Submitted:
12 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Eroom’s law and the innovation crisis.
2.1. The Traditional Drug Discovery Paradigm
2.2. Industry level challenges
2.2.1. Regulatory oversight
2.2.2. The drug “innovation chasm”
2.2.3. Mergers and Acquisitions
2.3. Science & technology challenges
2.3.1. Target-based discovery
2.3.2. Drug Promiscuity
2.3.3. The reproducibility crisis
2.3.4. The problem with model systems
3. The “First Principles” case for a Human Data Driven Discovery (HD3) paradigm
2.3. Human data as a driver for systems-based discovery
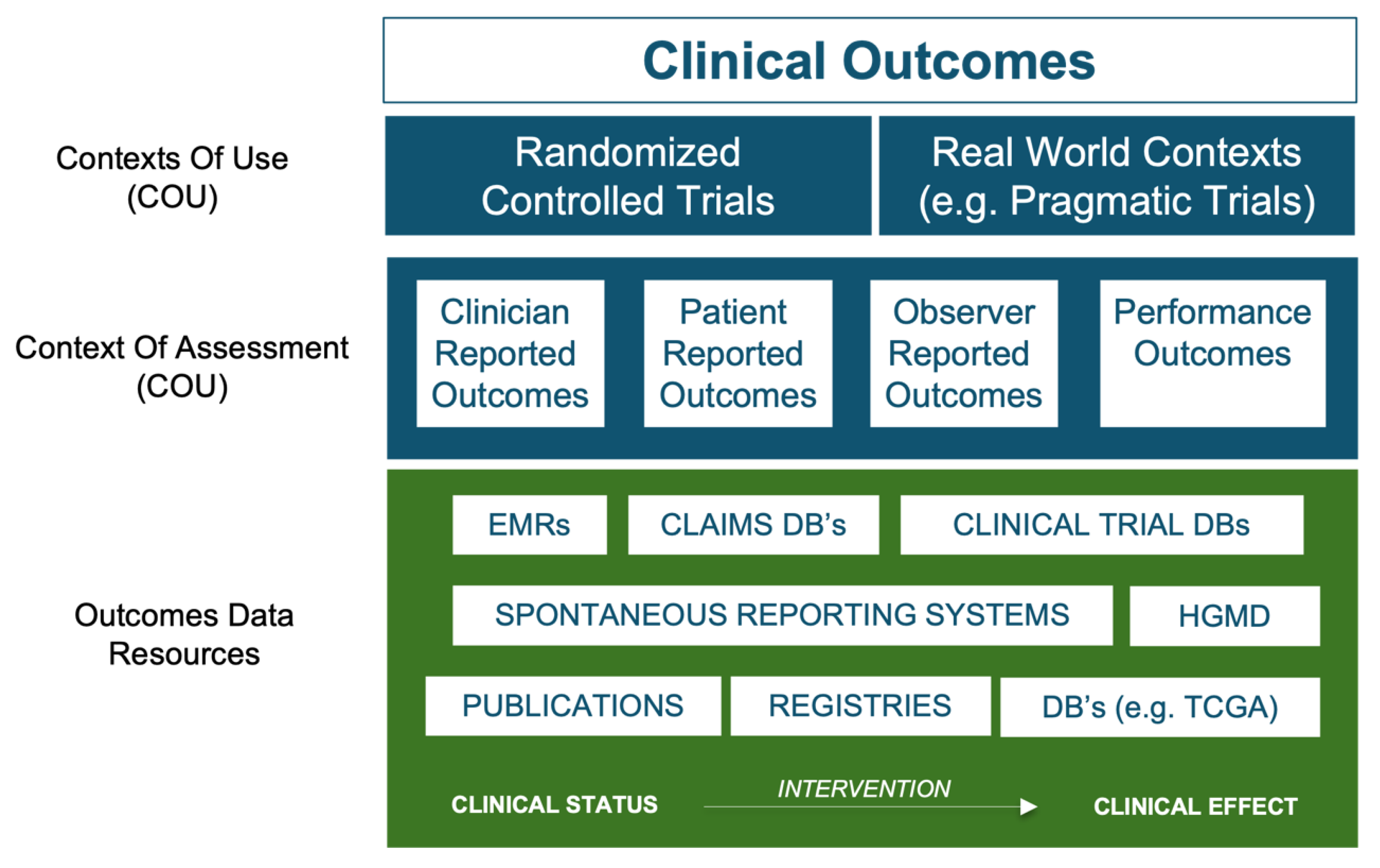
4. Current applications of the HD3 approach
4.1. Application to the analysis and prediction of Adverse Events
4.1.1. Examples from the FDA’s Division of Applied Regulatory Science.
4.2. Application of HD3 to drug repositioning and combinatorial therapy design.
5. Discussion
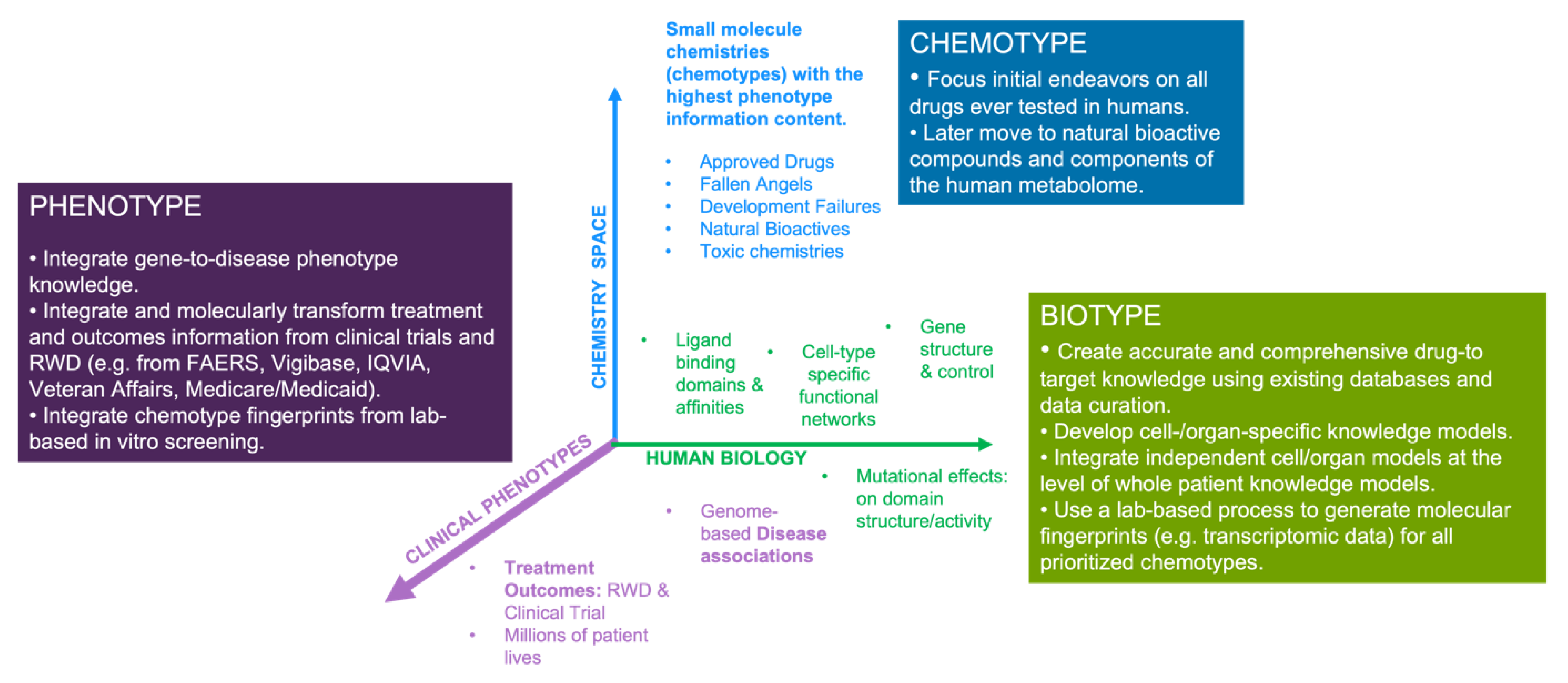
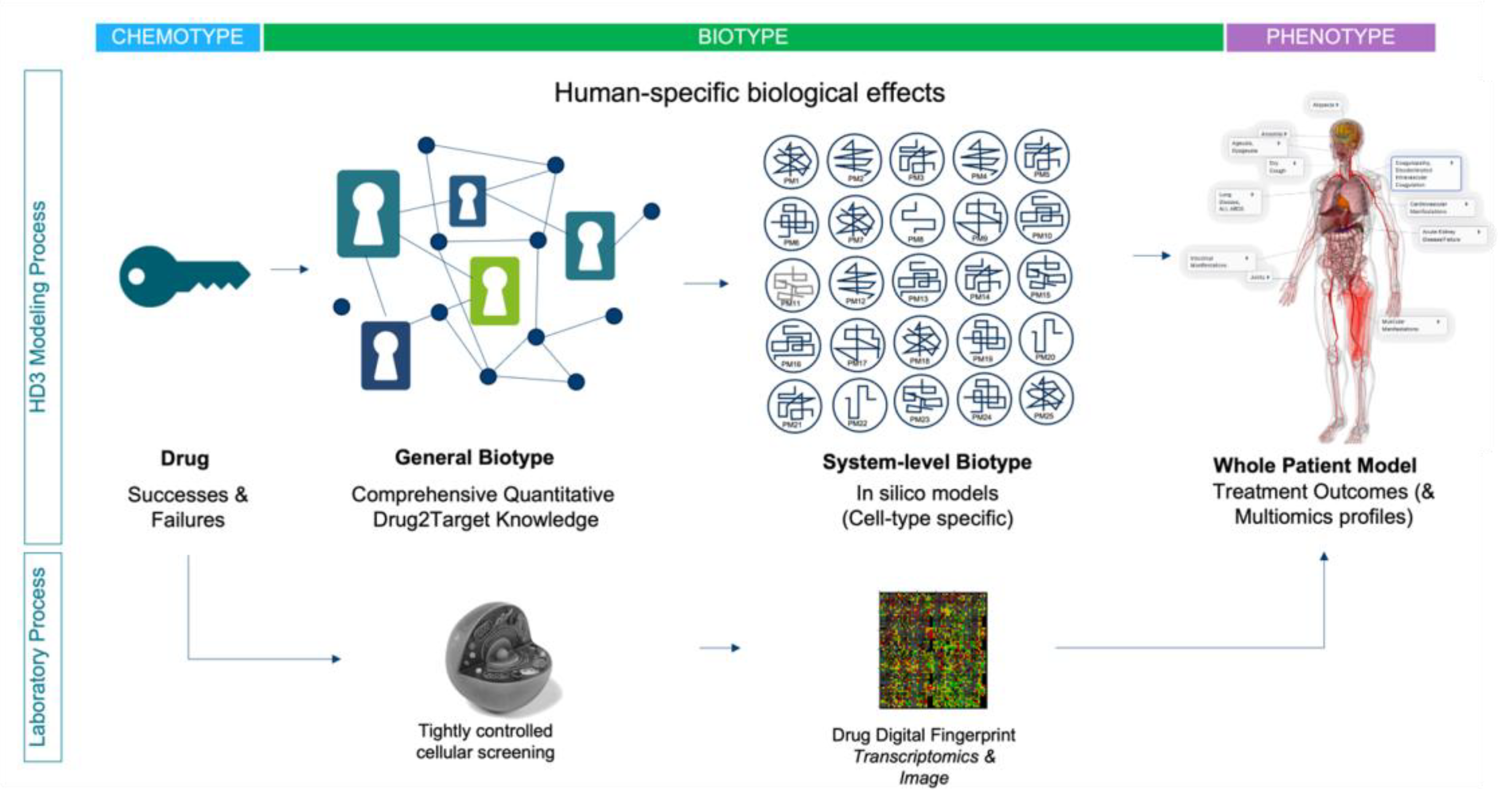
6. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Hutchinson L, Kirk R. High drug attrition rates--where are we going wrong? Nat Rev Clin Oncol. 2011 Mar 30;8(4):189-90. [CrossRef]
- Barton P, Riley RJ. A new paradigm for navigating compound property related drug attrition. Drug Discov Today. 2016 Jan;21(1):72-81. [CrossRef]
- Evaluate Vantage 2020 Preview. Evaluate Ltd. https://www.evaluate.com/thought-leadership/vantage/evaluate-vantage-2020-preview#download. 2023.
- Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020 Mar 3;323(9):844-853. [CrossRef]
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. 2016 May;47:20-33. [CrossRef]
- Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012 Mar 1;11(3):191-200. [CrossRef]
- Davis, RL. Mechanism of Action and Target Identification: A Matter of Timing in Drug Discovery. iScience. 2020 Aug 21;23(9):101487. [CrossRef]
- Wyatt PG, Gilbert IH, Read KD, Fairlamb AH. Target validation: linking target and chemical properties to desired product profile. Curr Top Med Chem. 2011;11(10):1275-83. [CrossRef]
- Morra G, Genoni A, Neves MA, Merz KM Jr, Colombo G. Molecular recognition and drug-lead identification: what can molecular simulations tell us? Curr Med Chem. 2010;17(1):25-41. [CrossRef]
- Wang S, Dong G, Sheng C. Structural simplification: an efficient strategy in lead optimization. Acta Pharm Sin B. 2019 Sep;9(5):880-901. [CrossRef]
- Haley B, Roudnicky F. Functional Genomics for Cancer Drug Target Discovery. Cancer Cell. 2020 Jul 13;38(1):31-43. Epub 2020 May 21. [CrossRef]
- Williams, M. Editorial overview: from Vioxx to Luckenbach: drug discovery at a crossroads. Curr Opin Investig Drugs. 2005 Jan;6(1):17-20.
- Nissen, S. Rosiglitazone: a disappointing DREAM. Future Cardiol. 2007 Sep;3(5):491-2. [CrossRef]
- Kaitin K, DiMasi J (2011) Pharmaceutical innovation in the 21st century: new drug approvals in the first decade, 2000–2009. Clin Pharmacol Ther 2011 Feb;89(2):183-8. [CrossRef]
- Thomas L (1996) Industrial Policy and International Competitiveness in the Pharmaceutical Industry. In: Helms R (ed) Competitive Strategies in the Pharmaceutical Industry. The American Enterprise Institute, Washington, D.C., pp 107–129.
- Tufts Center Report on Trial timelines: https://www.centerwatch.com/articles/25033-trend-of-longer-trial-timelines-is-likely-to-continue Accessed th 2022. 19 November.
- LaMattina, JL. The impact of mergers on pharmaceutical R&D. Nat Rev Drug Discov. 2011 Aug 1;10(8):559-60. [CrossRef]
- Szabo M, Svensson Akusjärvi S, Saxena A, Liu J, Chandrasekar G, Kitambi SS. Cell and small animal models for phenotypic drug discovery. Drug Des Devel Ther. 2017 Jun 28;11:1957-1967. [CrossRef]
- Ekins S, Mestres J, Testa B. In silico pharmacology for drug discovery: applications to targets and beyond. Br J Pharmacol. 2007 Sep;152(1):21-37. [CrossRef]
- Luo, J. CRISPR/Cas9: From Genome Engineering to Cancer Drug Discovery. Trends Cancer. 2016 Jun;2(6):313-324. [CrossRef]
- Bon M, Bilsland A, Bower J, McAulay K. Fragment-based drug discovery-the importance of high-quality molecule libraries. Mol Oncol. 2022 Nov;16(21):3761-3777. [CrossRef]
- Tewkesbury DH, Robey RC, Barry PJ. Progress in precision medicine in cystic fibrosis: a focus on CFTR modulator therapy. Breathe (Sheff). 2021 Dec;17(4):210112. [CrossRef]
- Carofiglio F, Lopalco A, Lopedota A, Cutrignelli A, Nicolotti O, Denora N, Stefanachi A, Leonetti F. Bcr-Abl Tyrosine Kinase Inhibitors in the Treatment of Pediatric CML. Int J Mol Sci. 2020 Jun 23;21(12):4469. [CrossRef]
- Santos, R et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2016; 16. [CrossRef]
- Mestres J, Gregori-Puigjane E, Valverde S, Sole RV. Data completeness – The Achilles heel of drug-target networks. Nat Biotechnol. 2008; 26(9):983–984. [CrossRef]
- Begley CG, Ellis L. Drug development: raise standards for preclinical research.Nature. 2012; 483:531–533. [CrossRef]
- Peers IS, Ceuppens PR, Harbron C. In search of preclinical robustness.Nat Rev Drug Discov. 2012; 11:733–734. [CrossRef]
- Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets?Nat Rev Drug Discov. 2011; 10:712. [CrossRef]
- Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA. 2006; 296:1731–1732. [CrossRef]
- Kaste, M. Use of animal models has not contributed to development of acute stroke therapies: pro. Stroke. 2005;36:2323-2324. [CrossRef]
- Horrobin, DF. Modern biomedical research: an internally self-consistent universe with little contact with medical reality? Nat Rev Drug Discov. 2003 Feb;2(2):151-4. [CrossRef]
- First Principles Thinking - https://www.csc.edu/media/website/content-assets/documents/pdf/tlpec/First-Principles-Thinking.pdf.
- Workman P, Al-Lazikani B, Clarke PA. Genome-based cancer therapeutics: targets, kinase drug resistance and future strategies for precision oncology. 2013 Aug;13(4):486-96. [CrossRef]
- Cui JJ, Tran-Dubé M, Shen H, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 2011; 54(18): 6342-63. [CrossRef]
- FDA Adverse Events Reporting System (FAERS) Public Dashboard. https://fis.fda.gov/sense/app/d10be6bb-494e-4cd2-82e4-0135608ddc13/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis.
- Sentinel Initiative. https://www.sentinelinitiative.org/. Accessed th, 2023. 12 January.
- Ball R, Robb M, Anderson SA, Dal Pan G. The FDA's sentinel initiative-A comprehensive approach to medical product surveillance. 2016 Mar;99(3):265-8. [CrossRef]
- European database of suspected adverse drug reaction reports. https://www.adrreports.eu/en/index.html. 20 December 2022.
- UMC | VigiBase. https://www.who-umc.org/vigibase/vigibase/. 28 December 2022.
- Amberger, J.S. , Bocchini C.A., Schiettecatte F., Scott A.F., Hamosh A. OMIM.org: Online Mendelian Inheritance in Man (OMIM(R)), an online catalog of human genes and genetic disorders. 2015 Jan;43(Database issue):D789-98. [CrossRef]
- Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, Groza T, Güneş O, Hall P, Hayhurst J, Ibrahim A, Ji Y, John S, Lewis E, MacArthur JAL, McMahon A, Osumi-Sutherland D, Panoutsopoulou K, Pendlington Z, Ramachandran S, Stefancsik R, Stewart J, Whetzel P, Wilson R, Hindorff L, Cunningham F, Lambert SA, Inouye M, Parkinson H, Harris LW. The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res. 2023 Jan 6;51(D1):D977-D985. [CrossRef]
- Parsa A, Fuchsberger C, Kottgen A et al.. Common variants in Mendelian kidney disease genes and their association with renal function. J Am Soc Nephrol 2013; 24: 2105–2117. [CrossRef]
- Pattaro C, Kottgen A, Teumer A et al.. Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet 2012;8(3):e1002584. [CrossRef]
- Jupp S, Klein J, Schanstra J, Stevens R. Developing a kidney and urinary pathway knowledge base. J Biomed Semantics. 2011 ;2 Suppl 2(Suppl 2):S7. 17 May. [CrossRef]
- Fernandes M, Husi H. Establishment of a integrative multi-omics expression database CKDdb in the context of chronic kidney disease (CKD). Sci Rep. 2017 Jan 12;7:40367. [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013 ;497(7447):67-73. 2 May. [CrossRef]
- Zhong Q, Simonis N, Li QR, Charloteaux B, Heuze F, Klitgord N, Tam S, Yu H, Venkatesan K, Mou D, Swearingen V, et al. Edgetic perturbation models of human inherited disorders. Mol Syst Biol. 2009; 5:321. [CrossRef]
- Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. [CrossRef]
- Southan C, Várkonyi P, Muresan S. Complementarity between public and commercial databases: new opportunities in medicinal chemistry informatics. Curr Top Med Chem. 2007;7(15):1502-8. [CrossRef]
- Zhou Y, Zhang Y, Lian X, Li F, Wang C, Zhu F, Qiu Y, Chen Y. Therapeutic target database update 2022: facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022 Jan 7;50(D1):D1398-D1407. [CrossRef]
- Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021 Jan 8;49(D1):D1388-D1395. [CrossRef]
- Hastings J, Owen G, Dekker A, Ennis M, Kale N, Muthukrishnan V, Turner S, Swainston N, Mendes P, Steinbeck C. ChEBI in 2016: Improved services and an expanding collection of metabolites. Nucleic Acids Res. 2016 Jan 4;44(D1):D1214-9. [CrossRef]
- He Y, Xiang Z, Zheng J, Lin Y, Overton JA, Ong E. The eXtensible ontology development (XOD) principles and tool implementation to support ontology interoperability. J Biomed Semantics. 2018 Jan 12;9(1):3. [CrossRef]
- Jiang L, Wang M, Lin S, Jian R, Li X, Chan J, Dong G, Fang H, Robinson AE; GTEx Consortium; Snyder MP. A Quantitative Proteome Map of the Human Body. Cell. 2020 Oct 1;183(1):269-283.e19. [CrossRef]
- Digre A, Lindskog C. The Human Protein Atlas - integrated omics for single cell mapping of the human proteome. Protein Sci. 2023 Jan 5:e4562. [CrossRef]
- Lam KHB, Faust K, Yin R, Fiala C, Diamandis P. The Brain Protein Atlas: A conglomerate of proteomics datasets of human neural tissue. Proteomics. 2022 Dec;22(23-24):e2200127. [CrossRef]
- Yamamoto, T. The 4th Human Kidney and Urine Proteome Project (HKUPP) workshop. , Toronto, Canada. Proteomics. 2010 Jun;10(11):2069-70. 26 September. [CrossRef]
- Lysenko A, Roznovăţ IA, Saqi M, Mazein A, Rawlings CJ, Auffray C. Representing and querying disease networks using graph databases. BioData Min. 2016 Jul 25;9:23. [CrossRef]
- Zitnik, M. , Agrawal, M., and Leskovec, J. (2018). Modeling polypharmacy side effects with graph convolutional networks. Bioinformatics 34, i457–i466. [CrossRef]
- Muñoz, E. , Nováček, V., and Vandenbussche, P.-Y. (2019). Facilitating prediction of adverse drug reactions by using knowledge graphs and multi-label learning models. Briefings in Bioinformatics 20, 190–202. [CrossRef]
- Bean, D.M. , Wu, H., Iqbal, E., Dzahini, O., Ibrahim, Z.M., Broadbent, M., Stewart, R., and Dobson, R.J.B. (2017). Knowledge graph prediction of unknown adverse drug reactions and validation in electronic health records. Sci Rep 7, 16416. [CrossRef]
- Joshi, P. , V, M., and Mukherjee, A. (2022). A knowledge graph embedding based approach to predict the adverse drug reactions using a deep neural network. Journal of Biomedical Informatics 132, 104122. [CrossRef]
- Bobed, C. , Douze, L., Ferré, S., and Marcilly, R. (2018). PEGASE: A Knowledge Graph for Search and Exploration in Pharmacovigilance Data. EKAW Posters and Demonstrations. https://hal.inria.fr/hal-01976818.
- Soldatos TG, Taglang G, Jackson DB. In silico Profiling of Clinical Phenotypes for Human Targets Using Adverse Event Data. High Throughput. 2018 Nov 23;7(4):37. [CrossRef]
- Jassal B, Matthews L, Viteri G, Gong C, Lorente P, Fabregat A, Sidiropoulos K, Cook J, Gillespie M, Haw R, Loney F, May B, Milacic M, Rothfels K, Sevilla C, Shamovsky V, Shorser S, Varusai T, Weiser J, Wu G, Stein L, Hermjakob H, D'Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2020 Jan 8;48(D1):D498-D503. [CrossRef]
- Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, Hannay T, Buetow KH. PID: the Pathway Interaction Database. Nucleic Acids Res. 2009 Jan;37(Database issue):D674-9. Epub 2008 Oct 2. [CrossRef]
- The ATC ontology [WHOCC—Structure and Principles. Available online: https://www.whocc.no/atc/structure_and_ principles/). Accessed th, 2022. 19 December.
- Rao MS, Gupta R, Liguori MJ, Hu M, Huang X, Mantena SR, Mittelstadt SW, Blomme EAG, Van Vleet TR. Novel Computational Approach to Predict Off-Target Interactions for Small Molecules. Front Big Data. 2019 Jul 17;2:25. [CrossRef]
- The OFF-X Platform - https://clarivate.com/products/biopharma/off-x Accessed th 2023. 8 January.
- Kim S, Lahu G, Vakilynejad M, Soldatos TG, Jackson DB, Lesko LJ, Trame MN. A case study of a patient-centered reverse translational systems-based approach to understand adverse event profiles in drug development. Clin Transl Sci. 2022 Apr;15(4):1003-1013. [CrossRef]
- Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007 May;7(5):332-44. [CrossRef]
- Grazette LP, Boecker W, Matsui T, Semigran M, Force TL, Hajjar RJ, Rosenzweig A. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol. 2004 Dec 7;44(11):2231-8. [CrossRef]
- Kim S, Lahu G, Vakilynejad M, Soldatos TG, Jackson DB, Lesko LJ, Trame MN. Application of a patient-centered reverse translational systems-based approach to understand mechanisms of an adverse drug reaction of immune checkpoint inhibitors. Clin Transl Sci. 2022 Jun;15(6):1430-1438. [CrossRef]
- Tafenoquine label https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210795s001lbl.pdf.
- Association Between Serotonin Syndrome and Second-Generation Antipsychotics via Pharmacological Target-Adverse Event Analysis. Racz R, Jackson DB, Soldatos, Burkhart K. TG Clin Transl Sci. 2018 May;11(3):322-329. [CrossRef]
- Target-Adverse Event Profiles to Augment Pharmacovigilance: A Pilot Study With Six New Molecular Entities. Schotland P, Racz R, Jackson DB, Strauss DG, Burkhart K. CPT Pharmacometrics Syst Pharmacol. 2018 Dec;7(12):809-817. [CrossRef]
- Schotland P, Racz R, Jackson DB, Soldatos TG, Levin R, Strauss DG, Burkhart K. Target Adverse Event Profiles for Predictive Safety in the Postmarket Setting. Clin Pharmacol Ther. 2021 May;109(5):1232-1243. [CrossRef]
- Daluwatte C, Schotland P, Strauss DG, Burkhart KK, Racz R. Predicting potential adverse events using safety data from marketed drugs. BMC Bioinformatics. 2020 Apr 29;21(1):163. [CrossRef]
- Armaiz-Pena GN, Allen JK, Cruz A, Stone RL, Nick AM, Lin YG, Han LY, Mangala LS, Villares GJ, Vivas-Mejia P, Rodriguez-Aguayo C, Nagaraja AS, Gharpure KM, Wu Z, English RD, Soman KV, Shahzad MM, Zigler M, Deavers MT, Zien A, Soldatos TG, Jackson DB, Wiktorowicz JE, Torres-Lugo M, Young T, De Geest K, Gallick GE, Bar- Eli M, Lopez-Berestein G, Cole SW, Lopez GE, Lutgendorf SK, Sood AK. Src activation by β-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4:1403. [CrossRef]
- De la Torre, A.N.; Castaneda, I.; Hezel, A.F.; Bascomb, N.F.; Bhattacharyya, G.S.; Abou-Alfa, G.K. Effect of coadministration of propranolol and etodolac (VT-122) plus sorafenib for patients with advanced hepatocellular carcinoma (HCC). J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Srinivasan, AV. Propranolol: A 50-Year Historical Perspective. Ann Indian Acad Neurol. 2019 Jan-Mar;22(1):21-26. [CrossRef]
- Fjæstad KY, Rømer AMA, Goitea V, Johansen AZ, Thorseth ML, Carretta M, Engelholm LH, Grøntved L, Junker N, Madsen DH. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene. 2022 Feb;41(9):1364-1375. [CrossRef]
- Amaya CN, Perkins M, Belmont A, Herrera C, Nasrazadani A, Vargas A, Khayou T, Montoya A, Ballou Y, Galvan D, Rivas A, Rains S, Patel L, Ortega V, Lopez C, Chow W, Dickerson EB, Bryan BA. Non-selective beta blockers inhibit angiosarcoma cell viability and increase progression free- and overall-survival in patients diagnosed with metastatic angiosarcoma. Oncoscience. 2018 Apr 29;5(3-4):109-119. [CrossRef]
- Shaghaghi Z, Alvandi M, Farzipour S, Dehbanpour MR, Nosrati S. A review of effects of atorvastatin in cancer therapy. Med Oncol. 2022 Dec 2;40(1):27. [CrossRef]
- Weng N, Zhang Z, Tan Y, Zhang X, Wei X, Zhu Q. Repurposing antifungal drugs for cancer therapy. J Adv Res. 2022 Sep 5:S2090-1232(22)00199-0. [CrossRef]
- Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, Reya T, Liu JO, Epstein EH, Stevens DA, Beachy PA. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010 Apr 13;17(4):388-99. [CrossRef]
- F. Cheng, C. Liu, J. Jiang, et al., Prediction of drug-target interactions and drug repositioning via network-based inference, PLoS Comput. Biol. 8 (5) (2012) e1002503,. [CrossRef]
- C.H. Choi, J.Y. Ryu, Y.J. Cho, et al., The anti-cancer effects of itraconazole in epithelial ovarian cancer, Sci. Rep. 7 (1) (2017) 6552. [CrossRef]
- L. Huang, S. Garrett Injac, K. Cui, et al., Systems biology-based drug repositioning identifies digoxin as a potential therapy for groups 3 and 4 medulloblastoma, Sci TranslMed. 10 (464) (2018). pii: eaat0150. [CrossRef]
- J. Sun, M. Zhao, P. Jia, et al., Deciphering signaling pathway networks to understand the molecular mechanisms of metformin action, PLoS Comput. Biol. 11 (6) (2015) e1004202, eCollection 2015 Jun. [CrossRef]
- Sheridan, C. Massive data initiatives and AI provide testbed for pandemic forecasting. Nat Biotechnol. 2020 Sep;38(9):1010-1013. [CrossRef]
- Domingo-Fernández D, Baksi S, Schultz B, Gadiya Y, Karki R, Raschka T, Ebeling C, Hofmann-Apitius M, Kodamullil AT. COVID-19 Knowledge Graph: a computable, multi-modal, cause-and-effect knowledge model of COVID-19 pathophysiology. Bioinformatics. 2021 Jun 9;37(9):1332-1334. [CrossRef]
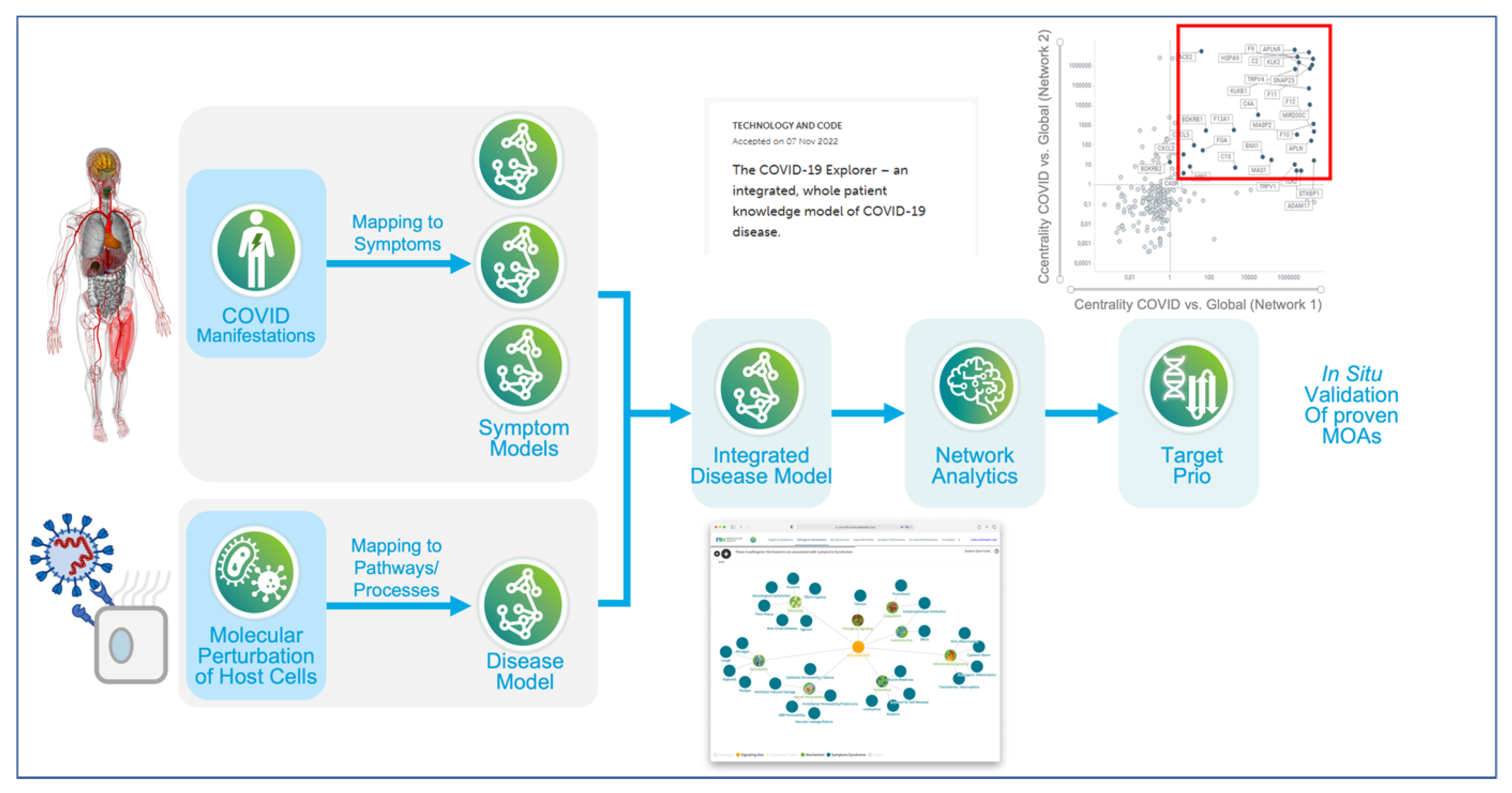
- Brock, S. , Soldatos, T. G., Jackson, D. B., Diella, F., Hornischer, K., Schäefer, A., et al. (2022). The COVID-19 Explorer - an integrated, whole patient knowledge model of COVID-19 disease. Front. Mol. Med. 2, 1035215. [CrossRef]
- Brock, S. , Jackson, D. B., Soldatos, T. G., Hornischer, K., Schäfer, A., Diella, F., et al. (2022). Whole patient knowledge modeling of COVID-19 symptomatology reveals common molecular mechanisms. Front. Mol. Med. 2:1035290. [CrossRef]
- Ringel MS, Scannell JW, Baedeker M, Schulze U. Breaking Eroom's Law. Nat Rev Drug Discov. 2020 Dec;19(12):833-834. [CrossRef]
- Scannell JW, Bosley J. When Quality Beats Quantity: Decision Theory, Drug Discovery, and the Reproducibility Crisis. PLoS One. 2016 Feb 10;11(2):e0147215. [CrossRef]
- Morgan P, Brown DG, Lennard S, Anderton MJ, Barrett JC, Eriksson U, Fidock M, Hamrén B, Johnson A, March RE, Matcham J, Mettetal J, Nicholls DJ, Platz S, Rees S, Snowden MA, Pangalos MN. Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nat Rev Drug Discov. 2018 Mar;17(3):167-181. [CrossRef]
- Bohacek RS, McMartin C, Guida WC. The art and practice of structure-based drug design: a molecular modeling perspective. Med Res Rev. 1996 Jan;16(1):3-50. [CrossRef]
- 230 AI-driven drug discovery start-ups https://blog.benchsci.com/startups-using-artificial-intelligence-in-drug-discovery.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




