Submitted:
11 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
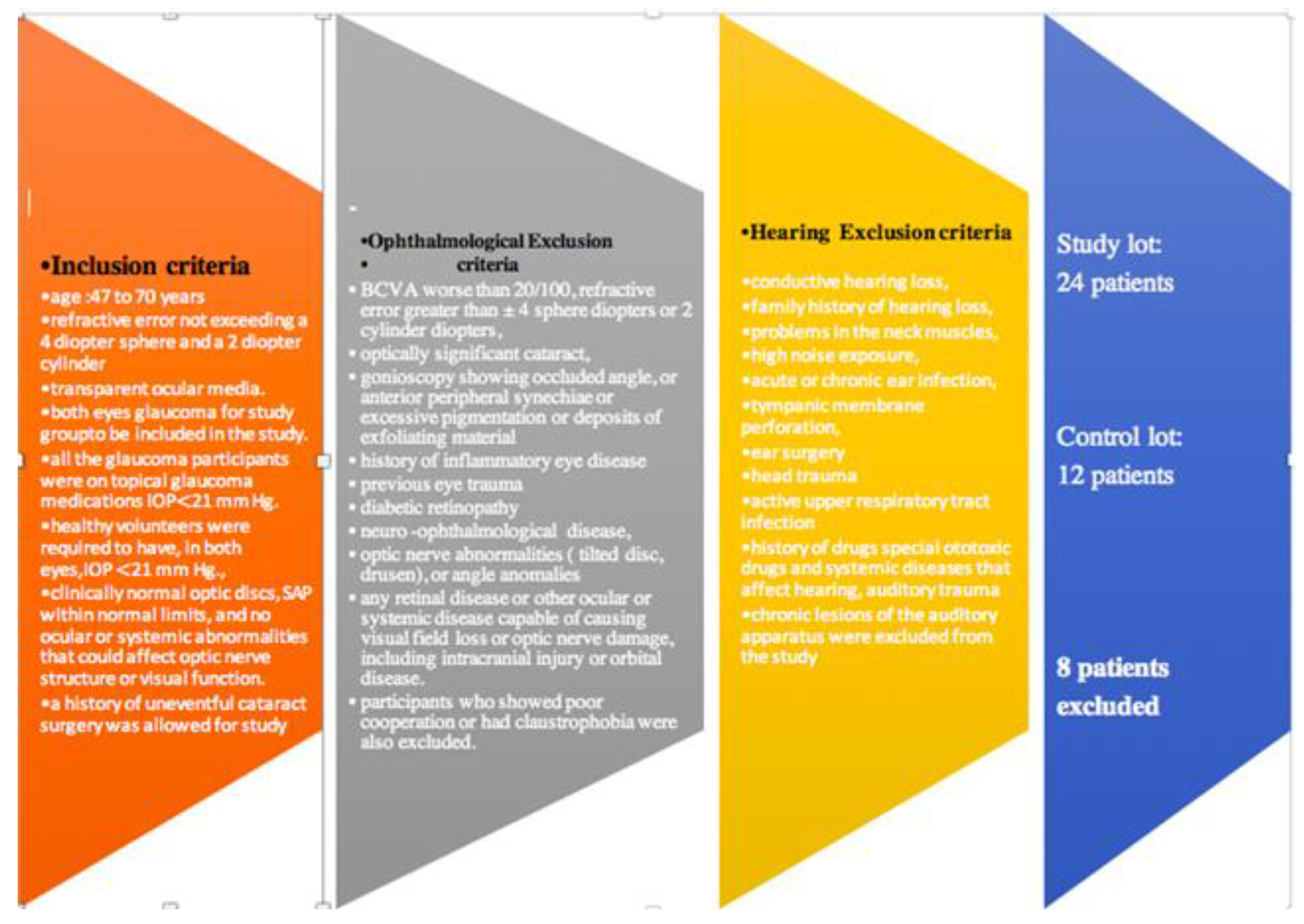
2.1. Study Design.
2.2. Evaluation protocol
2.3. Statistical Analysis
3. Results
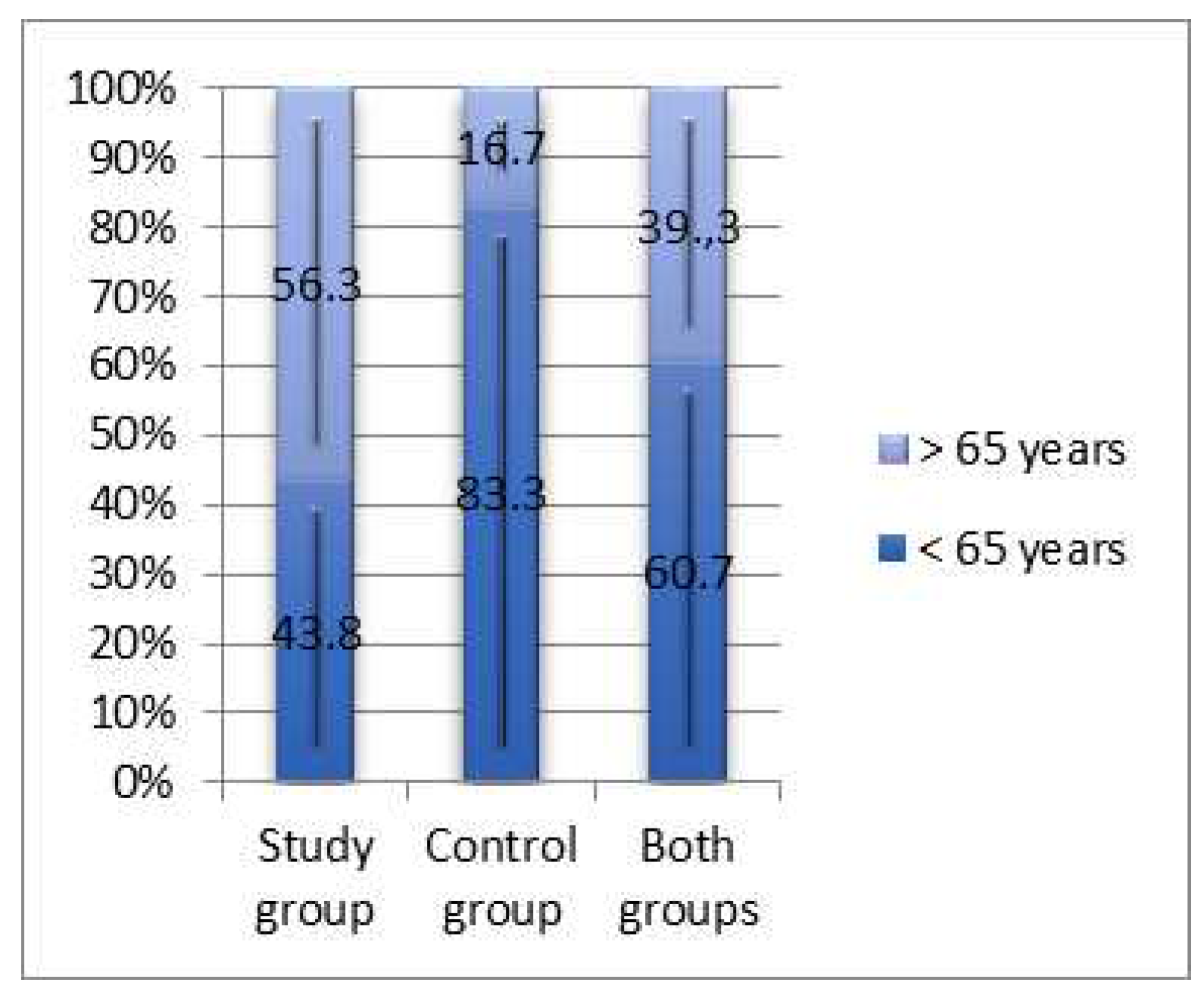
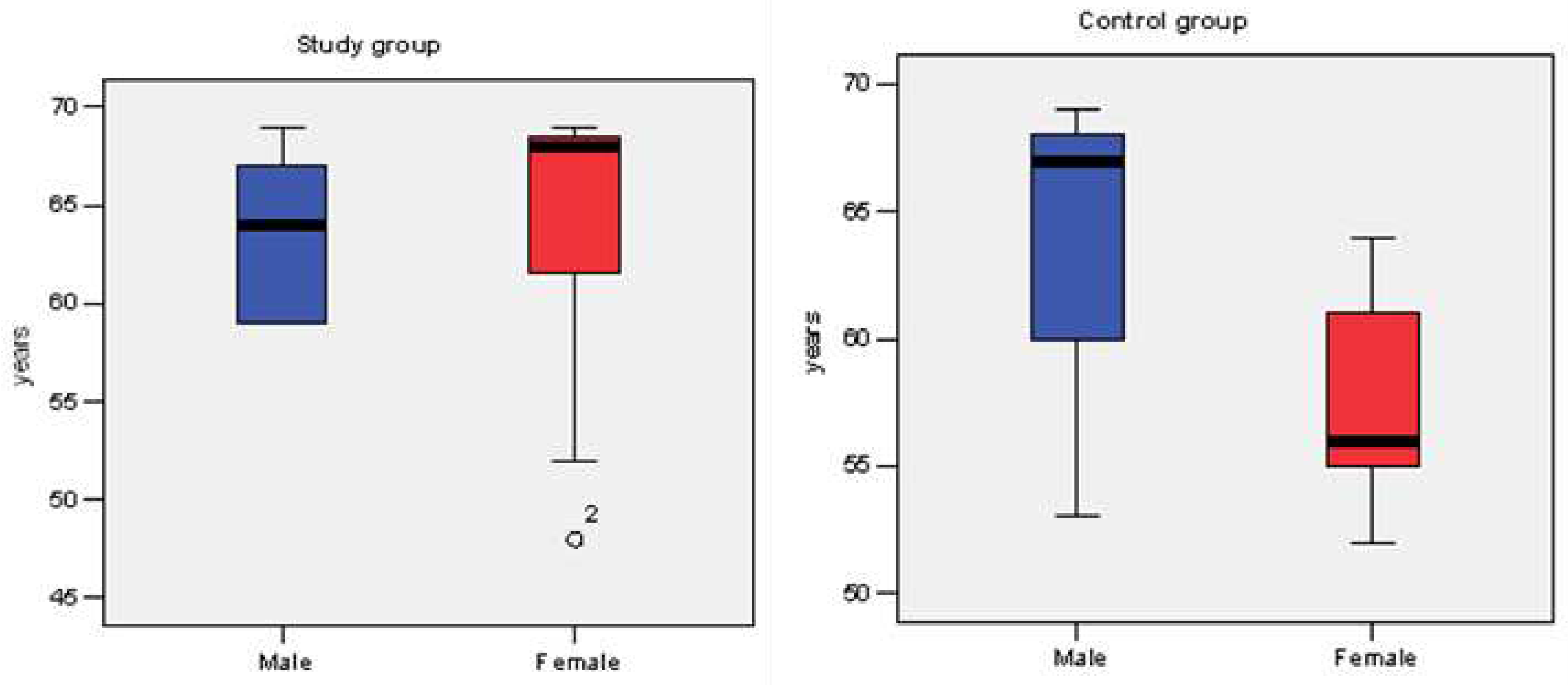
3.1. Epidemiologic characteristics.
3.2. Clinical parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pache, M. ,Flammer J. A Sick Eye in a Sick Body? Systemic Findings in Patients with Primary Open-Angle Glaucoma. Surv. of Ophthalmol. 2006, 51, 179–203. [Google Scholar] [CrossRef] [PubMed]
- Weinreb RN, Khaw PT. Primary Open-Angle Glaucoma. Lancet. 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye (Lond). 2007, 21, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Yucel Y, Gupta N. Glaucoma of the brain: a disease model for the study of transsynaptic neural degeneration. Prog Brain Res. 2008, 173, 465–478. [Google Scholar]
- Quigley, HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999, 18, 39–57. [Google Scholar] [CrossRef]
- Gupta, N. , Yucel, Y.H. What changes can we expect in the brain of glaucoma patients? Surv. Of Ophthalmol. 2007, 52, S122–S126. [Google Scholar]
- Gupta, N. , Greenberg G., Noel de Tilly L., Gray B., Polemidiotis M., Yucel Y.H. Atrophy of the lateral geniculate nucleus in 520 human glaucoma detected by magnetic resonance imaging. Br J Ophthalmology. 2009, 93, 56–60. [Google Scholar] [CrossRef]
- Jeffrey, L. Goldberg, Glaucoma research updates, 2018, 21 February.
- Jorge, A. Ghiso, PhD, Ivo Doudevski, PhD, Robert Ritch, PhD, and Agueda A. Rostagno, PhD Alzheimer’s Disease and Glaucoma: Mechanistic Similarities and Differences. J Glaucoma. 2013, 22, S36–S38. [Google Scholar]
- Gupta N, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opinion Opththalmol. 2007, 18, 110–114. [Google Scholar] [CrossRef]
- Ray K, Mookherjee S. Molecular complexity of primary open angle glaucoma: Current concepts. J Genet. 2009, 88, 451–467. [Google Scholar] [CrossRef]
- Nakamura T, Lipton SA. Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: Potential implications for Alzheimer’s and Parkinson’s diseases. Apoptosis. 2010, 15, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Ramirez,A. I.,deHoz,R.,Salobrar-Garcia,E.,Salazar,J.J.,Rojas,B.,Ajoy,D.,Lopez- Cuenca,I.,Rojas,P., Trivino, A., Ramirez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar]
- Stephane Melik Parsadaniantz, Annabelle Reaux-Le Goazigo, Anais Sapienza, Habas C., Baudouin C. Glaucoma: A De-generative Optic Neuropathy Related to Neuroinflammation? Cells, MDPI. 2020, 9, 535.
- Lam, D.Y.; Kaufman, P.L.; Gabelt, B.T.; To, E.C.; Matsubara, J.A. Neurochemical correlates of cortical plasticity after unilateral elevated intraocular pressure in a primate model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Kremmer S, Kreuzfelder E, Bachor E, Jahnke K, Selbach JM, Seidahmadi S. Coincidence of normal tension glaucoma, pro-gressive sensorineural hearing loss, and elevated antiphos-phatidylserine antibodies. Br JOphthalmol. 2004, 88, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- ShapiroA, Siglock TJ, Ritch R, Malinoff R. Lack of association between hearing loss and glaucoma. Am J Otol. 1997, 18, 172–174. [Google Scholar]
- Aydogan Ozkan B, Yuksel N, Keskin G, et al. Homocysteine levels in plasma and sensorineural hearing loss in patients with pseudoexfoliation syndrome. Eur J Ophthalmol. 2006, 16, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Cahill M, Early A, Stack S, Blayney AW, Eustace P. Pseudoexfoliation and sensorineural hearing loss. Eye. 2002, 16, 261–266. [Google Scholar] [CrossRef]
- Detorakis ET, Chrysochoou F, Paliobei V, et al. Evaluation of the acoustic function in pseudoexfoliation syndrome and exfo-liation glaucoma: audiometric and tympanometric findings. Eur J Ophthalmol. 2008, 18, 71–76. [Google Scholar] [CrossRef]
- Shaban RI, Asfour WM. Ocular pseudoexfoliation associated with hearing loss. Saudi Med J. 2004, 25, 1254–1257. [Google Scholar]
- Turacli ME, Ozdemir FA, Tekeli O, Gokcan K, Gerceker M, Duruk K. Sensorineural hearing loss in pseudoexfoliation. Can J Ophthalmol. 2007, 42, 56–59. [Google Scholar]
- Yazdani S, Tousi A, Pakravan M, Faghihi AR. Sensorineural hearing loss in pseudoexfoliation syndrome. Ophthalmo-lo-gy. 2008, 115, 425–429. [Google Scholar]
- Joe Walter Kutz, Ginger Mullin, Kathleen C M Campbel Audiology Pure-Tone Testing, English edition, drugs &disease, Clinical procedures.Medscape, 2018, August 27.
- Clark, J. G. Uses and abuses of hearing loss classification. Asha, 1981, 23, 493–500. [Google Scholar] [PubMed]
- ASHA recomandations online. Degree, and Configuration of Hearing Loss, 2023, February 5. Available online: https://www.asha.org.public/hearing/Type.
- Mustafa Deger Bilgec,Nagehan Erdogmus Kucukcan, Leman Birdane Armagan Incesulu, Nilgun Yildirim Evaluation of the Vestibulocochlear System in Patients with Pseudoexfoliation Syndrome. TurkJOphthalmol. 2021, 51, 156–160.
- Wenbin Huang, Jifa Kuang, Feilan Chen, Yu Fu Association of sensorineural hearing loss and pseudoexfoliation syn-drome: a meta-analysis. Annals of Eye Science. 2022, 7, 26. [CrossRef]
- Fleur O’Hare, Gary Rance, Jonathan G. Crowston, and Allison M. McKendrick Auditory and Visual Temporal Pro-cessing Disruption in Open Angle Glaucoma. Invest Ophthalmol Vis Sci. 2012, 53, 6512–6518. [CrossRef]
- Lucy, I. Mudie, Varshini Varadaraj, Prateek Gajwani, Beatriz Munoz, Pradeep Ramulu, Frank R. Lin, Bonnielin K. Swenor, 565 David S. Friedman, Nazlee Zebardas Dual sensory impairment: The association between glaucomatous vi-sion loss and hearing impairment and function Hearing and vision impairment: Effect on function. PLOS ONE, 2018, July 6.
- Joon Mo Kim,SeYoung Kim,Hee Seung Chin,Hyun Ji Kim and Na Rae Kim, on behalf of the Epidemiologic Survey Committee of the Korean Ophthalmological Society Relationships between Hearing Loss and the Prevalences of Cata-ract, Glaucoma, Diabetic Retinopathy, and Age-Related Macular Degeneration in Korea. J. Clin. Med. 2019, 8, 1078.
- Hsiang-Wen Chien, Pei-Hsuan Wu, Kai Wang, Chi-Chin Sun, Jing-Yang Huang, Shun-Fa Yang, Hung-Chi Chen and 573 Chia-Yi Lee Increased Incidence of Glaucoma in Sensorineural Hearing Loss: A Population-Based Cohort Study Int. J. Environ. Res. Public Health. 2019, 16, 2907.
- Jennifer, H. Madans, PhD., Julie D. Weeks, Ph.D., and Nazik Elgaddal, M.S. Hearing Difficulties Among Adults:. United States 2019. 2021, 414, 1–8. [Google Scholar]
- M Alzaher, N Vannson, O Deguine, M Marx, Pascal Barone, et al. Brain plasticity and hearing disorders. Revue Neu-rologique 2021, 177, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Eric, I. Knudsen, Phyllis F. KnudsenVision Guides the Adjustment of Auditory Localization in Young Barn Owls. KnudsenVision Guides the Adjustment of Auditory Localization in Young Barn Owls..Science 1985, Vol.230, 545–548. [Google Scholar]
- Chiara Valzolgher, Mariam Alzhaler, Elena Gessa, Michela Todeschini, Pauline Nieto, et al. . The impact of a visual spa-tial frame on real sound-source localization in virtual reality. Current Research in Behavioral Sciences. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Redon, Christine; Hay, Laurette Role of visual context and oculomotor conditions in pointing accuracy Cognitive neu-ro-science and neuropsychology. NeuroReport. 2005, 16, 2065–2067. [CrossRef]
- Grzegorz Zielinsk, Marcin Wojcicki,Maria Rapa, Anna Matysik-Wozniak, Michal Baszczowski Michal Ginszt,Monika Litko-Rola, Jacek Szkutnik, Ingrid Rozylo-Kalinowska, Robert Rejdak and Piotr Gawda Masticatory Muscle Thickness and Activity Correlates to Eyeball Length, Intraocular Pressure, Retinal and Choroidal Thickness in Healthy Women versus Women with Myopia. Pers. Med. 2022, 12, 626.
- Luthra, S. The role of the right hemisphere in processing phonetic variability between talkers. Neurobiology of Language. 2021, 2, 138–151. [Google Scholar] [CrossRef]
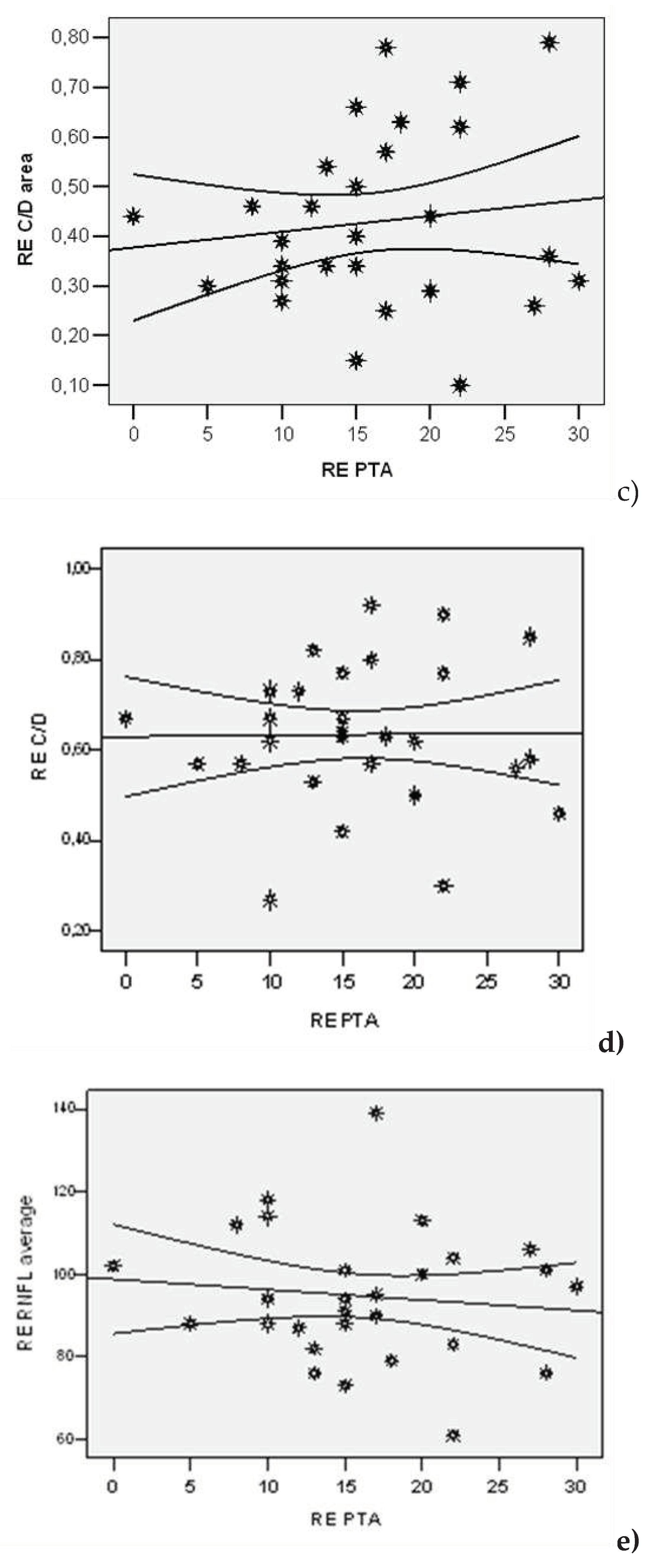
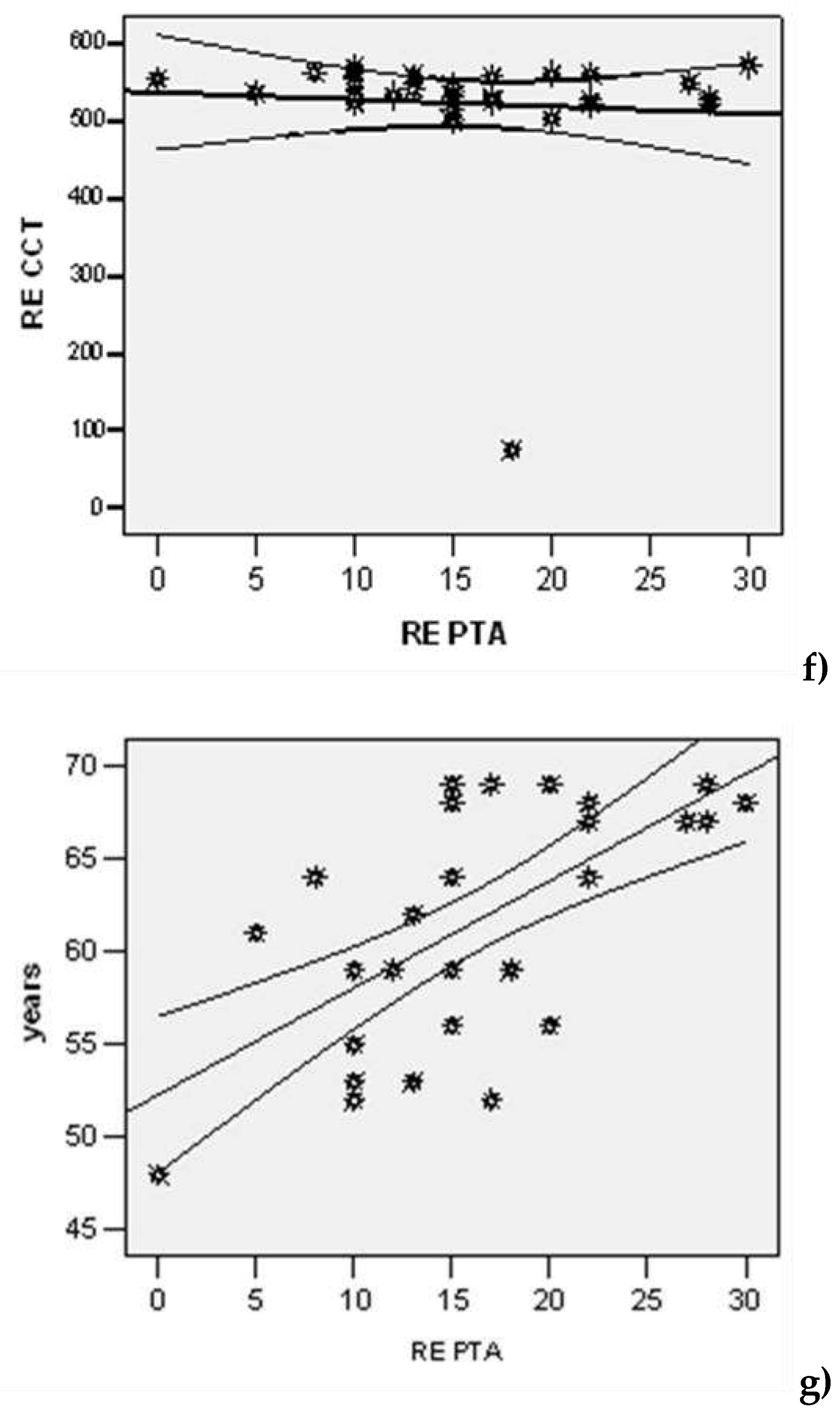
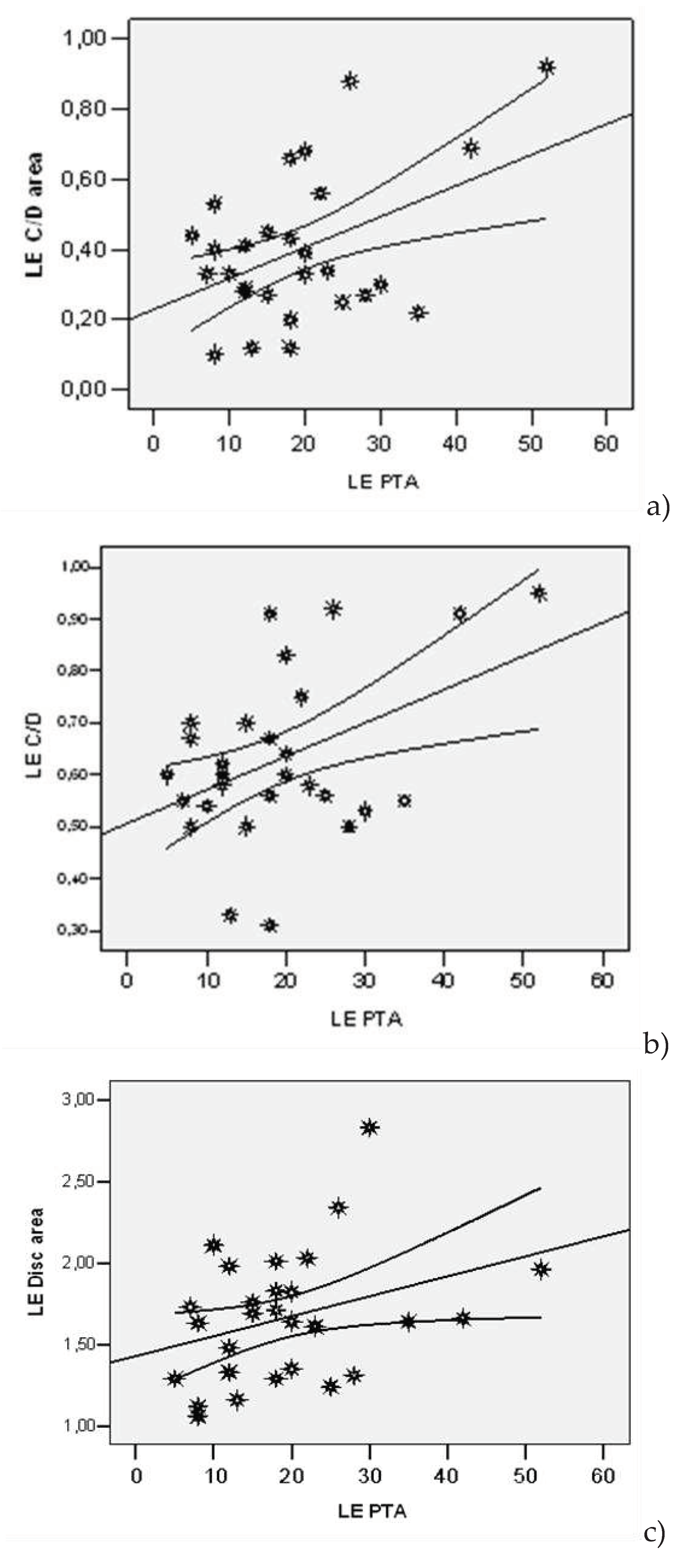
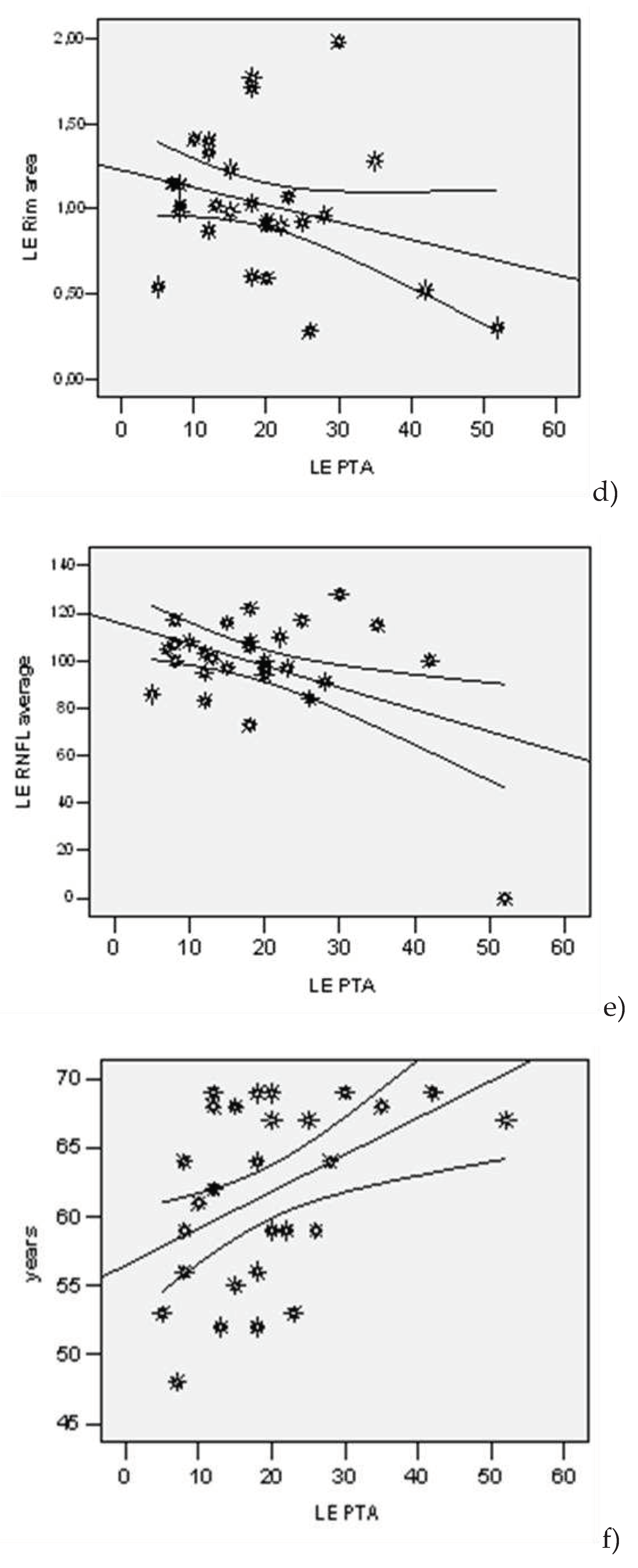
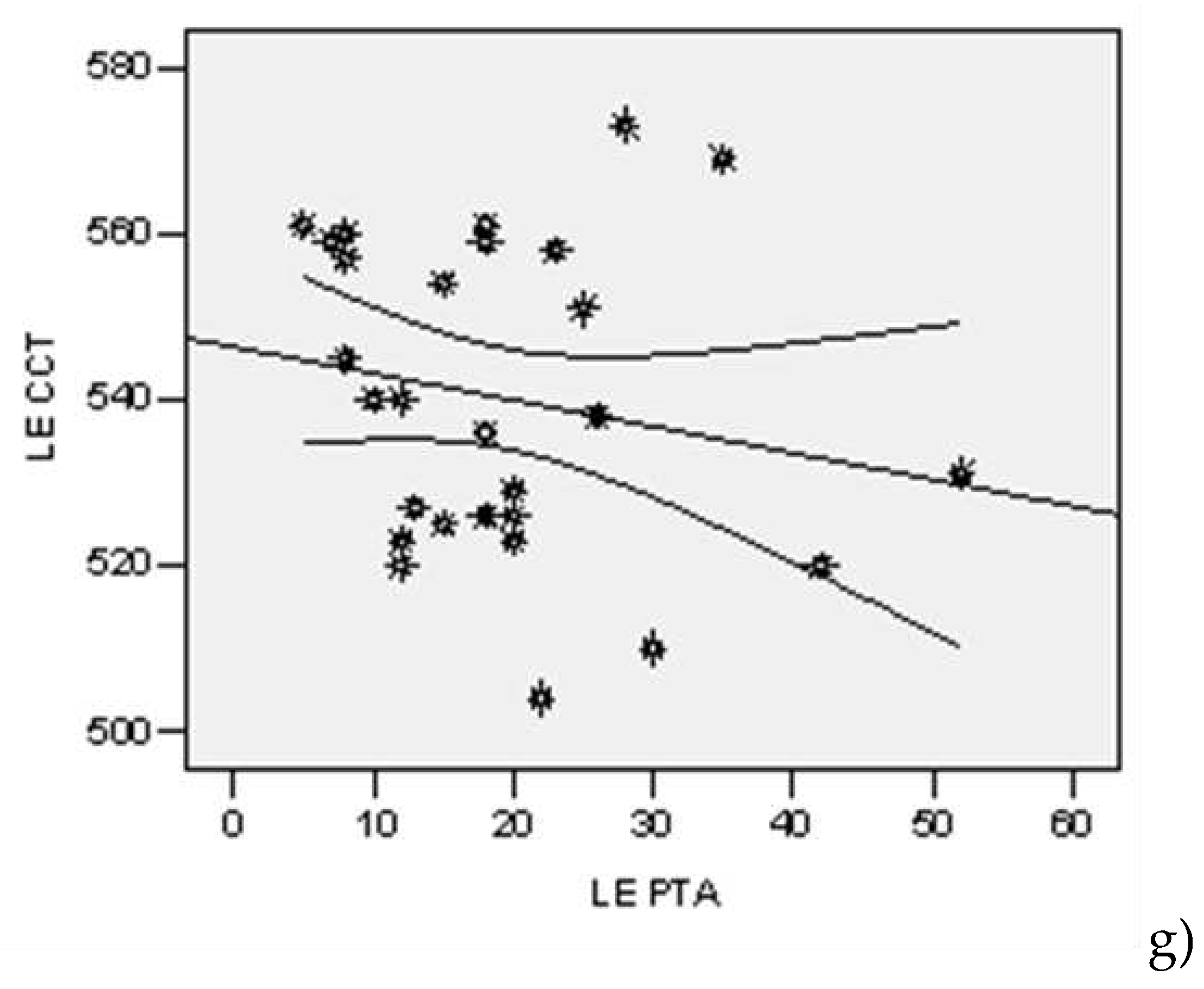
| Lot | N | Average | Std. Dev | Std.error | Confidence Interval 95% | Min | Max | t-Student Test ( p) | |
|---|---|---|---|---|---|---|---|---|---|
| -95%CI | +95%CI | ||||||||
| PTA (dB) right | |||||||||
| Study | 16 | 18.38 | 7.949 | 1.987 | 14.14 | 22.61 | 0 | 30 | 0.063 |
| Control | 12 | 13.33 | 4.793 | 1.384 | 10.29 | 16.38 | 5 | 22 | |
| PTA (dB) left | |||||||||
| Study | 16 | 23.25 | 12.283 | 3.071 | 16.70 | 29.80 | 7 | 52 | 0.023 |
| Control | 12 | 14.00 | 5.560 | 1.605 | 10.47 | 17.53 | 5 | 23 | |
| Model | R | R Square | Adjusted R Square | StandardError | Change Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|
| R Square | F | df1 | df2 | p | |||||
| The dependent variable PTA (dB) right | |||||||||
| 1 | 0,342(a) | 0,117 | 0.083 | 6.834 | 0.117 | 3.437 | 1 | 26 | 0,075 |
| 2 | 0,672(b) | 0,452 | 0.408 | 5.491 | 0.335 | 15.269 | 1 | 25 | 0,001 |
| 3 | 0,672(c) | 0,452 | 0.383 | 5.603 | 0.000 | 0,008 | 1 | 24 | 0,930 |
| 4 | 0,672(d) | 0,452 | 0.357 | 5.724 | 0,000 | 0.000 | 1 | 23 | 0,995 |
| The dependent variable PTA (dB) left | |||||||||
| 1 | 0,390(a) | 0,152 | 10.197 | 0.152 | 4.677 | 4,677 | 1 | 26 | 0,040 |
| 2 | 0,547(b) | 0,300 | 9.452 | 0.147 | 5.259 | 5.259 | 1 | 25 | 0,031 |
| 3 | 0,548(c) | 0,300 | 9.646 | 0.000 | 0.006 | 0,006 | 1 | 24 | 0,939 |
| 4 | 0,616(d) | 0,380 | 9.275 | 0.080 | 2.960 | 2.960 | 1 | 23 | 0,099 |
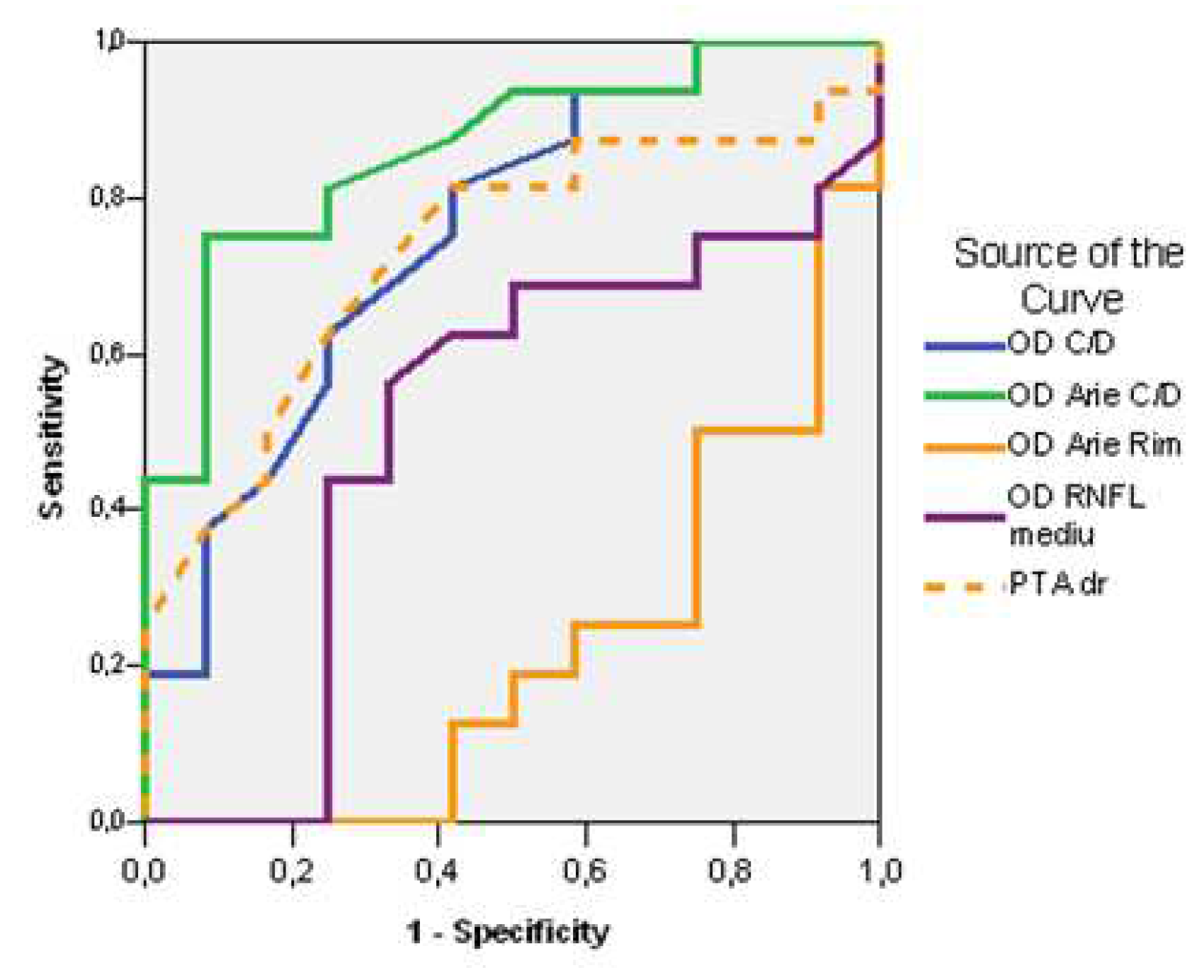
| Test Result Variable(s) | Area | Std. Error(a) | Asymptotic Sig.(b) | Asymptotic 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Cut off | Lower Bound | Upper Bound | ||||
| RE C/D ratio | 0,753 | 0,60 | 0,094 | 0,024 | 0,569 | 0,937 |
| RE C/D Area | 0,862 | 0,33 | 0,070 | 0,001 | 0,725 | 0,999 |
| RE Rim Area | 0,219 | 1,11 | 0,090 | 0,012 | 0,043 | 0,395 |
| RE RNFL average | 0,505 | 95,00 | 0,118 | 0,963 | 0,275 | 0,736 |
| PTA right | 0,732 | 14,00 | 0,097 | 0,039 | 0,542 | 0,922 |
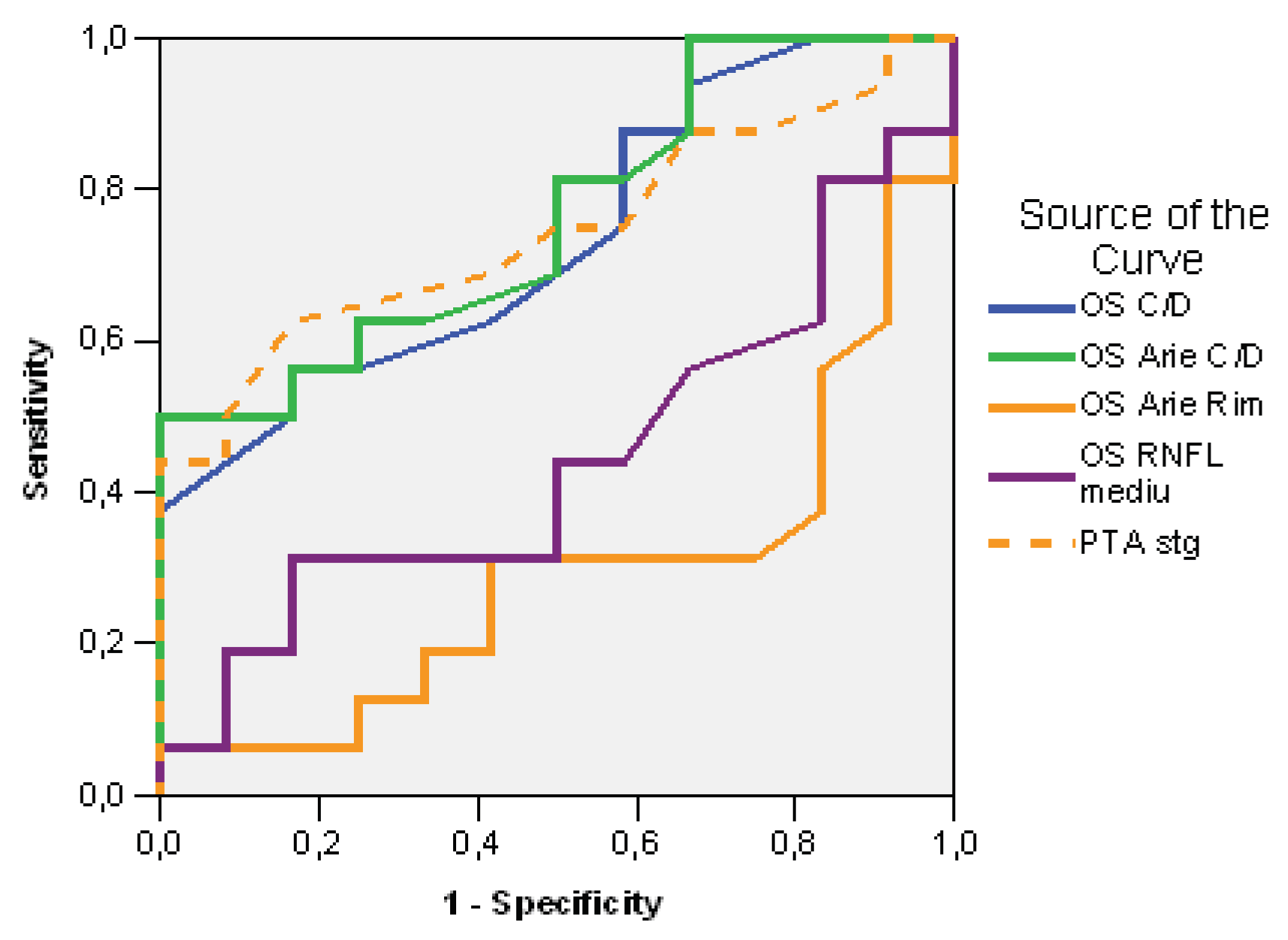
| Test Result Variable(s) | Area | Std. Error(a) | Asymptotic Sig.(b) | Asymptotic 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Cut off | Lower Bound | Upper Bound | ||||
| LE C/D ratio | 0,734 | 0,56 | 0,094 | 0,037 | 0,550 | 0,919 |
| LE C/D Area | 0,763 | 0,30 | 0,090 | 0,019 | 0,588 | 0,938 |
| LE Rim Area | 0,292 | 1,11 | 0,100 | 0,063 | 0,095 | 0,488 |
| LE RNFL average | 0,443 | 100,00 | 0,111 | 0,610 | 0,226 | 0,659 |
| PTA left | 0,745 | 14,00 | 0,093 | 0,029 | 0,562 | 0,928 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





