1. Introduction
Mandarins constitute the main group among citrus for fresh fruit consumption, covering several species of the genus Citrus and their hybrids, which have many physicochemical characteristics. The mandarin group, include common mandarins (C. reticulata), satsumas (C. x unshiu), clementines (C. x clementina), willow leaf ou mediterranean mandarins (C. x deliciosa), tangors (mandarin x sweet orange hybrids) and tangelos (mandarin x grapefruit hybrids) are part of the group, which share characteristics that make them suitable for in natura consumption, such as the ease of peeling, in general, looser peel than other citrus fruits, balanced flavor, among others.
Among the fruits produced worldwide, mandarins are the main ones in volume. According to FAO data for the year 2020 [
1], world production of this group of citrus was 38.60 million t. China leading as the world's largest producer, with a production of 23.13 million t, much higher than the other producers, Spain (2.17 mi t), Turkey (1.59 mi t), Morocco (1.37mi t), Brazil (1.03 mi t) and Egypt (0.971 mi t. In the fruit productivity world ranking, among the ten largest producers of mandarins, the USA is the first (33.7 t ha
-1), Brazil is 8th place (18.7 t ha-1), and China is 10th (10.4 t ha
-1) [
1]. Among the factors that led to this lower Brazilian productivity are those associated with phytosanitary problems [
2].
The State of São Paulo (SPS) leads the mandarin production, in 2020, around 340 thousand tons in just over 10 thousand ha in 2020, which corresponds to one-third of the country's total production. The states of Minas Gerais (MG), Paraná, and Rio Grande do Sul produced in the same year, respectively 244, 157, and 122 thousand tons of fruit [
3]. These data demonstrate that more than 60% of Brazilian production is concentrated in the country's southeastern region, mainly in the states of SP and MG.
Interestingly, when analyzing agricultural production data in the country, a decrease in area and production volume has been observed in recent years. In 2005, for example, production was more than 1,230.0 tons in 61.0 thousand ha. In 2020, there was a reduce on of 10% in the area (55.5 thousand ha) and 16% in the volume produced (1.03 thousand tons) [
3].
Among the factors involved in the cultivated area decline one can highlight the raise in production costs given the phytosanitary problems that occurred in the country during this period, mainly alternaria brown spot (ABS) and huanglongbing (HLB; ex-greening), which may have demotivated the maintenance of orchards, since the main mandarin varieties planted in the country are highly intolerant to these diseases.
ABS is caused by the fungus
Alternaria alternata (Fr.) Keissl, whose germinate spores infect an Alternaria citri toxin (ACT) that kills host cells, causing necrotic lesions on leaves, fruits, and young branches and consequently premature leaf and fruit drop [
4,
5]. The disease has made it impossible to maintain commercial variety orchards not only in Brazil but also in the main mandarin-producing regions of the world [
6,
7]. Symptoms under field conditions are particularly severe on some mandarins, grapefruit (
C. x
paradisii Macf.), and their hybrids, including important economic varieties such as 'Emperor,' 'Fortune,' 'Nova,' and 'Murcott' [
8,
9], among others.
The disease causes a reduction in production due to fruit drop and the presence of symptoms that depreciates the fruits for the fresh fruit market, which leads to the need for intensive control of the fungus, from 4 to 30 fungicides applications, increasing production costs [
2,
10]. Problems related to the loss of sensitivity of the pathogen to commercial fungicides of several active principles have already been reported [
11,
12] possibly due to constant use in orchards.
Huanglongbing was reported in Brazil in 2004 [
13], and today it is the most critical disease in citrus cropping systems worldwide. Caused by the bacteria '
Candidatus liberibacter spp'., transmitted by the psyllid
Diaphorina citri, it is a highly destructive and aggressive disease, causing damage, increasing production costs, and making citrus production unfeasible in several regions of the world.
The search for resistant varieties, which meet consumer demand, has been one of the greatest demands in citriculture. The Centro de Citricultura ‘Sylvio Moreira’ of the Instituto Agronômico (CCSM/IAC) has one of the largest Citrus Collections in the world [
14], enabling the establishment of dozens of accessions in the field for the selection of new commercial varieties. In this paper, we related results of three years of observations of on reaction of 173 mandarins to ABS and HLB.
2. Material and Methods
2.1. Experimental area and treatments description
The experiment was established in February 2015 and conducted for three agricultural years (2018 to 2020) at CCSM/SP, Cordeirópolis, State of São Paulo, Brazil (22°27'29.82"S and 47°24'16.62"W), with an average altitude of 709 m above sea level. The on-site climate is classified as subtropical (Cwa) and humid, with dry winters (temperatures below 18 °C) and hot summers (temperatures above 22 °C) [
15].The soil was classified as an Orthic Ferralsol (WRB/FAO: oxisol, Rhodic Hapludox, USDA Soil Taxonomy; Latossolo Vermelho distrófico, Brazilian classification), with 64.6% clay, 21.3% sand and 14.1% silt, and the average results of the last four years of the soil chemical analysis presented pH at 5.3 ± 0.4 and organic matter content at 3.5 ± 0.8%.
Accessions of mandarins (Citrus reticulata), satsumas (C. x unshiu), clementines (C. x clementina), willow leaf mandarins (C. x deliciosa Tenore), tangors (C. reticulata x C. sinensis) and tangelos (C. reticulata x C. x paradisi), composed of a total of 173 genotypes. The accessions came from the Active Citrus Germplasm Bank of the IAC (BAG Citrus IAC) and the Bebedouro Experimental Station (EEAB) (Supplementary Table S1). All plants were grafted onto Rangpur lime (C. x limonia Osbeck) and established in a randomized block design, with four replications and two plants per plot, spaced at 7.0 x 4.0 m, totaling 1424 plants and 39.872 m² of the experimental area.
2.2. Alternaria brown spot (ABS) evaluations
Alternaria brown spot (ABS) was evaluated for three consecutive years (2018 to 2020) in plants using a qualitative rating scale, ranging from zero (no symptoms) to 4 (plant with burnt shoots and leaf fall) [
16]. In fruits, ABS was evaluated through a diagrammatic scale for assessment of alternaria brown spot severity on mandarin leaves diagrammatic severity scale, corresponding to the relative fruit area with lesions [
17]. For each evaluated year, the means of the variables incidence and severity in plants and fruits were assessed, and the data was used to calculate the area under the disease progress curve (AUDPC) by the trapezoidal integration method [
18].
2.3. HLB evaluations
All plants in the experimental area were inspected for HLB symptoms for three consecutive years, starting in 2017. The plants were visually inspected, and the percentage of the canopy that had disease symptoms on leaves. was determined. A percentage was assigned to each plant, where 0 means the absence of symptomatic leaves and 100% means the absence of healthy leaves [
19]. As for ABS, assessments were used to calculate the AUDPC.
2.4. Fruit quality and ‘Candidatus Liberibacter asiaticus’ (CLas) detection through qPCR
After the HLB incidence assessments, in the 2019 harvest, four varieties with contrasting severity levels were selected and evaluated for the influence of the presence of the pathogen on fruit quality. Leaves and fruits of Murcott IAC 221 tangor, Ponkan IAC 172 mandarin, Dekopon IAC 2009 tangor and Rio IAC 194 willow leaf mandarin were sampled from symptomatic and asymptomatic branches, respectively for HLB detection through qPCR (leaves) and physical-chemical analysis (fruits). Analyzes were conducted on three samples of five fruits each. Were evaluated average mass (g), height/width ratio (cm) of the fruit, titratable acidity, obtained by titration with a standardized solution of 0.3125 N of NaOH, using phenolphthalein as an indicator, with estimates based on volume; total soluble solids determined by a refractometer and ratio calculated with the relationship soluble solids/acidity.
DNA samples were analyzed for the presence of the 16SrDNA from CLas with HLBaspr primer/probe (5'FAM/BHQ1, Macrogen, Seoul, South Korea) through qPCR [
19]. For each reaction, 1.0 μL of DNA was used, Master Mix [1x], 0.5 μM of HLBas and HLBr primers, and 0.2 μM HLBp probe, in a final reaction volume of 12 μL. The polymerase chain reaction consisted of the denaturation phase at 50 ºC (2 minutes) and 95 ºC (10 minutes), followed by 45 cycles of 15 seconds at 95 ºC and 45 seconds at 59 ºC. The control used were healthy (absence of CLas), positive (DNA with the presence of CLas), and negative (water – absence of DNA). Fluorescence assessment of qPCR was carried out with StepOnePlus™ Real-Time PCR System (Applied Biosystems) at each amplification cycle and, after, analyzed through StepOne™ Software v2.3. CLas titer values were obtained by Threshold Cycle (Ct) values, obtained for qPCR targeting the 16S ribosomal DNA sequence below 36 were considered CLas positives (CLas+). Log10 of CLas genomes per gram of tissue were estimated [
20].
2.5. Data analysis
All data were submitted to the normality analysis, variance analysis (ANOVA), and when necessary, to the multiple mean comparisons test by Tukey's honestly significant difference test (HSD) (ɑ = 0.05). The correlations between the variables were submitted by Pearson's linear correlation test (ɑ = 0.05). Normality, Pearson's correlation, and ANOVA tests were provided by R software (v. 4.1.0). Multivariate analyses through the principal component analysis (PCA) were made via correlation matrix, using the ABS and HLB data to identify the response of the citrus groups. PCA data were normalized and demonstrated by the principal components' distance biplot by their average values [
21]. PCAs were performed by
FactoMineR and
Factoextra packages [
22,
23], while means separation tests were performed using the
agricolae package [
24].
3. Results
3.1. Alternaria brown spot (ABS) severity and incidence
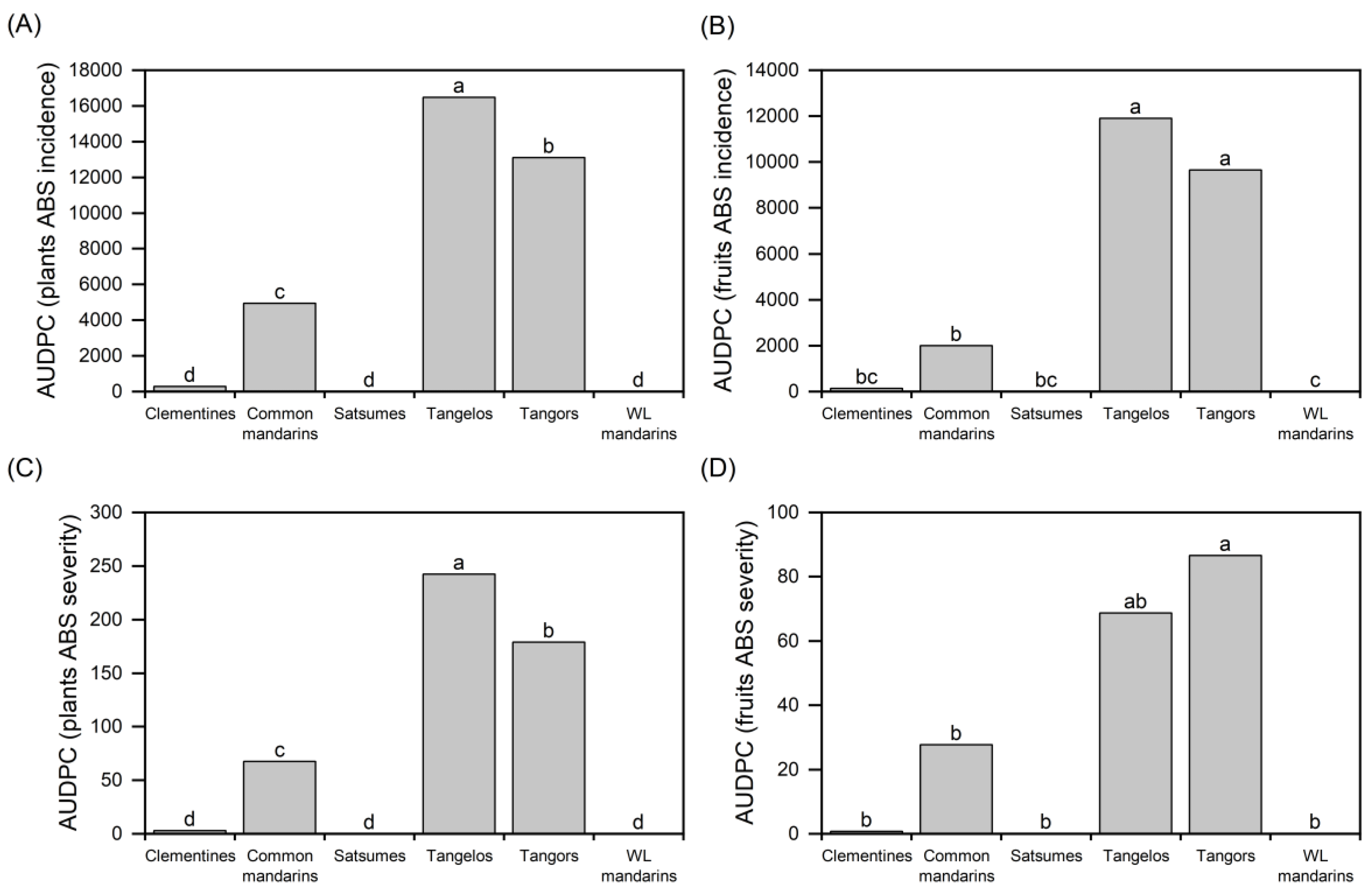
The first symptoms of ABS were observed in the field after three years of planting. Rating carried out in the years after the emergence of symptoms observed that there was a higher incidence (% of genotypes with symptoms) in plants (leaves and branches) and fruits in the tangelo and tangor groups when compared to the common mandarins and hybrids, while clementine, willow leaf (WL) mandarin and satsuma plants and fruits remained asymptomatic (p <0.001) (
Figure 1 A-C). The same was observed for ABS severity in plants (p <0.001) (
Figure 1D).
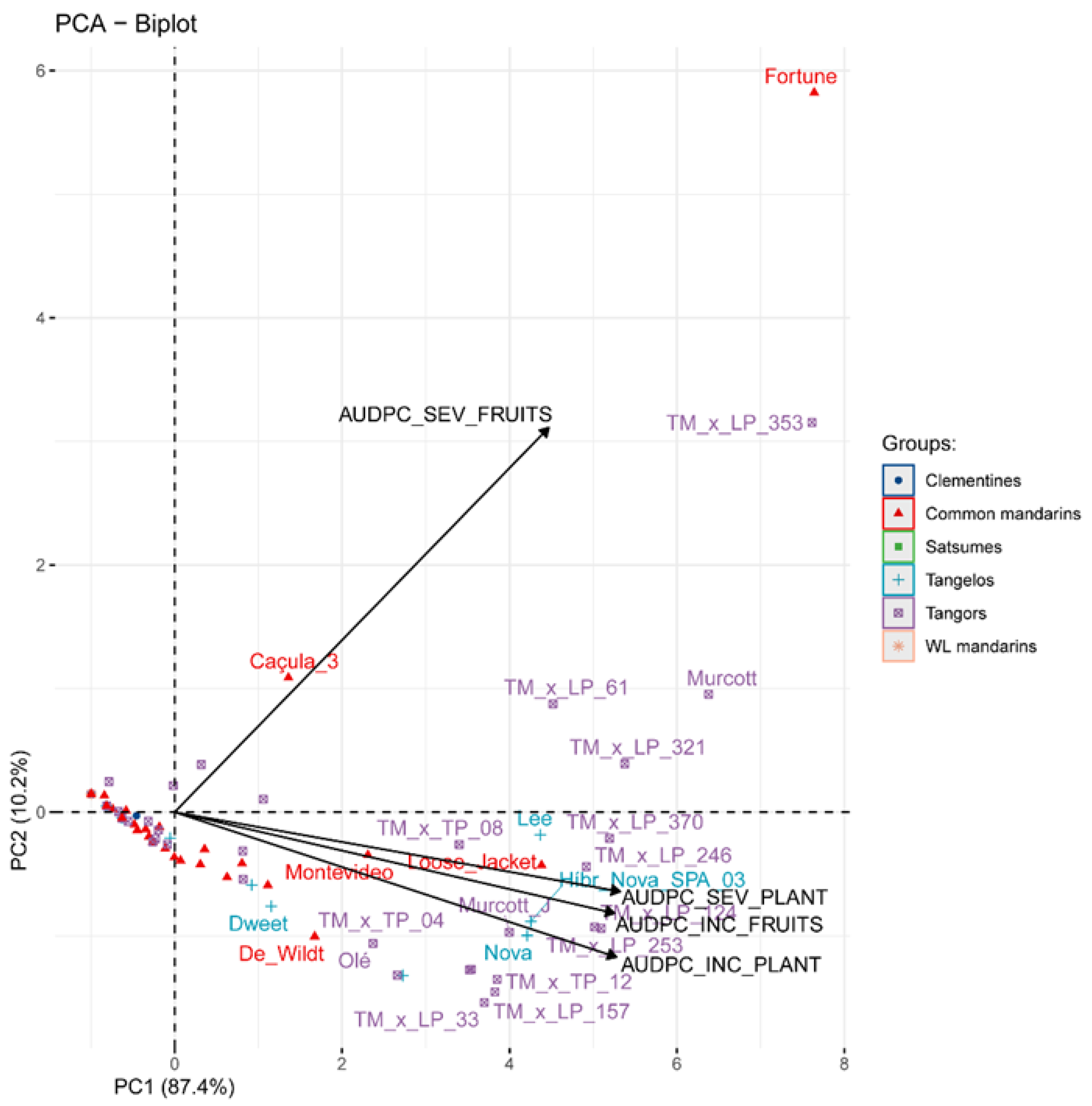
The main citrus varieties among the horticultural groups were used in the principal component analysis (PCA) to distinguish the varieties regarding their susceptibility to ABS. It was possible to identify one main component that comprised most of the variance in this study (PC1: 87.4% of the total variance) (
Figure 2). This component showed a strong positive correlation with plant ABS incidence (r = 0.969) and severity (r = 0.977) and with fruit incidence (r = 0.965) and severity (r = 0.821). This result was expected since the leaves, branches, and fruits are sources of A. alternata inoculum.
Among all varieties, Fortune (common mandarin group) showed the highest levels of severity and incidence in both plants and fruits, followed by the Murcott and TMxLP (Murcott tangor x Pera sweet orange) hybrids, such as the 61, 124, 246, 253, 321, 353, 370 (tangor group), Nova, Nova SPA and Lee (tangelo group), and Loose Jacket (common man-darin group) also showed high ABS susceptibility (
Figure 2).This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.2. HLB severity and incidence
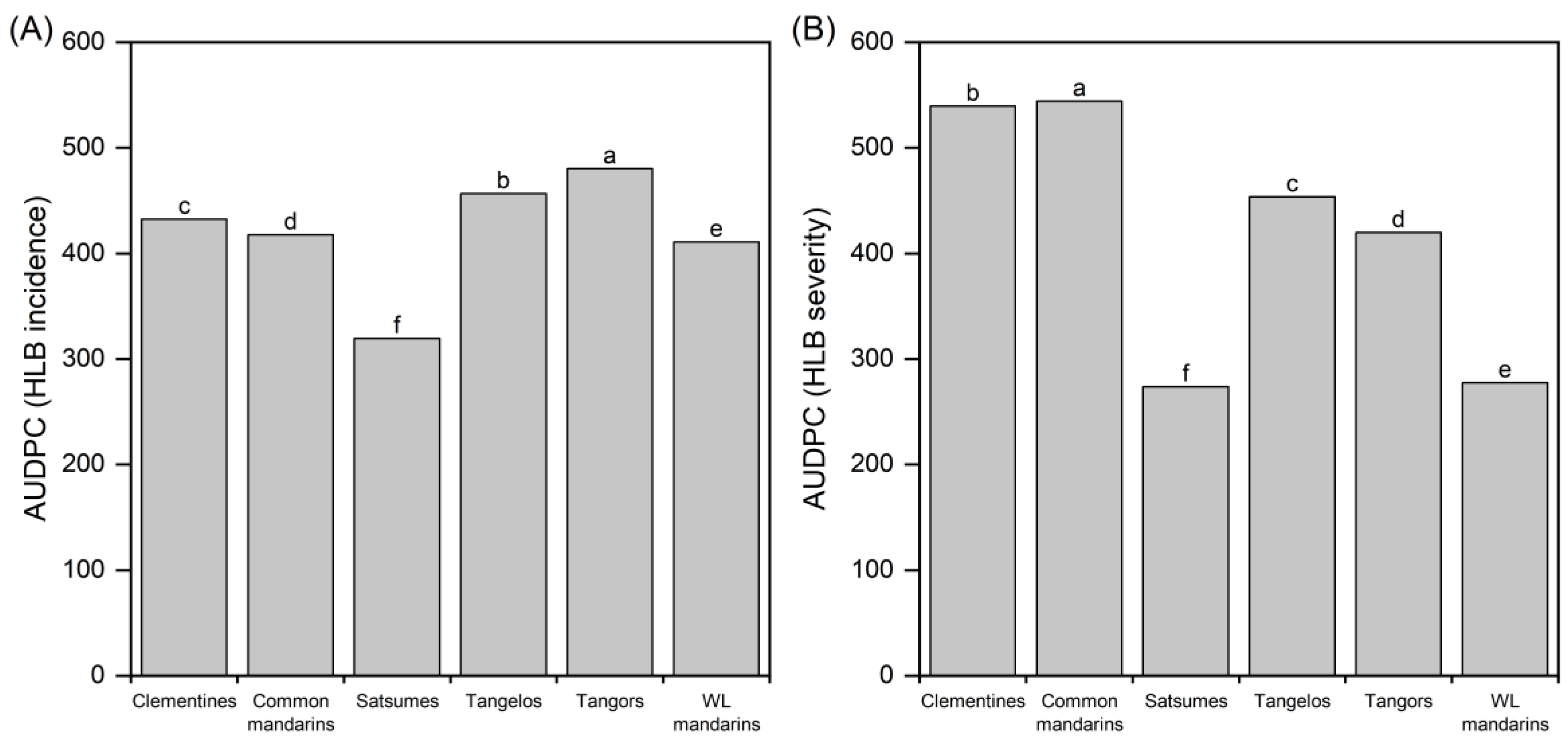
The AUDPC for HLB incidence and severity in different mandarin groups demonstrates significant differences in the incidence and symptoms severity among the common mandarin group (p <0.05) (
Figure 3). Higher HLB incidence (% of infected plants) was observed in tangors and tangelos, followed by common mandarins, clementines, and willow leaf mandarins (WL). Field observations showed that the HLB symptoms are more expressed in common mandarins and clementines, followed by tangelos and tangors. The Satsumes group was the less affected by both HLB-incidence and HLB-severity, followed by WL mandarins for this last.
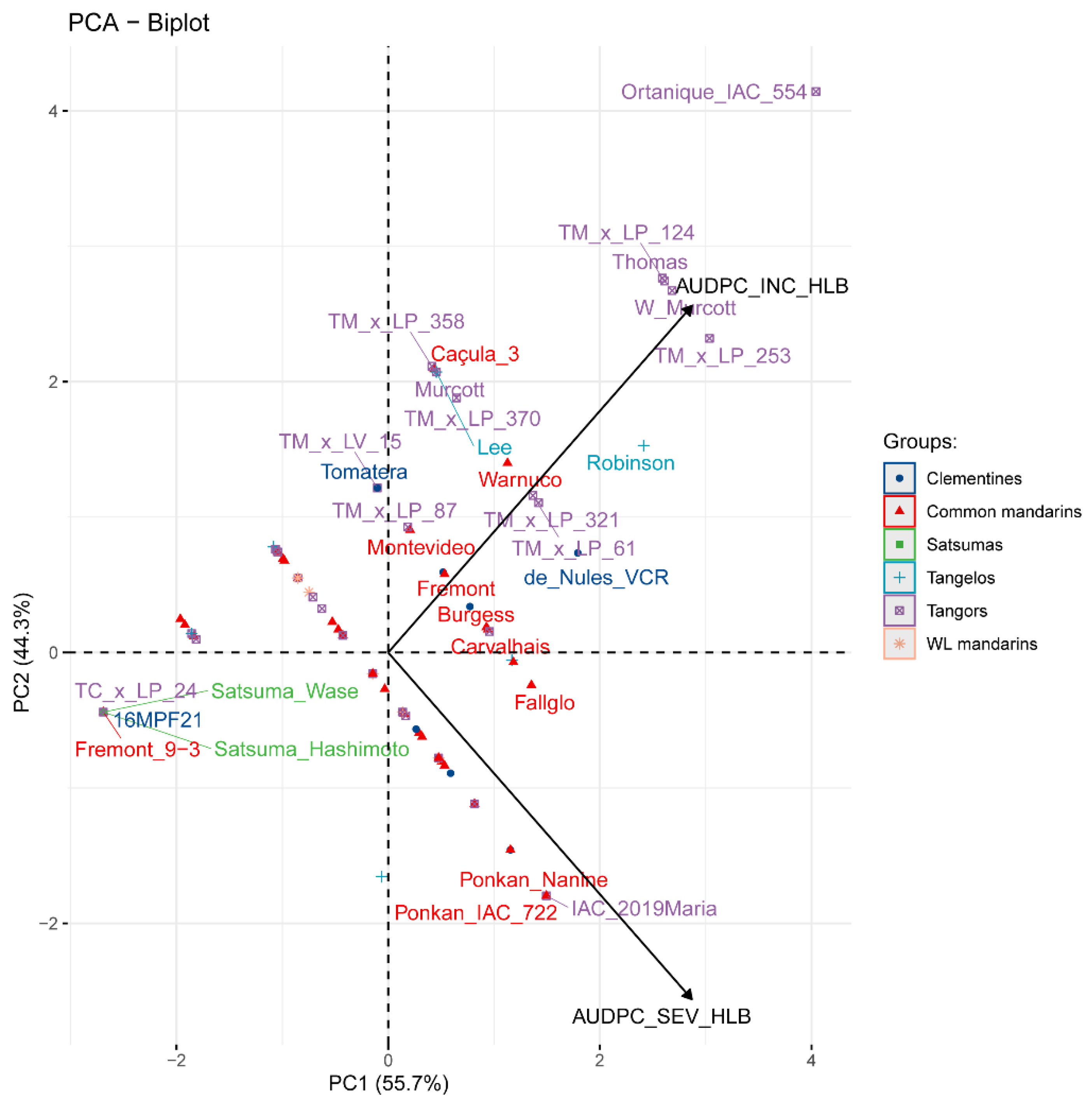
By the PCA of the HLB data, the two main components comprised all the variance (100.0%) (
Figure 4). This component positively correlated (r = 0.746) with HLB incidence and severity. Interestingly, HLB incidence data did not correlate with HLB severity (r = 0.114), as the common mandarin varieties that were shown to be infected by HLB did not always show high severity values. The Ponkan IAC 722 and Ponkan Nanine (common mandarin group), and IAC 2019Maria (tangor group) demonstrated higher HLB severity symptoms, as Ortanique IAC 554, TMxLP 253, W Murcott, TMxLP 124, and Thomas (tangor group) demonstrated higher HLB incidence. Contrarily, Freemont 9-3 common mandarin, TCxLP 24 tangor, Hashimoto and Wase satsumas, and 16MPF21 clementine showed lower HLB incidence values.
3.3. Fruit quality and CLas detection through qPCR
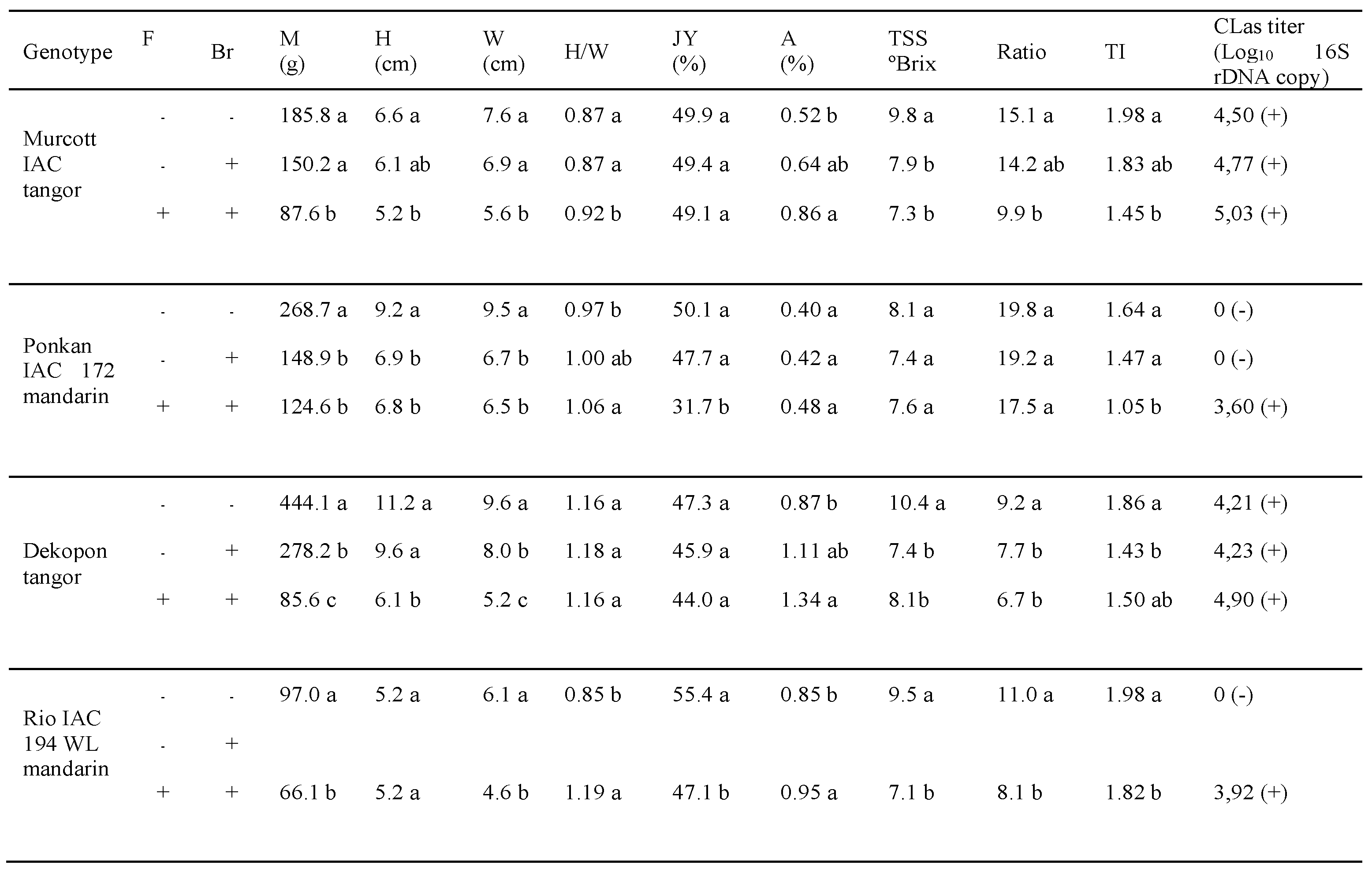
In the analysis of symptomatic and asymptomatic fruits and branches, a decrease in mass, fruit size, total solu-ble solids, ratio, and, consequently, a lower technological index between healthy fruits collected from healthy branches and symptomatic fruits from symptomatic branches was observed for all the varieties studied (
Table 1). These results demonstrate that the bacterium negatively influences fruit quality, limiting the cultivation of mandarins in the presence of HLB disease.
4. Discussion
4.1. Citrus varieties responses to ABS
The willow leaf or mediterranean mandarins and satsumas remained asymptomatic to ABS. Although initially symptoms were observed in satsumas under in vitro inoculation conditions [
25], the resistance of these two mandarin groups was later reported in several studies [
26,
27,
28].
Among the common mandarins, six clones of 'Fremont' obtained from irradiated buds of the Fremont mandarin [
29], IAC 2019Maria mandarin, the first variety developed and protected by the IAC, and the hybrid TMxTP 09 hybrid remained asymptomatic to ABS. The particularity of Fremont clones is their great commercial potential, as they are seedless and have excellent fruit organoleptic characteristics, in addition to smaller plant size; therefore ABS resistance adds even more value to a new cultivar. Some authors have reported the tolerance of Fremont mandarin to
A. alternata [
5,
30], this ABS resistance probably inherited from the parent Clementina, since it is a hybrid of this variety.
Likewise, IAC219 Maria mandarin, is a new variety resulting from the IAC Breeding Program, recently licensed (2019) and commercially planted. The TMxLP 09 hybrid is another variety resulting from the Program, whose fruits meet the demand of the Brazilian consumer, with characteristics similar to 'Ponkan', the most consumed variety, but with a later maturation period.
All clementine accessions remained asymptomatic, confirming the tolerance of this group to ABS [
31], however, clementine hybrids were very susceptible to the disease, such as Fortune an Caçula 3 IAC 1330 and Caçula 4 IAC 1318 (
Figure 2). The fact that the origin of these last three materials are hybrids of crosses between a clementine and Cravo mandarin, both tolerant to disease, shows that other factors may be involved in the inheritance of resistance/susceptibility to MMA [
31,
32].
The identification of tolerance to ABS in mandarin genotypes makes them potentially interesting for use in commercial orchards or as parents in breeding programs. Recent studies indicate the potential of using molecular markers in assisted selection in breeding progenies [
5,
33], since they allow the early selection of hybrids resistant from directed crosses, an affordable and efficient strategy to obtain new resistant varieties.
The severity observed among all accessions was very variable. ´'Fortune' mandarin was one of the most susceptible varieties to ABS in our study. Among the mandarins’ varieties and their cultivated hybrids 'Dancy' and 'Fortune' have been reported as the most susceptible [
34], as well as the 'Minneola', 'Orlando', 'Sunburst' and 'Nova' tangelos [
35]. This high susceptibility of the Fortune' has been widely reported and has been cited as the probable causes of its reduction in planting in Spain over the years, where it is currently no longer found in orchards [
6,
7]. Recently characterized by molecular markers as a clementine x Orlando tangelo hybrid [
36], Fortune mandarin appears to have inherited the high susceptibility of tangelo. The four tangelos abovementioned (Minneola, Orlando, Sumbusrt and Nova) have Dancy mandarin as parental. This fact can be the the reason for their extreme susceptibility to ABS. Unfortunately, 'Dancy' was not evaluated in this study, but it is known that all these tangelos.
In this study, dozens of mandarins and hybrids were evaluated under field conditions. Diferences on ABS incidence and susceptibility observed wonder natural inoculation of pathogen, reducing production costs and damage. environmental damage caused by the intensive application of fungicides, currently dispensed with for the cultivation of susceptible varieties. The evaluation of fruit production and quality may indicate potential varieties for the Brazilian citrus industry.
4.2. Citrus varieties responses to HLB
Although no known HLB-resistant citrus species or varieties have been identified, some citrus accessions are reportedly tolerant [
37,
38]. Differential anatomical responses of tolerant and susceptible citrus species were observed to the infection of 'Candidatus Liberibacter asiaticus' (37). In an experiment conducted in a greenhouse McCollum et al. [
38], showed good agreement with trends observed in the orchard for susceptibility to HLB. The authors observed that
Citrus macrophylla e
C. medica trees were most susceptible to 'Ca.L.asiaticus', with 100% infection by the end of the test period, while the complex genetic hybrids' US 1-4-59' and 'Fallglo' consistently were the east susceptible, with approximately 30% infection.
Recently, some citrus cultivars released by the UF-CREC breeding program have exhibited tolerance to HLB, specifically LB8-9" (Sugar Belle) and 7-6-27" mandarin. Gmitter et al [
39] studying some hybrids populations of mandarins observed that the tolerant trees represent very low percentage (5.3%) comparing to the other categories in the field evaluation for symptoms of HLB disease. Based on the number of trees in every cross, we can notice that more than 30 % of cross {(Clementine x Temple) x
C. ichangensis} were tolerant trees, followed by {(Clementine x Temple) x Swingle} with ratio of 22.72% tolerant trees. The cross {(Clementine x Temple) x
C. ichangensis} includes most of HLB tolerant trees in visual evaluation. In the present work, Field observations showed that the symptoms are more expressed in tangors, tangelos, and mandarins than clementines and willow leaf mandarins (WL mandarin), as these seem to be less affected by the disease. Interestingly, HLB incidence data did not correlate with HLB severity, as the citrus varieties that were shown to be infected by HLB did not always show high severity values. A decrease in mass, fruit size, total soluble solids, ratio and, consequently, a lower technological index between healthy fruits collected from healthy branches and symptomatic fruits from symptomatic branches was observed for all the varieties studied. These results demonstrate that the bacterium negatively influences fruit quality, limiting the cultivation of mandarins in the presence of HLB disease.
5. Conclusion
Evaluations of the incidence and severity of the diseases showed that there is a range of genotypes tolerant to ABS with potential to replace the current commercial varieties, mainly within the clementina and Willow leaf mandarin groups. Although with differences in incidence and susceptibility to HLB, there are no varieties tolerant to this disease, which significantly impacts the loss of fruit quality.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, M.B, F.A.A. and M.CY; methodology, M.B, F.A.A., F.T.D., E.S.S, R.V.F and M.CY and M.B.; formal analysis, , M.B, F.A.A., F.T.D, R.M. and M.CY ; investigation, F.T.D and F.A.A.; resources, , F.T.D and F.A.A.; data curation, F.T.D..; writing —original draft preparation, R.M., M.B, F.A.A., F.T.D., E.S.S, R.V.F and M.CY.; writing—review and editing, , R.M., M.B, F.A.A., F.T.D., E.S.S, R.V.F and M.CY. All authors have read and agreed to the published version of the manuscript.
Funding
Financial Support: This study was partially funded by the research projects: Fapesp nº 2014/50880-0, nº 2017/24564-1, nº2020/07045-3, and CNPq nº 465-440/2014-2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization. FAOSTAT: Statistical database. Disponível em: <http://faostat.fao.org/site/567/default.aspx>. Acessado em: 06 de setembro, 2022.
- Azevedo, F. A.; Milaneze, T. F.; da Conceição, P. M.; de Andrade Pacheco, C.; Martinelli, R.; Bastianel, M. Winter pruning: option for management against alternaria brown spot ('Alteraria alternata'f. sp.'citri') in Honey Murcott tangor ['Citrus reticulata 'Blanco x 'C. sinensis'(L.) Osbeck]. Australian Journal of Crop Science, 2019, 13(10), 1631-1637.
- IBGE. Brazilian Institute of Geography and Statistics - IBGE. World agricultural production. Available online: <https://www.ibge.gov.br/estatisticas-novoportal/economicas/agricultura-e-pecuaria/9201-levantamento-sistematico-da-producao-agricola.html?=&t=o-que-e:> Accessed on: 10 april 2023.
- Timmer, L. W.; Peever, T. L.; Solel, Z.; Akimitsu, K. Alternaria diseases of citrus - Novel pathosystems. Phytopathol. Mediterr., 2003, 42(3), 16.
- Arlotta, C.; Ciacciulli, A.; Strano, M.C.; Cafaro, V., Salonia; F., Caruso; P., Caruso; M. Disease resistant citrus breeding using newly developed high resolvution melting and CAPS protocols for Alternaria brown spot marker assisted selection. Agronomy 2020, 10(9), 1368.
- Bassimba, D. D. M.; Mira, J.L.; Vicent, A. Evaluation of models for Alternaria brown spot of mandarin under Mediterranean conditions by partial receiver operating characteristic curve analysis. Phytopathology, 2017, 107, 721–731.
- De-Miguel, M.D.; Caballero, P.; Fernández-Zamudio, M.A. Varietal Change Dominates Adoption of Technology in Spanish Citrus Production. Agronomy, 2019, 9, 631.
- Stuart, R. M.; Bastianel, M.; de Azevedo, F. A.; Machado, M. A. Alternaria brown spot. Citrus Research & Technology, 2017, 30(1-2), 29-44.
- Timmer, L. W.; Darhower, H. M.; Zitko, S. E.; Peever, T. L.; Ibanez, A. M.; Bushong, P. M. Environmental factors affecting the severity of Alternaria brown spot of citrus and their potential use in timing fungicide applications. Plant Disease, 2000, 84(6), 638-643.
- Vicent, A., Armengol, J., and García-Jiménez, J. Rain fastness and persistence of fungicides for control of Alternaria brown spot of citrus. Plant Dis., 2007, 91, 393-399. https://doi.org/10.1094/PDIS-91-4-0393.
- Chitolina, G.M ; Silva-Junior, G.J. ; Feichtenberger, E. ; Pereira, Rosana G. ; Amorim, Lilian . Distribution of Alternaria alternata isolates with resistance to quinone outside inhibitor (QoI) fungicides in Brazilian orchards of tangerines and their hybrids. CROP PROTECTION , v. 141, p. 105493, 2021.
- Baraldi, A. L. M.; Poloni, N. M.; Fischer Filho, J. A.; Pereira, F. D.; de Góes, A. Sensibilidade de Alternaria alternata, agente causal da mancha marrom de alternária a fungicidas do grupo químico das estrobilurinas. Citrus Research & Technology, 2022, 42, 0-1.
- Coletta-Filho, H. D.; Targon, M. L. P. N.; Takita, M. A.; De Negri, J. D.; Pompeu Jr, J.; Machado, M. A.; Muller, G. W. First report of the causal agent of Huanglongbing (“Candidatus Liberibacter asiaticus”) in Brazil. Plant Disease, 2004, 88(12), 1382-1382.
- Volk, GM; Gmitter Jr.,F; Krueger, R.R. Conserving Citrus diversity: From Vavilov´s early explorations to Gnebanks around the world. Plants, 2023, 12, 814.
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [CrossRef]
- Bastianel, M.; Simonetti, L. M.; Schinor, E. H.; Giorgi, R. O. D.; Negri, J. D. D.; Gomes, D. N.; Azevedo, F. A. D. Avaliação do banco de germoplasma de mexericas com relação às características físico-químicas e suscetibilidade à mancha marrom de alternária. Bragantia, 2014, 73, 23-31.
- Renaud, M.S.A.; Amorin, L.; Lourenço, S.A.; Spósito, M.B.. Diagrammatic scale for assessment of Alternaria Brown Spot of citrus. Summa Phytopathologica, 2008, 34, 270–272.
- Shaner, G., & Finney, R. E. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology, 1977, 67(8), 1051-1056.
- Gottwald. T. R., B. Aubert, X.-Y. Zhao. Preliminary analysis of citrus greening (Huanglonbing) epidemics in the People's Republic of China and French Reunion Island. Phytopathology, 1989, 79, 687-693.
- Lopes, S. A.; Luiz, F. Q. B. F.; Martins, E. C.; Fassini, C. G.; Sousa, M. C.; Barbosa, J. C.; Beattie, G. A. C. ‘Candidatus Liberibacter asiaticus’ titers in citrus and acquisition rates by Diaphorina citri are decreased by higher temperature. Plant disease, 2013, 97(12), 1563-1570.
- Zuur, A. F.; Ieno, E. N.; Smith, G. M. Principal component analysis and redundancy analysis. In Zuur, A., Ieno, E. N., Smith, G. M. Analysing ecological data. New York: Springer, 2007, 193-224.
- Lê S.; Josse J.; Husson F. FactoMineR: A Package for Multivariate Analysis.”Journal of Statistical Software, 2008, 25(1), 1–18.
- Kassambara, A. and Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7, 2020.
- De Mendiburu, F. Agricolae: statistical procedures for agricultural research. R package version 1.3.5, 2021, 157.
- Soleil, Z.; Kimchi, M. Susceptibility and Resistance of Citrus Genotypes to Alternaria alternata pv. citri. J. Phytopathol. 1997, 145, 389–391.
- Vicent, A.; Badal, J.; Asensi, M.J.; Sanz, N.; Armengol, J.; Garc, J. Laboratory evaluation of citrus cultivars susceptibility and influence of fruit size on Fortune mandarin to infection by Alternaria alternata pv. citri. Eur. J. Plant Pathol. 2004, 110, 245–251.
- Reis, R. F.; De Almeida, T. F.; Stuchi, E. S.; De Goes, A. Susceptibility of citrus species to Alternaria alternata, the causal agent of the Alternaria brown spot. Scientia Horticulturae, 2007, 113(4), 336-342.
- Cuenca, J.; Navarro, L.; Aleza, P. Advances in mandarin breeding. In Achieving sustainable cultivation of tropical fruits. Burleigh Dodds Science Publishing, 2019, 1, 183-212.
- Bastianel, M.; Barros, V.L.N.P., Tulmann Neto, A.; Souza, P. S.; Pio, R. M.; Latado, R. R. Induction and Selection of Mandarin Mutants with Fruits Containing Low Number of Seeds. In: Sivasankar S.; Ellis, N.; Jankuloski, L.; Ingelbrecht, I.. (Org.). Mutation Breeding, Genetic Diversity and Crop Adaptation to Climate Change. 1ed.Oxfordshire: CAB International, 2019, 379-385.
- Azevedo, F. A.; Polydoro, D. A.; Bastianel, M.; Kupper, K. C.; Stuart, R. M.; Costa, F. P.; Pio, R. M. Resposta de diferentes genótipos de tangerinas e seus híbridos à inoculação in vitro e in vivo de Alternaria alternata. Revista Brasileira de Fruticultura, 2010, 32, 944-951.
- Cuenca, J.; Aleza, P.; Vicent, A.; Brunel, D.; Ollitrault, P.; Navarro, L. Genetically Based Location from Triploid Populations and Gene Ontology of a 3.3-Mb Genome Region Linked to Alternaria Brown Spot Resistance in Citrus Reveal Clusters of Resistance Genes. PLoS ONE, 2013, 8, e76755.
- Michielin, T. H. V.; Cristofani-Yaly, M.; Campos, K. A. F. D.; Schinor, E. H.; Azevedo, F. A. D.; Bastianel, M. Reação de híbridos de citros à inoculação com Alternaria alternata. Summa Phytopathologica, 2016, 42, 313-320.
- Cuenca, J.; Navarro, L.; Aleza, P. Advances in mandarin breeding. In Achieving sustainable cultivation of tropical fruits. Burleigh Dodds Science Publishing, 2019, 1, 183-212.
- Nemsa, I., Hernández, M. A., Lacasa, A., Porras, I., García-Lidón, A., Cifuentes, D., ... & Del Río, J. A. Pathogenicity of Alternaria alternata on fruits and leaves of ‘Fortune’mandarin (Citrus clementina× Citrus tangerina). Canadian journal of plant pathology, 2012, 34(2), 195-202.
- Peever, T. L.; Olsen, L.; Ibanez, A.; Timmer, L. W. Genetic differentiation and host specificity among populations of Alternaria spp. causing brown spot of grapefruit and tangerine× grapefruit hybrids in Florida. Phytopathology, 2000, 90(4), 407-414.
- Barry, G.; Gmitter, F.; Chen, C.; Roose, M.; Federici, C.; McCollum, G. T. Investigating the parentage of 'Orri’and ‘Fortune’mandarin hybrids. Acta Hortic, 2015, 1065, 449-456.
- Fan, J., Chen, C., Achor, D. S., Brlansky, R. H., Li, Z.-G., and Gmitter, F. G. Differential anatomical responses of tolerant and susceptible citrus species to the infection of ‘Candidatus Liberibacter asiaticus’. Physiol. Mol. Plant P., 2013, 83, 69–74.
- McCollum, G.; Hilf, M.; Irey, M.; Luo, W.; Gottwald, T. Susceptibility of Sixteen Citrus Genotypes to ‘Candidatus Liberibacter asiaticus’. Plant Disease, 2016, 100(6), 1080-1086.
- Deng, H., Achor, D., Exteberria, E., Yu, Q., Du, D., Stanton, D., Liang, G., Gmitter Jr., F.G., Phloem regeneration is a mechanism for Huanglongbing-tolerance of “Bearss” lemon and “LB8-9” Sugar Belle® mandarin. Front. Plant Sci., 2019, 10.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).