1. Introduction
Khat is the common name for the plant known by its scientific name, Catha edulis (Vahl) Forssk. ex Endl. Typically, its leaves are chewed to produce stimulant effects such as alertness, euphoria, and increased motor activity [
1]. The two major active components of khat are cathine and cathinone. They are listed in controlled substances act as psychotropic substances Schedule III and I, respectively, and khat plant is listed as a controlled plant [
2]. Despite the low concentrations of cathine and cathinone in khat leaves (which range from 0.1% to 0.2% and 0.1% to 0.3%, respectively), long-term consumption of large amounts of khat leaves can cause toxicity [
3,
4]. In addition to those compounds, khat leaves also contain sterols, tannins, cathedulins (polyhydroxylated sesquiterpenes), triterpenes, and flavonoids [
5].
According to Kalix, the most significant toxic effects of khat on humans are hyperthermia, insomnia, anorexia, constipation, urinary retention, hypertension, and arrhythmia [
6]. Moreover, myocardial infarction has been reported among khat abusers [
7,
8]. Hepatitis was also reported in two khat users which was resolved by discontinuing khat use but relapsed when khat use was resumed in both cases [
9]. There are also reported cases of cerebral hemorrhage, psychoactive disorders, and cancer [
5,
10]. Chewers of khat typically consume 50-200 g of fresh khat leaves per day [
11]. This amount of khat leaves consumed daily induces psychological dependence and tolerance, leading to an increased daily consumption [
12,
13,
14]. The metabolic cytochrome P450 enzymes 3A4, 2D6, 2C19, and 1A2 are affected by khat consumption [
15,
16]. Hence, the inhibitory effects of khat on metabolic enzymes may alter plasma concentrations, which may impact the efficacy and safety of drug therapy.
Both cathine and cathinone are basic compounds with pKa values of 9.37 and 7.55, respectively, and are susceptible to post-mortem redistribution [
17]. As a parent drug, cathine and cathinone are excreted in human urine. Furthermore, they each have a minor metabolite, pseudoephedrine and diethylpropion [
18,
19]. Cathinone was also rapidly converted to norephedrine and nor-pseudoephedrine [
20]. Consequently, the aforementioned metabolites may indicate Khat consumption [
21].
The toxicity of khat exposure has been demonstrated by previous studies, and the possibility of lethality cannot be ruled out. To rule out an overdose as the cause of death, toxicologists must evaluate and interpret the substance concentrations present in postmortem samples. Substance concentrations must be quantified, reported, and published to expand the limited published evidence on toxicological findings for khat-related fatalities. The autopsy findings and other circumstances surrounding death are also important indicators of substance-related deaths. The results of an autopsy finding will also be important for figuring out what concentrations of cathine and cathinone are dangerous and which are fatal. Studying the prevalence of khat-related fatalities is also important for prevention, treatment, and education. The purpose of this study were to a) analyze khat-related fatalities in Jazan, Saudi Arabia, and b) explore the disposition of cathine and cathinone in postmortem tissues.
2. Materials and Methods
2.1. Study design and data collection
All fatal forensic cases received in the Poison Control and Medical Forensic Chemistry Center in Jazan, Saudi Arabia, from the 1st of January 2018 to the 31st of December 2021 were retrospectively evaluated. The data was collected from the OTARR electronic system using the data collection form. All acquired data regarding to the toxicological study results and accident summary, including the manner of death in the forensic cases involving khat were analyzed. These data included forensic cases that are confirmed positive for the presence of cathine and cathinone and excluded the forensic cases that are negative for the presence of cathine and cathinone.
All information regarding to the autopsy finding, manner and causes of death in khat-related fatalities within the period of study was revised, defined and recorded by the medical forensic examiner experts in Jazan Forensic Medicine Center. The autopsy finding data in the autopsied cases is clearly defined and tabulated. The manner of death is classified as follows: suicidal, homicidal, accidental, and undetermined. The identification of hazards for cathine and cathinone, as well as forensic cases involving their exposure, have been evaluated.
2.2. Toxicological analysis
Drugs of abuse including amphetamines, cocaine, cannabinoids, opiates, barbiturates, and benzodiazepines in all autopsied samples are primarily screened by immunoassay analysis using the RANDOX system (Evidence Plus; Randox Laboratories, Crumlin, UK). Volatiles including methanol, ethanol, isopropanol and acetone are routinely analyzed using headspace-gas chromatography. Positive cathine and cathinone results were confirmed and quantified by using liquid chromatography mass-spectrometry, whereas gas chromatography mass-spectrometry was used for non-targeted analysis.
Cathine and cathinone concentrations were identified and quantified by liquid chromatography ion trap mass spectrometry (LCQ Fleet Ion Trap LC/MS) method (Thermo Fisher Scientific, Waltham, Massachusetts, USA). For sample preparation, one gram of each organ tissue was homogenized in one milliliter of deionized water, then centrifuged for 15 minutes at 3000×g using a Heraeus Labofuge 400 centrifuge (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The supernatant was then mixed with 1 mL of phosphate buffer pH 6 and vortexed for solid phase extraction (SPE) using DAU extraction columns (UCT, Bristol, Pennsylvania, USA). One milliliter of each sample homogenate was spiked with 1000 ng/mL of 3,4-methylenedioxymethamphetamine (MDMA) as an internal standard prior to the extraction method. SPE columns were preconditioned with 3 mL methanol and 3 mL deionized water and equilibrated with 1 mL phosphate buffer at pH 6. Upon sample load completion, SPE columns were washed with 3 mL of deionized water, 1 mL of 0.1 M acetic acid and 3 ml of methanol and dried for 10 min under nitrogen stream. Next, cathine, cathinone and MDMA were eluted into 12 mL glass tubes with 3 mL of a mixture of dichloromethane, isopropanol, ammonium hydroxide (78:20:2, v:v:v). Then one drop of 0.1 M hydrochloric acid (HCl) was added into each tube, and all elutions were evaporated to dryness under nitrogen stream. Finally, all samples were reconstituted with 100 µL of the aqueous proportion of the mobile phase (10 mM ammonium formate with 0.11% formic acid) for LCQ Fleet Ion Trap LC/MS analysis.
Calibration samples were prepared by spiking 1 ml of blank tissue homogenates with cathine, cathinone, and MDMA (Lipomed, Arlesheim, Switzerland). Six calibration points were prepared at concentrations of 50, 100, 250, 500, 1000, 2000, 3000, 4000, and 6000 ng/ml of cathine and cathinone and 1000 ng/ml MDMA as the internal standard. Postmortem samples were diluted prior extraction and reanalyzed when concentrations exceed the upper limits of quantification. Three quality control samples were prepared by spiking 1 mL of the blank homogenate tissues with cathine and cathinone at the concentrations of 100, 250 and 500 ng/mL and the concentration of 1000 ng/mL of MDMA as internal standard.
LCQ Fleet Ion Trap LC/MS system (Thermo Fisher Scientific, Waltham, Massachusetts, USA), employing LCQ fleet mass analyzer coupled with Surveyor Auto-Sampler and Surveyor Quaternary Pump and managed by X-Caliber Software (Themo Scientific, USA). Briefly, 10 µl of each sample was injected by an autosampler. The chromatographic separation of cathine, cathinone and MDMA was achieved by HPLC column (Hypersil GOLD, 5 μm, 150 x 4.6 mm, Thermo Fisher Scientific, Waltham, Massachusetts, USA), using mobile phase (A) [ ammonium formate (10 Mm; 0.639 mg ammonium formate in 1 L HPLC water] and mobile phase (B) [ formic acid in acetonitrile (0.1%; 1 ml formic acid in 999 ml acetonitrile]. Gradient elution was performed as follows: 0–1 min, 100% A; and 1–7.5 min, 80% A; 7.5–8.5 min, 50% A, 8.5–9.5 min, 0% A; 9.5–10.5 min, 50% A; and 10.5–11.5 min 100% A. Flow rate was 300 µL/min, and the injection volume was 5 µL. The electrospray ion source (ESI), as optimized tuning profile of ATS, runs in positive ionization mode with 5 kV spraying voltage, 275 ºC capillary temperature and sheath gas value is 30. The mass analyzer runs in scan mode scanning for m/z 152 for cathine, m/z 150 for cathinone and m/z 194 for MDMA. Cathine, cathinone and MDMA are fragmented in the collision cell with helium gas by Pulsed q collision-induced dissociation (PQD) mode into m/z 134 and 117 for cathine, m/z 132 and 105 for cathinone and m/z 163 and 135 for MDMA. The PQD value for cathine and cathinone was 19 and 22 for MDMA. Qualitative and quantitative analyses were performed by X-Caliber Software. The analytical method employed in this study was developed and validated in our laboratory, and it is already routinely used to analyze amphetamine and related substances, with in-house modifications [
22].
The general unknown screening analysis was determined by gas chromatography-mass spectrometry (GC/MS) analysis (GC/MS Agilent Technologies, Santa Clara, California, USA). Each sample (one ml of homogenate sample) was mixed with 1 ml phosphate buffer (pH 6). 1 ml of homogenate sample was extracted by solid phase extraction (SPE) technique using DAU extraction columns (UCT, Bristol, Pennsylvania, USA) according manufacture instruction. For instant, columns were used conditioned with 3 ml methanol then 3 ml deionized water and equilibrated with 1 ml phosphate buffer (pH 6). 2 ml of each sample was loaded and allowed to pass slowly. Then, columns were washed with 3 ml of deionized water followed by 1 ml of 0.1 M acetic acid and allowed to dry for 15 minutes under a flow of air. First elution was collected by adding 2 ml of (ethyl acetate : hexane; 50:50, v:v). Thereafter, columns were washed with 3 ml methanol and 2 ml of the second elution (dichloromethane : isopropanol : ammonium hydroxide; 78:20:2, v:v:v) was added and the sample was dried under nitrogen. All samples were reconstituted with methanol (100 µl), then vortexed and transferred to GC/MS autosampler vials for GC/MS analysis.
Thermo Fisher Scientific (TR-5MS) separation column had the following properties: 30 m length, internal diameter (ID) 0.25 mm and film thickness 0.25 µm utilized. Helium was used as the carrier gas with 1 ml/min flow rate. A total of 2 µl of each sample injected into splitless mode at an injection port with a temperature of 260 ℃. The GC thermal program started at 80 ℃ and lasted 1.5 min. The thermal program then increased the temperature at the initial ramp to 210 ℃ at a rate of 30 ℃/min, then slowed to 20 ℃/min to reach the final temperature of 320 ℃, which held for 11 min. Electron ionization (EI) used as the ion source in MS and the analysis is carried out in scanning mode with an electron energy of 70 eV. The temperature of the ion source and transfer line is set at 230 ℃. The mass spectral libraries from Wiley and the National Institute of Standards and Technology (NIST) were used to identify the GC/MS mass spectra of unknown substances.
2.3. Statistical analysis
All variables were categorized and tabulated using descriptive statistics. Means, standard error of mean (SEM), and median were presented. SigmaPlot 11 for Windows was used to analyze all of the data.
3. Results
The Forensic Medicine Center in Jazan, Saudi Arabia, investigated 651 fatal cases over four-year period. Thirty of the cases had postmortem samples positive for cathinone and cathine, the active ingredients in khat. They were all male ranging in age from 23 to 45. Firearm injuries (10 cases), hanging (7 cases), road traffic accidents (2 cases), head injury (2 cases), stab wounds (2 cases), poisoning (2 cases), unknown (2 cases), ischemic heart disease (1 case), brain tumor (1 case), and choking (1 case) were responsible for deaths. The toxicological analysis of cathinone and cathine in khat-related fatalities was investigated and summarized (
Table 1 and
Table 2).
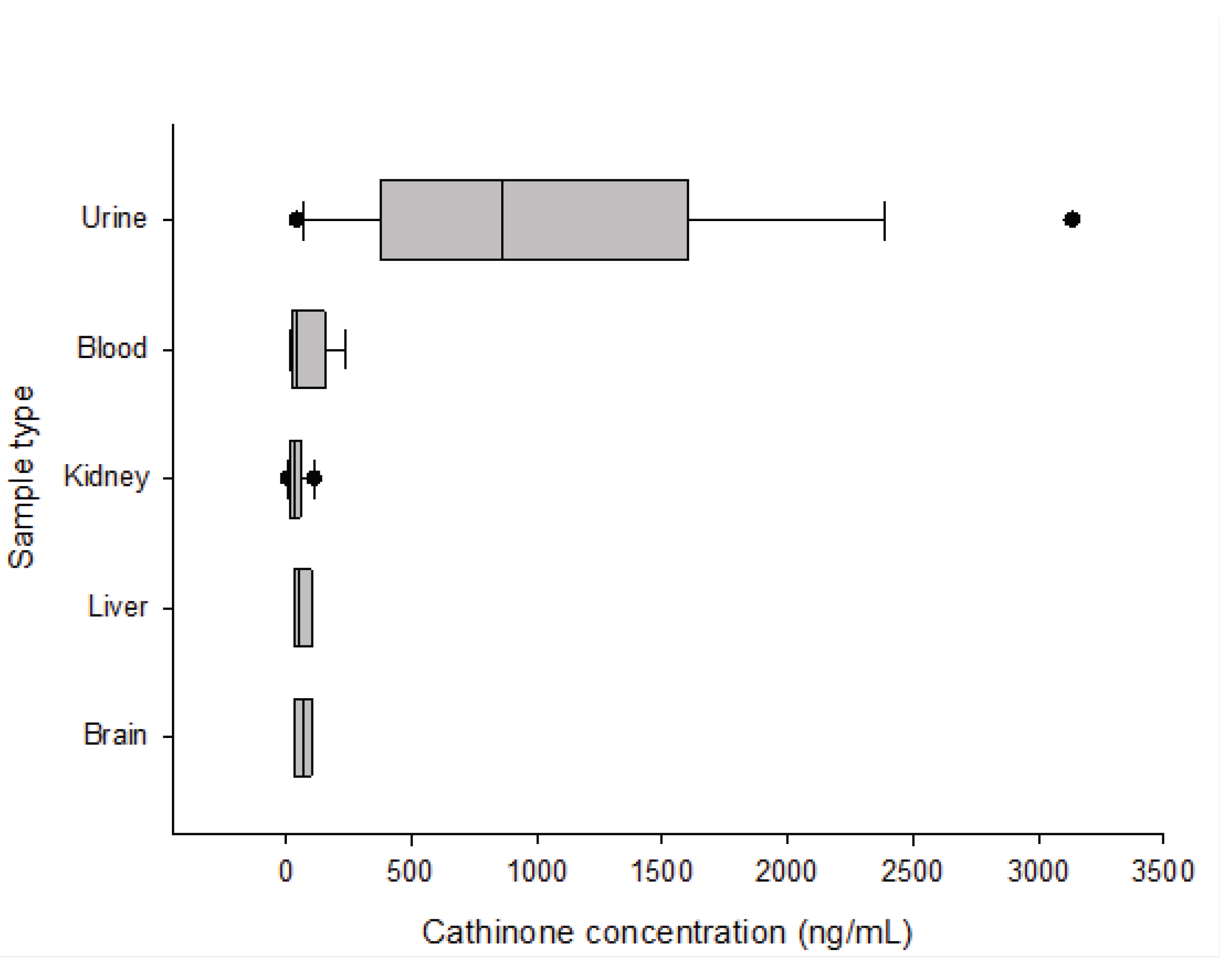
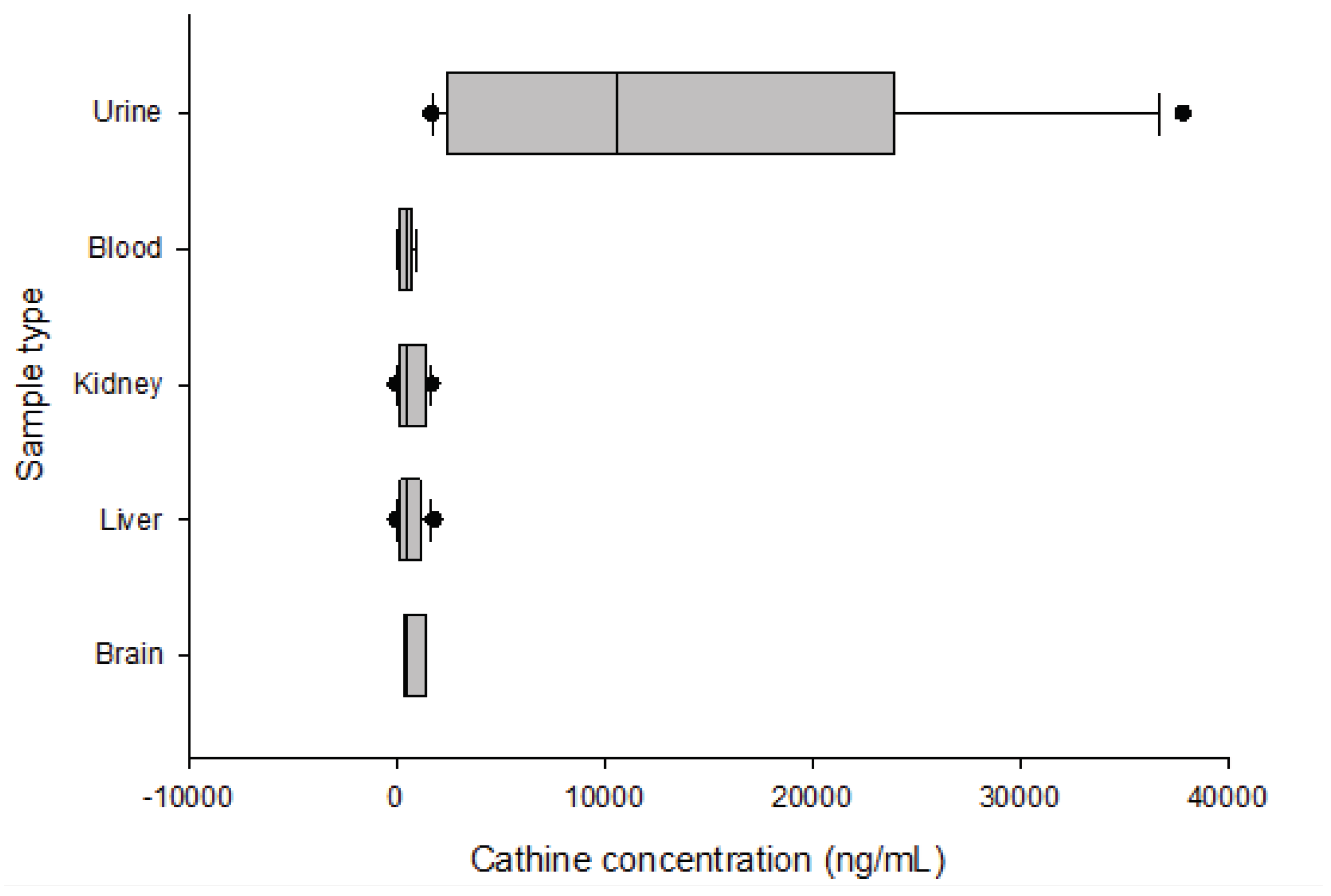
Cathinone and cathine average concentrations were 85 and 486 ng/mL in blood, 1009 and 12616 ng/mL in urine, 69 and 682 ng/mL in the brain, 64 and 635 ng/mL in the liver, and 43 and 758 ng/mL in the kidney. The blood concentrations of cathinone and cathine ranged from 18 to 218 ng/mL and 222 to 843 ng/mL, respectively. In 90% of khat-related fatalities, cathinone concentrations greater than 18 ng/mL and cathine concentrations greater than 222 ng/mL were detected. The retention time for Cathine, cathinone and MDMA were 4.4, 4.5, and 5.1 minutes, respectively. The limit of quantification (LOQ) and detection (LOD) were both 36 ng/mL. Precision and accuracy were within 20% standard deviation and 20% bias, respectively. The extraction recovery ranged from 80 to 90%, with less than 20% carryover. At doses of 100 ng/mL and 6000 ng/mL, matrix effects (suppression/enhancement) are within acceptable limits (25%).
Urine samples had the highest 90th percentile concentrations of cathinone (2134 ng/mL) and cathine (36400 ng/mL).
Figure 1 and
Figure 2 show box plot diagrams of the median and interquartile range of cathinone and cathine concentrations detected in khat-related fatalities. According to the current study, the number of khat-related fatalities in the last four years increased from 3% in 2018 and 2019 to 4% and 9% in 2020 and 2021, respectively.
Table 3 summarizes the findings of the autopsy. Homicide was the major manner of death (13 of 30 fatalities) and firearm injuries are the leading cause of death among homicide victims (10 of 13 fatalities).
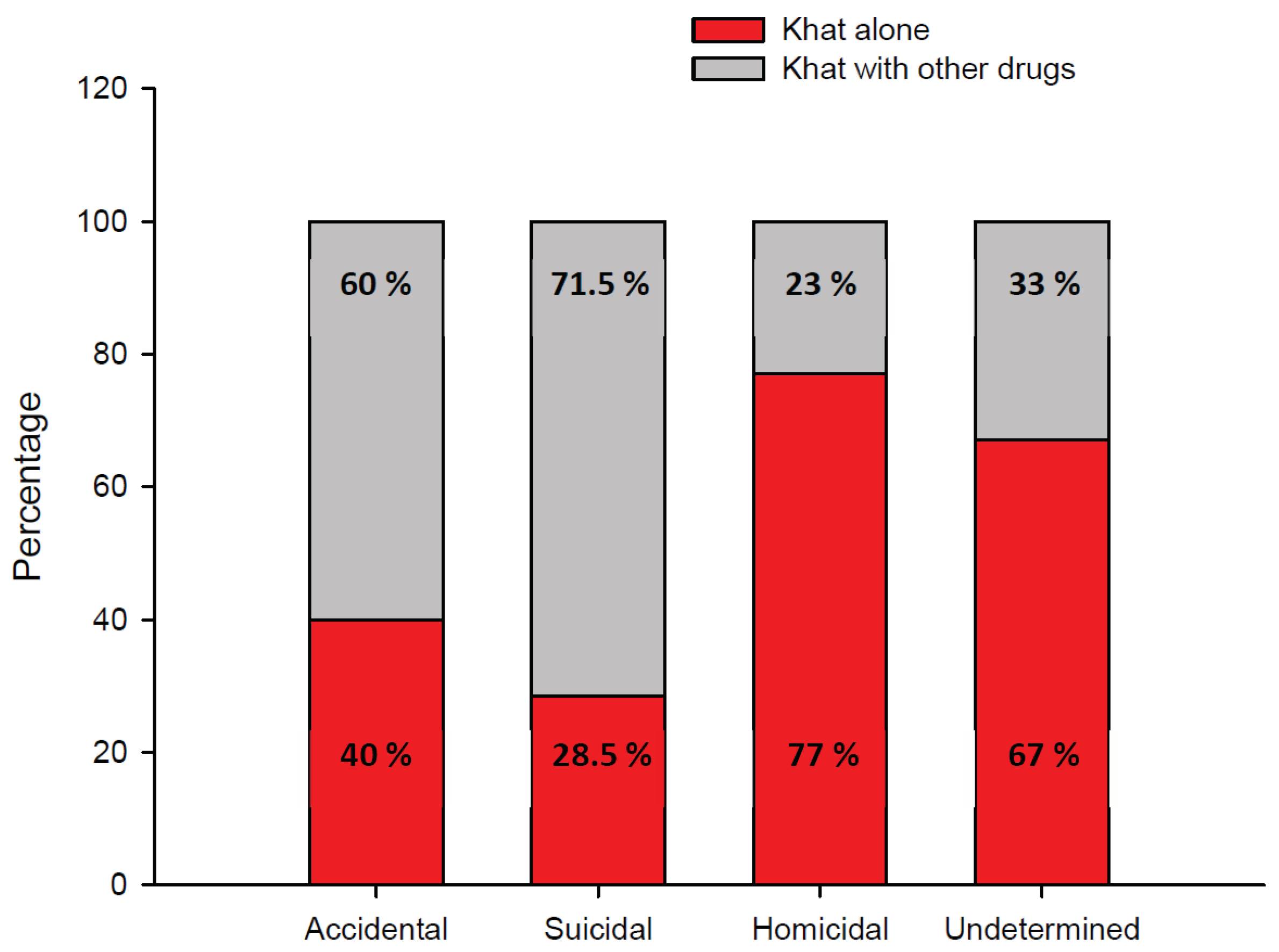
A stacked bar chart of fatalities involving khat alone or khat in combination with other drugs in different manners of death cases is presented in
Figure 3. Homicide was more likely in fatalities involving khat alone (77%) than in fatalities involving khat in combination with other drugs (23%). Suicidality was more likely in fatalities involving khat in combination with other drugs (71.5%) than in fatalities involving khat alone (28.5%).
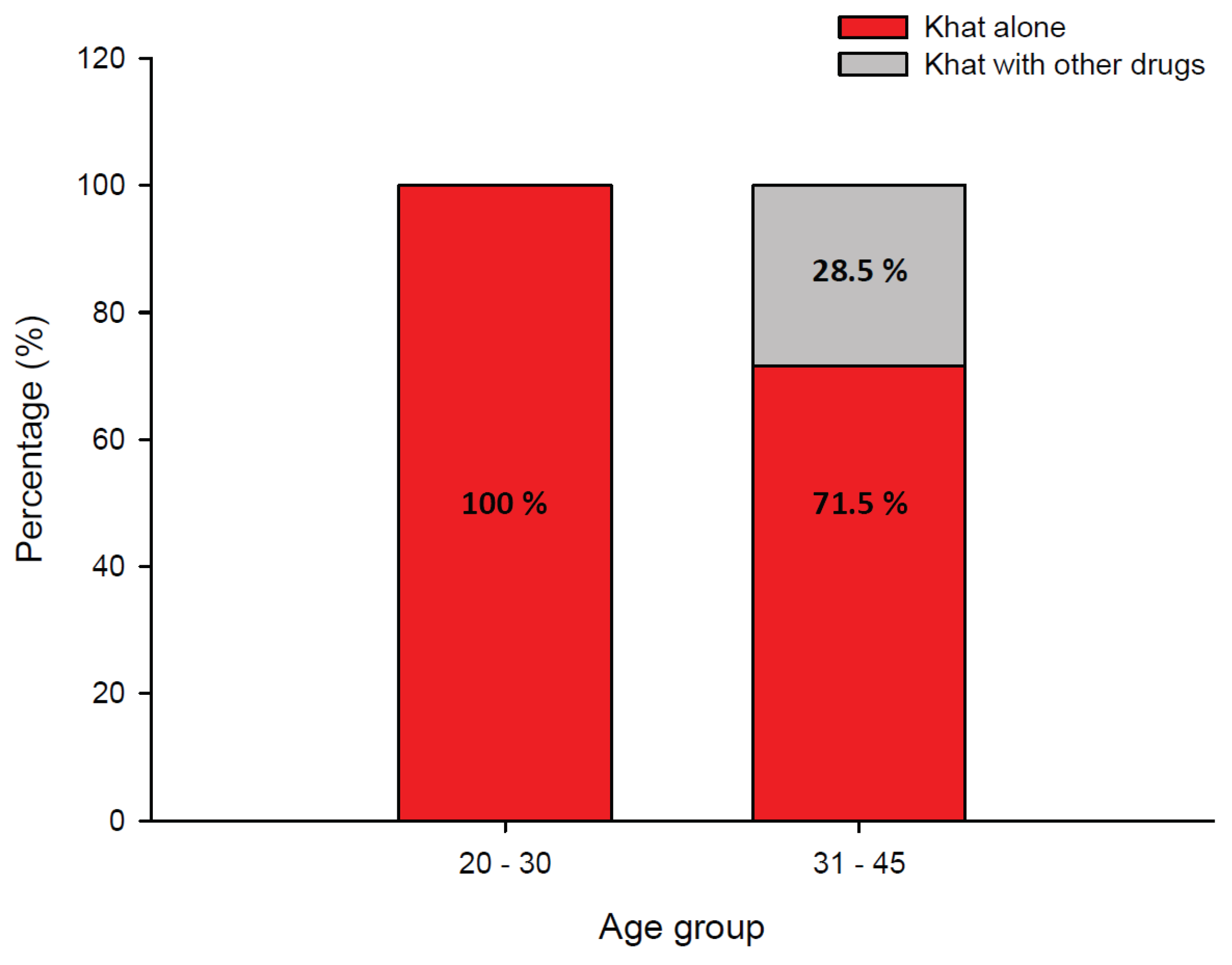
Figure 4 is a stacked bar chart showing the occurrence of firearm injuries in khat-related fatalities (khat alone and khat with other drugs) by age group. Remarkably, all fatalities in the 20- to 30-year-old age range and more than 70 percent of fatalities in the 31- to 45-year-old age group were caused by firearm injuries in homicides involving only khat. Another remarkable finding is that five of the seven hanging suicides involved khat in combination with other drugs, and amphetamine is frequently involved in khat-related fatalities (3 of 7 suicide victims).
4. Discussion
The khat plant (Catha edulis (Vahl) Forssk. ex Endl.) is listed by the Saudi Food and Drugs Authority (SFDA) as a prohibited plant under the Act, whereas cathinone and cathine are listed in Schedules I and III, respectively, of the 1971 United Nations Convention on Psychotropic Substances. Khat, on the other hand, is cultivated in Yemen, where it is widely consumed. However, in the Jazan region, which is close to the Yemeni border, 21.4% of students and 28.7% of all individuals surveyed reported that they were currently chewing khat. In Yemen, khat is commonly cultivated and consumed by 68% of the Yemenis surveyed [
23,
24,
25].
In 2020, khat was the most frequently seized plant-based substance, according to the United Nations Office on Drugs and Crime (UNODC, 2022). Khat accounts for 55% of all plant-based substances seized between 2016 and 2020. Saudi Arabia accounted for the most total khat seizures in 2019 [
26]. In addition, chewing khat has become increasingly illegal in Europe, not because it is associated with toxicity, but because it has fallen into the hands of organized crime networks [
27]. There is a significant risk of toxicity for those who consume khat excessively, a problem that is common among the majority of khat users who consume large amounts of khat on a regular basis. According to a recent study, 73.5 percent of people who are dependent on khat chewing have consumed half a bundle or more of khat, and 55.9 percent of those people chew khat more than three times per week for an average of more than six hours each session [
28]. Approximately 200 grams of khat leaves are contained within each bundle.
Few articles have documented fatalities associated with khat, and there is currently no established reference range for cathinone and cathine that is toxic and lethal [
21,
29,
30]. Cathinone and cathine were found in the blood, urine, vitreous humour, brain, liver, kidney, and stomach in previous studies on khat-related fatalities. Although there is no conclusive evidence that khat caused death, and its presence in the body does not necessarily imply that it did, however, it is expected that khat will cause death when consumed in large quantities [
31]. As a result, it is critical to record and publish postmortem toxicology levels in khat fatalities.
The median blood concentrations of cathinone and cathine were 40 and 470 ng/mL, respectively, exceeding the median blood levels of cathinone and cathine in previously published forensic non-fatal road traffic accidents (median 33 and 129 ng/mL, respectively; N=19) [
32]. They conclude that chewing khat may severely impair driving ability. While the median urine concentrations of cathinone and cathine were 860 and 6240 ng/mL, respectively in our study, were lesser than the median levels of cathinone and cathine in the study mentioned (median 8000 and 38600 ng/mL, respectively; N=19). This may be because cathinone is less stable and has a shorter elimination half-life (1.5 ± 0.8 h) than cathine (5.2 ± 3.4 h) [
33,
34]. As a result, cathine has a longer detectable period than cathinone. Nevertheless, urine concentrations indicate chemical exposure, but not acute toxicity. On other hand, cathinone blood concentrations ranged from 19 to 122 ng/mL in forensic postmortem cases [
30]. These levels were lower than the wide range observed in our study (18 - 218 ng/mL). While cathine blood concentration was 1447 ng/mL in one forensic postmortem case, which exceeding the range observed in our study (222 – 843 ng/mL). The average concentrations of cathinone and cathine in the brain, liver, and kidney are 69 and 682 ng/mL, 64 and 635 ng/mL, and 43 and 758 ng/mL, respectively. The concentrations of cathine were roughly 10 times higher than those of cathinone.
Cathine and cathinone should be included in all routine toxicological investigations, especially in homicidal cases, because the number of khat-related fatalities has already increased. This study reveals the number of deaths associated with khat use, both alone and in combination with other drugs. Which demonstrate that homicidal patterns were observed in a greater proportion of cases involving khat alone (77%) than cases involving khat in combination with other drugs (23%), and that suicidal pattern was observed in a greater proportion of cases involving khat in combination with other drugs (71.5%) than cases involving khat alone (28.5%).
Khat use has been associated with a higher risk of myocardial infarction [
8,
35,
36,
37]. It has been shown that khat induces tachycardia and hypertension, which may be increased by a higher dose, hence raising the risk of myocardial infarction [
33,
38,
39]. Cathine and cathinone were the only substances detected in Case 1 of the current study, which showed ischemic heart disease with coronary partial occlusion.
Hepatotoxicity, on the other hand, has been observed in multiple studies, particularly among chronic khat chewers. Multilobular necrosis, canalicular cholestasis, an enlarged liver with decreased echogenicity, cirrhotic and portal fibrosis, jaundice with elevated liver enzymes, and severe liver injury with an elevated international normalized ratio were all characteristics of khat-related hepatic toxicity [
40,
41,
42,
43]. Hepatotoxicity was cured in two cases by discontinuing khat usage [
9]. In the current study, cathine and cathinone were detected in the liver at average concentrations of 635±132 and 64±16 ng/mL, respectively. However, no evidence of liver toxicity was found.
khat use has also been associated with mental disorders including anxiety, irritability, aggression, psychosis, paranoia, dysphoria, depression, insomnia, hallucinations, and delusions [
44,
45,
46]. In addition, anxiety, trembling, lethargy, depression, and nightmares could be withdrawal symptoms [
21,
47,
48]. On the other hand, khat intake may aggravate schizophrenia symptoms and diminish the therapeutic efficacy of antipsychotic drugs [
49]. With both khat use and withdrawal condition, incidents of violence have been reported. In addition, anxiousness and poor decision-making have been observed following khat use [
28,
50,
51]. Repeated oral administration of khat extract, according to Banjaw et al. (2006) report, makes experimental rats more aggressive [
52]. Moreover, studies show that khat affects driving, social behavior, and work performance. It has been associated with homicide, notably in mentally ill people [
10,
45,
50,
53,
54], and two reported homicides have been linked to khat consumption [
53,
55].
In the current study, homicide and suicide were the most leading manner of death in fatalities involving khat alone and khat in combination with other drugs, respectively. In fatalities involving khat in combination with other drugs, particularly amphetamine, suicide was the most common manner of death. The increase in deaths relating to khat use is cause for concern. It is necessary to consider khat abuse among individuals with a higher propensity for homicide and firearm injuries among young people. Implying that khat may contribute to criminal activity in the Jazan region. Therefore, any health-based crime prevention strategy in Jazan should take khat use into account.
5. Conclusions
This study determined the average levels of cathinone and cathine in 30 cases of fatalities involving khat. This information may help forensic toxicologists and pathologists interpret toxicological investigations, but it should not be interpreted independently of other evidence, such as the death investigation and autopsy results. To create the body of literature necessary for more precise determinations of the role of khat in crimes and death investigations, additional research is required, particularly involving toxicological investigative and autopsy findings.
Author Contributions
Conceptualization, G.S., I.A., M.G. and A.A.; methodology, M.O., S.A., M.G., I.A. and M.G.; software, I.A. and M.O.; validation, M.O., M.G. and S.A.; formal analysis, M.O. and I.A. ; investigation, G.S., A.A., M.O., S.A., M.G., A.H., M.G., M.T., M.A. and M.F.; resources, I.K., M.T. and I.A.; data curation, G.S., A.A., M.G. and I.A.; writing—original draft preparation, I.A.; writing—review and editing, G.S., M.A., M.G. and I.A.; visualization, M.G. and I.A.; supervision, I.A. and G.S.; project administration, I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study has been reviewed and approved by Jazan Health Ethics Committee, Saudi Arabia (No. 2238).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant details are included in the article.
Acknowledgments
This study would not have been possible without the exceptional efforts of the Poison Control & Medical Forensic Chemistry Center and Forensic Medicine Center, Jazan Health Affairs, Ministry of Health, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nencini, P.; Ahmed, A.M. Khat consumption: a pharmacological review. Drug Alcohol Depend. 1989, 23, 19–29. [Google Scholar] [CrossRef] [PubMed]
- D. E. Administration, Drugs of Abuse: A Dea Resource Guide: CreateSpace Independent Publishing Platform, 2020.
- P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach: Wiley, 2009.
- Halbach, H. Medical aspects of the chewing of khat leaves. . 1972, 47, 21–9. [Google Scholar] [PubMed]
- Al-Habori, M. The potential adverse effects of habitual use ofCatha edulis(khat). Expert Opin. Drug Saf. 2005, 4, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Kalix, P. Khat: Scientific knowledge and policy issues. Br. J. Addict. 1987, 82, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Dollery, C. Severe Ischaemic Cardiomyopathy Associated with Khat Chewing. J. R. Soc. Med. 2006, 99, 316–318. [Google Scholar] [CrossRef]
- Al-Motarreb, A.; Briancon, S.; Al-Jaber, N.; Al-Adhi, B.; Al-Jailani, F.; Salek, M.S.; Broadley, K.J. Khat chewing is a risk factor for acute myocardial infarction: a case-control study. Br. J. Clin. Pharmacol. 2005, 59, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.G.; Handslip, R.; Kumar, M.; Mahadeva, U.; Lucas, S.; Yamamoto, T.; Wood, D.M.; Wong, T.; I Dargan, P. Reversible khat-induced hepatitis: two case reports and review of the literature. Front. Gastroenterol. 2013, 4, 278–281. [Google Scholar] [CrossRef]
- G. Cox and H. Rampes, “Adverse effects of khat: a review,” Advances in psychiatric treatment, vol. 9, pp. 456-463, 2003.
- S. Geisshüsler and R. Brenneisen, “The content of psychoactive phenylpropyl and phenylpentenyl khatamines in Catha edulis Forsk. of different origin,” J Ethnopharmacol, vol. 19, pp. 269-277, 1987.
- P. Kalix, “Khat: a plant with amphetamine effects,” J Subst Abuse Treat, vol. 5, pp. 163-169, 1988.
- Kalix, P. Cathinone, a Natural Amphetamine. Basic Clin. Pharmacol. Toxicol. 1992, 70, 77–86. [Google Scholar] [CrossRef]
- Nencini, P.; Ahmed, A.M.; Amiconi, G.; Elmi, A.S. Tolerance Develops to Sympathetic Effects of Khat in Humans. Pharmacology 1984, 28, 150–154. [Google Scholar] [CrossRef]
- Bedada, W.; de Andrés, F.; Engidawork, E.; Pohanka, A.; Beck, O.; Bertilsson, L.; Llerena, A.; Aklillu, E. The Psychostimulant Khat (Catha edulis) Inhibits CYP2D6 Enzyme Activity in Humans. J. Clin. Psychopharmacol. 2015, 35, 694–699. [Google Scholar] [CrossRef]
- Bedada, W.; de Andrés, F.; Engidawork, E.; Hussein, J.; Llerena, A.; Aklillu, E. Effects of Khat (Catha edulis) use on catalytic activities of major drug-metabolizing cytochrome P450 enzymes and implication of pharmacogenetic variations. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Pélissier-Alicot, A.-L.; Gaulier, J.-M.; Champsaur, P.; Marquet, P. Mechanisms Underlying Postmortem Redistribution of Drugs: A Review. J. Anal. Toxicol. 2003, 27, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.L.; Shieh, M.-H.; Kuo, F.-H. Metabolites of ephedrines in human urine after administration of a single therapeutic dose. Forensic Sci. Int. 2006, 157, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Pokrajac, M.; Miljković, B.; Bisailović, B. Mass spectrometric investigation of 2-aminopropiophenones and some of their metabolites. Rapid Commun. Mass Spectrom. 1991, 5, 59–61. [Google Scholar] [CrossRef] [PubMed]
- R. R. Scheline, Handbook of Mammalian Metabolism of Plant Compounds: CRC Press, 2017.
- J. M. Corkery, F. J. M. Corkery, F. Schifano, A. Oyefeso, A. H. Ghodse, T. Tonia, V. Naidoo, and J. Button, “Overview of literature and information on” khat-related” mortality: a call for recognition of the issue and further research,” Ann Ist Super Sanita, vol. 47, pp. 445-464, 2011.
- Alamir, A.; Watterson, J.; Attafi, I. Development and Validation of a Uplc-Qtof-Ms Method for Blood Analysis of Isomeric Amphetamine-Related Drugs. Separations 2022, 9, 285. [Google Scholar] [CrossRef]
- R. M. Alsanosy, M. S. R. M. Alsanosy, M. S. Mahfouz, and A. M. Gaffar, “Khat chewing habit among school students of Jazan region, Saudi Arabia,” PLoS One, vol. 8, p. e65504, 2013.
- Ageely, H.M. Prevalence of Khat chewing in college and secondary (high) school students of Jazan region, Saudi Arabia. Harm Reduct. J. 2009, 6, 11–7. [Google Scholar] [CrossRef]
- Numan, N. Exploration of adverse psychological symptoms in Yemeni khat users by the Symptoms Checklist-90 (SCL-90). Addiction 2003, 99, 61–65. [Google Scholar] [CrossRef]
- UNODC, “Drug market trends of Cocaine, Amphetamine-type stimulants and New Psychoactive Substances,” The United Nations Office on Drugs and Crime (UNODC), United Nations publication 9789211483758, 2022.
- S. B. Karch and O. Drummer, Karch’s Pathology of Drug Abuse: Taylor & Francis, 2015.
- M. El-Setouhy, R. M. M. El-Setouhy, R. M. Alsanosy, A. Alsharqi, and A. A. Ismail, “Khat dependency and psychophysical symptoms among chewers in Jazan Region, Kingdom of Saudi Arabia,” BioMed research international, vol. 2016, 2016.
- Attafi, I.M.; Albeishy, M.Y.; Oraiby, M.E.; Khardali, I.A.; Shaikhain, G.A.; Fageeh, M.M. Postmortem Distribution of Cathinone and Cathine in Human Biological Specimens in a Case of Death Associated with Khat Chewing. Arab. J. Forensic Sci. Forensic Med. 2018, 1, 922–930. [Google Scholar] [CrossRef]
- J. M. Corkery, F. J. M. Corkery, F. Schifano, A. Oyefeso, A. H. Ghodse, T. Tonia, V. Naidoo, and J. Button, “‘Bundle of fun’or ‘bunch of problems’? Case series of khat-related deaths in the UK,” Drugs: education, prevention and policy, vol. 18, pp. 408-425, 2011.
- J. M. Corkery, “Khat–chewing it over: continuing ‘cultural cement’, cardiac challenge or catalyst for change,” Forensic Toxicology–Drug Use and Misuse14 July. London: Royal Society of Chemistry, pp. 165-207, 2016.
- Toennes, S.W.; Kauert, G.F. Driving under the influence of khat—alkaloid concentrations and observations in forensic cases. Forensic Sci. Int. 2004, 140, 85–90. [Google Scholar] [CrossRef]
- Toennes, S.W.; Harder, S.; Schramm, M.; Niess, C.; Kauert, G.F. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. Br. J. Clin. Pharmacol. 2003, 56, 125–130. [Google Scholar] [CrossRef]
- Widler, P.; Mathys, K.; Brenneisen, R.; Kalix, P.; Fisch, H.-U. Pharmacodynamics and pharmacokinetics of khat: A controlled study. Clin. Pharmacol. Ther. 1994, 55, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Al-Motarreb, A.; Shabana, A.; El-Menyar, A. Epicardial Coronary Arteries in Khat Chewers Presenting with Myocardial Infarction. Int. J. Vasc. Med. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Alkadi, H.; A Noman, M.; Al-Thobhani, A.K.; Al-Mekhlafi, F.S.; Raja’A, Y. Clinical and experimental evaluation of the effect of Khat-induced myocardial infarction. . 2002, 23. [Google Scholar]
- F. H. Al-Hashem, M. A. F. H. Al-Hashem, M. A. Dallak, L. O. Nwoye, I. M. Bin-Jaliah, H. S. Al-Amri, M. H. Rezk, H. F. Sakr, A. S. Shatoor, and M. Al-Khateeb, “Acute exposure to Catha edulis depresses contractility and induces myocardial infarction in spontaneously contracting, isolated rabbit’s heart,” Saudi J Biol Sci, vol. 19, pp. 93-101, 2012.
- L. Al-Motarreb and K. J. Broadley, “Coronary and aortic vasoconstriction by cathinone, the active constituent of khat,” Auton Autacoid Pharmacol, vol. 23, pp. 319-26, Oct-Dec 2003.
- Al-Motarreb, A.; Al-Kebsi, M.; Al-Adhi, B.; Broadley, K.J. Khat chewing and acute myocardial infarction. Hear. 2002, 87, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Peevers, C.G.; Moorghen, M.; Collins, P.L.; Gordon, F.H.; McCune, C.A. Liver disease and cirrhosis because of Khat chewing in UK Somali men: a case series. Liver Int. 2010, 30, 1242–1243. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.H.; Kajihara, M.; Borges, G.; O’Beirne, J.; Patch, D.; Dhillon, A.P.; Crozier, A.; Morgan, M.Y. Severe, Acute Liver Injury and Khat Leaves. New Engl. J. Med. 2010, 362, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- J. M. Brostoff, C. J. M. Brostoff, C. Plymen, and J. Birns, “Khat--a novel cause of drug-induced hepatitis,” Eur J Intern Med, vol. 17, p. 383, Aug 2006.
- J. Brostoff, C. J. Brostoff, C. Plymen, and J. Birns, “Khat—a novel cause of drug-induced hepatitis,” European Journal of Internal Medicine, vol. 17, p. 383, 2006.
- E. E. Balint, G. E. E. Balint, G. Falkay, and G. A. Balint, “Khat–a controversial plant,” Wiener Klinische Wochenschrift, vol. 121, pp. 604-614, 2009.
- Pantelis, C.; Hindler, C.G.; Taylor, J.C. Use and abuse of khat(Catha edulis): a review of the distribution, pharmacology, side effects and a description of psychosis attributed to khat chewing. Psychol. Med. 1989, 19, 657–668. [Google Scholar] [CrossRef]
- Odenwald, M. Chronic khat use and psychotic disorders: A review of the literature and future prospects. SUCHT 2007, 53, 9–22. [Google Scholar] [CrossRef]
- Wabel, N. Psychopharmacologic aspect of Catha edulis khat and consequence of long term use: a review. J. Mood Disord. 2011, 1, 1. [Google Scholar] [CrossRef]
- Giannini, A.J.; Castellani, S. A Manic-like Psychosis Due to Khat Catha edulis Forsk. J. Toxicol. Clin. Toxicol. 1982, 19, 455–459. [Google Scholar] [CrossRef]
- Hakami, T.; Mahmoud, M.; Mohammed, B.; El-Setouhy, M. Effects of khat use on response to antipsychotic medications in patients with newly diagnosed schizophrenia: a retrospective study. East. Mediterr. Heal. J. 2021, 27, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.; Neuner, F.; Schauer, M.; Elbert, T.; Catani, C.; Lingenfelder, B.; Hinkel, H.; Häfner, H.; Rockstroh, B. Khat use as risk factor for psychotic disorders: A cross-sectional and case-control study in Somalia. BMC Med. 2005, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, B.; Philpart, M.; Goshu, M.; Berhane, Y.; Fitzpatrick, A.L.; Williams, M.A. Anger expression, negative life events and violent behaviour among male college students in Ethiopia. Scand. J. Public Heal. 2008, 36, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Banjaw, M.Y.; Miczek, K.; Schmidt, W.J. Repeated Catha edulis oral administration enhances the baseline aggressive behavior in isolated rats. J. Neural Transm. 2005, 113, 543–556. [Google Scholar] [CrossRef]
- Alem, A.; Shibre, T. Khat induced psychosis and its medico-legal implication: a case report. . 1997, 35. [Google Scholar]
- Stefan, J.; Mathew, B. Khat Chewing: An Emerging Drug Concern in Australia? Aust. New Zealand J. Psychiatry 2005, 39, 842–843. [Google Scholar] [CrossRef]
- Tesfaye, E.; Krahl, W.; Alemayehu, S. Khat induced psychotic disorder: case report. Subst. Abus. Treat. Prev. Policy 2020, 15, 1–5. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).