanov, V.P.; Fitilev, S.B.; Maksimov, S.L.; Smolyarchuk, E.A.; Manuylov, V.A.; et al. Safety and Immunogenicity of the GamTBvac, the Recombinant Subunit Tuberculosis Vaccine Candidate: A Phase II, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Study. Vaccines 2020, 8, 652. [Google Scholar] [CrossRef]
- Vasina, D.V.; Kleymenov, D.A.; Manuylov, V.A.; Mazunina, E.P.; Koptev, E.Y.; Tukhovskaya, E.A.; Murashev, A.N.; Gintsburg, A.L.; Gushchin, V.A.; Tkachuk, A.P. First-In-Human Trials of GamTBvac, a Recombinant Subunit Tuberculosis Vaccine Candidate: Safety and Immunogenicity Assessment. Vaccines 2019, 7, 166. [Google Scholar] [CrossRef]
- Ning, H.; Zhang, W.; Kang, J.; Ding, T.; Liang, X.; Lu, Y.; Guo, C.; Sun, W.; Wang, H.; Bai, Y.; et al. Subunit Vaccine ESAT-6:c-di-AMP Delivered by Intranasal Route Elicits Immune Responses and Protects Against Mycobacterium tuberculosis Infection. Frontiers in Cellular and Infection Microbiology 2021, 11. [Google Scholar] [CrossRef]
- Zhao, R.; Luo, T.; Ma, P.; Ge, L.; Chen, Z.; Wang, X.; Liao, W.; Bao, L. Improvement of the immunogenicity of ESAT-6 via fusion with the dodecameric protein dodecin of Mycobacterium tuberculosis. Microb. Pathog. 2021, 155, 104890. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-G.; Choi, S.; Woo Back, Y.; Paik, S.; Park, H.-S.; Sik Kim, W.; Kim, H.; Bin Cha, S.; Hee Choi, C.; Jae Shin, S.; et al. Rv2299c, a novel dendritic cell-activating antigen of Mycobacterium tuberculosis, fused-ESAT-6 subunit vaccine confers improved and durable protection against the hypervirulent strain HN878 in mice. Oncotarget 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.; Ruggiero, A.; Esposito, L.; Choi, H.-G.; Kim, H.-J.; Berisio, R. Structural features of HtpGMtb and HtpG-ESAT6Mtb vaccine antigens against tuberculosis: Molecular determinants of antigenic synergy and cytotoxicity modulation. Int. J. Biol. Macromol. 2020, 158, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Choi, H.-G.; Barra, G.; Squeglia, F.; Back, Y.W.; Kim, H.-J.; Berisio, R. Structure based design of effective HtpG-derived vaccine antigens against M. tuberculosis. Frontiers in Molecular Biosciences 2022, 9. [Google Scholar] [CrossRef]
- Moguche, A.O.; Musvosvi, M.; Penn-Nicholson, A.; Plumlee, C.R.; Mearns, H.; Geldenhuys, H.; Smit, E.; Abrahams, D.; Rozot, V.; Dintwe, O.; et al. Antigen Availability Shapes T Cell Differentiation and Function during Tuberculosis. Cell Host & Microbe 2017, 21, 695–706. [Google Scholar] [CrossRef]
- Pai, M.; Denkinger Claudia, M.; Kik Sandra, V.; Rangaka Molebogeng, X.; Zwerling, A.; Oxlade, O.; Metcalfe John, Z.; Cattamanchi, A.; Dowdy David, W.; Dheda, K.; et al. Gamma Interferon Release Assays for Detection of Mycobacterium tuberculosis Infection. Clin. Microbiol. Rev. 2014, 27, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Aggerbeck, H.; Ruhwald, M.; Hoff, S.T.; Borregaard, B.; Hellstrom, E.; Malahleha, M.; Siebert, M.; Gani, M.; Seopela, V.; Diacon, A.; et al. C-Tb skin test to diagnose Mycobacterium tuberculosis infection in children and HIV-infected adults: A phase 3 trial. PLoS One 2018, 13, e0204554. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO operational handbook on tuberculosis: module 5: management of tuberculosis in children and adolescents; World Health Organization: Geneva, 2022. [Google Scholar]
- Vidyarthi, A.; Khan, N.; Agnihotri, T.; Siddiqui, K.F.; Nair, G.R.; Arora, A.; Janmeja, A.K.; Agrewala, J.N. Antibody response against PhoP efficiently discriminates among healthy individuals, tuberculosis patients and their contacts. PLoS One 2017, 12, e0173769. [Google Scholar] [CrossRef] [PubMed]
- Araujo, Z.; Fernández de Larrea, C.; López, D.; Isern-Kebschull, J.; de Waard, J.H.; Hagel, I.; Camargo, M.; Vanegas, M.; Patarroyo, M.A. ESAT-6 and Ag85A Synthetic Peptides as Candidates for an Immunodiagnostic Test in Children with a Clinical Suspicion of Tuberculosis. Dis. Markers 2021, 2021, 6673250. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




