Submitted:
10 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
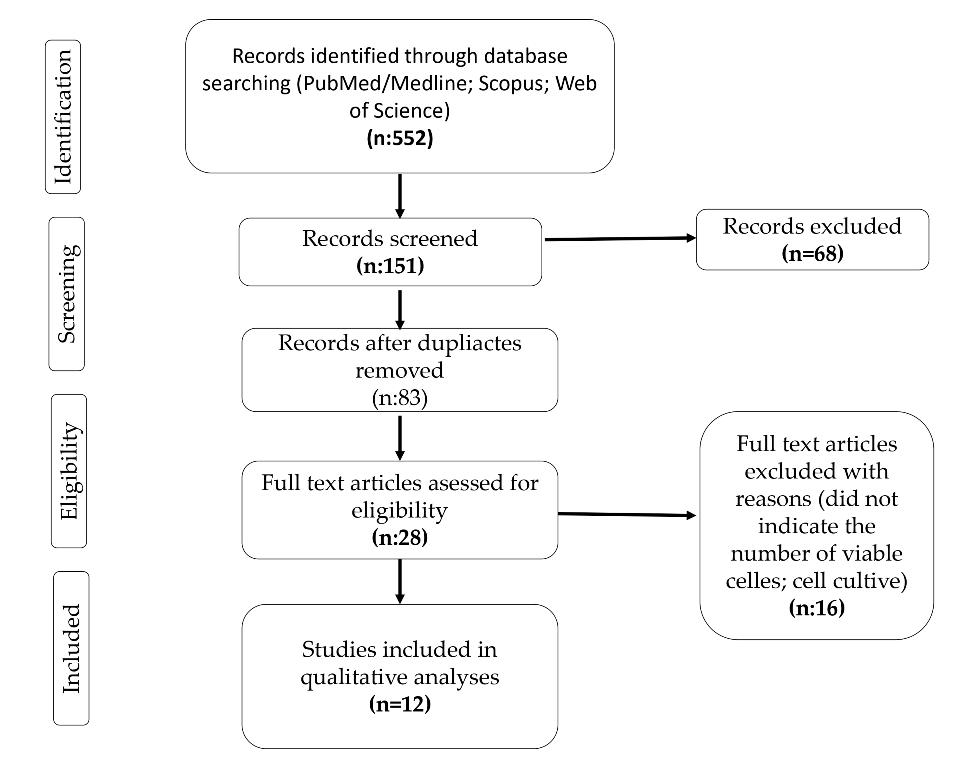
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mejia, P.; De la Hoz, K. Medios de almacenamiento para dientes avulsionados. Una revisión. Salud Uninorte. 2017, 33, 517–531. [Google Scholar]
- Rajakeerthi, R.; Nivedhitha, M. Natural Product as the Storage Medium for an Avulsed Tooth. Cumhur. Dent. J. 2019, 22, 249–256. [Google Scholar]
- Chellammal, R.; et al. Naturally Available Transport Medium for Avulsed Teeth- AReview Indian, J. Forensic Med. Toxicol. 2020, 14, 1076–1080. [Google Scholar]
- Cruz, G.; et al. Citotoxicidad de soluciones recomendadas para el almacenamiento de dientes avulsionados en cultivo con células del ligamento periodontal. Gac Med Mex. 2018, 15, 217–221. [Google Scholar]
- Adnan, S.; Lone, M.; Khan, F.; Hussain, S.; Nagi, S. Which is the most recommended medium for the storage and transport of avulsed teeth? A Review. Dent Traumatol. 2018, 34, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Sheth, P.; Lolayekar, N.; Hegde, A.; Shetty, V. Evaluation of Periodontal Ligament Cell Viability in Honey as a Storage Media at Different Time Intervals: An In Vitro Study. World J. Dent. 2020, 11, 310–315. [Google Scholar]
- Abraham, B.; Kumaran, P.; Varma, B. R.; XAVIER, A.; KUMAR, S. J. Comparative evaluation of the efficacy of aloe vera gel with milk and Hank’s balanced salt solution in maintaining the viability of PDL cells in avulsed teeth. J. Clin. Diagnostic Res. 2019, 13, 11–15. [Google Scholar] [CrossRef]
- Dhimole, P.; Bhayya, D.; Gupta, S.; Kumar, P.; Tiwari, S.; Pandey, S. Evaluation of the efficacy of neem (Azadirachta indica) and turmeric (Curcuma longa) as storage media in maintaining periodontal ligament cell viability: An in vitro study. J Indian Soc Pedod Prev Dent. 2019, 37, 140–145. [Google Scholar] [CrossRef]
- Babaji, P.; Melkundi, M.; Devanna, R.; Suresh, B.; Chaurasia, V.; Gopinath, P. In vitro comparative evaluation of different storage media (hank's balanced salt solution, propolis, Aloe vera, and pomegranate juice) for preservation of avulsed tooth. Eur J Dent. 2017, 11, 71–75. [Google Scholar] [CrossRef]
- D'Costa, V.F.; Bangera, M.K.; Kini, S.; Kutty, S.M.; Ragher, M. An In vitro Comparison of Coconut Water, Milk, and Saline in Maintaining Periodontal Ligament Cell Viability. J Pharm Bioallied Sci. 2017, 9, 107–111. [Google Scholar] [CrossRef]
- Saini, D.; Gadicherla, P.; Chandra, P.; Anandakrishna, L. Coconut milk and probiotic milk as storage media to maintain periodontal ligament cell viability: an in vitro study. Dent Traumatol. 2017, 33, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.; Garcia, L.; Bortoluzzi, E.; Felippe, W.; Felippe, M. Effects of several storage media on viability and proliferation capacity of periodontal ligament cells. Eur Arch Paediatr Dent. 2020, 21, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kokkali, V.; Bendgude, V.; Sharangpan, G. Comparative evaluation of post-traumatic periodontal ligament cell viability using three storage media. Eur Arch Paediatr Dent. 2017, 18, 209–214. [Google Scholar] [CrossRef]
- Cobo, K.; Unapanta, J. Viabilidad de las células del ligamento periodontal usando Solución Genérica De Hank. Estudio in vitro. MED. FCM-UCSG. 2019, 23, 47–56. [Google Scholar]
- Madhusudhan, K.; Monisha, K.; Nayak, N. Store in nature- A médium for avulsed tooth. Int. Dent. J. Stud. Res. 2021, 9, 1–5. [Google Scholar]
- Kaur, C.; Neetika, S.; Dharmani, U. Medios de almacenamiento para dientes avulsionados: Una visión general. J. Med. Dent. Sci. 2017, 16, 138–142. [Google Scholar]
- Mariano, C. Guidelines for Reporting Pre-clinical In Vitro Studies on Dental Materials. J. Evid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar]
- Fina, B.; Lombarte, M.; Rigalli, A. Investigación de un fenómeno natural: ¿estudios in vivo, in vitro o in silico? Actual. Osteol 2013, 9, 239–240. [Google Scholar]
- Singh, D.; Jain, A.; Govila, S. Use of Herbs-A natural approach in dentistry. Indian J Dent. 2016, 4, 16–21. [Google Scholar] [CrossRef]
- Gamboa, D.; et al. Medios de almacenamiento tras avulsión dental. Cont Od. 2021, 11, 5–13. [Google Scholar]
- Talebi, M.; Parisay, I.; Tavakol, J.; Shajiei, A.; Sofiani, M. Viability and Reproducibility of Periodontal Ligament Cells on Avulsed Teeth Stored in Ham’s F-10 Solution. J Clin Pediatr Dent. 2018, 42, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Roma, M. Current developments in transport media for avulsed teeth: An Update. Asian J Pharm Clin Res. 2017, 10, 43–46. [Google Scholar]
- Jain, D.; Dasar, P.; Nagarajappa, S. Natural products as storage media for avulsed tooth. Saudi Endod J. 2015, 5, 107–113. [Google Scholar] [CrossRef]
- Mueen, S.; et al. Comparative Evaluation of Aloe Vera, Green Tea, Histidine tryptophan Ketoglutarate Solution, and Propolis Storage Media on Viability of Periodontal Ligament Cell. Ann. Romanian Soc. Cell Biol. 2021, 25, 11450–11458. [Google Scholar]
- Pasupuleti, V.; Sammugam, L.; Ramesh, N.; Gan, S. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid Med Cell Longev. 2017, 1, 1–21. [Google Scholar] [CrossRef]
- Misurya, R.; et al. An in vitro Evaluation of Efficacy of ViaSpan, Aloe vera, Gatorade Solution, and Propolis Storage Media for Maintaining the periodontal ligament cell viability. Ann Afr Med. 2022, 21, 34–38. [Google Scholar]
- Mahesh, C. Evaluating the effectiveness of rehydrating solutions in preserving periodontal ligament cells vitality: An in vitro study. Saudi Endod J. 2018, 8, 19–24. [Google Scholar]
- Mousavi, B.; et al. Solución de rehidratación oral estándar como nuevo medio de almacenamiento para dientes avulsionados. Int Dent J. 2010, 60, 379–82. [Google Scholar]
- Shetty, A.; et al. Comparative Evaluation of Efficacy of Platelet-Rich Fibrin and Hank’s Balanced Salt Solution as a Storage Medium for Avulsed Teeth: An In Vitro Study. EUR Endod J. 2019, 3, 118–21. [Google Scholar]
- Godoy, C.; Deg, C.; Zhang, Y.; Liu, W.; Chen, G. Roles of vitamins in stem cells. Rev. Cellular and Molecular Life Sciences. Cell Mol Life Sci. 2020, 77, 1771–1791. [Google Scholar] [CrossRef]
- Khinda, V.; Kaur, G.; Brar, G.; Kallar, S.; Khurana, H. Clinical and Practical Implications of Storage Media used for Tooth Avulsion. Int J Clin Pediatr Dent. 2017, 10, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sharma, M. Comparison of efficacy of different storage media for an avulsed tooth. Int J Appl Dent Sci. 2020, 6, 528–531. [Google Scholar] [CrossRef]
| Title and year | n | Storage media | Time | Periodontal viable cells (%) | p value | Level of BIAS |
|---|---|---|---|---|---|---|
| An In vitro comparison of coconut water, milk, and saline in maintaining periodontal ligament cell viability. (Vivian Flourish, et al. 2017) |
40 | Coconut Water | 45 min | 82.00* | 0.0000 | MEDIUM |
| Milk | 59.00 | |||||
| Saline Solution | 15.00 | |||||
| Evaluation of Periodontal Ligament Cell Viability in Honey as a Storage Media at Different Time Intervals: An In Vitro Study (Sheth P, et al. 2020) |
50 | Honey - Immediately | 3 hours | 96.43 ± 3.83* | a-b: p=<0.001 a-c: p=0.339 b-c: p=<0.001 |
MEDIUM |
| Honey - Extraoral drying b | 84.76 ± 2.00 | |||||
| Hank’s balanced salt solution (HBSS) c | 98.89 ± 1.07* | |||||
| Nature's Benefaction as a Life Saver for an Avulsed Tooth: An In vitro Study (Saumya Navit et al. 2017) |
58 | Hank’s balanced salt solution (HBSS) | 45 min | 87.33* | <0.001 | LOW |
| Coconut water | 79.87 | |||||
| Aloe Vera | 70.59 | |||||
| Salina | 50.56 | |||||
| Evaluation of the efficacy of neem (Azadirachta indica) and turmeric (Curcuma longa) as storage media in maintaining periodontal ligament cell viability: An in vitro study. (Dhimole P et al. 2019) |
90 | Neem extract | 30 min | 88,00 ± 5.85 * | <0.001 | MEDIUM |
| Turmeric extract | 81,63 ±7.12 | |||||
| Efficiency of Castor oil as a Storage medium for avulsed teeth in maintaining the viability of periodontal ligament cells (Navavizadeh M et al. 2018) |
40 | Castor oil a | 30 min | 46,93 ±3,24 | a-b: p=<0.05 a-c: p=<0.05 b-c: p=>0.05 |
MEDIUM |
| Hank's balanced salt solution (HBSS) b | 52,85±4,04* | |||||
| Milk with 2.5% fat c | 61,02±2,55* | |||||
| Viability and reproducibility of periodontal ligament cells on avulsed teeth stored in ham's f-10 solution (Talebi M et al. 2018) |
60 | Ham’s F-10 solution | 1 hour | 91.27 ± 4.75* | <0.001 | MEDIUM |
| Pasteurized skim milk | 68.33 ± 8.47 |
| Title and year | n | Storage media | Time | Viable periodontal cells (cells/mm3) | p value | Level of BIAS |
|---|---|---|---|---|---|---|
| In vitro comparative evaluation of different storage media (Hank's balanced salt solution, propolis, Aloe vera, and pomegranate juice) for preservation of avulsed tooth. (Babaji P et al. 2017) |
50 | Hank’s balanced salt solution (HBSS) |
45 min | 282,000 | > 0.05 | MEDIUM |
| Aloe vera gel | 45 min | 226,000 | ||||
| Propolis | 45 min | 285,000* | ||||
| Pomegranate juice | 45 min | 214,000 | ||||
| Comparative Evaluation of Efficacy of Platelet-Rich Fibrin and Hank's Balanced Salt Solution as a Storage Medium for Avulsed Teeth: An In Vitro Study (Shetty et al. 2019) |
30 | Hank’s balanced salt solution (HBSS) | 45 min | 76,800 | 0.001 | MEDIUM |
| Platelet Rich Fibrin | 79,072* | |||||
| Evaluating the effectiveness of rehydrating solutions in preserving periodontal ligament cells vitality: An in vitro study. (Mahesh C, et al. 2018) |
60 | Distilled water + Electrolyte powder | 1 hour | 496.00 ± 31.62 | <0,001 | LOW |
| Ringer's Lactate Solution | 906.40 ± 60.57* | |||||
| Oral rehydration saline solution | 385.60 ± 31.32 | |||||
| Tender Coconut Water | 555.20 ± 36.49 | |||||
| Coconut milk and probiotic milk as storage media to maintain periodontal ligament cell viability: an in vitro study. (Saini D et al. 2017) |
69 | Coconut milk | 1 hour | 8.75 ± 3.166 | 000 | MEDIUM |
| Probiotic milk | 143.25 ±1.616* | |||||
| Comparative evaluation of posttraumatic periodontal ligament cell viability using three storage media (Kokkali V et al. 2017) |
55 | Cow's milk | 45 min | 23,213.33 ± 2664.56* | <0,001 | LOW |
| Tender coconut water | 13,920.00± 2094.61 | |||||
| Whey | 10,566.67 ± 1415.05 | |||||
| Comparative evaluation of the efficacy of aloe vera gel with milk and hank's balanced salt solution in maintaining the viability of PDL cells in avulsed teeth. (Baren A et al.2019) |
40 | Hank’s balanced salt solution (HBSS). | 45 min | 921.40 ± 608.438* | <0,001 | LOW |
| Low-fat pasteurized cow's milk | 812.70 ± 449.170 | |||||
| Aloe vera gel | 241.00 ± 194.572 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





