1. Introduction
Corneal pathologies such as keratoconus, stromal dystrophies or scarring present indications for corneal transplantation. Penetrating keratoplasty (pKP) replaces all corneal layers including the endothelium and oftentimes remains the only therapeutic option for deep central traumatic scars, dystrophies with full-thickness corneal involvement or ulcerations up to perforation [
1,
2]. However, if the disease is confined to the epithelium and/or stromal corneal layers, lamellar keratoplasty can be applied. Deep Anterior Lamellar Keratoplasty (DALK) is a surgical technique that preserves the host Descemet’s Membrane (DM) and endothelial layer when both are healthy and intact. The big-bubble technique, as first described by Anwar et al. in 2002 [
3], is the most commonly used and likely most standardized technique.
DALK has been shown to be associated with significantly less endothelial graft rejection compared to pKP [
4,
5,
6]. Secondary glaucoma has been observed less frequently in DALK as compared to pKP [
7]. Unrejected grafts [
5] following DALK have exhibited comparable graft clarity and visual acuity outcome [
8,
9] in comparison to pKP. However, the crucial step in DALK is precise air injection at a sufficient depth, with research suggesting successful bubble formation at a depth traversing ≥80% of the corneal stromal thickness [
10,
11,
12], while depths greater than 90% are believed to be beneficial. Naturally, these are associated with greater risk of DM perforation and make DALK a technically demanding procedure with a relatively high failure rate. Intraoperative conversion to pKP is necessary in such cases [
5,
7,
13]. Conversion to pKP is reportedly ranging between 4 and 39,2% in big-bubble DALK procedures [
14,
15,
16]. (One more recent study reported a relatively high conversion rate of 42,5% [
17]).
Two key parameters can be changed in order to facilitate cannula insertion to sufficient depth. Firstly, depth perception throughout the procedure can be enabled via Optical Coherence Tomography (OCT); secondly, accuracy can be improved by utilizing robotic assistance to manage tool-tip oscillation, which can occur due to physiological hand tremor.
In several studies, intraoperative OCT has been used for cannula monitoring throughout the big-bubble DALK procedure, allowing for higher depth perception accuracy [
18,
19]. Izatt et al. examined the combination of intraoperative OCT and robotic assistance during big-bubble DALK cannula insertion in two recent studies [
20,
21]. Insertions were performed by inexperienced surgeons on ex-vivo human corneas. It was found that the use of a cooperative system allowed for a 31% increase in reached depth compared to manual trials. In addition, perforation rates in the cooperative trials were lower compared to manual trials without image guidance.
In this work, the feasibility and implications of using a surgical robot for the cannula insertion phase of DALK are studied. To achieve this, a custom-built robot in a master-slave setup is utilized. Simulated DALK procedures are performed on ex-vivo porcine eyes using surgical microscopes equipped with intraoperative continuous OCT capture capabilities. As opposed to the setup used by Draelos et al. [
20], intraoperative OCT is implemented throughout both manual and robotic trials, enabling the surgeons to intraoperatively assess cannula depth. Furthermore, each cannula insertion is followed by air injection to immediately evaluate the airtightness of the formed tunnel and demarcation of the achieved injection plane by OCT. While the approach by Draelos focuses on the reached depth without perforation by performing up to 8 insertions into each cornea, this work aims to evaluate successful depth achievement of the demarcated plane at over80% of the stromal thickness. Quantitative measures such as the number of perforations and the reached stromal depth are assessed for comparison.
2. Materials and Methods
2.1. Microscope and Intraoperative OCT
A standard ophthalmic surgery microscope equipped with an OCT engine (LUMERA 700 with RESCAN 700, Carl Zeiss Meditec) is used for all experiments. Two OCT B-scans in a cross formation are continuously captured by the microscope at a desired location controlled by the surgeon using a foot control pedal or by the assistant using the auxiliary screen. The surgical field is directly observed through the microscope’s eyepiece with the two B-scans and an OCT acquisition location marker overlaid onto the scene.
2.2. Robot
A custom-made hybrid parallel-serial robot with prismatic piezo actuators is used for the robotic trials (
Figure 1). The robot has five degrees of freedom and is controlled in a master-slave fashion using a 3D mouse in this experiments. The syringe is mounted on the last joint of the robot. Operation of the robot is internally handled through optimized collective movements of joints.
2.3. Instruments
A rubber base imitating the orbit was used to secure porcine eyes throughout the procedure. Pins were used to fixate eyes by the lateral conjunctive tissue onto the base to enable passive movements simulating elevation and depression along the transversal axis. For insertion and injection, 1ml syringes were tipped with sharp disposable 30G cannulas of 12 mm length.
2.4. Surgical Technique
Big-bubble pneumodissection technique in DALK aimed for posterior stroma detachment by air injection at a sufficient depth of at least 80%, causing an air bubble to form between DM and posterior stroma [
11]. If the air bubble formation succeeded, access to and removal of stroma located anterior to DM was facilitated. Typically, the procedure started with the insertion of a thin 27G or 30G straight or bent cannula into the host cornea with its beveled side facing towards the DM. Traversing the cornea, the cannula produced an airtight tunnel. At the desired depth, air was injected into the deep corneal stroma leading to detachment of posterior stroma from DM and endothelium. The defective posterior stroma was then removed and replaced with a donor graft [
3,
22].
2.5. Method
Two novice surgeons with no prior experience in DALK were trained to perform the procedure by receiving verbal instructions and supervision from an experienced ophthalmic surgeon. Thereafter, each surgeon performs manual and robot-assisted DALK practiced trials on ex-vivo porcine corneas. The main big-bubble DALK cannula insertion followed by air injection experiments were simulated on ex-vivo porcine eyes as well. Although the actual detachment of the stroma from DM in these specimens was not reproducible, a porcine model was chosen for two reasons. Firstly, parameters ensuring successful bubble formation and pneumodissection in DALK could be replicated reliably in porcine models. Secondly, a large sample size could be gathered without potential waste of human donor material.
Operators were randomly assigned to trial order and setting; the other operator respectively assumes the assistant role. For each manual and robot-assisted trial, a 1-mL syringe filled with air was tipped with a disposable sharp 12 mm long 30G cannula. In half of the trials, the air-filled syringe was held and guided manually by the surgeon. In this setting, the cannula was bent approximately at the base at an angel of about 60 degrees for better ergonomics corresponding to the state of the art [
22]. In the other half of the trials, the air-filled tipped syringe was mounted onto the robot which was then remotely controlled by the surgeon. In this setting, bending of the 30G cannula was omitted since no effect on ergonomics could be achieved.
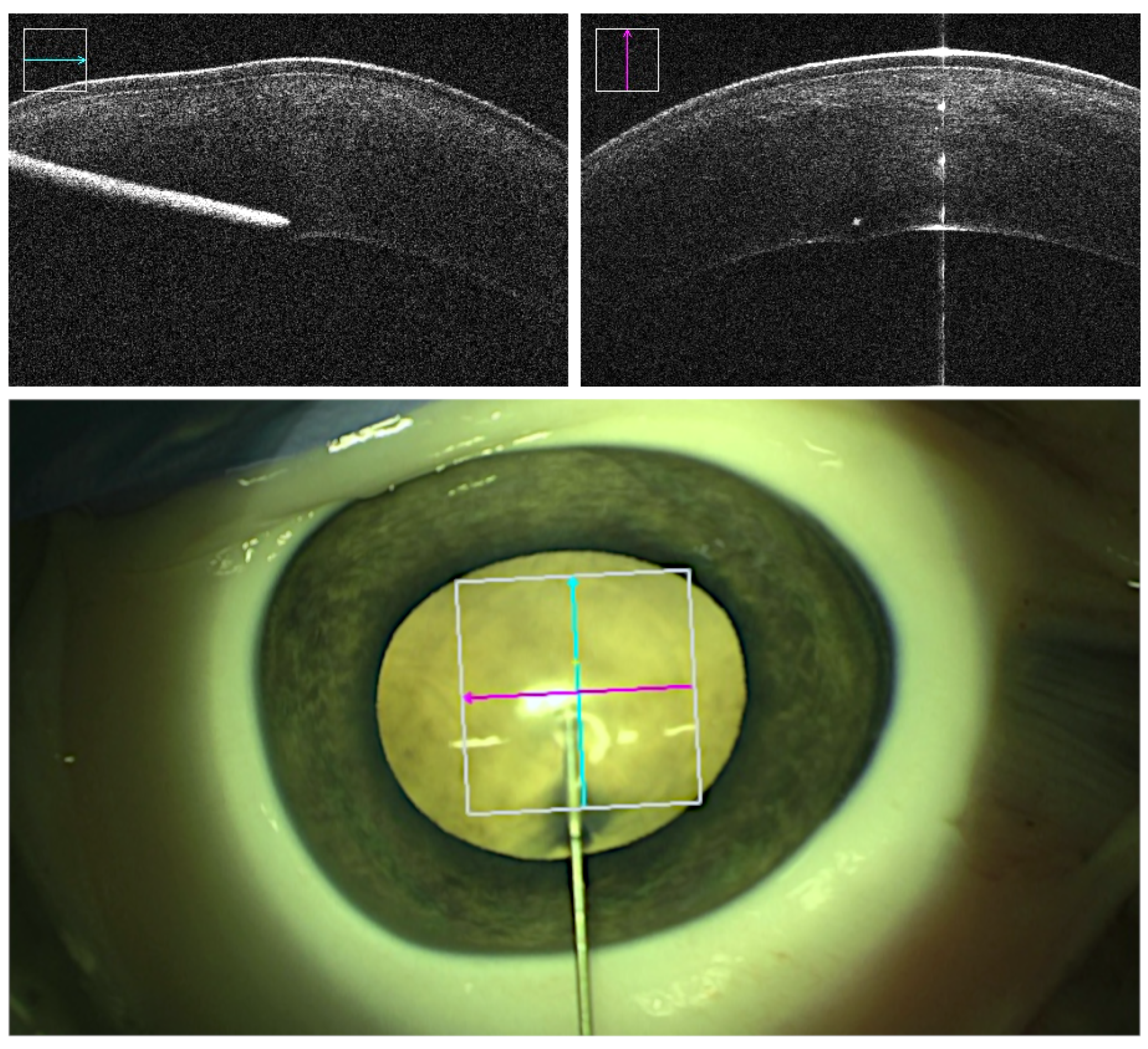
The point of entrance within the cornea was chosen close to the limbus to ensure sufficient tunnel lengths. In robot-assisted trials, the robot with the syringe mounted onto it was positioned to a distance of around 2 cm of the intended entrance point. Intraoperative OCT was enabled and focused by the assistant so both the corneal stroma and the cannula were displayed in cross and longitudinal sections. The cannula was then aligned following the intended insertion angle relative to the corneal central surface. The alignment was realized by remote-controlled fine adjustments continuously done by the assistant. Insertion was executed aiming for an estimated depth of 90% or greater while avoiding perforation into the DM (
Figure 2). Depth information was obtained from projected real-time intraoperative OCT images and, on inquiry, confirmed or reevaluated by the assistant. At the desired depth, air injection was performed, and demarcation plane was observed and recorded.
2.6. Parameters
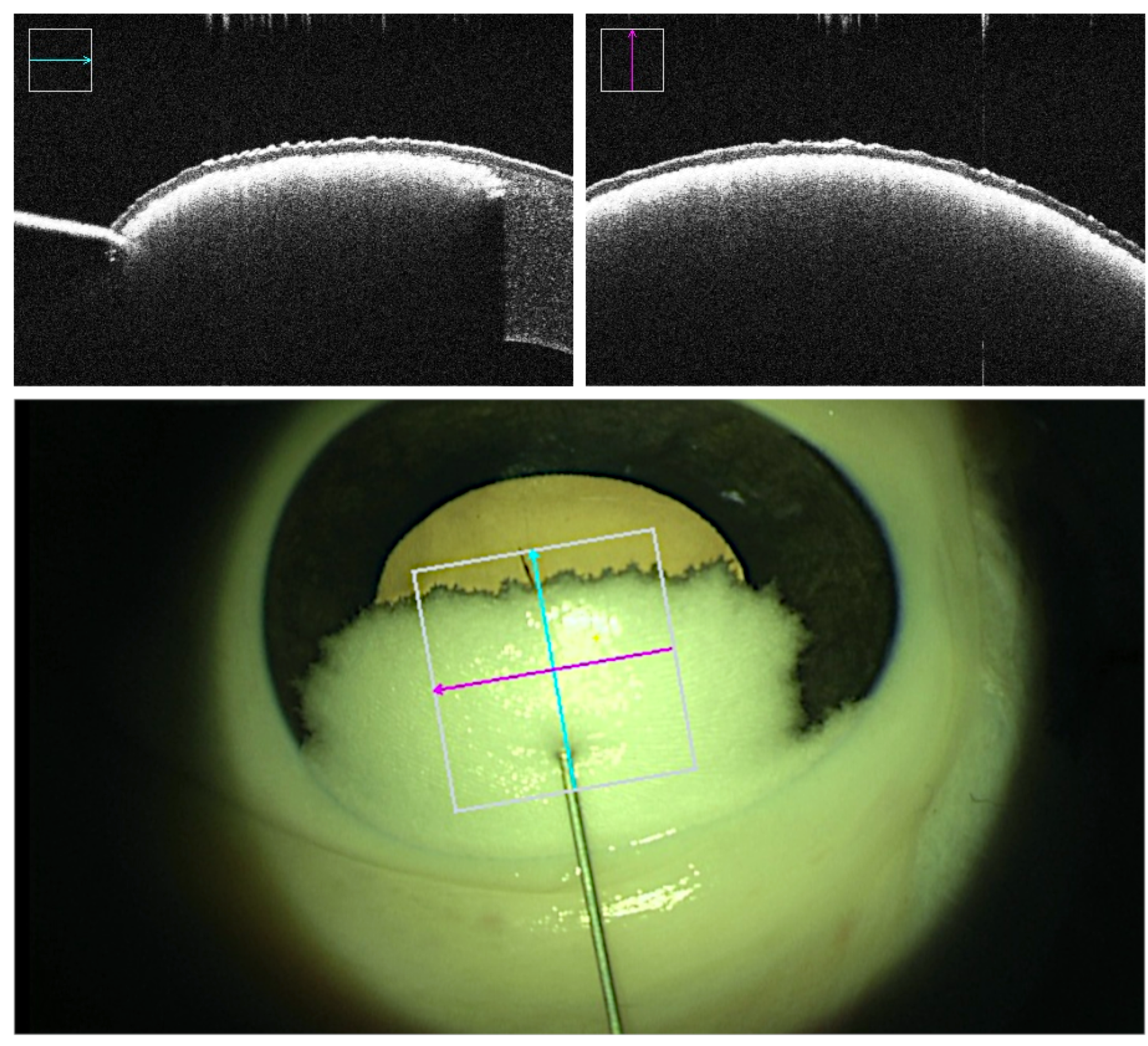
Real-time intraoperative OCT and microscopic visual field was recorded throughout all trials. 3D OCT volumes of the cannula tip and the corneal stroma were captured at multiple milestones: prior to cannula insertion, prior to air injection at the desired depth, post air injection displaying demarcation plane representative of potential successful bubble formation, and post cannula retrieval displaying the caused tunnel and the final outcome. Success or failure was determined based on the outcome of the injection. Failed trials consist of unsuccessful demarcation plane due to air leakage, formation of superficial small bubbles rather than a demarcation line and perforations in which the cannula tip punctures the DM at any point in time along the procedure. Failed big-bubble formation was indicated by the observation of small air bubbles throughout the entire stromal layer [
23,
24] instead of a homogenous demarcation layer. Superficial intrastromal air was reflecting the OCT rays and thus appeared as a cloudy layer directly below the corneal epithelium (
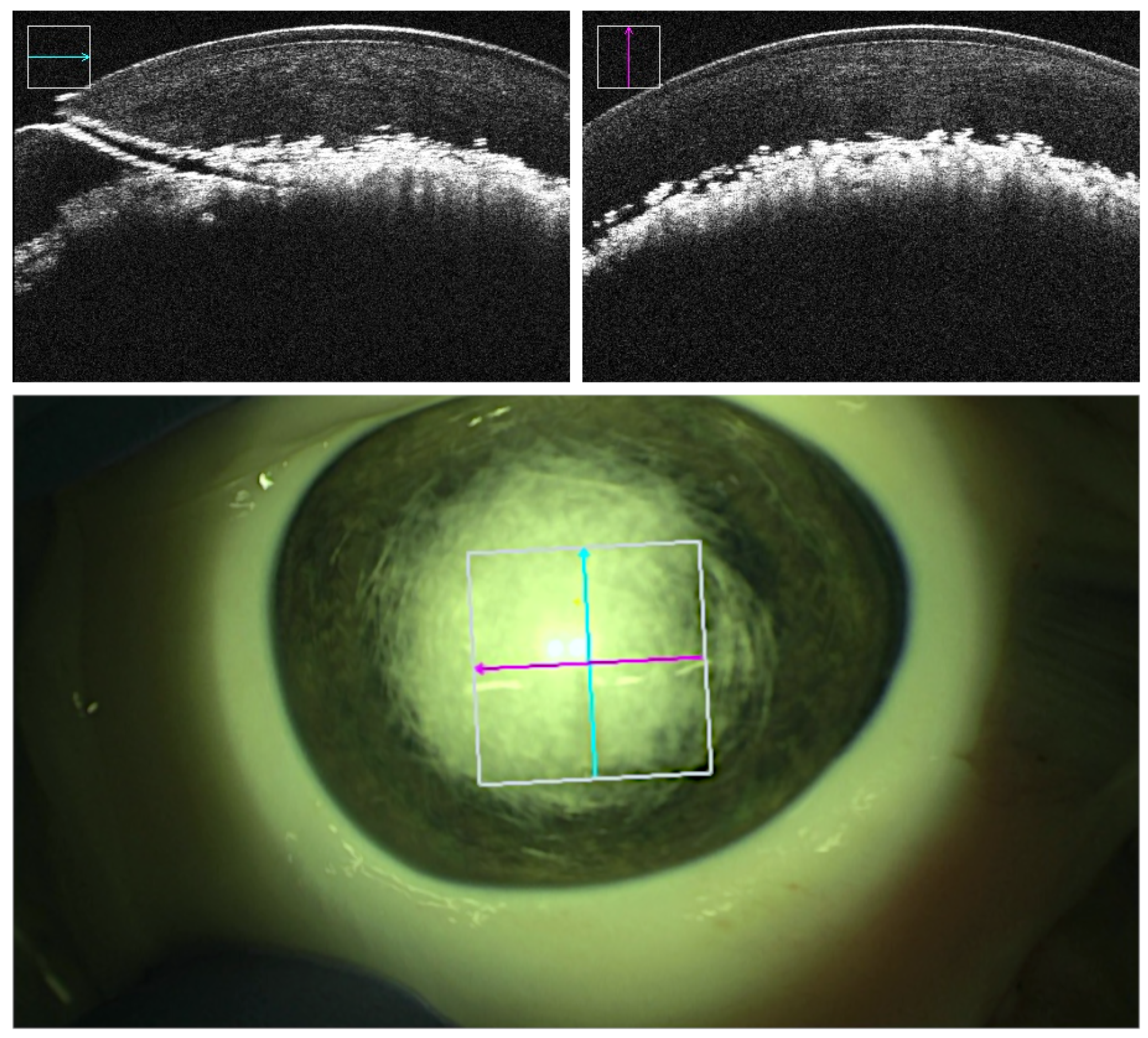
Figure 3). In supposedly successful bubble formation however, the injected air caused a sharp homogenous demarcation layer right above the DM without penetrating more superficially located stromal layers (
Figure 4). Additionally, the insertion angle, tunnel length and reached depth were measured. The insertion angle referred to the angle comprising the longitudinal section of the cannula and corneal central surface prior to insertion. It was calculated by analysing the volumetric OCT captures immediately before insertion. The tunnel length referred to the length of the tunnel produced by the inserted cannula and was calculated by analysis of the volumetric OCT captures immediately before injection. The reached depth was calculated via analysis of volumetric OCT captures immediately before insertion and immediately after cannula retraction in successful trials. It corresponded to the deepest the needle tip which has been in the cornea during the procedure and was reported in percentage of corneal stroma traversed by the cannula tip (
Table 1).
3. Results
DALK cannula insertion was simulated on 48 ex-vivo porcine eyes obtained from healthy rearing pigs. The corneal thickness of each eye was measured using OCT. The mean observed corneal thickness was 998 micrometers with a standard deviation of 105 micrometers. Due to low intraocular pressure in two eyes, only 46 trials, 23 manual and 23 robot-assisted, were included in the evaluation. Success and failure rates could be obtained from
Table 1. Although in robotic trials, less perforation occurred (1 versus 3 cases in manual trials), failure due to unsuccessful generation of a sharp, sufficiently deep demarcation layer was present in both settings. Both the differences in perforation rates as well as in success rates in robotic and manual trials were not significant (two-tail t-test with significance of p < 0.01).
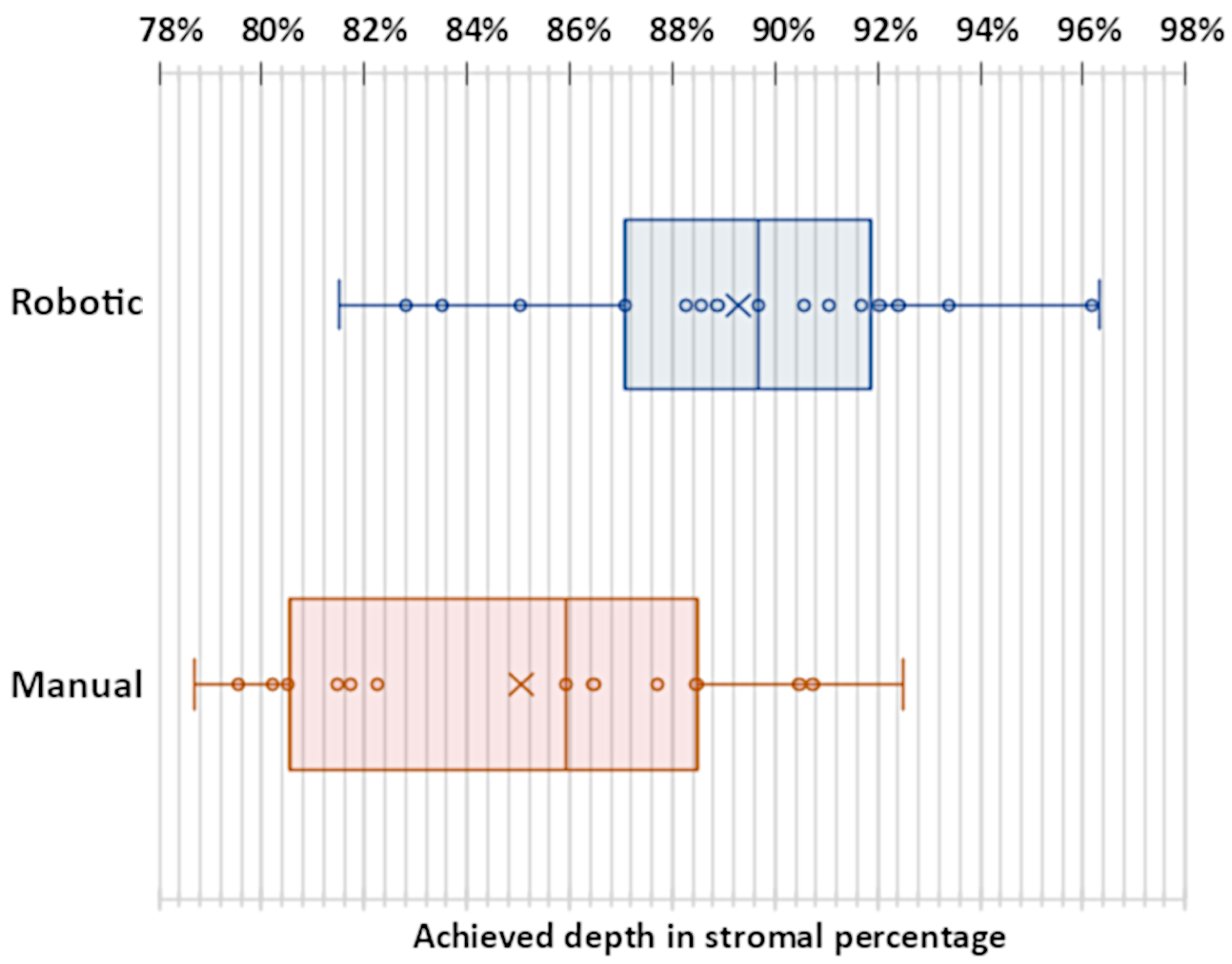
The mean tunnel length in all successful trials was 1.76 mm with a standard deviation of 0.38 mm and did only differ slightly in robotic (mean 1.72 mm, standard deviation 0.45 mm) and manual (mean 1.82 mm, standard deviation 0.27 mm) trials. The mean reached depth in successful cases was greater in robotic trials (89.29% of the cornea with a standard deviation of 4.01%) than in manual trials (85.05% of the cornea with a standard deviation of 4.52%). This difference was significant (two-tail t-test with significance of p < 0.01). The reached depths were plotted in
Figure 5. In all cases in which no perforation occurred, an average volume of 0.24 mL of air was injected. The mean duration of surgery in successful cases performed with robot assistance was 261 seconds with a standard deviation of 96 seconds which was considerably longer than manual surgery with 212 seconds with a standard deviation of 96 seconds. Surgical time did not include robot preparations and calibrations.
4. Discussion
This work showed that novice surgeons with no prior experience in performing DALK, were capable of generating an airtight tunnel in the porcine cornea under the given conditions, resulting in the successful generation of a sharp, deep stromal demarcation plane representing sufficient depth reached for big-bubble generation in most cases. The combination of intraoperative OCT and robotic assistance in this study resulted in a significant increase in the depth of achieved detachment in non-perforated cases, comprising a mean of 89% as opposed to 85% of the cornea in manual trials. As a reached depth of greater than 79% has been reported to likely result in successful bubble formation, the mean depths reached in this study both in manual and robotic trials are to be considered sufficient to assume a high probability of full pneumo-dissection in human eyes. Pasricha et al. [
11] reported thatdepths surpassing 90% are not conditional for successful pneumo-dissection. However, depths up to a maximum of 96% are reached in robotic trials without perforation. It was conceivable that incorporating robotic assistance in DALK or similar ophthalmic procedures may be beneficial by increasing the accuracy without compromising safety, although at the cost of extended surgery duration.
The mean reached depth in the robot-assisted setting in the performed experiments was greater (89%) than the average depth reported in an equivalent study (71%) conducted by Draelos et al. As in their study where air injection did not follow the cannula insertion, it cannot be assessed whether perforation would have occurred during or after the injection. It was important to note that the mean depth measured in this study only comprises of cases in which the air injection was successfully evaluated by the analysis of OCT scans captured after air injection. By doing so, the tunnel could be proven to be airtight indicating a precise advancement of the cannula. However, as can be observed in
Figure 4, DM detachment was not visible despite of successful bubble formation, which concurs with previous observation in porcine models. Tunnel lengths were assessed after injection showing a mean of 1.76mm in successful trials, not differing significantly from tunnel lengths in failed cases with a mean of 1.88mm. However, the sample size of failed cases was too small to study potential differences in tunnel lengths. To our knowledge, the tunnel length in DALK has not yet been investigated, making it a topic of interest for further research.
It was essential to consider the following aspects regarding the validity of the study. Firstly, ex-vivo porcine eyes instead of human eyes are used. It is known that the constitution and properties of the porcine cornea do not fully resemble those of the human cornea. Moreover, it is impossible to generate a true bubble formation in these specimens. Therefore, repeating this successful ex-vivo proof-of-concept with human corneas [
25], similar to the setting of the work by Draelos et al. is favorable. Secondly, as indicated by previous studies, the usage of intraoperative OCT provided a fairly accurate estimation of the cannula tip location during any point in the procedure. Inexperienced surgeons have been demonstrated to successfully perform simulated DALK guided by intraoperative OCT [
26]. Successful cannula insertion could likely resulted from precise visualization provided by the intraoperative OCT feedback [
20]. However, improved tremor management and ergonomics may follow from employing surgical robots.
5. Conclusions
In summary, the application of robotic assistance allows for significantly greater depth achievement in DALK without compromising safety. The study of this work suggests that in order to reliably reach a sufficient depth of at least 80% of the cornea, intraoperative OCT along with robotic assistance is feasible and beneficial especially for novice or inexperienced surgeons, albeit being slightly more time-consuming. Further potential applications of the designed robot and its proven increased precision such as assisting in subretinal injections are interesting and evolving fields for investigation.
Author Contributions
Conceptualization, M.A.N. and Y.Z.; methodology, M.A.N. and Y.Z.; validation, M.A.N., Y.Z., A.J., N.A.M., H.R., A.E., M.M. and D.Z.; formal analysis, M.A.N., Y.Z., A.J., N.A.M., M.M. and D.Z.; investigation, M.A.N. and Y.Z.; resources, M.A.N.; data curation, H.R. and A.E.; writing—original draft preparation, Y.Z.; writing—review and editing, M.A.N.; visualization, M.A.N. and Y.Z.; supervision, M.A.N. and D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the state of the Bavaria.
Data Availability Statement
No applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krysik, K.; Wroblewska-Czajka, E.; Lyssek-Boron, A.; Wylegala, E.A.; Dobrowolski, D. Total penetrating keratoplasty: indications, therapeutic approach, and long-term follow-up. Journal of ophthalmology 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Krysik, K.; Dobrowolski, D.; Lyssek-Boron, A.; Jankowska-Szmul, J.; Wylegala, E.A. Differences in surgical management of corneal perforations, measured over six years. Journal of ophthalmology 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Teichmann, K.D. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. Journal of Cataract & Refractive Surgery 2002, 28, 398–403. [Google Scholar]
- Feizi, S.; Javadi, M.A.; Jafarinasab, M.R.; Karimian, F. Penetrating keratoplasty versus lamellar keratoplasty for mustard gas–induced keratitis. Cornea 2013, 32, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.C.; Murthy, S.I.; Vaddavalli, P.K.; Garg, P.; Ramappa, M.; Chaurasia, S.; Rathi, V.; Sangwan, V.S. Clinical outcomes and risk factors for graft failure after deep anterior lamellar keratoplasty and penetrating keratoplasty for macular corneal dystrophy. Cornea 2015, 34, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.L.; Ramsay, A.; Dart, J.K.; Bunce, C.; Craig, E. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology 2004, 111, 1676–1682. [Google Scholar] [CrossRef]
- Gadhvi, K.A.; Romano, V.; Cueto, L.F.V.; Aiello, F.; Day, A.C.; Allan, B.D. Deep anterior lamellar keratoplasty for keratoconus: multisurgeon results. American Journal of Ophthalmology 2019, 201, 54–62. [Google Scholar] [CrossRef]
- Javadi, M.A.; Feizi, S.; Yazdani, S.; Mirbabaee, F. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for keratoconus: a clinical trial. Cornea 2010, 29, 365–371. [Google Scholar] [CrossRef]
- Razmju, H.; Shams, M.; Abtahi, M.A.; Abtahi, S.H. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus: A clinical trial study. Journal of Isfahan Medical School 2011, 29, 798–803. [Google Scholar]
- Scorcia, V.; Busin, M.; Lucisano, A.; Beltz, J.; Carta, A.; Scorcia, G. Anterior segment optical coherence tomography–guided big-bubble technique. Ophthalmology 2013, 120, 471–476. [Google Scholar] [CrossRef]
- Pasricha, N.D.; Shieh, C.; Carrasco-Zevallos, O.M.; Keller, B.; Cunefare, D.; Mehta, J.S.; Farsiu, S.; Izatt, J.A.; Toth, C.A.; Kuo, A.N. Needle depth and big bubble success in deep anterior lamellar keratoplasty: An ex vivo microscope-integrated OCT study. Cornea 2016, 35, 1471. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, R.C.; Bogoni, A.; Ghanem, V.C. Pachymetry-guided intrastromal air injection (“pachy-bubble”) for deep anterior lamellar keratoplasty: results of the first 110 cases. Cornea 2015, 34, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Javadi, M.A.; Jamali, H.; Mirbabaee, F. Deep anterior lamellar keratoplasty in patients with keratoconus: big-bubble technique. Cornea 2010, 29, 177–182. [Google Scholar] [CrossRef]
- Fogla, R.; Padmanabhan, P. Results of deep lamellar keratoplasty using the big-bubble technique in patients with keratoconus. American journal of ophthalmology 2006, 141, 254–259. [Google Scholar] [CrossRef]
- Al-Torbak, A.A.; Al-Motowa, S.; Al-Assiri, A.; Al-Kharashi, S.; Al-Shahwan, S.; Al-Mezaine, H.; Teichmann, K. Deep anterior lamellar keratoplasty for keratoconus. Cornea 2006, 25, 408–412. [Google Scholar]
- Michieletto, P.; Balestrazzi, A.; Balestrazzi, A.; Mazzotta, C.; Occhipinti, I.; Rossi, T. Factors predicting unsuccessful big bubble deep lamellar anterior keratoplasty. Ophthalmologica 2006, 220, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Koçluk, Y.; Sukgen, E.A.; Burcu, A. Comparison of outcomes in patients who underwent deep anterior lamellar keratoplasty and those converted to penetrating keratoplasty. Turkish journal of ophthalmology 2017, 47, 63. [Google Scholar] [CrossRef]
- Tao, Y.K.; LaBarbera, M.; Ehlers, J.P.; Srivastava, S.K.; Dupps Jr, W.J. Image-guided modified deep anterior lamellar keratoplasty (DALK) corneal transplant using intraoperative optical coherence tomography. In Proceedings of the Ophthalmic Technologies XXV. SPIE; 2015; Volume 9307, pp. 11–14. [Google Scholar]
- Roodaki, H.; di San Filippo, C.A.; Zapp, D.; Navab, N.; Eslami, A. A surgical guidance system for big-bubble deep anterior lamellar keratoplasty. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2016: 19th International Conference, Athens, Greece, 17–21 October 201; Proceedings, Part I 19. Springer, 2016; pp. 378–385. [Google Scholar]
- Draelos, M.; Keller, B.; Tang, G.; Kuo, A.; Hauser, K.; Izatt, J. Real-time image-guided cooperative robotic assist device for deep anterior lamellar keratoplasty. In Proceedings of the 2018 IEEE international conference on robotics and automation (ICRA). IEEE; 2018; pp. 4013–4018. [Google Scholar]
- Draelos, M.; Tang, G.; Keller, B.; Kuo, A.; Hauser, K.; Izatt, J.A. Optical coherence tomography guided robotic needle insertion for deep anterior lamellar keratoplasty. IEEE Transactions on Biomedical Engineering 2019, 67, 2073–2083. [Google Scholar] [CrossRef]
- Nanavaty, M.A.; Vijjan, K.S.; Yvon, C. Deep anterior lamellar keratoplasty: A surgeon’s guide. Journal of current ophthalmology 2018, 30, 297–310. [Google Scholar] [CrossRef]
- Steven, P.; Le Blanc, C.; Lankenau, E.; Krug, M.; Oelckers, S.; Heindl, L.M.; Gehlsen, U.; Huettmann, G.; Cursiefen, C. Optimising deep anterior lamellar keratoplasty (DALK) using intraoperative online optical coherence tomography (iOCT). British Journal of Ophthalmology 2014, 98, 900–904. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, H.J.; Tsaousis, K.T.; Tabin, G.C. Salvaging deep anterior lamellar keratoplasty with microbubble incision technique in failed “big bubble” cases: an update study. European Journal of Ophthalmology 2016, 26, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, J.; Huang, K.; Lee, Z.; Lee, X. A comparison of biomechanical properties between human and porcine cornea. Journal of biomechanics 2001, 34, 533–537. [Google Scholar] [CrossRef] [PubMed]
- De Benito-Llopis, L.; Mehta, J.S.; Angunawela, R.I.; Ang, M.; Tan, D.T. Intraoperative anterior segment optical coherence tomography: a novel assessment tool during deep anterior lamellar keratoplasty. American journal of ophthalmology 2014, 157, 334–341. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).