Submitted:
06 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ag-OLA solution
2.3. Synthesis of Cs2CuCl4 NCs
2.4. Synthesis of the Ag passivation reagent
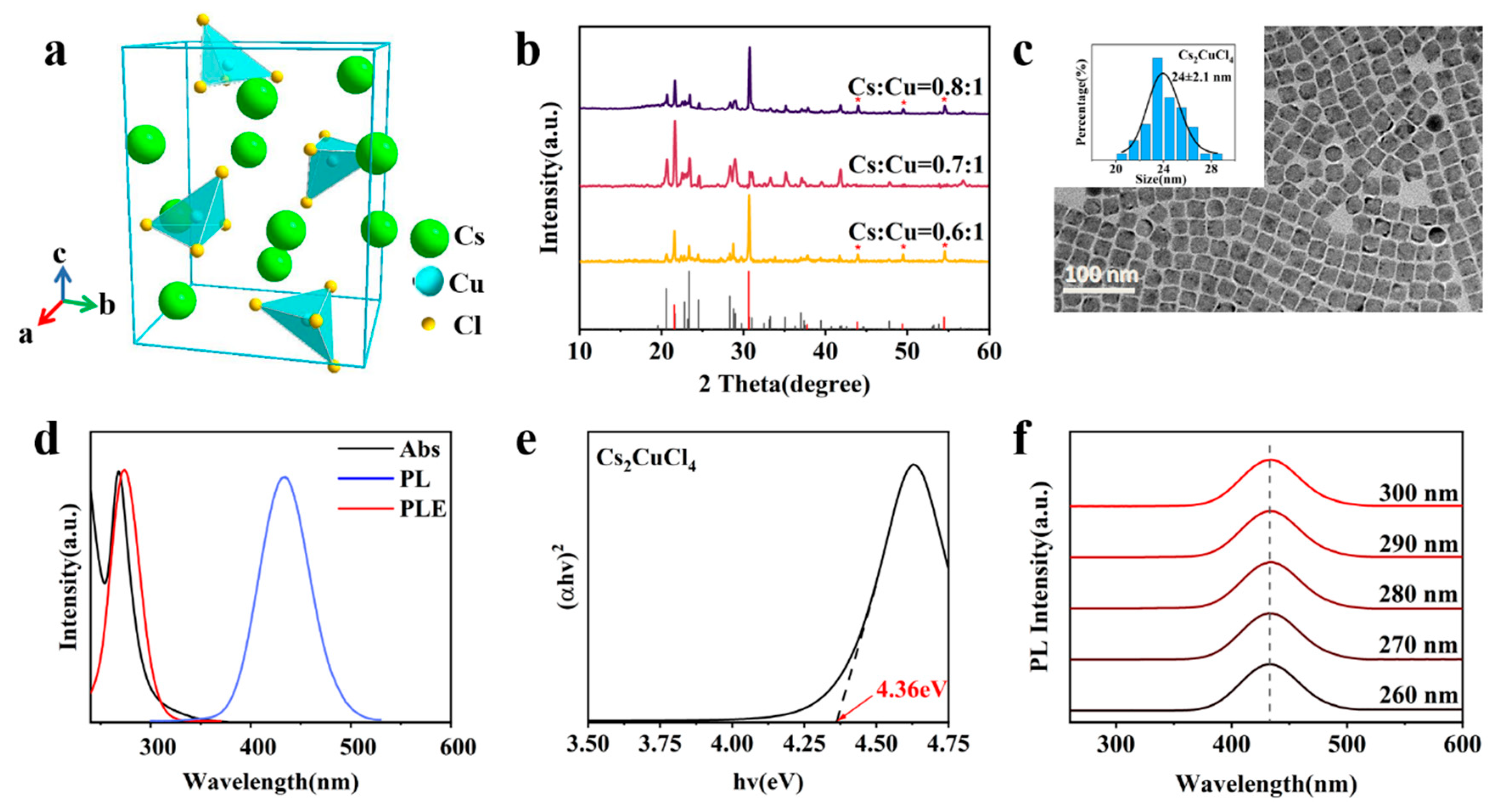
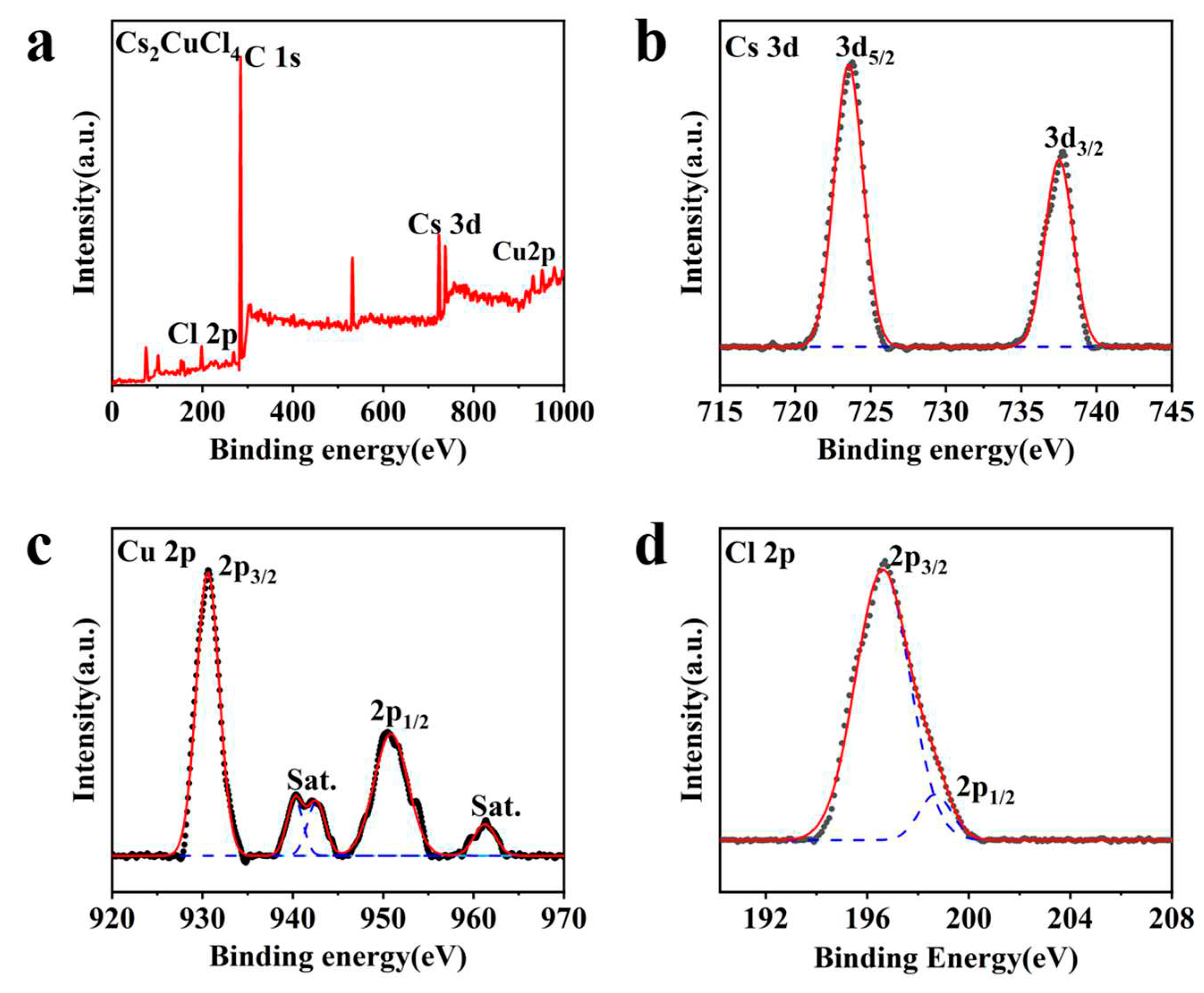
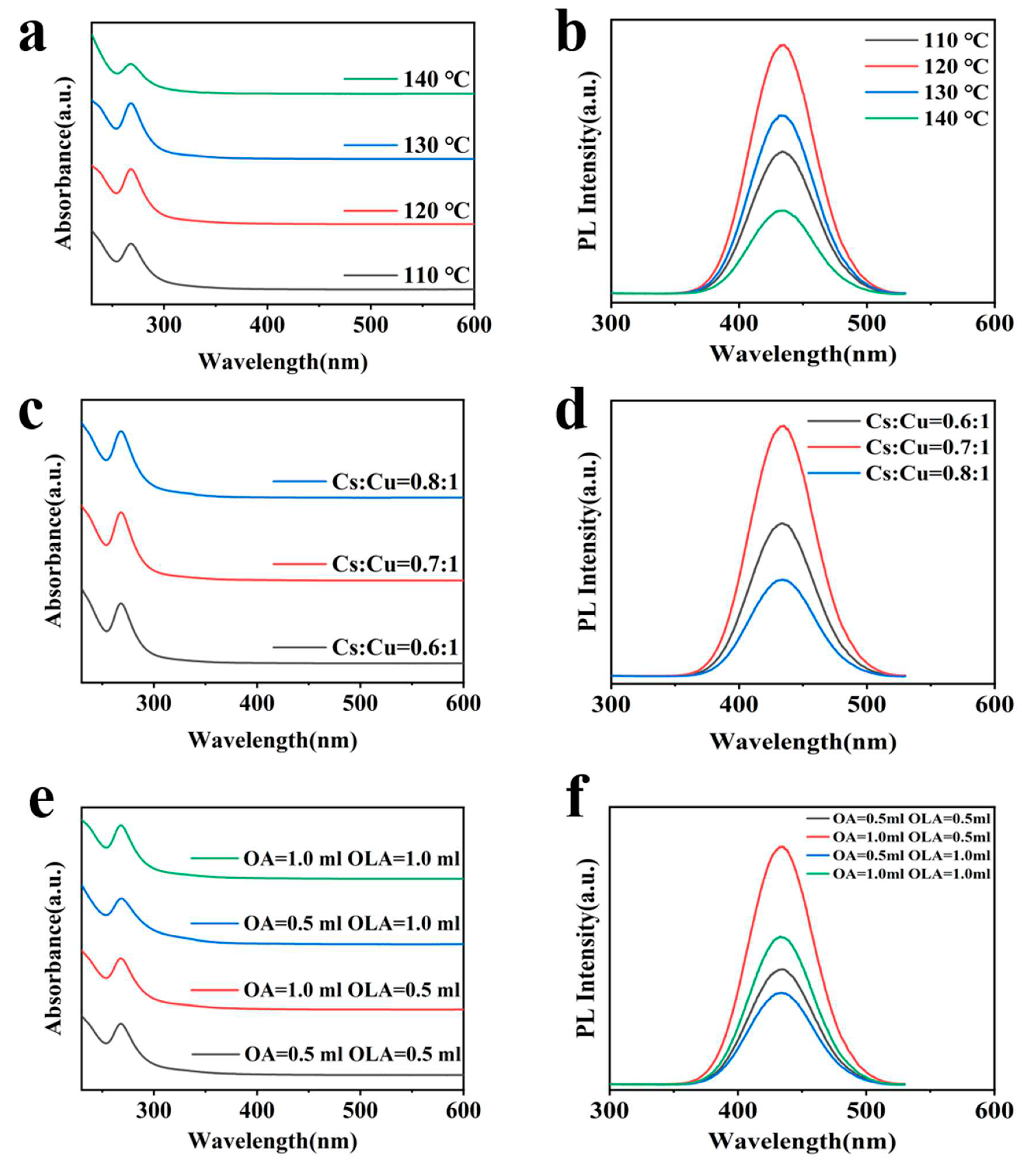
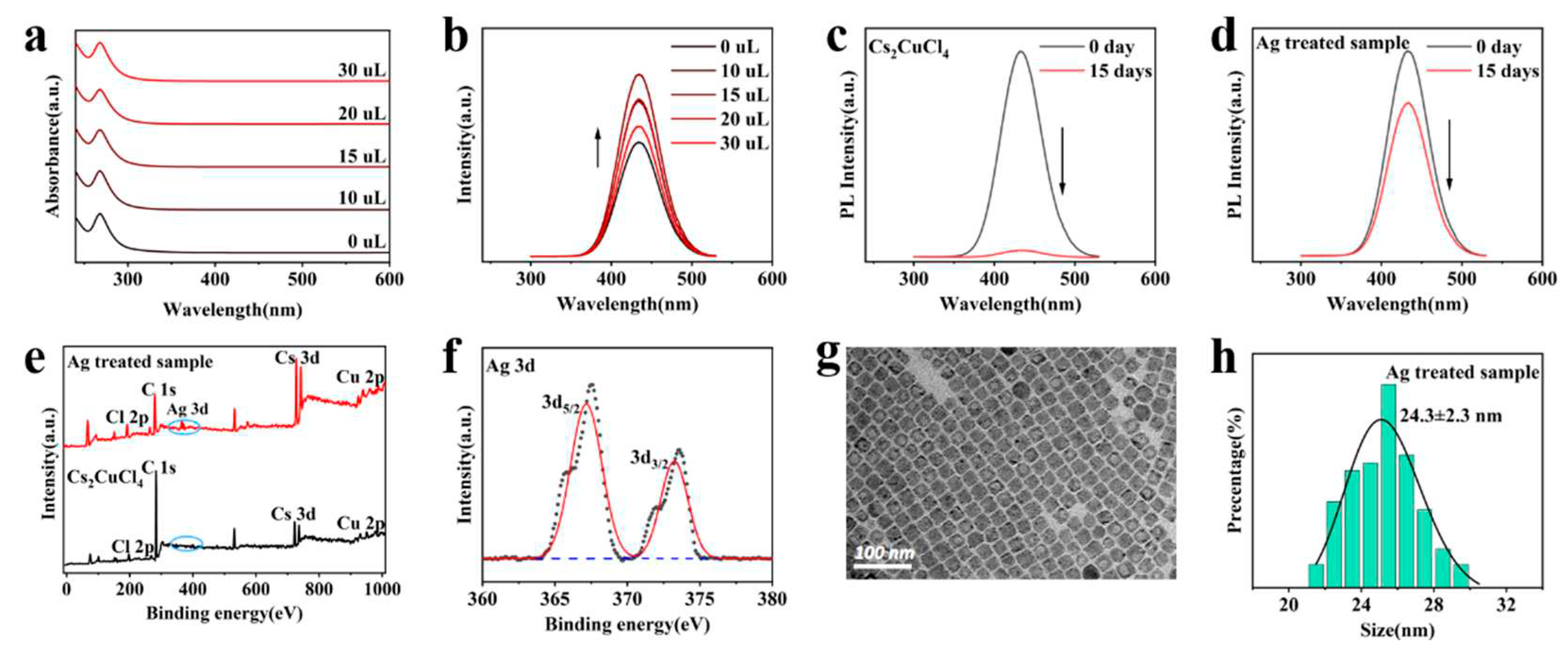
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ling, X.; Zhou, S.; Yuan, J.;Shi, J.; Qian, Y.; Larson, B. W.; Zhao, Q.; Qin, C.; Li, F.; Shi, G.; Stewart, C.; Hu, J.; Zhang, X.; Luther, J. M.; Duhm, S.; Ma, W. 14.1% CsPbI3 Perovskite Quantum Dot Solar Cells via Cesium Cation Passivation. Adv. Energy Mater. 2019, 9, 1900721. [CrossRef]
- Li, C.; Zhou, Z.; Vashishtha, P.; Halpert, J. E. The Future Is Blue (LEDs): Why Chemistry Is the Key to Perovskite Displays. Chem. Mater. 2019, 31, 6003−6032. [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M. V.; Trinh, M. T.; Jin, S.; Zhu, X. Y. Lead Halide Perovskite Nanowire Lasers with Low Lasing Thresholds and High Quality Factors. Nat. Mater. 2015, 14, 636−642. [CrossRef]
- Kim, Y. H.; Kim, S.; Kakekhani, A.; Park, J.; Park, J.; Lee, Y. H.; Xu, H.; Nagane, S.; Wexler, R. B.; Kim, D. H.; Jo,S. H.;Sarti, L. M.; Tan, P.; Sadhamala, A.;Park, G. S.; Kim, Y. W.; Hu, B.; Bolink, H. J.; Yoo, S.; Friend, R. H.; Rappe, A. M., Lee, T. W. Comprehensive defect suppression in perovskite nanocrystals for high-efficiency light-emitting diodes. Nat. Photonics. 2021, 15, 148−155. [CrossRef]
- Zhu, L.; Cao, H.; Xue, C.; Zhang, H.; Qin, M.; Wang, J.; Wen, K.; Fu, Z.; Jiang, T.; Xu, L.; Zhang, Y.; Cao, Y.; Tu, C.; Zhang, J.; Liu, D.; Zhang, G.; Kong, D.; Fan, N.; Li, G.; Yi, C.; Peng, Q.; Chang, J.; Lu, X.; Wang, N.; Huang, W.; Wang, J. Unveiling the additive-assisted oriented growth of perovskite crystallite for high performance light-emitting diodes. Nat. Commun. 2021, 12, 5081. [CrossRef]
- Zhou, Y. H.; Wang, C.; Yuan, S.; Zou, C.; Si, Z.; Wang, K.; Xia, Y.; Wang, B.; Di, D.; Wang, Z. K.; Liao, L.S. J. Am. Chem. Soc. 2022, 144, 18470−18478.
- Zhang, C.; Wan, Q.; Ono, L.K.; Liu, Y.; Zheng, W.; Zhan, Q.; Liu, M.; Kong, L.; Li, L.; Qi, Y.; ACS Energy Lett. 2021, 6, 3545−3554.
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162−7167. [CrossRef]
- Zheng, X.; Yuan, S.; Liu, J.; Yin, J.; Yuan, F.; Shen, W. S.; Yao, K.; Wei, M.; Zhou, C.; Song, K.; Zhang, B. B.; Lin, Y.; Hedhili, M. N.; Wehbe, N.; Han, Y.; Sun, H. T.; Lu, Z. H.; Anthopoulos, T. D.; Mohammed, O. F.; Sargent, E. H.; Liao, L. S.; Bakr, O. M. Chlorine Vacancy Passivation in Mixed Halide Perovskite Quantum Dots by Organic Pseudohalides Enables Efficient Rec. 2020 Blue Light-Emitting Diodes. ACS Energy Lett. 2022, 5, 793−798. [CrossRef]
- Pan, G.; Bai, X.; Xu, W.; Chen, X.; Zhai, Y.; Zhu, J.; Shao, H.; Ding, N.; Xu, L.; Dong, B.; Mao, Y.; Song, H. Bright Blue Light Emission of Ni2+ Ion-Doped CsPbClxBr3-x Perovskite Quantum Dots Enabling Efficient Light-Emitting Devices. ACS Appl. Mater. Interfaces. 2020, 12, 14195−14202.
- Hou, S.; Gangishetty, M. K.; Quan, Q.; Congreve, D. N. Efficient Blue and White Perovskite Light-Emitting Diodes via Manganese Doping. Joule. 2018, 2, 2421−2433. [CrossRef]
- Locardi, F.; Cirignano, M.; Baranov, D.; Dang, Z.; Prato, M.; Drago, F.; Ferretti, M.; Pinchetti, V.; Fanciulli, M.; Brovelli, S.; Trizio, L. D.; Manna, L. Colloidal Synthesis of Double Perovskite Cs2AgInCl6 and Mn-Doped Cs2AgInCl6 Nanocrystals. J. Am. Chem. Soc. 2018, 140, 12989−12995.
- Lou, Y.; Fang, M.; Chen, J.; Zhao, Y. Formation of highly luminescent cesium bismuth halide perovskite quantum dots tuned by anion exchange. Chem. Comm. 2018, 54, 3779−3782. [CrossRef]
- Li, T.; Ma, J.; Qiao, M.; Kuang, Y.; He, Y.; Ran, X.; Guo, L.; Wang, X. Enhanced blue photoluminescence and photostability of Cs3Bi2Br9 perovskite quantum dots via surface passivation with silver ions. CrystEngComm. 2021, 23, 7219−7227.
- Ma, Z.; Shi, Z.; Yang, D.; Zhang, F.; Li, S.; Wang, L.; Wu, D.; Zhang, Y.; Na, G.; Zhang, L.; Li, X.; Zhang, Y.; Shan, C.; Electrically-Driven Violet Light-Emitting Devices Based on Highly Stable Lead-Free Perovskite Cs3Sb2Br9 Quantum Dots. ACS Energy Lett. 2020, 5, 385−394.
- Wang, L.; Shi, Z.; Ma, Z.; Yang, D.; Zhang, F.; Ji, X.; Wamg, M.; Chen, X.; Na, G.; Chen, S.; Wu, D.; Zhang, Y.; Li, X.; Zhang, L.; Shan, C. Colloidal Synthesis of Ternary Copper Halide Nanocrystals for High-Efficiency Deep-Blue Light-Emitting Diodes with a Half-Lifetime above 100 h. Nano Lett. 2020, 20, 3568−3576.
- Li, Y.; Zhou, Z.; Tewari, N.; Ng, M.; Geng, P.; Chen, D.; K, P. K.; Qammar, M.; Guo, L.; Halpert, J. E. Progress in copper metal halides for optoelectronic applications. Mater. Chem. Front. 2021, 5, 4796−4820. [CrossRef]
- Cheng, P.; Sun, L.; Feng, L.; Yang. S.; Yang, Y.; Zheng, D.; Zhao, Y.; Sang, Y.; Zhang, R.; Wei, D.; Deng, W.; Han, K. Colloidal Synthesis and Optical Properties of All-Inorganic Low-Dimensional Cesium Copper Halide Nanocrystals. Angew. Chem. Int. Ed. 2019, 58, 16087−16091. [CrossRef]
- Zhang, C.; Wan, Q.; Ono, L. K.; Liu, Y. Zheng, W.; Zhang, Q.; Liu, M.; Kong, L.; Li, L.; Qi, Y. Narrow-Band Violet-Light-Emitting Diodes Based on Stable Cesium Lead Chloride Perovskite Nanocrystals. ACS Energy Lett. 2021, 6, 3545−3554.
- Yang, P.; Liu, G.; Liu, B.; Liu, X.; Lou, Y.; Chen, J.; Zhao, Y. All-inorganic Cs2CuX4 (X=Cl, Br, and Br/I) perovskite quantum dots with blue-green luminescence. Chem. Comm. 2018, 54, 11638. [CrossRef]
- Shankar, H.; Jha, A.; Kar, P. Water-assisted synthesis of lead-free Cu based fluorescent halide perovskite nanostructures. Mater. Adv. 2022, 3, 658−664. [CrossRef]
- Cortecchia, D.; Dewi, H. A.; Yin, J.; Bruno, A.; Chen, S.; Baikie, T.; Boix, P. P.; Gratzel, M.; Mhaisalkar, S.; Soci, C.; Mathews, N. Lead-Free MA2CuClxBr4-x Hybrid Perovskites. Inorg. Chem. 2016, 55, 3, 1044−1052.
- Li, H.; Qian, Y.; Xing, X.; Zhu, J.; Huang, X.; Jing, Q.; Zhang, W.; Zhang, C.; Lu, Z. Enhancing Luminescence and Photostability of CsPbBr3 Nanocrystals via Surface Passivation with Silver Complex. J. Phys. Chem. C. 2018, 122, 12994−13000. [CrossRef]
- Zhu, D.; Zaffalon, M. L.; Pinchetti, V.; Brescia, R.; Moro, F.; Fasoli, M.; Fanciulli, M.; Tang, A.; Infante, I.; Trizio, L.D.; Brovelli, S.; Manna, L. Bright Blue Emitting Cu-Doped Cs2ZnCl4 Colloidal Nanocrystals. Chem. Mater. 2020, 32, 5897−5903.
- Xu, C.; Sun, X.; Zhang, X.; Ke, L.; Chua, S. Photoluminescent properties of copper-doped zinc oxide nanowires. Nanotechnology. 2004, 15, 856. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




