Submitted:
08 May 2023
Posted:
10 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
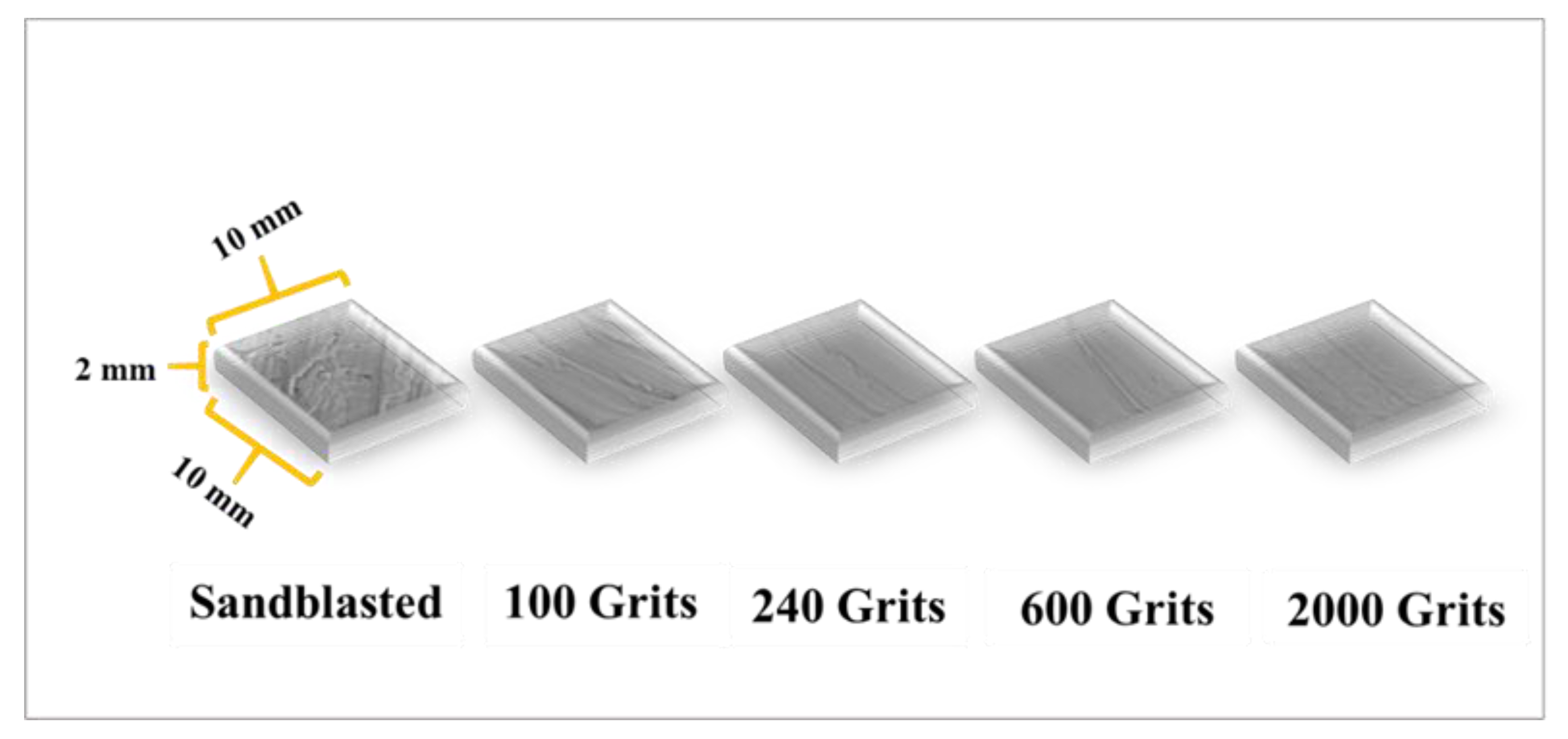
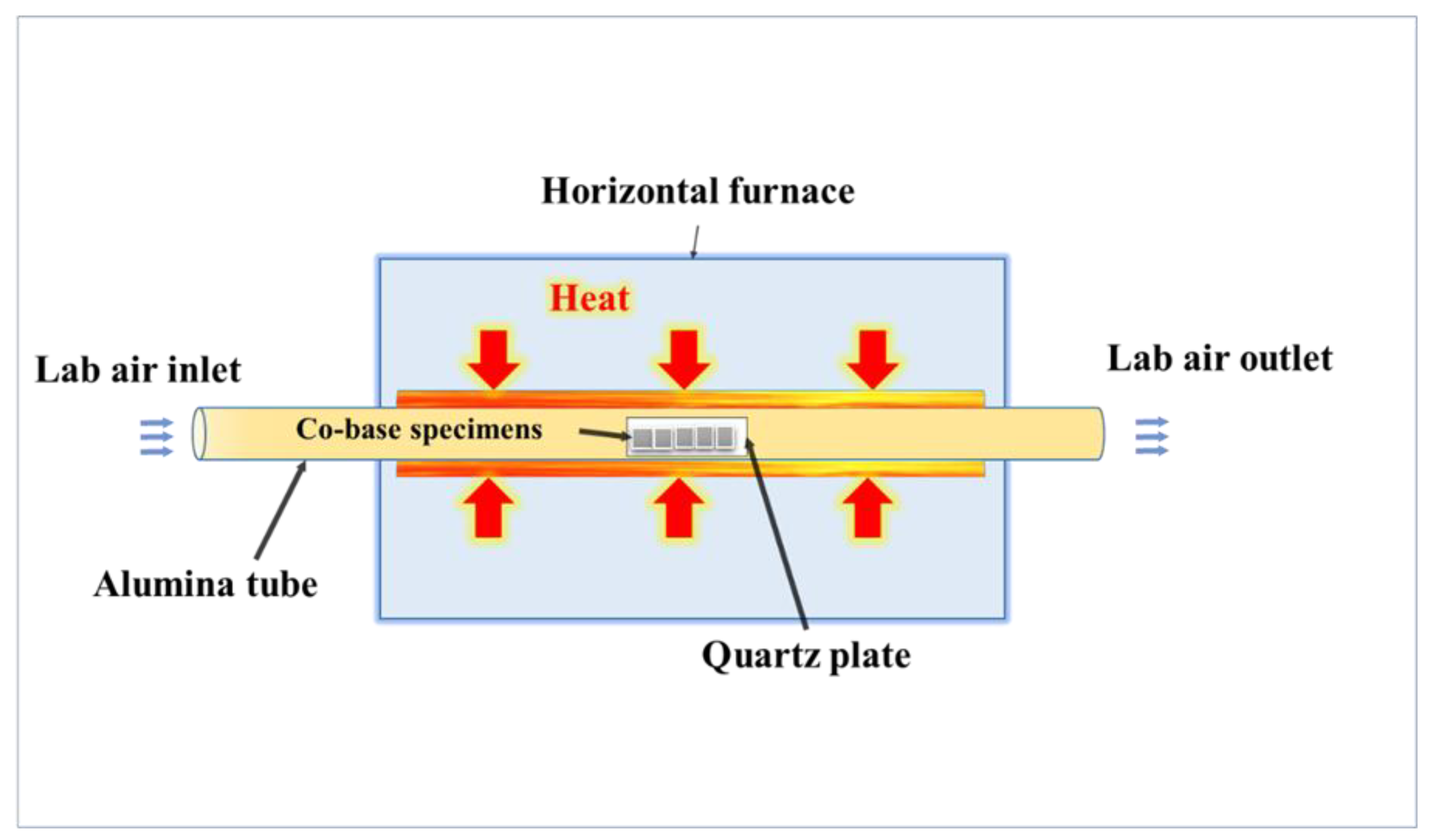
2. Material and Methods
3. Results
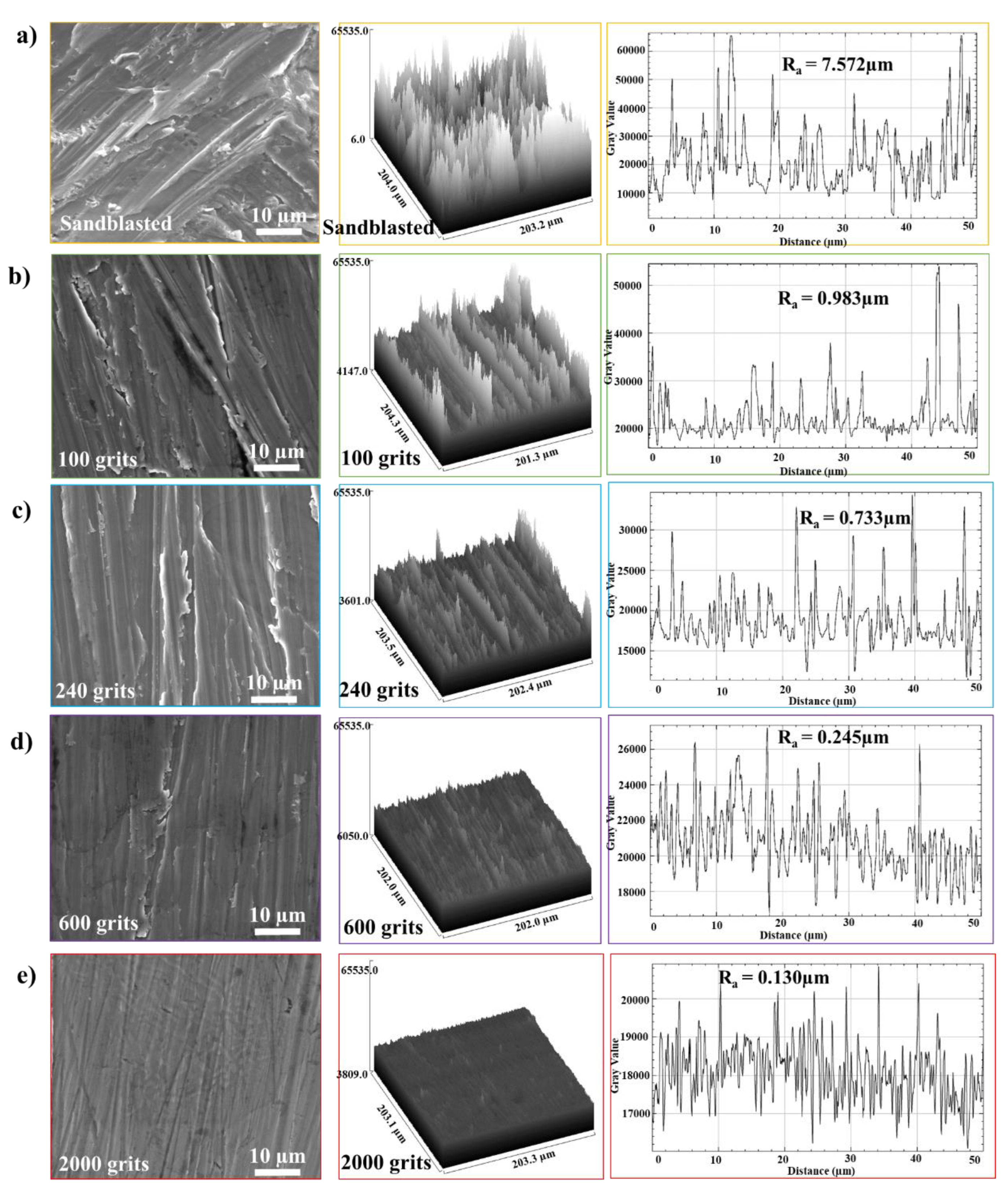
3.1. Surface Roughness
3.2. Isothermal Oxidation Test at 1050 °C
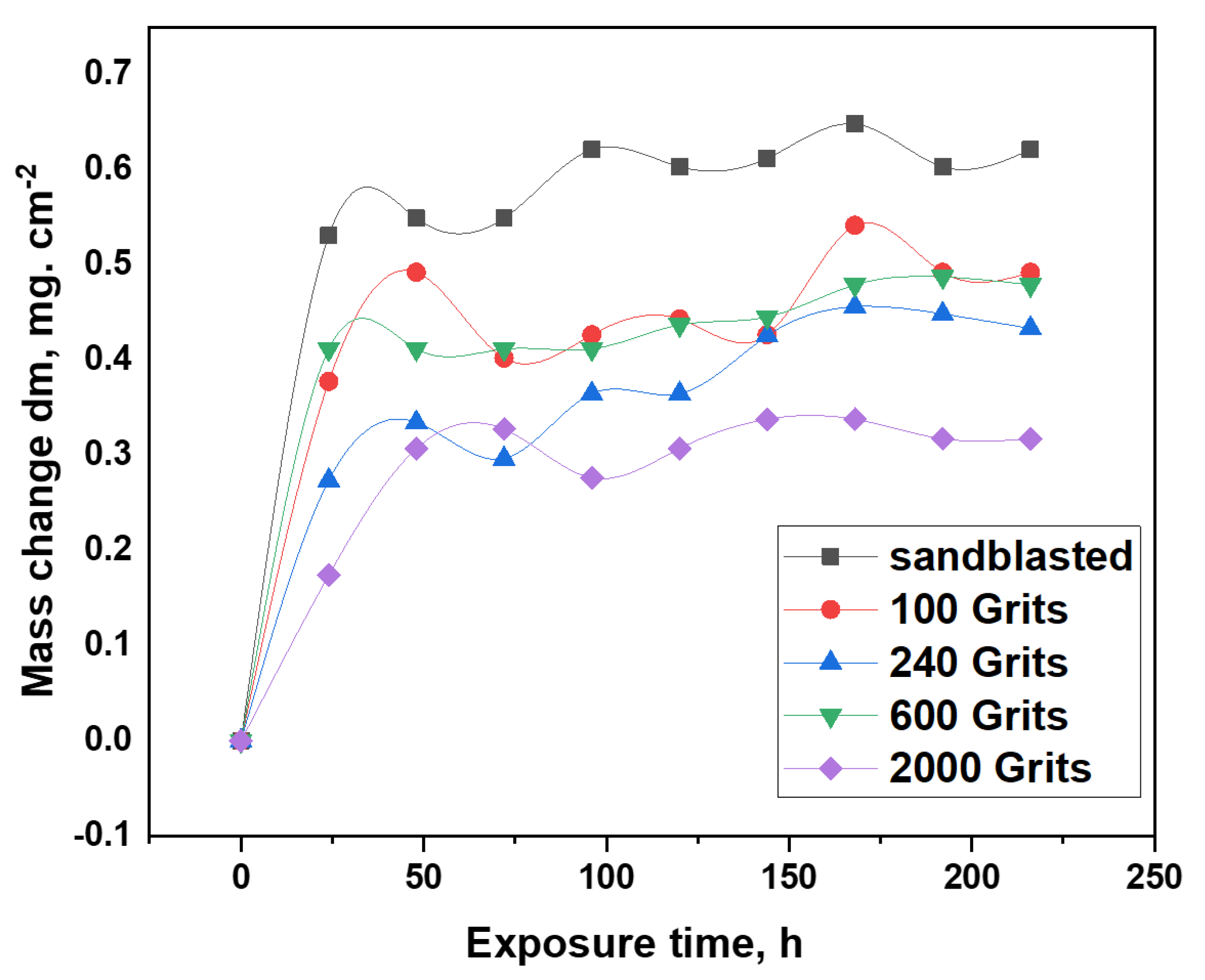
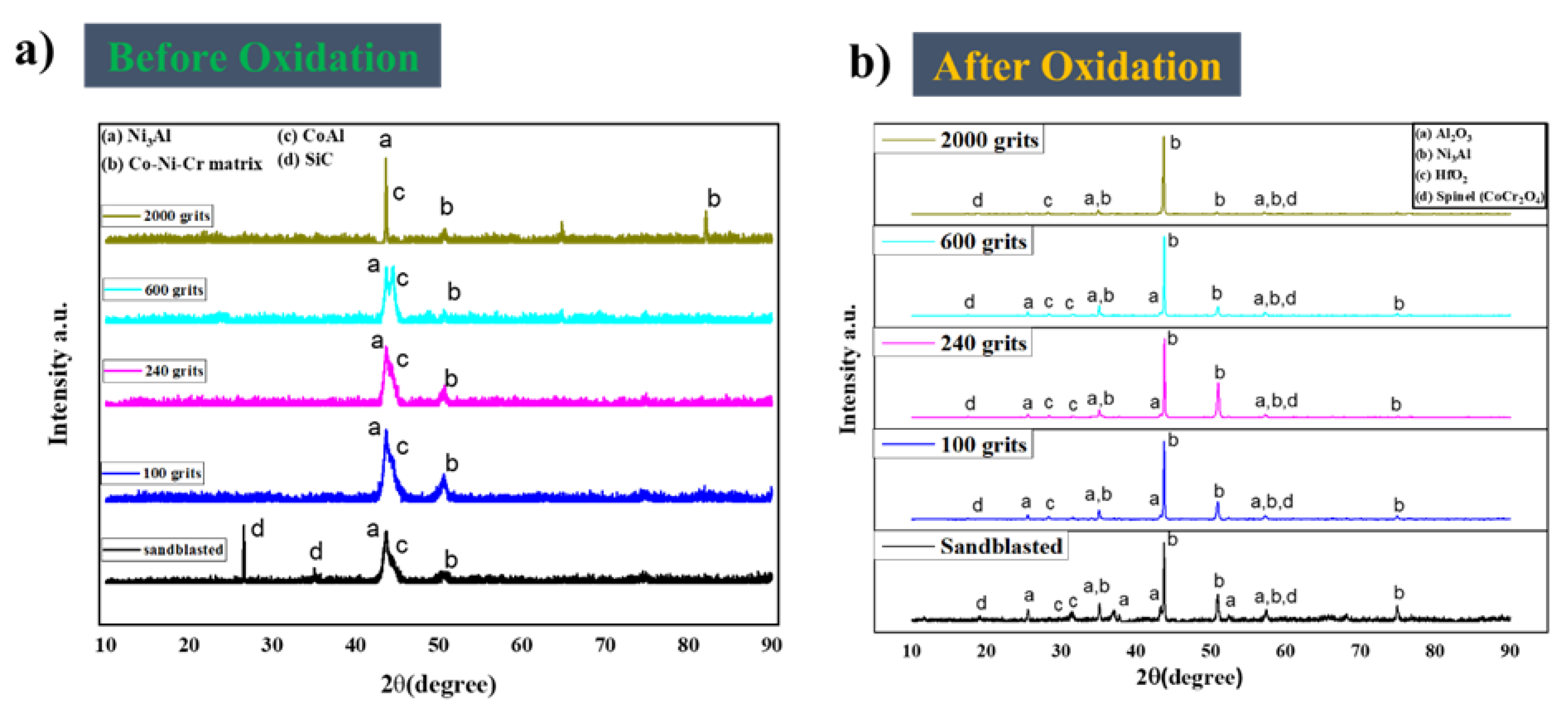
3.3. Oxide phase composition after 216 h of oxidation
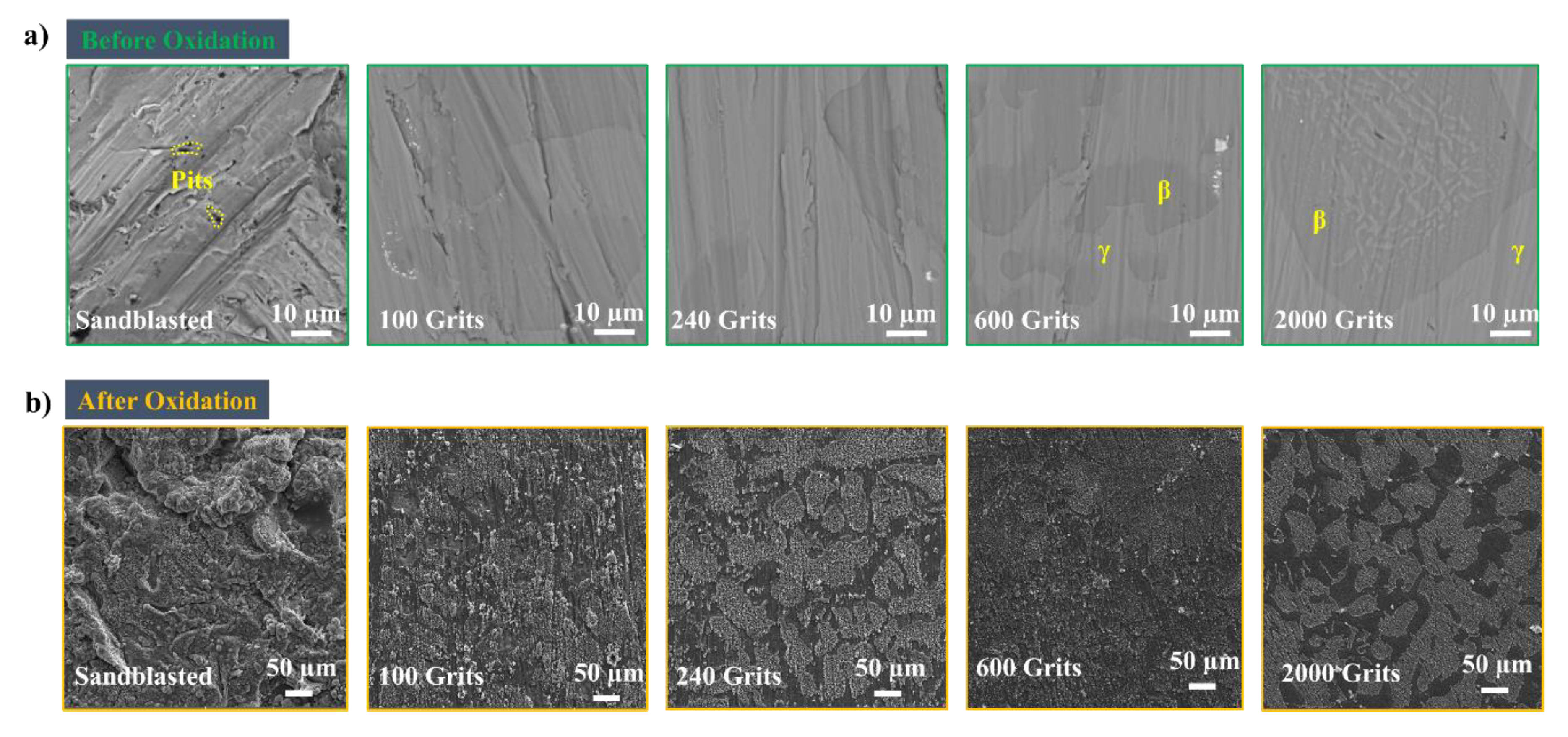
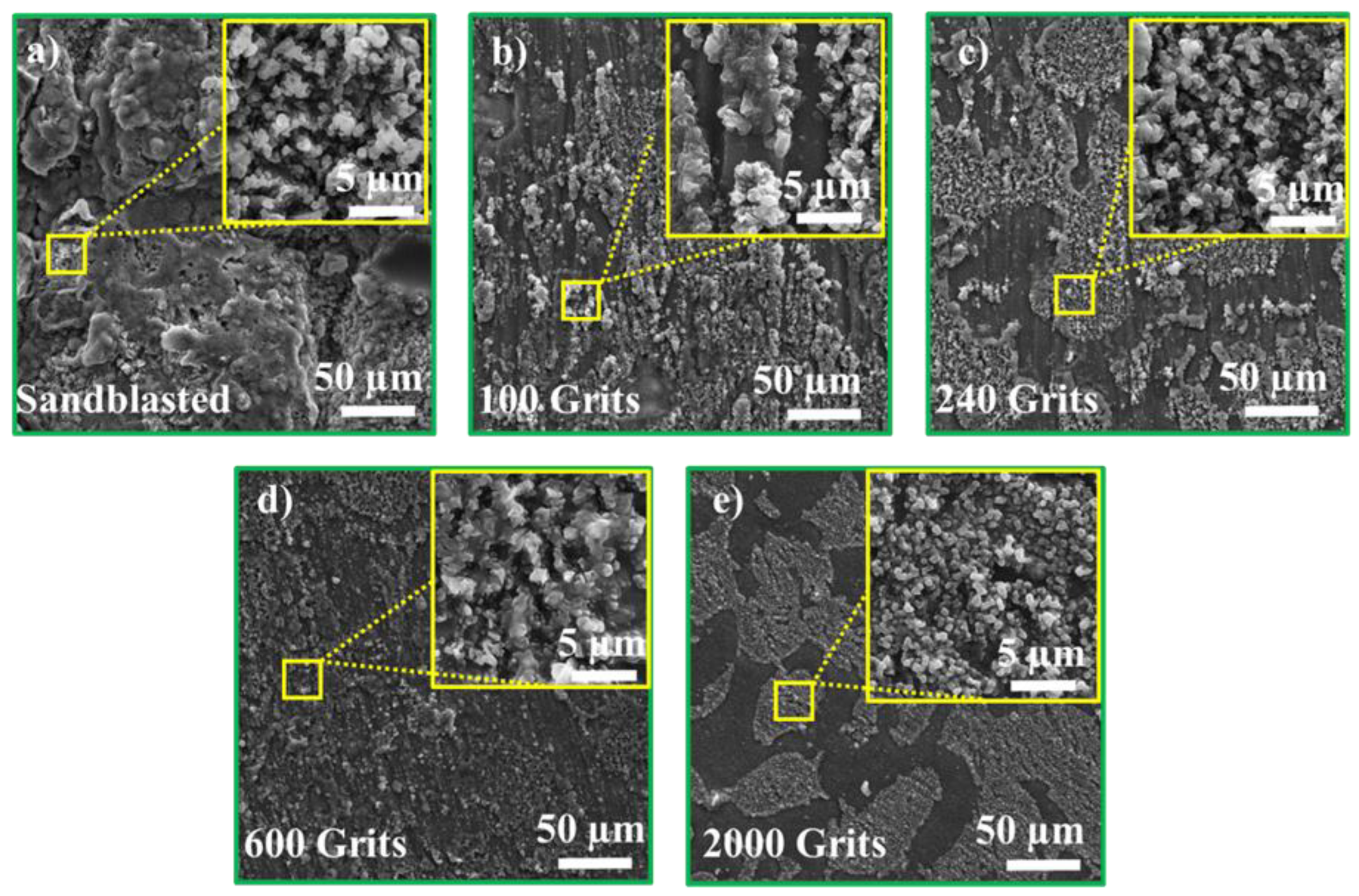
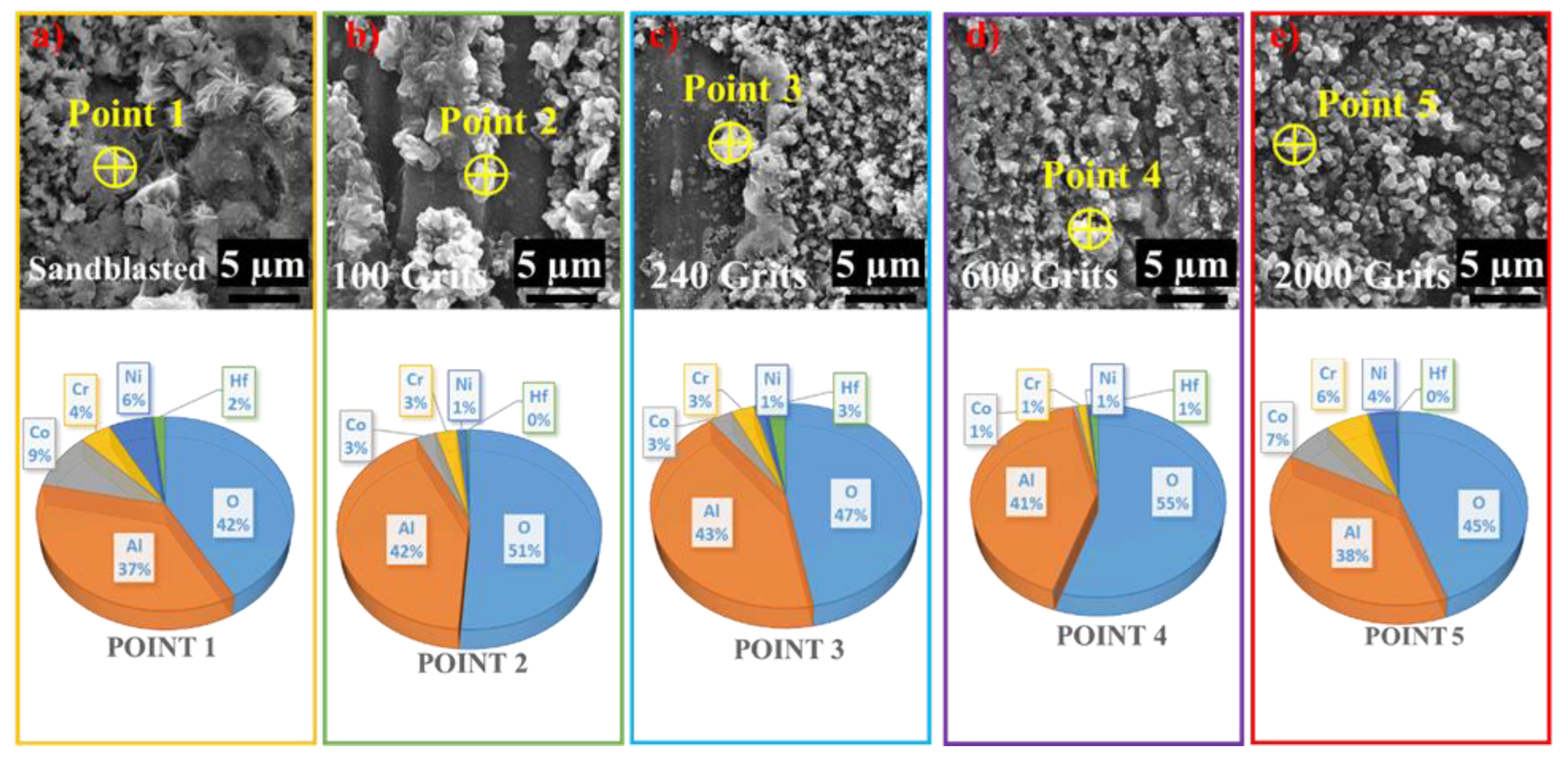
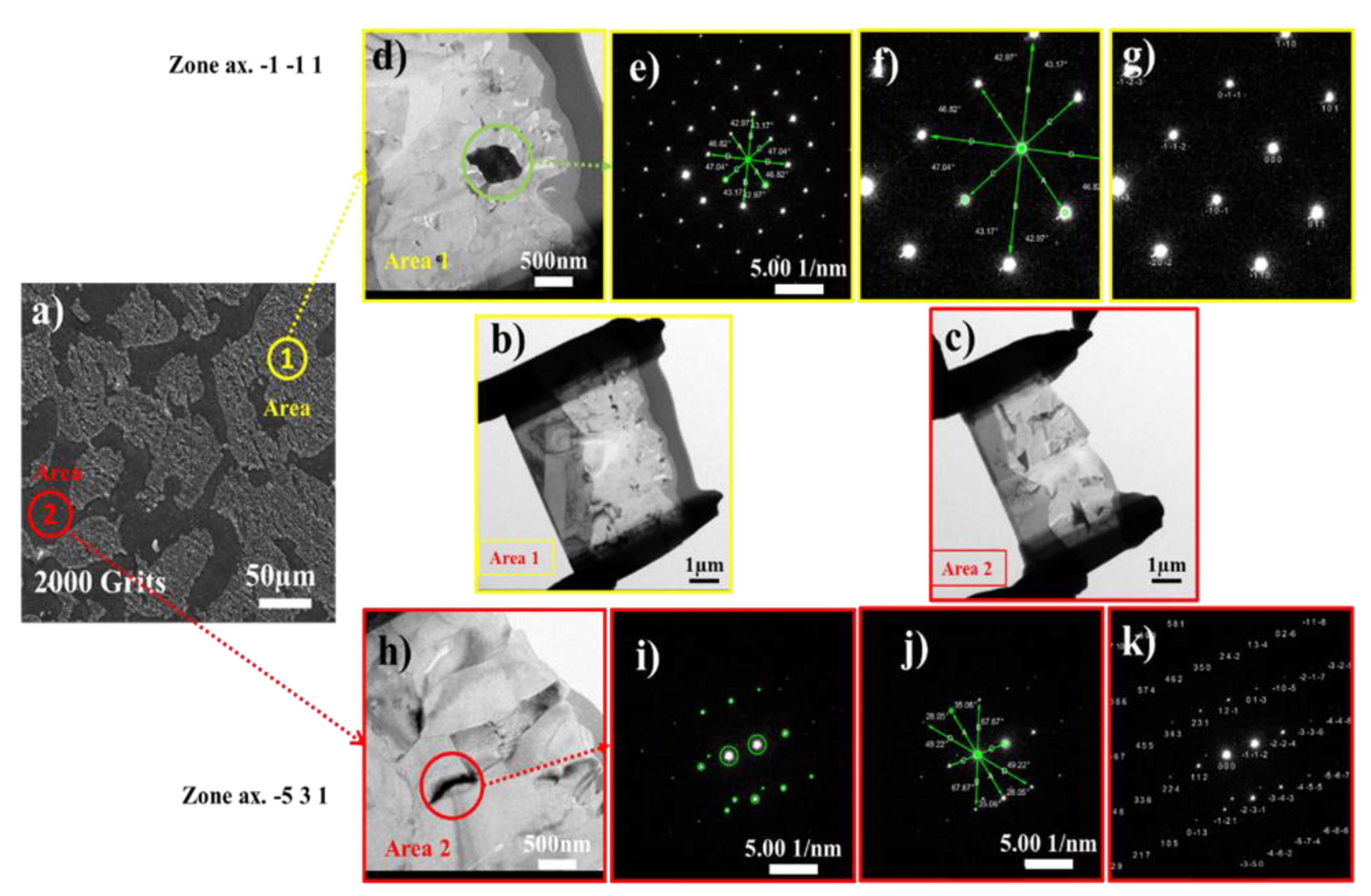
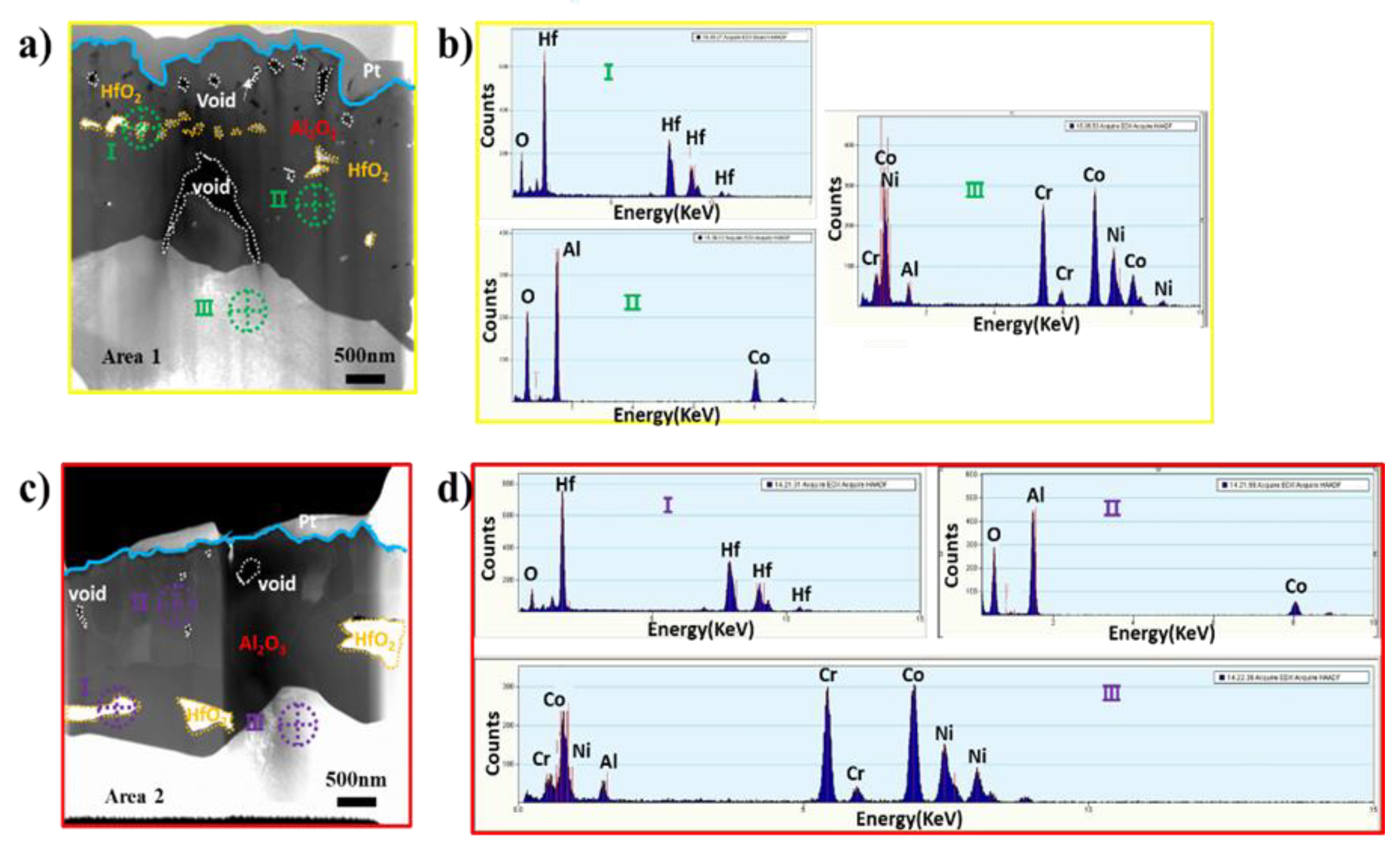
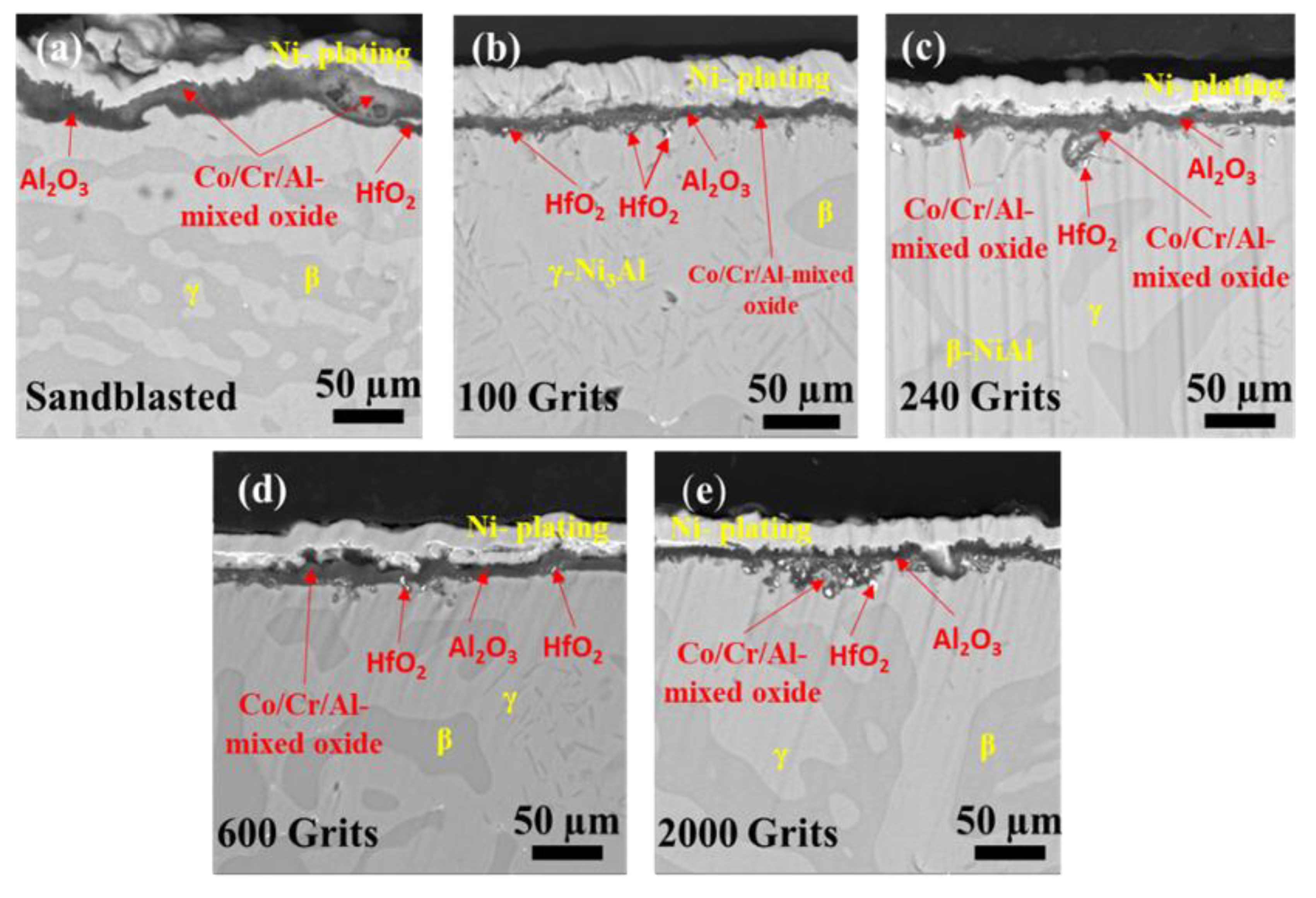
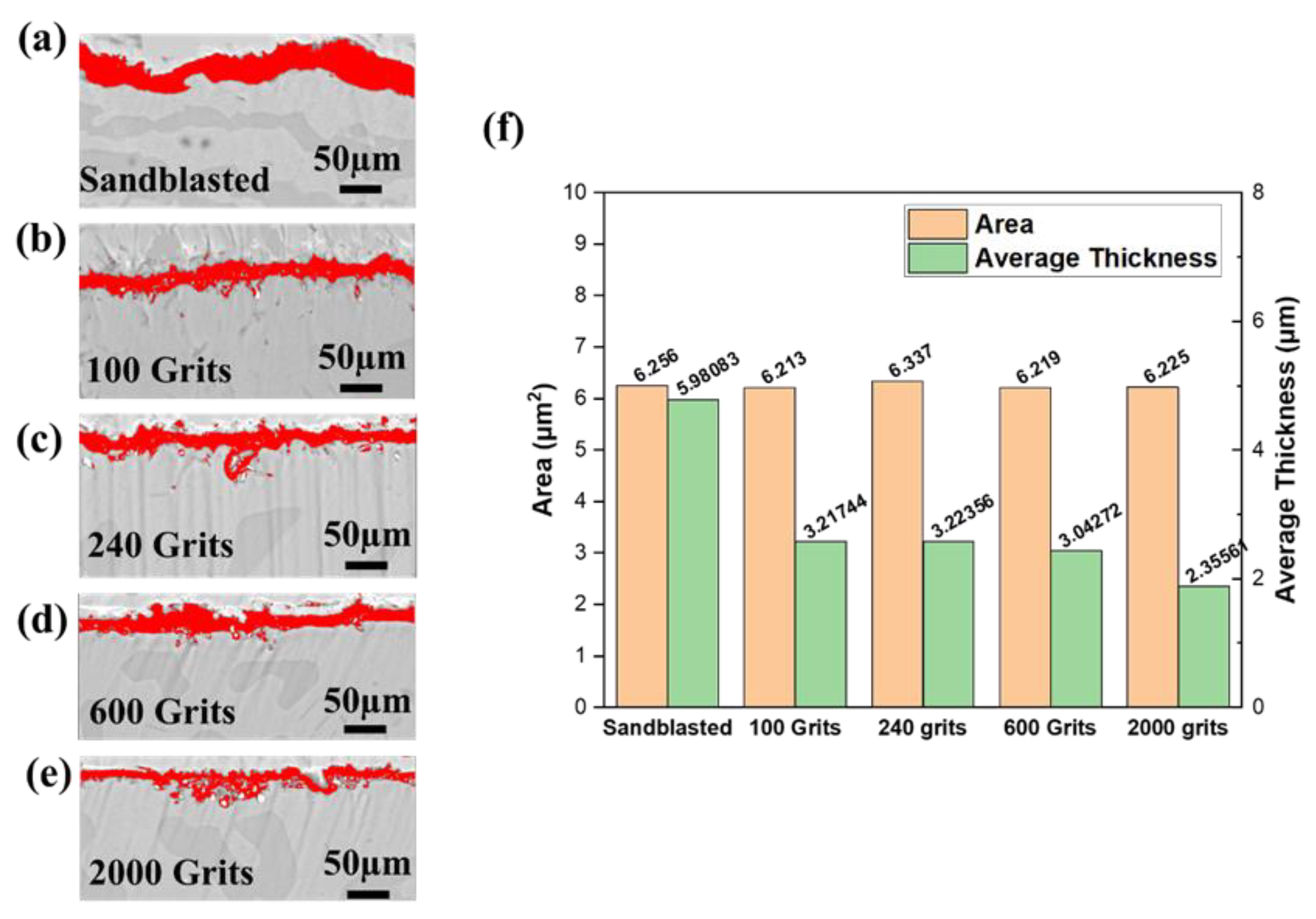
3.4. Surface Microstructural and Cross-Section Analyses
4. Discussion
4.1. Effects of Surface Roughness on the Oxidation Behavior of Free-Standing CoNiCrAlYHf Oxide Scale
5. Conclusions
- Differences in the oxidation behavior of the free-standing CoNiCrAlYHf coating at 1050 °C were observed at different surface roughness.
- The samples with a surface roughness of 0.130 μm and polished with 2000 grits demonstrated the highest oxidation resistance because of their small exposed surface areas and thin work-hardening layers.
- The identical surface preparation process that resulted in a rough surface caused the formation of a thick oxide layer in the near-surface area of the material. A higher surface roughness led to the formation of a more protective oxide scale. In contrast, the surface with a smoother roughness exhibited a thin oxide scale; the interior oxide was closer to the surface. An increase in the tensile tension contributed to a shift in the oxidation behavior of the investigated free-standing CoNiCrAlYHf coating.
- A simple mechanical surface preparation method demonstrated that the free-standing CoNiCrAlYHf coating could move from the alumina formation region to the cobalt-chromium formation region. Polishing improved the resistance of the investigated materials to oxidation at high temperatures.
Author Contributions
Acknowledgments
References
- Young, D.J. High temperature oxidation and corrosion of metals. Elsevier: 2008; Vol. 1.
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the high temperature oxidation of metals. Cambridge university press: 2006.
- Giggins, C.; Pettit, F. Oxidation of Ni-Cr-Al alloys between 1000° and 1200° C. Journal of the Electrochemical Society 1971, 118, 1782. [Google Scholar] [CrossRef]
- Evans, J.L. Effect of surface roughness on the oxidation behavior of the Ni-base superalloy ME3. Journal of materials engineering and performance 2010, 19, 1001–1004. [Google Scholar] [CrossRef]
- Forsik, S.A.; Polar Rosas, A.O.; Wang, T.; Colombo, G.A.; Zhou, N.; Kernion, S.J.; Epler, M.E. High-temperature oxidation behavior of a novel Co-base superalloy. Metallurgical and Materials Transactions A 2018, 49, 4058–4069. [Google Scholar] [CrossRef]
- J. Nowak, W.; Siemek, K.; Ochał, K.; Kościelniak, B.; Wierzba, B. Consequences of different mechanical surface preparation of Ni-base alloys during high temperature oxidation. Materials 2020, 13, 3529. [Google Scholar] [CrossRef] [PubMed]
- Nowak, W.J. Effect of surface roughness on oxidation resistance of stainless steel AISI 316Ti during exposure at high temperature. Journal of Materials Engineering and Performance 2020, 29, 8060–8069. [Google Scholar] [CrossRef]
- Bensch, M.; Preußner, J.; Hüttner, R.; Obigodi, G.; Virtanen, S.; Gabel, J.; Glatzel, U. Modelling and analysis of the oxidation influence on creep behaviour of thin-walled structures of the single-crystal nickel-base superalloy René N5 at 980 C. Acta Materialia 2010, 58, 1607–1617. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.; Chen, R.; Ye, Y.; Hua, Y. High temperature oxidation behavior of Ni-based superalloy GH202. Materials Characterization 2016, 118, 122–128. [Google Scholar] [CrossRef]
- El-Awadi, G.; Abdel-Samad, S.; Elshazly, E.S. Hot corrosion behavior of Ni based Inconel 617 and Inconel 738 superalloys. Applied surface science 2016, 378, 224–230. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, X.; Gao, M.; Liu, K. High-Temperature oxidation behavior of a Ni-Cr-W-Al alloy. Journal of Materials Science & Technology 2011, 27, 841–845. [Google Scholar]
- Pei, H.; Wen, Z.; Zhang, Y.; Yue, Z. Oxidation behavior and mechanism of a Ni-based single crystal superalloy with single α-Al2O3 film at 1000° C. Applied Surface Science 2017, 411, 124–135. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Lei, S.; Yuan, W.; Pei, Y.; Zhang, X. Effects of Ta and Y additions on the high temperature oxidation mechanisms of Ni–10Al alloy at 1100° C. Vacuum 2023, 112074. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, S.-M.; Yoo, Y.-S.; Jeong, H.-W.; Jang, H. Effects of Al and Ta on the high temperature oxidation of Ni-based superalloys. Corrosion Science 2015, 90, 305–312. [Google Scholar] [CrossRef]
- Wu, R.; Kawagishi, K.; Harada, H.; Reed, R. The retention of thermal barrier coating systems on single-crystal superalloys: effects of substrate composition. Acta Materialia 2008, 56, 3622–3629. [Google Scholar] [CrossRef]
- Yun, D.W.; Seo, S.M.; Jeong, H.W.; Yoo, Y.S. Effect of refractory elements and Al on the high temperature oxidation of Ni-base superalloys and modelling of their oxidation resistance. Journal of Alloys and Compounds 2017, 710, 8–19. [Google Scholar] [CrossRef]
- Sato, A.; Chiu, Y.-L.; Reed, R. Oxidation of nickel-based single-crystal superalloys for industrial gas turbine applications. Acta Materialia 2011, 59, 225–240. [Google Scholar] [CrossRef]
- Jiang, D.; Tian, Y.; Zhu, Y.; Huang, A. Investigation of surface roughness post-processing of additively manufactured nickel-based superalloy Hastelloy X using electropolishing. Surface and Coatings Technology 2022, 441, 128529. [Google Scholar] [CrossRef]
- Paknahad, H.; Nogorani, F.S. Effects of substrate roughness on the surface morphology and corrosion properties of Fe-and Ni-aluminide coatings on martensitic stainless steel. Surface and Coatings Technology 2020, 392, 125761. [Google Scholar] [CrossRef]
- Song, P.; Naumenko, D.; Vassen, R.; Singheiser, L.; Quadakkers, W. Effect of oxygen content in NiCoCrAlY bondcoat on the lifetimes of EB-PVD and APS thermal barrier coatings. Surface and Coatings Technology 2013, 221, 207–213. [Google Scholar] [CrossRef]
- Cheng, C.-Q.; Hu, Y.-B.; Cao, T.-S.; Zhang, L.; Zhu, Y.-W.; Zhao, J. Two typical oxidation models on nickel-based superalloys under different initial surface roughness. Corrosion Science 2020, 176, 108942. [Google Scholar] [CrossRef]
- Yang, S.-J.; Song, W.-J.; Dingwell, D.B.; He, J.; Guo, H.-B. Surface roughness affects metastable non-wetting behavior of silicate melts on thermal barrier coatings. Rare Metals 2022, 41, 469–481. [Google Scholar] [CrossRef]
- Pei, H.; Wen, Z.; Li, Z.; Zhang, Y.; Yue, Z. Influence of surface roughness on the oxidation behavior of a Ni-4.0 Cr-5.7 Al single crystal superalloy. Applied Surface Science 2018, 440, 790–803. [Google Scholar] [CrossRef]
- Pfennig, A.; Fedelich, B. Oxidation of single crystal PWA 1483 at 950° C in flowing air. Corrosion science 2008, 50, 2484–2492. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, C.; Zhang, L.; Cao, T.; Guo, G.; Meng, X.; Zhao, J. Microstructural evolution of oxidation film on a single crystal nickel-based superalloy at 980° C. Oxidation of Metals 2018, 89, 303–317. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, L.; Li, Z.; Sun, F.; Dong, X.; Shan, A. The effect of surface machining on the high-temperature oxidation of a single crystal Ni-based superalloy. Materials transactions 2014, 55, 1540–1546. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, W.-G.; Li, X.-W.; Dong, J.-S.; Zheng, W.; Feng, H.; Lou, L.-H. Effect of surface roughness on the oxidation behavior of a directionally solidified Ni-based superalloy at 1,100 C. Acta Metallurgica Sinica (English Letters) 2015, 28, 381–385. [Google Scholar] [CrossRef]
- Sheng, N.; Horke, K.; Meyer, A.; Gotterbarm, M.R.; Rettig, R.; Singer, R.F. Surface recrystallization and its effect on oxidation of superalloy C263. Corrosion Science 2017, 128, 186–197. [Google Scholar] [CrossRef]
- Montero, X.; Ishida, A.; Meißner, T.; Murakami, H.; Galetz, M. Effect of surface treatment and crystal orientation on hot corrosion of a Ni-based single-crystal superalloy. Corrosion Science 2020, 166, 108472. [Google Scholar] [CrossRef]
- Cruchley, S.; Taylor, M.; Ding, R.; Evans, H.; Child, D.; Hardy, M. Comparison of chromia growth kinetics in a Ni-based superalloy, with and without shot-peening. Corrosion Science 2015, 100, 242–252. [Google Scholar] [CrossRef]
- Dong, C.; Shang, M.; Ma, H.; Wang, Y.; Ma, H. Effect of substrate surface roughness on interfacial reaction at Sn-3.0 Ag/(001) Cu interface. Vacuum 2022, 197, 110816. [Google Scholar] [CrossRef]
- Ha, Y.; Baeg, J.-H.; Park, S.; Cho, Y.-R. Effect of substrate roughness and film thickness on the magnetic properties of CoFeB films on polymer substrate. Vacuum 2021, 191, 110399. [Google Scholar] [CrossRef]
- Ju, J.; Shen, Z.; Kang, M.; Zhang, J.; Wang, J. On the preferential grain boundary oxidation of a Ni-Co-based superalloy. Corrosion Science 2022, 199, 110203. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Tavangar, R.; Akhtari, F.; Hejazi, S. High-temperature oxidation behavior of GTD-111 Ni-based superalloy with an ultrafine-grained surface at 900 C. Corrosion Science 2023, 212, 110935. [Google Scholar] [CrossRef]
- Kupka, M.; Marut, J.; Aniołek, K.; Barylski, A. The effect of oxide scale roughness on the plasticity of iron aluminide alloy. Vacuum 2016, 132, 111–118. [Google Scholar] [CrossRef]
- Ali, S.; Lü, J.; Song, P.; Li, C.; Ali, R.; Lu, J. Effect of external pressure on β-NiAl phase transformation of Co-base alloy at 1323 K. Materials Research Express 2019, 6, 1265b2. [Google Scholar] [CrossRef]
- De Heer, J. The principle of le chatelier and braun. Journal of Chemical Education 1957, 34, 375. [Google Scholar] [CrossRef]
- Yu, H.; Ukai, S.; Hayashi, S.; Oono, N. Effect of Al content on the high-temperature oxidation of Co-20Cr-(5, 10) Al oxide dispersion strengthened superalloys. Corrosion Science 2017, 118, 49–59. [Google Scholar] [CrossRef]
| Element (wt. %) | Ni | Co | Cr | Al | Y | Hf |
| Sample 1 | 28 | 37.1 | 24 | 10 | 0.6 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





