Submitted:
28 April 2023
Posted:
29 April 2023
You are already at the latest version
Abstract
Keywords:
Introduction
II.1- Putative epigenetic drivers in ulcer recurrence
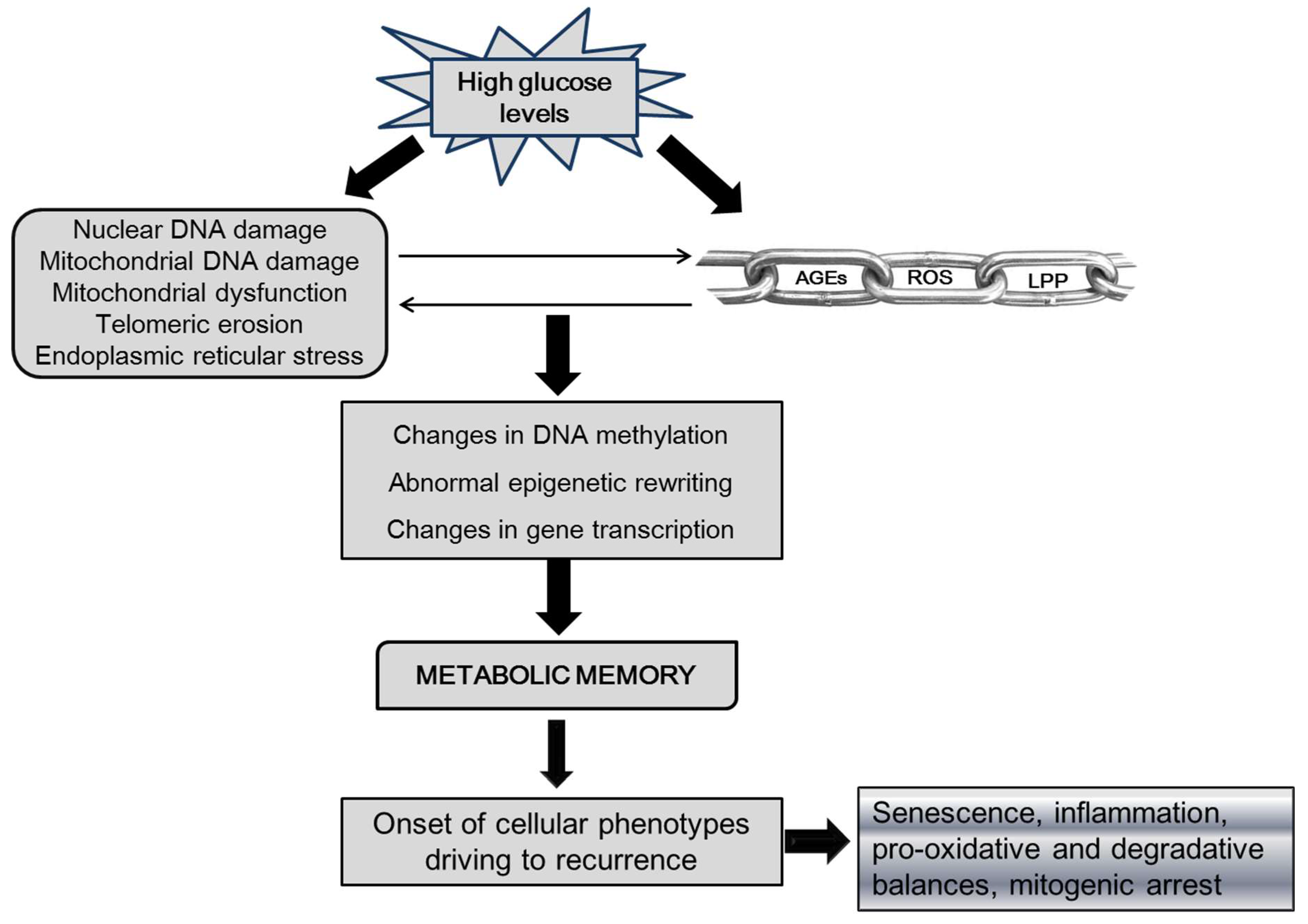
II.2- Dermal matrix, fibroblasts, and keratinocytes in ulcer recurrence
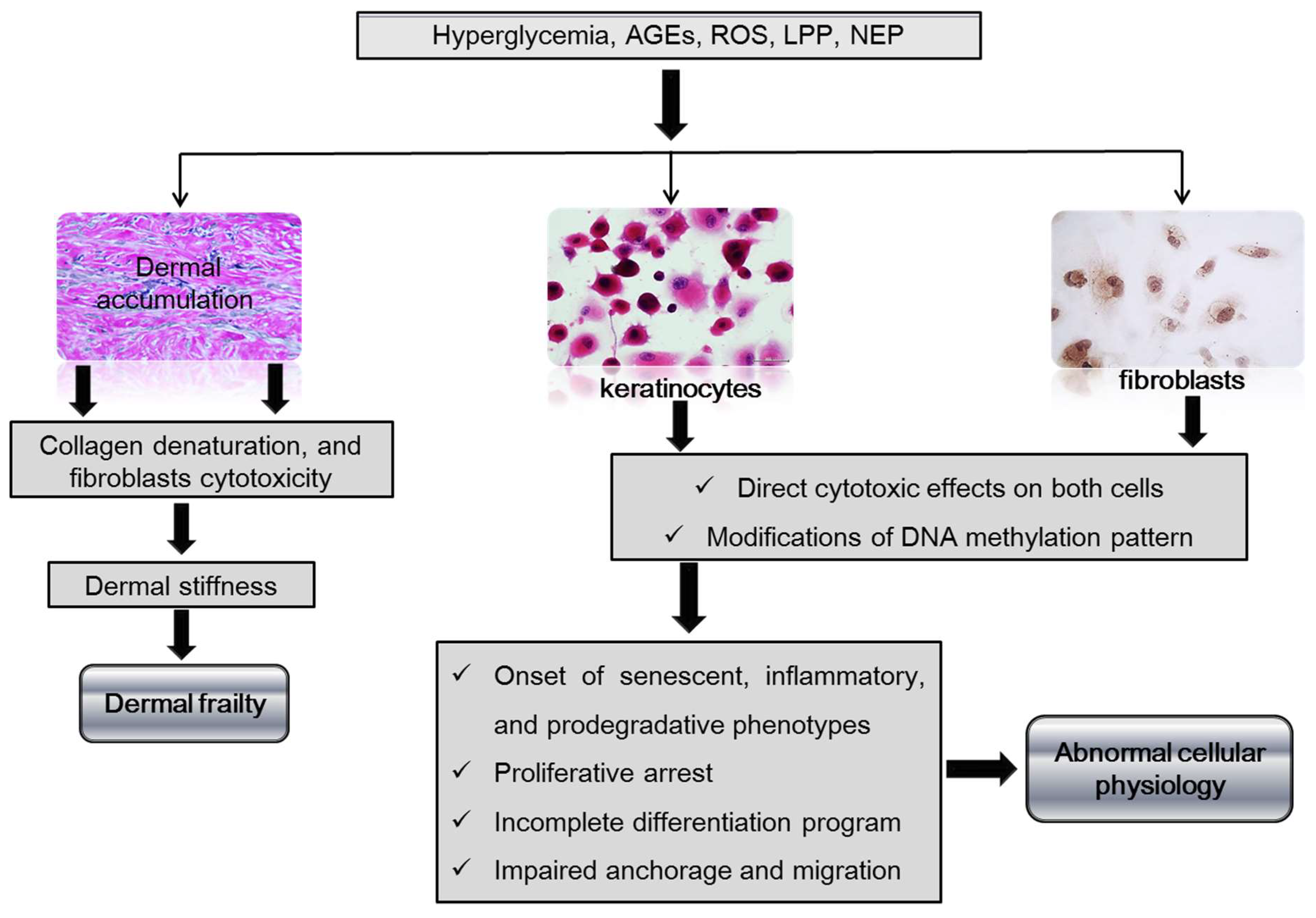
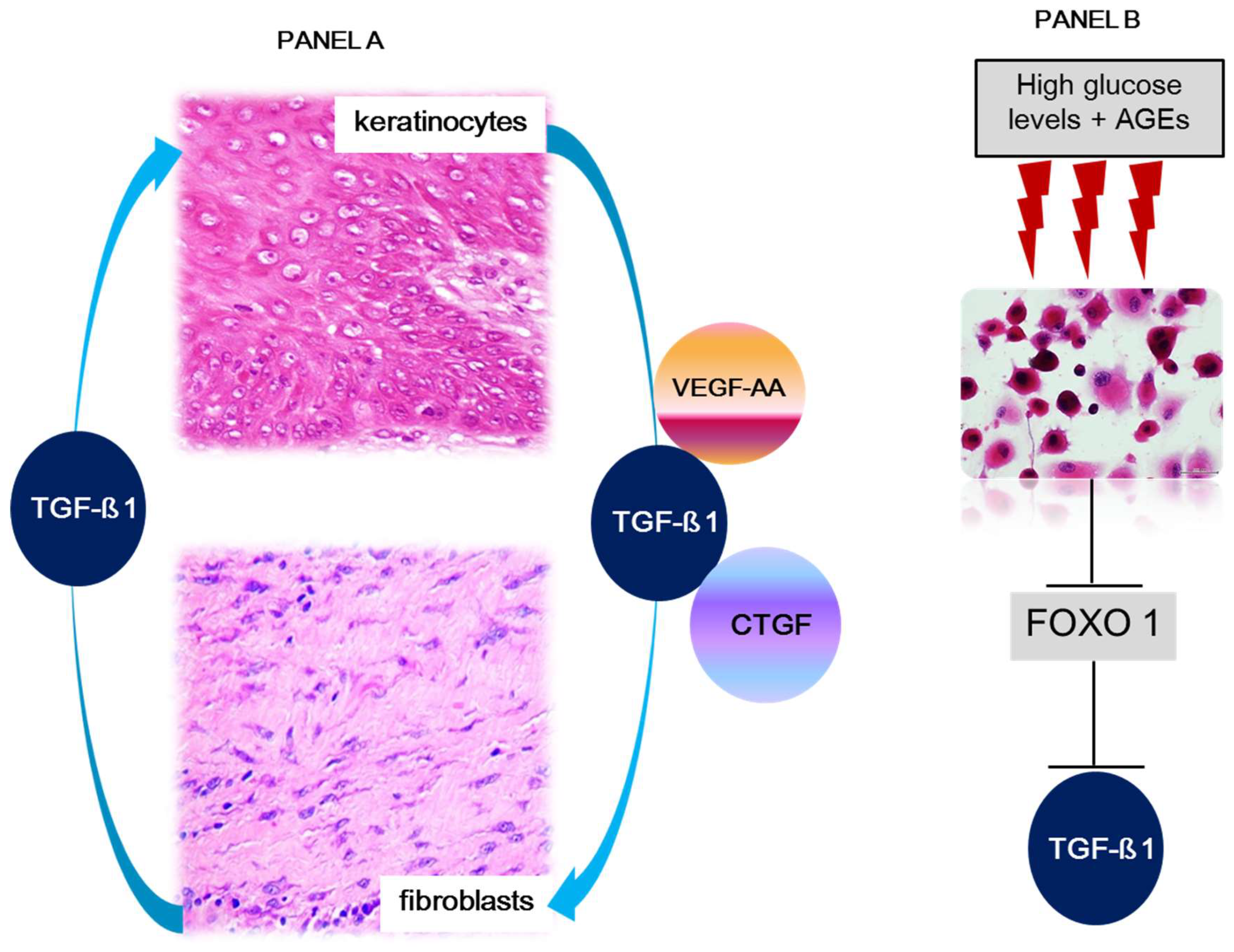
III- Ulcer recurrences in the clinical arena
IV- Concluding remarks and future directions
Authors contributions
Funding
Conflicts of Interest
References
- Cheng, H.T.; Xu, X.; Lim, P.S.; Hung, K.Y. Worldwide Epidemiology of Diabetes-Related End-Stage Renal Disease, 2000-2015. Diabetes Care. 2021, 44, 89–97. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Z.H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; et al. Trends in the incidence of diabetes mellitus: results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health. 2020, 20, 1415. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; et al. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020, 21. [Google Scholar] [CrossRef]
- Magliano, D.J.; Sacre, J.W.; Harding, J.L.; Gregg, E.W.; Zimmet, P.Z.; Shaw, J.E. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol. 2020, 16, 321–31. [Google Scholar] [CrossRef]
- Rosengren, A.; Dikaiou, P. Cardiovascular outcomes in type 1 and type 2 diabetes. Diabetologia. 2023, 66, 425–37. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, D.; Alonso, N.; Gratacos, M. Chronic Diabetes Complications: The Need to Move beyond Classical Concepts. Trends Endocrinol Metab. 2020, 31, 287–95. [Google Scholar] [CrossRef]
- Natarajan, R. Epigenetic Mechanisms in Diabetic Vascular Complications and Metabolic Memory: The 2020 Edwin Bierman Award Lecture. Diabetes. 2021, 70, 328–37. [Google Scholar] [CrossRef]
- Deng, J.Y.; Wu, X.Q.; He, W.J.; Liao, X.; Tang, M.; Nie, X.Q. Targeting DNA methylation and demethylation in diabetic foot ulcers. J Adv Res. 2023. [CrossRef]
- Dogruel, H.; Aydemir, M.; Balci, M.K. Management of diabetic foot ulcers and the challenging points: An endocrine view. World J Diabetes. 2022, 13, 27–36. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Gao, Y.; Ran, X. Global mortality of diabetic foot ulcer: A systematic review and meta-analysis of observational studies. Diabetes Obes Metab. 2023, 25, 36–45. [Google Scholar] [CrossRef]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care. 2023, 46, 209–21. [Google Scholar] [CrossRef]
- Boulton, A.J.M.; Armstrong, D.G.; Londahl, M.; Frykberg, R.G.; Game, F.L.; Edmonds, M.E.; et al. New Evidence-Based Therapies for Complex Diabetic Foot Wounds. Arlington (VA) 2022. [CrossRef] [PubMed]
- Rai, V.; Moellmer, R.; Agrawal, D.K. Clinically relevant experimental rodent models of diabetic foot ulcer. Mol Cell Biochem. 2022, 477, 1239–47. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, N.; Martinez-Jimenez, I.; Garcia-Ojalvo, A.; Mendoza-Mari, Y.; Guillen-Nieto, G.; Armstrong, D.; et al. Wound chronicity, impaired immunity and infection in diabetic patients. MEDICC Review. 2022, 24, 44–58. [Google Scholar] [CrossRef]
- Leal, E.C.; Carvalho, E. Heme Oxygenase-1 as Therapeutic Target for Diabetic Foot Ulcers. Int J Mol Sci. 2022, 23. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.A.; Guillen-Nieto, G.E.; Rodriguez-Rodriguez, N.; Mendoza-Mari, Y.; Bringas-Vega, M.L.; Berlanga-Saez, J.O.; et al. Cellular Senescence as the Pathogenic Hub of Diabetes-Related Wound Chronicity. Front Endocrinol (Lausanne). 2020, 11, 573032. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, P.; Vasishta, S.; Balakrishnan, A.; Joshi, M. Mechanistic insights into glucose induced vascular epigenetic reprogramming in type 2 diabetes. Life Sciences. 2022, 298, 120490. [Google Scholar] [CrossRef]
- Bouly, M.; Laborne, F.X.; Tourte, C.; Henry, E.; Penfornis, A.; Dardari, D. Post-healing follow-up study of patients in remission for diabetic foot ulcers Pied-REM study. PLoS One. 2022, 17, e0268242. [Google Scholar] [CrossRef]
- Guo, Q.; Ying, G.; Jing, O.; Zhang, Y.; Liu, Y.; Deng, M.; et al. Influencing factors for the recurrence of diabetic foot ulcers: A meta-analysis. Int Wound J. 2023, 20, 1762–75. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017, 376, 2367–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Li, S.Q.; Kou, Y.; Huang, L.; Yu, T.; Hu, A. Risk factors for the recurrence of diabetic foot ulcers among diabetic patients: a meta-analysis. Int Wound J. 2019, 16, 1373–82. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.J.; Rothenberg, G.M.; Lakhani, P.J.; Zhou, M.; Linders, D.R.; Bloom, J.D.; et al. Ulcer metastasis? Anatomical locations of recurrence for patients in diabetic foot remission. J Foot Ankle Res. 2020, 13, 1. [Google Scholar] [CrossRef]
- Engberg, S.; Kirketerp-Moller, K.; Ullits Andersen, H.; Rasmussen, A. Incidence and predictors of recurrent and other new diabetic foot ulcers: a retrospective cohort study. Diabet Med. 2019, 36, 1417–23. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Canner, J.K.; Mathioudakis, N.; Lippincott, C.; Sherman, R.L.; Abularrage, C.J. Incidence and Risk Factors Associated With Ulcer Recurrence Among Patients With Diabetic Foot Ulcers Treated in a Multidisciplinary Setting. J Surg Res. 2020, 246, 243–50. [Google Scholar] [CrossRef]
- Gazzaruso, C.; Gallotti, P.; Pujia, A.; Montalcini, T.; Giustina, A.; Coppola, A. Predictors of healing, ulcer recurrence and persistence, amputation and mortality in type 2 diabetic patients with diabetic foot: a 10-year retrospective cohort study. Endocrine. 2021, 71, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Wang, Y.; Zheng, M.; Wang, Q.; Lin, H.; Zhang, L.; et al. Transcriptomic study of high-glucose effects on human skin fibroblast cells. Mol Med Rep. 2016, 13, 2627–34. [Google Scholar] [CrossRef]
- Cugalj Kern, B.; Trebusak Podkrajsek, K.; Kovac, J.; Sket, R.; Jenko Bizjan, B.; Tesovnik, T.; et al. The Role of Epigenetic Modifications in Late Complications in Type 1 Diabetes. Genes (Basel). 2022, 13. [Google Scholar] [CrossRef]
- Ramteke, P.; Deb, A.; Shepal, V.; Bhat, M.K. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers (Basel). 2019, 11. [Google Scholar] [CrossRef]
- Rehman, S.; Aatif, M.; Rafi, Z.; Khan, M.Y.; Shahab, U.; Ahmad, S.; et al. Effect of non-enzymatic glycosylation in the epigenetics of cancer. Semin Cancer Biol. 2022, 83, 543–55. [Google Scholar] [CrossRef]
- Chen, Z.; Miao, F.; Braffett, B.H.; Lachin, J.M.; Zhang, L.; Wu, X.; et al. DNA methylation mediates development of HbA1c-associated complications in type 1 diabetes. Nat Metab. 2020, 2, 744–62. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Stefan-Lifshitz, M.; Tomer, Y. Genetic and environmental factors regulate the type 1 diabetes gene CTSH via differential DNA methylation. J Biol Chem. 2021, 296, 100774. [Google Scholar] [CrossRef]
- Park, L.K.; Maione, A.G.; Smith, A.; Gerami-Naini, B.; Iyer, L.K.; Mooney, D.J.; et al. Genome-wide DNA methylation analysis identifies a metabolic memory profile in patient-derived diabetic foot ulcer fibroblasts. Epigenetics. 2014, 9, 1339–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luan, Y.; Feng, Q.; Chen, X.; Qin, B.; Ren, K.D.; et al. Epigenetics and Beyond: Targeting Histone Methylation to Treat Type 2 Diabetes Mellitus. Front Pharmacol. 2021, 12, 807413. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.R.; Habib, S.; Uddin, M. Recent advances in histone glycation: emerging role in diabetes and cancer. Glycobiology. 2021, 31, 1072–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Omans, N.D.; Leicher, R.; Osunsade, A.; Agustinus, A.S.; Finkin-Groner, E.; et al. Reversible histone glycation is associated with disease-related changes in chromatin architecture. Nat Commun. 2019, 10, 1289. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, M.; Liu, X.; Xiao, S.; Cai, Y.; Li, F.; et al. The progress, prospects, and challenges of the use of non-coding RNA for diabetic wounds. Mol Ther Nucleic Acids. 2021, 24, 554–78. [Google Scholar] [CrossRef]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008, 205, 2409–17. [Google Scholar] [CrossRef]
- Bhamidipati, T.; Kumar, M.; Verma, S.S.; Mohanty, S.K.; Kacar, S.; Reese, D.; et al. Epigenetic basis of diabetic vasculopathy. Front Endocrinol (Lausanne). 2022, 13, 989844. [Google Scholar] [CrossRef]
- Goodarzi, G.; Maniati, M.; Qujeq, D. The role of microRNAs in the healing of diabetic ulcers. Int Wound J. 2019, 16, 621–33. [Google Scholar] [CrossRef]
- Ozdemir, D.; Feinberg, M.W. MicroRNAs in diabetic wound healing: Pathophysiology and therapeutic opportunities. Trends Cardiovasc Med. 2019, 29, 131–7. [Google Scholar] [CrossRef]
- Lou, R.; Chen, J.; Zhou, F.; Wang, C.; Leung, C.H.; Lin, L. Exosome-cargoed microRNAs: Potential therapeutic molecules for diabetic wound healing. Drug Discov Today. 2022, 27, 103323. [Google Scholar] [CrossRef] [PubMed]
- Ross, K. MiR equal than others: MicroRNA enhancement for cutaneous wound healing. J Cell Physiol. 2021, 236, 8050–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, C.; Wang, X.Y.; Zhou, L.Y.; Lao, G.J.; Liu, D.; et al. MicroRNA-129 and -335 Promote Diabetic Wound Healing by Inhibiting Sp1-Mediated MMP-9 Expression. Diabetes. 2018, 67, 1627–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, W.; Zhang, L.; Dorset-Martin, W.; Morris, M.W.; Mitchell, M.E.; et al. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes. 2012, 61, 2906–12. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Tang, Y.; Zhao, X.; Xie, D.; Chen, M. Increased Expression of miR-155 in Peripheral Blood and Wound Margin Tissue of Type 2 Diabetes Mellitus Patients Associated with Diabetic Foot Ulcer. Diabetes Metab Syndr Obes. 2022, 15, 3415–28. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Shu, B.; Wang, P.; Tang, J.; Chen, L.; et al. Quantification of the differential expression levels of microRNA-203 in different degrees of diabetic foot. Int J Clin Exp Pathol. 2015, 8, 13416–20. [Google Scholar] [PubMed]
- Wu, T.; Xie, D.; Zhao, X.; Xu, M.; Luo, L.; Deng, D.; et al. Enhanced Expression of miR-34c in Peripheral Plasma Associated with Diabetic Foot Ulcer in Type 2 Diabetes Patients. Diabetes Metab Syndr Obes. 2021, 14, 4263–73. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Kuai, L.; Jiang, J.S.; Li, W.; Li, B.; Yin, S.Y. Long non-coding RNAs in diabetic wound healing: Current research and clinical relevance. Int Wound J. 2022, 19, 583–600. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, X.; Zhong, Y.; Yang, P.; Gao, P.; Wu, X.; et al. Exosomal ncRNAs: The pivotal players in diabetic wound healing. Front Immunol. 2022, 13, 1005307. [Google Scholar] [CrossRef]
- Pirola, L. Epigenetics of Diabetic Microvascular Disease. Microvascular Disease in Diabetes2020. p. 45-57.
- Legiawati, L. The Role of Oxidative Stress, Inflammation, and Advanced Glycation End Product in Skin Manifestations of Diabetes Mellitus. Current Diabetes Reviews. 2022, 18, 87–92. [Google Scholar] [CrossRef]
- Rai, V.; Moellmer, R.; Agrawal, D. Role of fibroblast plasticity and heterogeneity in modulating angiogenesis and healing in the diabetic foot ulcer. Molecular Biology Reports. 2023, 50, 1913–29. [Google Scholar] [CrossRef] [PubMed]
- Liechty, C.; Hu, J.; Zhang, L.; Liechty, K.W.; Xu, J. Role of microRNA-21 and Its Underlying Mechanisms in Inflammatory Responses in Diabetic Wounds. International Journal of Molecular Sciences. 2020, 21, 3328. [Google Scholar] [CrossRef]
- Choi, L.-S.; Ahmed, K.; Kim, Y.-S.; Yim, J.-E. Skin accumulation of advanced glycation end products and cardiovascular risk in Korean patients with type 2 diabetes mellitus. Heliyon. 2022, 8, e09571. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients. 2022, 14, 4588. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; et al. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Medicine and Cellular Longevity. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Al-Rikabi, A.H.A.; Tobin, D.J.; Riches-Suman, K.; Thornton, M.J. Dermal fibroblasts cultured from donors with type 2 diabetes mellitus retain an epigenetic memory associated with poor wound healing responses. Sci Rep. 2021, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Kashyap, S.; Chini, E.; Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. Journal of Clinical Investigation 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. American Journal of Physiology-Endocrinology and Metabolism. 2019, 316, E268–E85. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death & Disease. 2020, 11, 444. [Google Scholar]
- Kashpur, O.; Smith, A.; Gerami-Naini, B.; Maione, A.G.; Calabrese, R.; Tellechea, A.; et al. Differentiation of diabetic foot ulcer-derived induced pluripotent stem cells reveals distinct cellular and tissue phenotypes. FASEB J. 2019, 33, 1262–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, S.; Bjorklund, M.; Xu, S. Mitochondrial fragmentation and ROS signaling in wound response and repair. Cell Regen. 2022, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Lu, X.; Yang, Y.; Yang, Y.; Li, Y.; Kuai, L.; et al. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv Sci (Weinh). 2023, 10, e2203308. [Google Scholar] [CrossRef] [PubMed]
- Cabal Mirabal, C.; González Dalmau, E.; Berlanga-Acosta, J.; Zayas, D.; Herrera, L.; Lopez-Saura, P.; et al. Quantitative Studies of the Evolution of Diabetic Foot Lesions under EGF Treatment by Magnetic Resonance Imaging. Journal of Radiology Research and Practice 2014, 2014. [Google Scholar]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules. 2022, 12. [Google Scholar] [CrossRef]
- Tatmatsu-Rocha, J.C.; Tim, C.R.; Avo, L.; Bernardes-Filho, R.; Brassolatti, P.; Kido, H.W.; et al. Mitochondrial dynamics (fission and fusion) and collagen production in a rat model of diabetic wound healing treated by photobiomodulation: comparison of 904 nm laser and 850 nm light-emitting diode (LED). J Photochem Photobiol B. 2018, 187, 41–7. [Google Scholar] [CrossRef]
- Sacco, A.M.; Belviso, I.; Romano, V.; Carfora, A.; Schonauer, F.; Nurzynska, D.; et al. Diversity of dermal fibroblasts as major determinant of variability in cell reprogramming. J Cell Mol Med. 2019, 23, 4256–68. [Google Scholar] [CrossRef]
- Kirk, T.; Ahmed, A.; Rognoni, E. Fibroblast Memory in Development, Homeostasis and Disease. Cells. 2021, 10. [Google Scholar] [CrossRef]
- Li, B.; Bian, X.; Hu, W.; Wang, X.; Li, Q.; Wang, F.; et al. Regenerative and protective effects of calcium silicate on senescent fibroblasts induced by high glucose. Wound Repair Regen. 2020, 28, 315–25. [Google Scholar] [CrossRef]
- Phang, S.J.; Arumugam, B.; Kuppusamy, U.R.; Fauzi, M.B.; Looi, M.L. A review of diabetic wound models-Novel insights into diabetic foot ulcer. J Tissue Eng Regen Med. 2021, 15, 1051–68. [Google Scholar] [CrossRef]
- Maitra, S.; Dutta, D. Downregulation of hexose sugar metabolism in diabetes decreases the rate of wound healing. 2020. p. 259-70.
- Soydas, T.; Sayitoglu, M.; Sarac, E.Y.; Cinar, S.; Solakoglu, S.; Tiryaki, T.; et al. Metformin reverses the effects of high glucose on human dermal fibroblasts of aged skin via downregulating RELA/p65 expression. J Physiol Biochem. 2021, 77, 443–50. [Google Scholar] [CrossRef] [PubMed]
- Vujcic, S.; Kotur-Stevuljevic, J.; Vekic, J.; Perovic-Blagojevic, I.; Stefanovic, T.; Ilic-Mijailovic, S.; et al. Oxidative Stress and Inflammatory Biomarkers in Patients with Diabetic Foot. Medicina (Kaunas). 2022, 58. [Google Scholar]
- Theocharidis, G.; Baltzis, D.; Roustit, M.; Tellechea, A.; Dangwal, S.; Khetani, R.S.; et al. Integrated Skin Transcriptomics and Serum Multiplex Assays Reveal Novel Mechanisms of Wound Healing in Diabetic Foot Ulcers. Diabetes. 2020, 69, 2157–69. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.C.; Lan, C.E. The Epidermal Keratinocyte as a Therapeutic Target for Management of Diabetic Wounds. Int J Mol Sci. 2023, 24, 4290. [Google Scholar] [CrossRef] [PubMed]
- Dhariwala, M.O.; Scharschmidt, T.C. Baby's skin bacteria: first impressions are long-lasting. Trends Immunol. 2021, 42, 1088–99. [Google Scholar] [CrossRef]
- Haftek, M. 'Memory' of the stratum corneum: exploration of the epidermis' past. Br J Dermatol. 2014, 171 Suppl 3, 6–9. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Huang, S.M.; Cheng, Y.W.; Yen, M.C.; Hsu, Y.L.; Lan, C.E. Investigation of the keratinocyte transcriptome altered in high-glucose environment: An in-vitro model system for precision medicine. J Dermatol Sci. 2023, 109, 37–46. [Google Scholar] [CrossRef]
- Cibrian, D.; de la Fuente, H.; Sanchez-Madrid, F. Metabolic Pathways That Control Skin Homeostasis and Inflammation. Trends Mol Med. 2020, 26, 975–86. [Google Scholar] [CrossRef]
- Hosseini Mansoub, N. The role of keratinocyte function on the defected diabetic wound healing. Int J Burns Trauma. 2021, 11, 430–41. [Google Scholar] [CrossRef]
- Lan, C.C.; Huang, S.M.; Wu, C.S.; Wu, C.H.; Chen, G.S. High-glucose environment increased thrombospondin-1 expression in keratinocytes via DNA hypomethylation. Transl Res. 2016, 169, 91–101e1-3. [Google Scholar] [CrossRef]
- Li, D.; Kular, L.; Vij, M.; Herter, E.K.; Li, X.; Wang, A.; et al. Human skin long noncoding RNA WAKMAR1 regulates wound healing by enhancing keratinocyte migration. Proc Natl Acad Sci U S A. 2019, 116, 9443–52. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, C.; Wu, D.; Chen, G.; Tan, R.; Ran, J. AGEs-induced MMP-9 activation mediated by Notch1 signaling is involved in impaired wound healing in diabetic rats. Diabetes Res Clin Pract. 2022, 186, 109831. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, W.; Yang, C.; Zhang, J.; Sun, K.; Luo, H.C.; et al. alpha-ketoglutarate is associated with delayed wound healing in diabetes. Clin Endocrinol (Oxf). 2016, 85, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, P.; Liu, D.; Gao, M.; Wang, J.; Wang, X.; et al. c-Myc Upregulated by High Glucose Inhibits HaCaT Differentiation by S100A6 Transcriptional Activation. Front Endocrinol (Lausanne). 2021, 12, 676403. [Google Scholar] [CrossRef]
- Stojadinovic, O.; Brem, H.; Vouthounis, C.; Lee, B.; Fallon, J.; Stallcup, M.; et al. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005, 167, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Graves, D.T. Keratinocyte Function in Normal and Diabetic Wounds and Modulation by FOXO1. J Diabetes Res. 2020, 2020, 3714704. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.L.; Ding, H.; Miao, W.W.; Mao, C.X.; Zhan, M.Q.; Chen, H.L. Global recurrence rates in diabetic foot ulcers: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2019, 35, e3160. [Google Scholar] [CrossRef]
- Bus, S.A.; van Netten, J.J.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Jubiz, Y.; et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab Res Rev. 2016, 32 Suppl 1, 16–24. [Google Scholar] [CrossRef]
- Mahdipour, E.; Sahebkar, A. The Role of Recombinant Proteins and Growth Factors in the Management of Diabetic Foot Ulcers: A Systematic Review of Randomized Controlled Trials. J Diabetes Res. 2020, 2020, 6320514. [Google Scholar] [CrossRef]
- Tsang, M.W.; Wong, W.K.; Hung, C.S.; Lai, K.M.; Tang, W.; Cheung, E.Y.; et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care. 2003, 26, 1856–61. [Google Scholar] [CrossRef]
- Hong, J.P.; Jung, H.D.; Kim, Y.W. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg. 2006, 56, 394–8, discussion 9-400. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Han, S.H.; Hong, J.P.; Han, S.K.; Lee, D.H.; Kim, B.S.; et al. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: A phase III multicenter, double-blind, randomized, placebo-controlled trial. Diabetes Res Clin Pract. 2018, 142, 335–44. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Pendsey, S.; Sekar, N.; Murthy, G.S.R. A phase III study to evaluate the safety and efficacy of recombinant human epidermal growth factor (REGEN-D™ 150) in healing diabetic foot ulcers. Wounds. 2006, 18, 186–96. [Google Scholar]
- Tuyet, H.L.; Nguyen Quynh, T.T.; Vo Hoang Minh, H.; Thi Bich, D.N.; Do Dinh, T.; Le Tan, D.; et al. The efficacy and safety of epidermal growth factor in treatment of diabetic foot ulcers: the preliminary results. Int Wound J. 2009, 6, 159–66. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Montequin, J.I.; Valenzuela-Silva, C.M.; Diaz, O.G.; Savigne, W.; Sancho-Soutelo, N.; Rivero-Fernandez, F.; et al. Intra-lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo-controlled, double-blind study. Int Wound J. 2009, 6, 432–43. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Villa, R.; Aguilar-Rebolledo, F.; Lozano-Platonoff, A.; Teran-Soto, J.M.; Fabian-Victoriano, M.R.; Kresch-Tronik, N.S.; et al. Response to Uzun G et al. Wound Repair Regen. 2014, 22, 767. [Google Scholar] [CrossRef]
- Bartın, M.; Okut, G. The effect of human recombinant epidermal growth factor in the treatment of diabetic foot ulcers. Journal of Clinical and Investigative Surgery. 2022, 7, 38–42. [Google Scholar] [CrossRef]
- Yera-Alos, I.B.; Alonso-Carbonell, L.; Valenzuela-Silva, C.M.; Tuero-Iglesias, A.D.; Moreira-Martinez, M.; Marrero-Rodriguez, I.; et al. Active post-marketing surveillance of the intralesional administration of human recombinant epidermal growth factor in diabetic foot ulcers. BMC Pharmacol Toxicol. 2013, 14, 44. [Google Scholar] [CrossRef]
- López-Saura, P.A.; Yera-Alos, I.B.; Valenzuela-Silva, C.M.; González-Díaz, O.; del Río-Martín, A.; Berlanga-Acosta, J.; et al. Medical Practice Confirms Clinical Trial Results of the Use of Intralesional Human Recombinant Epidermal Growth Factor in Advanced Diabetic Foot Ulcers. Advances in Pharmacoepidemiology & Drug Safety 2013, 25. [Google Scholar]
- Kahraman, M.; Misir, A.; Kizkapan, T.B.; Ozcamdalli, M.; Uzun, E.; Mutlu, M. The Long-Term Outcomes Following the Application of Intralesional Epidermal Growth Factor in Patients With Diabetic Foot Ulcers. J Foot Ankle Surg. 2019, 58, 282–7. [Google Scholar] [CrossRef] [PubMed]
- Embil, J.M.; Papp, K.; Sibbald, G.; Tousignant, J.; Smiell, J.M.; Wong, B.; et al. Recombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open-label clinical evaluation of efficacy. Wound Repair Regen. 2000, 8, 162–8. [Google Scholar] [CrossRef] [PubMed]
- Smiell, J.M.; Wieman, T.J.; Steed, D.L.; Perry, B.H.; Sampson, A.R.; Schwab, B.H. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999, 7, 335–46. [Google Scholar] [CrossRef]
- Wieman, T.J.; Smiell, J.M.; Su, Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998, 21, 822–7. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hernandez, M.A.; Kirkpatrick, V.E.; Liang, L.J.; Nouvong, A.L.; Gordon, I.I. Topical platelet-derived growth factor vs placebo therapy of diabetic foot ulcers offloaded with windowed casts: a randomized, controlled trial. Wounds. 2015, 27, 83–91. [Google Scholar]
- Veves, A.; Falanga, V.; Armstrong, D.G.; Sabolinski, M.L.; Apligraf Diabetic Foot Ulcer, S. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001, 24, 290–5. [Google Scholar] [CrossRef]
- Marston, W.A.; Hanft, J.; Norwood, P.; Pollak, R.; Dermagraft Diabetic Foot Ulcer Study, G. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003, 26, 1701–5. [Google Scholar] [CrossRef]
- Zelen, C.M.; Serena, T.E.; Gould, L.; Le, L.; Carter, M.J.; Keller, J.; et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int Wound J. 2016, 13, 272–82. [Google Scholar] [CrossRef]
- Zelen, C.M.; Serena, T.E.; Fetterolf, D.E. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: A long-term follow-up study. Wound Medicine. 2014, 4, 1–4. [Google Scholar] [CrossRef]
- Blume, P.A.; Walters, J.; Payne, W.; Ayala, J.; Lantis, J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008, 31, 631–6. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; Diabetic Foot Study, C. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005, 366, 1704–10. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.Q.; Bui, Q.V.P.; Nemeth, D.; Hegyi, P.; Szakacs, Z.; Rumbus, Z.; et al. Epidermal Growth Factor is Effective in the Treatment of Diabetic Foot Ulcers: Meta-Analysis and Systematic Review. Int J Environ Res Public Health. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; fernandez montequin, J.; Santana, H.; Pérez, C.; Savigne, W.; Mendoza-Mari, Y.; et al. A narrative review on Epidermal Growth Factor (EGF) intralesional infiltrations for diabetic complex wounds: The rational of an innovative delivery route. Vascular Diseases and Therapeutics. 2022, 7, 1–9. [Google Scholar] [CrossRef]
- Al-Jalodi, O.; Kupcella, M.; Breisinger, K.; Serena, T.E. A multicenter clinical trial evaluating the durability of diabetic foot ulcer healing in ulcers treated with topical oxygen and standard of care versus standard of care alone 1 year post healing. Int Wound J. 2022, 19, 1838–42. [Google Scholar] [CrossRef] [PubMed]
| Reference | Major outcome | Administration route | Follow up period | Recurrences |
|---|---|---|---|---|
| Recombinant Epidermal Growth Factor (EGF) | ||||
| Tsang MW et al. 2003 (93) | 20 of 21 diabetic foot ulcers healed with daily application of 0.04% (wt/wt) hEGF for 12 weeks | Topical | 6 months | ND |
| Hong JP et al. 2006 (94) | The study suggests that topical treatment with EGF combined with advanced dressing may have positive effects in promoting healing | Topical | 6 months | No recurrences were observed in EGF group |
| Park KH et al. 2018 (95) | The phase III study supports the efficacy and safety of spray-applied EGF treatment for DFUs, by significantly increasing healing velocity and decreasing time to complete healing | Topical | ND | ND |
| Viswanathan V et al. 2006 (96) | It took 13 weeks for ulcers to heal in the control group versus 9 weeks in the test group. In the test group, 90% of ulcers healed in 15 weeks compared with 22 weeks in the control group | Topical | ND | ND |
| Tuyet HL et al. 2009 (97) | Easyef (topical EGF spray) has positive effects on healing of moderate-to-severe foot ulcers and demonstrated being safe to diabetic patients | Topical | ND | ND |
| Fernández-Montequin J et al. 2009 (98) | Locally infiltrated EGF at 75 µg enhanced granulation tissue growth and wound closure | Intralesional injection | 12 months | No recurrences reported for EGF groups |
| Gomez-Villa R et al. 2014 (99) | Patients with DFU who received intralesional rhEGF application resulted in complete healing, larger epithelialization of the wound bed, and reduction of ulcer area | Intralesional injection | ND | ND |
| Bartın M & Okut G 2022 (100) | The study shows that intralesional administration of EGF in T2DM can prevent amputations in DFU and also accelerate wound healing | Intralesional injection | 6 months | Two cases in the group receiving EGF |
| Yera-Alos IB et al. 2013 (101) | Post-marketing study including 1788 patients treated with intraulcer injected EGF. 1835 DFU (81% Wagner’s grades 3 or 4; 43% ischemic) were treated. Re-epithelization was documented in 61% of the 1659 followed-up cases | Intralesional injection | 14 months | 5% / year |
| López-Saura PA et al., 2013 (102) | A review summarizing the clinical information about intralesional use of EGF for high grade DFU, in more than 2000 subjects. It confirms the results of the clinical trials, with 75% probability of complete granulation response, 61% healing, and a 16% absolute, and 71% relative reduction of amputation risk | Intralesional injection | 12 months | The frequency of relapses at any momentwas significantly lower (p<0.001) in patients that received rhEGF: 2.0% person-years of follow-up |
| Kahraman M et al. 2019 (103) | Study aimed to investigate the long-term outcomes after intralesional epidermal growth factor injections in the treatment of 34 diabetic patients with foot ulcers. | Intralesional injection | 60 months | Of 29 patients involved in the 5-years follow up, 27 were ulcer free |
| Regranex or Becaplermin (rh-PDGF-BB) | ||||
| Embil JM et al. 2000 (104) | Results of the study further confirm the efficacy and safety of becaplermin gel for the treatment of lower extremity diabetic ulcers | Topical | 6 months | 21% of recurrence in Becaplermin- treated patients |
| Smiell JM et al. 1999 (105) | Treatment with becaplermin gel at a dose of 100 μg/g once daily, in conjunction with good ulcer care, is effective in patients with full thickness lower extremity diabetic ulcers | Topical | 3 months | ND |
| Wieman TJ et al. 1998 (106) | Becaplermin gel 100 μg/g significantly increased the incidence of complete wound closure by 43% (50 vs. 35%, P=0.007) and decreased the time to achieve complete wound closure by 32% | Topical | 3 months | The incidence of ulcer recurrence was ≈30% in all treatment groups |
| Ma C et al. 2015 (107) | Topical platelet derived growth factor does not appear to significantly improve healing of Wagner grade I diabetic foot ulcers | Topical | 6 months | No difference was observed between groups in recurrence followed by amputation |
| Cellular and tissue-based products | ||||
| Veves A et al. 2001 (108) | At the 12-week follow-up visit, 63 (56%) Graftskin-treated patients achieved complete wound healing compared with 36 (38%) in the control group (P=0.0042). | Topical –bioengineered skin substitutes | 6 months | The incidence of ulcer recurrence was similar for Graftskin and control groups |
| Marston WA et al. 2003 (109) | Patients with chronic diabetic foot ulcers of >6 weeks duration experienced a significant clinical benefit when treated with Dermagraft versus patients treated with conventional therapy alone. | Topical-bioengineered skin substitutes | ND | ND |
| Zelen CM et al. 2016 (110) | EpiFix® (dehydrated human amnion/chorion membrane) is superior to standard wound care SWC and Apligraf®, in achieving complete wound closure within 4–6 weeks. | Topical –bioengineered skin substitutes | ND | ND |
| Zelen CM et al. 2014 (111) | Study addressed to evaluate recurrence rates of DFU healed with use of dehydrated human amnion/chorion membrane (EpiFix) in 18 available subjects with healed DFU. Wound median size of 1.7 cm2. | Topical –bioengineered skin substitutes | 9-12 months | 17 wounds remained healed |
| Vacuum Assisted Closure (VAC)/ Negative Pressure Wound Therapy (NPWT) | ||||
| Blume P et al. 2008 (112) | A greater proportion of foot ulcers achieved complete ulcer closure with NPWT (73 of 169, 43.2%) than with advanced moist therapy within the 112-day active treatment | Topical- sub atmospheric pressure over the wound area | ND | ND |
| Armstrong DG et al. 2005 (113) | More patients healed in the NPWT group than in the control group (43 [56%] vs 33 [39%], p=0·040) | Topical- sub atmospheric pressure over the wound area | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





