1. Introduction & Background
The inhibition of Bruton's tyrosine kinase (BTK) has provided an array of therapeutic options for the effective treatment of chronic lymphocytic leukemia (CLL) [1,2]. In 2014 the first-in-class oral BTK inhibitor (BTKi), ibrutinib, was licensed and was a major advance in CLL treatment. Responses to treatment with ibrutinib were also favorable in the treatment patients of high-risk CLL which historically had relatively poor response rates to chemo-immunotherapy (CIT) [3–9]. BTKi treatment, though highly effective, does not generally achieve deep responses in terms of the attainment of low levels of measurable residual disease (MRD), which results in a therapeutic paradigm whereby indefinite, continuous treatment is required to maintain clinical responses. Consequentially, patients are exposed to BTKi therapy for prolonged periods and are therefore prone to developing treatment-related toxicities. Common BTKi-related toxicities include bleeding, infections, diarrhea, arthralgias, arrhythmias, and hypertension [10].
Randomized clinical trials have investigated the efficacy of second-generation BTKis such as acalabrutinib and found reduced toxicity due to off-target kinase effects when compared with ibrutinib. In a phase III open-label, randomized, prospective study (ELEVATE-RR trial), acalabrutinib had equivalent efficacy but enhanced safety when compared to ibrutinib, with fewer episodes of atrial fibrillation and reduced rates of drug discontinuation due to side effects [11]. Accordingly, acalabrutinib was the first covalent, second-generation BTKi, to receive Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval for CLL treatment [12,13].
Zanubrutinib (zanu) is another, next-generation small molecule BTKi that forms a covalent bond with cysteine residues in the BTK active binding site, leading to potent inhibition of kinase activity [14]. Though initially approved for the treatment of patients with relapsed mantle cell lymphoma (MCL) and relapsed/refractory (R/R) marginal zone lymphoma (MZL) in the USA in 2021, zanu was then licensed for the treatment of Waldenstrom's macroglobulinemia (WM), based upon the results of the phase 3 ASPEN study which compared the efficacy and safety of this agent with ibrutinib[15–18].
Finally, in January 2023, the FDA and EMA both approved zanu for the treatment of patients with CLL or small lymphocytic lymphoma (SLL) based upon the results of the pivotal phase 3 SEQUOIA (NCT03336333) and ALPINE (NCT03734016) trials [19–22]. Due to the increased selectivity, improved efficacy, and superior toxicity profile of zanu this agent is listed as a preferred treatment option for patients with CLL in the recently updated National Comprehensive Cancer Network (NCCN) (v2.2023) guidelines and newly released German algorithm [23,24].
2. Earlier Studies of Zanubrutinib in CLL
The first-in-human, open-label, multicenter, phase I/II study of zanu (AU-003 study) included R/R patients with B-cell malignancies who received zanu at doses of 40, 80, 160, or 320 mg once daily or 160 mg twice daily. Safety, tolerability, and the maximum tolerated dose were primary endpoints [25]. The expansion study enrolled a further 94 treatment-naïve (TN) or R/R CLL/ SLL patients who received zanu at the maximum tolerated dose continued until disease progression, loss of clinical benefit, or dose-limiting toxicity. Patients were given zanu at doses of 160 mg twice daily (81 patients), 320 mg once daily (40 patients), or 160 mg once daily (40 patients). After a median duration of follow-up of 13.7 months, 89 patients with CLL/SLL (94.7%) were still on study. The overall response rate (ORR) was 96.2% for 78 evaluable patients while the estimated 12-month PFS was 100%. The majority of toxicities were grade 1/2; neutropenia was the only grade 3-4 toxicity observed in more than two patients, while a grade 3 subcutaneous hemorrhage occurred in one patient [25]. These relatively favorable safety results were in keeping with the reduced affinity of zanu for off-target kinases including epidermal growth factor receptor (EGFR), Janus kinase 3 (JAK3), human EGFR2, interleukin-2 (IL2), inducible T-cell kinase (ITK), and TEC when compared to ibrutinib [26].
Data generated in this phase I/II study suggests that:-
The twice-daily dosing of zanu achieves 8-fold higher plasma drug exposure than ibrutinib and a longer half-life than acalabrutinib (4 vs. 1 hour)[25,27].
Zanu shows complete and sustained occupancy of the BTK binding site across lymph nodes and in peripheral blood mononuclear cells [25,27].
Consistent with the favorable oral bioavailability observed in preclinical studies, oral administration of zanu achieves therapeutic plasma drug concentrations using the recommended phase II dose of 160 mg twice daily, with maintenance of drug levels above the IC50 required for full occupancy of the BTK binding site [25,27].
Zanu is less prone to pharmacological interactions from food, drug-drug interactions with strong or moderate CYP3A inhibitors, and proton pump inhibitors [PPIs] leading to more consistent, sustained therapeutic exposures and improved dosing convenience. In addition, the clinical use of zanu is less sensitive than ibrutinib to impairments of liver function [28].
Cull et al [29] recently reported updated safety and efficacy data of the AU-003 study involving123 patients with a median follow-up of 47.2 months. The ORR was 95.9% (TN, 100%; R/R, 95%), with 18.7% obtaining a complete response (CR). In 16 patients with a del(17p)/TP53 mutation, the ORR was 87.5% (CR 16.7%). The estimated 3-year PFS was 83% (TN, 81%; R/R, 83%, respectively). Discontinuations due to adverse events (AEs) or disease progression were rare. The efficacy results of this updated analysis are also consistent with those of a single-arm Chinese study (NCT03206918) that reported an ORR of 84·6% (CR 3·3%) in 91 R/R CLL patients, with 87·2% of patients still alive and progression-free at 12·9 months. Finally, in relation to safety, the results of the AU-003 study indicate that neutropenia (15.4%), pneumonia (9.8%), hypertension (8.9%), and anemia (6.5%) were the most commonly reported Grade 3 AEs. Furthermore, the annual incidence of atrial fibrillation, major hemorrhage, grade 3 neutropenia, and grade 3 infection decreased over time [29]. The single-arm AU-003 study which included a significant proportion of patients with TN and R/R CLL/SLL treated with single-agent zanu for 4 years provides significant evidence of long-lasting efficacy and safety and paved the way for subsequent phase III trials.
3. Phase III Clinical Trials of Zanubrutinib in CLL
The recent FDA and EMA approvals of zanu for the treatment of CLL/SLL patients were mainly on the basis of results derived from the phase III SEQUOIA and ALPINE trials which demonstrated significant therapeutic efficacy and a favorable safety profile for zanu in both first-line and R/R setting.
3.1. Sequoia Trial
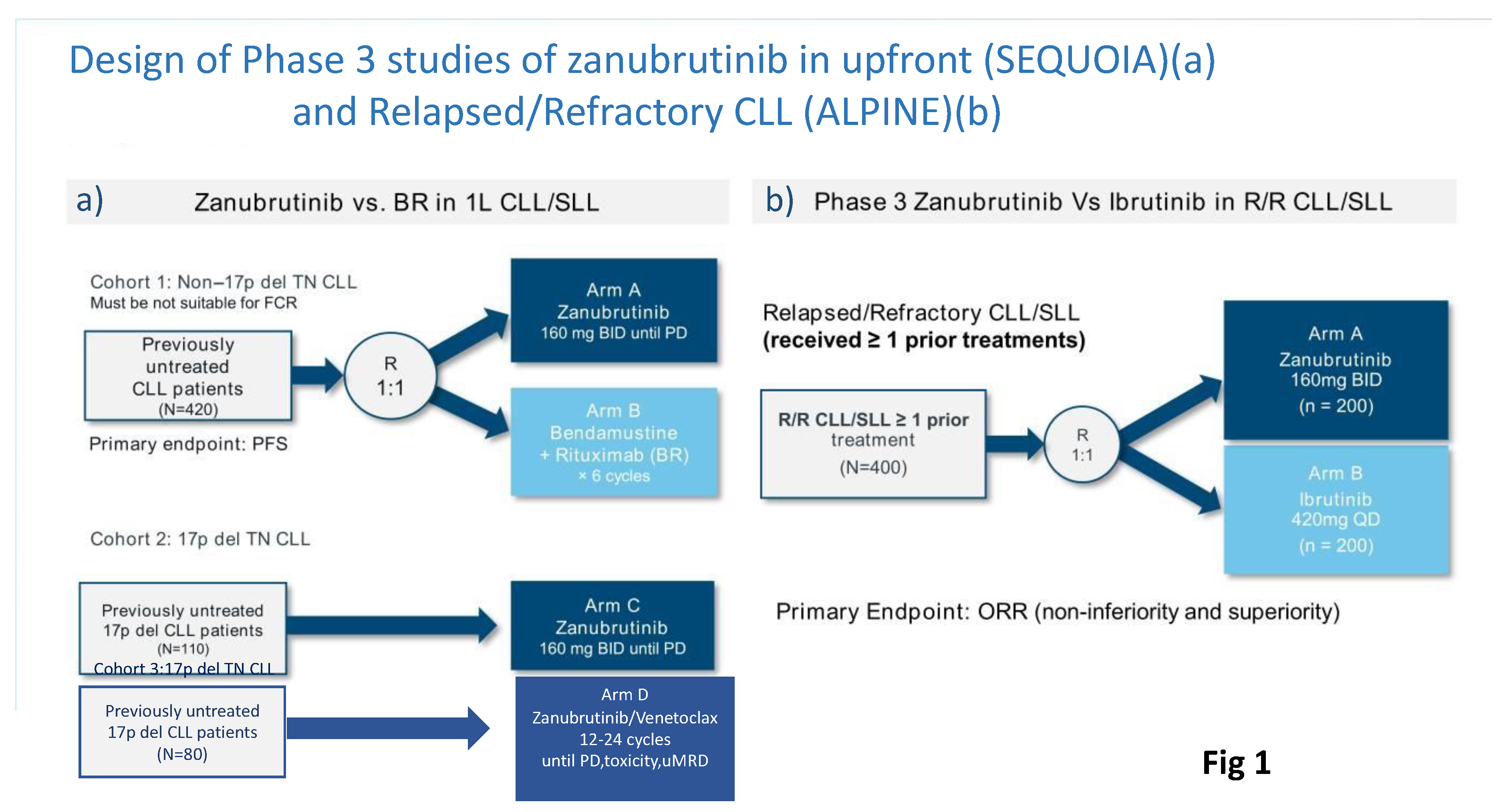
SEQUOIA was a randomized, multicenter, global, Phase III trial (NCT03336333) that evaluated the efficacy and safety of zanu in patients with TN CLL or SLL. This trial consisted of three cohorts (
Figure 1A)[30]:-
− Cohort 1 comprised 479 patients without del(17p) who were randomized 1:1 and either assigned to receive zanu (n=241)(until disease progression or unacceptable toxicity) or bendamustine and rituximab (BR) (n=238) for up to 6 cycles.
− Cohort 2 comprised 110 patients with del(17p) who were assigned to receive zanu monotherapy as it was deemed unethical to randomize patients with del(17p) to BR.
− Cohort 3 comprised 80 patients with del(17p) or TP53 aberrations who were assigned to receive zanu in combination with venetoclax (ZV). This cohort was opened when Cohort 2 was fully enrolled to provide non-randomized treatment for patients with del(17p).
Cohort 1 of the SEQUOIA trial enrolled CLL patients older than 65 who were ineligible for fludarabine, cyclophosphamide, and rituximab (FCR). Here the primary endpoint, as assessed by an independent review committee, was PFS in the intention-to-treat population [19]. With a median follow-up of 26.2 months, the estimated 24-month PFS rates for the zanu and BR groups in cohort 1 were 85.5% and 69.5%, respectively. In prespecified subgroup analyses, PFS was consistently superior with zanu as opposed to BR, regardless of age, sex, or high-risk disease status, such as Binet stage C, bulky disease, or the presence of IGHV unmutated status.
Indirect treatment comparisons between first- and second-generation BTKis can be limited by cross-trial differences, however, outcomes of patients assigned to receive zanu in the SEQUOIA study, ibrutinib single agent in the ALLIANCE trial, and acalabrutinib in the ELEVATE-TN trial totally coincided ( PFS 87% at 24 months) [5,19,31].
With respect to side effects encountered with BTKis, it is noteworthy that atrial fibrillation of any grade occurred in only 3% of patients treated with zanu, which is significantly lower than the 12.6% rate reported with ibrutinib in the Alliance trial. In contrast, the 5% major bleeding rate observed with zanu was equivalent to that reported with other BTKis [5,19,31]. The rate of discontinuation of zanu due to adverse events (AEs) was also relatively low at 13.7% [31]. Within the SEQUOIA study, it was evident that the safety profile of zanu favorably impacted the quality of life and patient-reported outcomes (PRO) which indicates that zanu is associated with a greater improvement of Health-Related Quality of Life (HRQoL) when compared to BR [32].
It may be argued that, in the era of targeted therapies, the SEQUOIA control arm of BR has reduced clinical relevance given the evidence base which consistently demonstrates the superiority of BTKi therapy over CIT-based approaches [3–6,31]. However, SEQUOIA trial enrollment began in 2017 before the widespread availability of data showing that ibrutinib-based therapies outperformed CIT in TN CLL. Hopefully, the SEQUOIA results in relation to the efficacy and safety of zanu will be validated in future studies enrolling different populations of CLL patients such as those with a higher comorbidity burden or those patients who are fit for FCR but who were not included in this trial. Moreover, well-designed head-to-head comparisons in the TN CLL population between zanu and other second-generation covalent or non-covalent BTK inhibitors, such as pirtobrutinib, would be useful to inform the choice of optimal BTKi therapy for individual patients in the future.
In the non-randomized arm 3 of the SEQUOIA trial, which enrolled TN, del(17p) CLL/SLL patients, the median PFS and OS were not reached, with a 18-month PFS and OS of 88.6% and 95.5% respectively. Four patients in this cohort (3.7%) discontinued treatment due to AEs, while atrial fibrillation/flutter was reported in 2.8% of patients [33]. Thus, the results obtained in arm 3 of the SEQUOIA trial in patients with del(17p) compare favorably with previous studies in similar patient cohorts. [32].
The SEQUOIA trial included an arm cohort 3 in which patients with del(17p) received ZV with discontinuation of treatment when a deep response was achieved based upon the attainment of CR and undetectable MRD (uMRD)_in peripheral blood and bone marrow. This arm was designed and initiated when cohort 2 became fully enrolled. The ORR at a median follow-up of 12.0 months was 97.2% in 36 evaluable patients. Preliminary safety data also indicate that ZV was well tolerated, with no cases of clinical tumor lysis syndrome (TLS) and relatively low incidences of neutropenia (all grades, 20.6 %), diarrhea (14.7%), and nausea (14.7%). In addition, 38.2% of ZV patients experienced at least one AE at grade 3 or greater. AEs were responsible for dose interruptions in 29.4 % of ZV patients but there was no need for dose reduction or treatment discontinuation with no fatal AEs encountered [34]. Longer follow-up is needed however to fully assess the depth of response and the safety of ZV in this high-risk population of CLL patients.
3.2. ALPINE Trial
The ALPINE trial was a randomized phase III study comparing the second-generation BTKi zanu to the first-generation BTKi ibrutinib (
Figure 1B). This study was designed on the assumption that complete/sustained occupancy of the BTK binding site by zanu may improve efficacy outcomes and minimize off-target inhibition-related toxicities due to increased specificity for BTK of the drug. In both arms, study treatment was given until disease progression or intolerance in 652 patients with R/R CLL. In the ALPINE study, included patients had received a median of one prior line of therapy and approximately 23% of participants had a 17p deletion or
TP53 mutation [35].
The primary endpoint of the ALPINE study was ORR defined as either CR or partial response (PR). Earlier data showed that zanu was associated with a significantly improved ORR compared with ibrutinib (78.3% versus 62.5%)[35]. The superiority of zanu over ibrutinib in terms of ORR was also confirmed when PR with lymphocytosis was included in the definition of ORR (89.9% versus 82.5%). These results paved the way for the assessment of PFS differences in the ALPINE trial by a hierarchical statistical analysis once 205 events had occurred [35-36].
In more detail, zanu significantly prolonged PFS when compared to ibrutinib, as assessed by both the independent review committee and the local investigators (hazard ratio [HR] for disease progression or death, 0.65). Furthermore, even in the highest-risk del(17p) and/or aberrant TP53 patient group, a pre-planned analysis showed that zanu improved the PFS by 22% [36,37]. One potential explanation for the observed superior efficacy of zanu may relate to its favorable pharmacokinetic properties compared to ibrutinib, with persistence of zanu above the IC50 of BTK throughout the entire dosing interval, thereby providing continuous coverage against newly synthesized BTK in the CLL cells [38].
Fewer patients discontinued zanu than ibrutinib due to AEs, and fewer discontinued zanu due to progressive disease when compared to the ibrutinib-treated group. With a median follow-up of 29.6 months of treatment the rate of treatment cessation was lower for zanu (26.3%) than ibrutinib (41.2%) with most discontinuations due to AEs (16.2 vs 22.8%) or progressive disease (7.3 vs 12.9%). These discontinuation rates were somewhat higher than expected when compared to those reported in previously published trials [32]. This probably relates to the fact that the ALPINE study was an international clinical trial, enrolling patients from many countries, and thus may better reflect real-world situations in terms of BTKi being discontinued for AEs [36].
In the ALPINE study zanu patients had fewer serious AEs and serious cardiac AEs leading to drug discontinuation when compared to ibrutinib-treated patients (1 vs. 14). Of note, no cardiac deaths were observed in zanu-treated persons vs 6 encountered in the ibrutinib group. Atrial fibrillation or flutter of any grade was reduced in the zanu-treated group compared to those in the ibrutinib group (5.2% versus 13.3%) with reduced atrial fibrillation or flutter at grade 3 or higher (2.5% versus 4.0%). Similar rates of hypertension were reported in the zanu (23.5%) and ibrutinib groups (22.8%). Neutropenia of any grade was recorded in 29.3% of patients in the zanu group vs 24.4% of those in the ibrutinib group. Infections of any grade were documented in 71.3% of patients in and in 73.1% in the ibrutinib group, while rates of infections of grade 3 or higher were 26.5% and 28.1%, respectively [36].
The 18-month PFS of patients treated with ibrutinib in the RESONATE trial was similar to the PFS of patients who received ibrutinib in the ALPINE trial (78% vs. 75%)[3,36]. However, different patient populations and different stratification factors make cross-trial comparisons between these two studies difficult. For example, patients enrolled in the RESONATE trial had received more lines of prior therapies and included more cases of high-risk CLL [17p deletion] than individuals enrolled in the ALPINE trial. [3,36]. Finally, in the ALPINE trial, patients with R/R CLL/SLL treated with zanu monotherapy reported improvements in key HRQOL endpoints in comparison with those who received ibrutinib monotherapy [39].
4. Specific Aspects of Zanubrutib Therapy in CLL
4.1. Is It Possible to Simplify the Zanubrutinib Treatment Schedule?
Zanu dose selection has been a matter of contention during the clinical development of this drug. This is an important issue as the simplification of the zanu dosing regimen to 320-mg once daily from 160-mg twice daily may improve medication adherence and thereby maintain overall dose intensity. To better understand the outcomes of different dose regimens of zanu in MCL, Ou et al. reviewed data from a single-arm, open-label multicenter phase II study in which patients were treated with zanu at 160 mg twice daily until disease progression or unacceptable toxicity [40]. The investigators also assessed data from another multicenter phase II first-in-human study of zanu administered in dose increments of 40 mg, 80 mg, 160 mg, or 320 mg once daily or 160 mg twice daily in patients with B-cell malignancies. A total of 86 patients were enrolled in the phase II study and 37 in the phase I study (of whom 32 were treated at the recommended phase 2 dose of either 320 mg once daily [n = 18] or 160 mg twice daily [ n= 14]). For both dosing regimens, the median BTK occupancy in peripheral blood mononuclear cells was 100% across all time points. However, the median BTK occupancy in nodal tissue was higher for 160 mg twice daily than 320 mg once daily (100% versus 93%). There were no notable differences in the safety and tolerability profiles of the two zanu dosing schedules. Overall, a similar degree of plasma exposure and BTK inhibition was achieved with the two zanu doses; thus, any differences in the trough and maximum plasma concentrations between the two regimens are unlikely to have a meaningful impact on efficacy and safety endpoints [40]. In contrast , Shadman et al. have suggested that there may be a difference in efficacy and toxicity of different zanu schedules used in patients with BTKi intolerance [41]. However, the relatively small number of patients included in these studies and the short periods of follow-up prevent drawing firm conclusions in relation to the relative efficacy and safety of the 320 mg once daily zanu regimen. The issue of whether once daily dose has an impact on efficacy or adverse effects, therefore, needs to be carefully addressed in future post-marketing or real-world studies.
4.2. Zanubrutinib after Discontinuation of a Covalent BTKi Because of Toxicity
A real-world analysis of ibrutinib treatment in CLL revealed that 21% of treated patients discontinued this drug due to toxicity [42]. Although acalabrutinib has a greater selectivity for BTK than ibrutinib, this agent and its metabolite M27, continue to bind to other kinases during therapy leading to adverse events with the potential for subsequent discontinuation of treatment. In a phase I/II study of acalabrutinib in CLL patients, 9% of participants discontinued this treatment due to adverse effects[43]. Although acalabrutinib is a safe and effective option for patients with R/R CLL who cannot tolerate ibrutinib, patients who are intolerant of either ibrutinib or acalabrutinib currently have very few BTKis treatment options [44].
Shadman and colleagues [41] recently published the results of a phase II study of zanu treatment in 67 patients with B-cell malignancies (CLL/SLL, MCL, or MZL) who were previously intolerant to a BTKi. Fifty-seven of these patients were intolerant to ibrutinib, while 10 were intolerant to acalabrutinib or acalabrutinib plus ibrutinib. After a median duration of 11.6 months of zanu treatment, most of the prior intolerance events (81 of 115 [70%] for ibrutinib; 15 of 18 [83%] for acalabrutinib) did not recur with the zanu therapy. Of the recurrent intolerance events, seven (21%) of 34 ibrutinib and two (67%) of three acalabrutinib intolerance events recurred with the same degree severity with zanu whilst 27 (79%) ibrutinib intolerance events and one (33%) acalabrutinib intolerance event recurred at a lower severity with zanu. Among 64 efficacy-evaluable patients, the disease control rate was 93·8% and the ORR rate was 64·1% with an 18-month PFS of 83·8% [41]. This study is the first clinical trial to assess the safety and efficacy of the next-generation BTK inhibitor, zanu, in patients with previously treated B-cell malignancies who are intolerant to ibrutinib, acalabrutinib, or both. These results suggest that patients who are unable to tolerate ibrutinib and/or acalabrutinib or both may benefit from switching to zanu [41].
4.3. Combining Zanubrutinib with Monoclonal Antibodies or Anti-BCL2 Agents
Due to its substantial toxicity and other theoretical concerns, ibrutinib may not be the ideal BTKi to use in combination with an anti-CD20 antibody for the treatment of CLL/SLL [5,45]. In this respect, zanu may be a better option to use in combination, as it does not inhibit interleukin-2 inducible T-cell kinase (ITK), which is essential for the antibody-dependent cell cytotoxicity (ADCC) that is induced by anti-CD20 antibodies. In a phase I study, by Tam et al, zanu was used in combination with obinutuzumab (ZO) to treat patients with CLL/SLL [
46]. The ORR to ZO was 100% (n = 20) in patients with TN and 92% (n = 23) in patients with R/R CLL/SLL. Upper respiratory tract infection (51%) and neutropenia (44%), were the most common AEs and neutropenia was the most common grade 3-4 AE (31%)[46]. A phase II trial from the MD Anderson Cancer Center trials group is currently assessing the efficacy and safety of zanu in association with rituximab in previously untreated patients with CLL/SLL (NCT04458610). Another phase II study is also assessing the safety and efficacy of zanu with tafasitamab, a humanized monoclonal anti-CD19 antibody, recently approved by the FDA in combination with lenalidomide for the treatment of R/R diffuse large B-cell lymphoma (DLBCL) not eligible for stem cell transplantation (TaZa CLL Study; NCT05718869).
ZV was studied in cohort 3 of the SEQUOIA trial to treat patients with TN CLL/SLL who had the del(17p) mutation [34]. Early results suggest that this combination was effective and well tolerated. Another open-label, non-randomized phase II trial is assessing ZV in patients with CLL/SLL who have relapsed after at least one prior therapy. In this trial, patients are stratified into 3 groups: Cohort A, patients who have never received a BTK or BCL-2 inhibitor; Cohort B, patients who have received prior treatment with a BTK or BCL-2 inhibitor and discontinued treatment for any reason other than disease progression and cohort C which includes patients who have experienced disease progression whilst treated with a prior BTK inhibitor (NCT05168930)).
4.4. Three Drug Zanubrutinib Combinations
The three drug combination of zanu, obinutuzumab and venetoclax (BOVen) has been investigated in 37 TN CLL patients [47]. This regimen, which included a 2-month lead-in with zanu and obinutuzumab prior to the commencement of venetoclax, was attractive in that the treatment duration is limited to between 8 and 24 cycles of therapy. The duration of BOVen is determined by the timing of the attainment of uMRD in the peripheral blood and bone marrow. After a median of 25.8 months follow-up, 33 (89%) of 37 patients had uMRD and thus met the predetermined criteria for therapy discontinuation. Thrombocytopenia (59%) was the most common AE of any grade, followed by fatigue (54%) and neutropenia (51%, whilst easy bruising (51%) was the most common AE of grade 3 or worse. A patient died of an intracranial hemorrhage on day 1 of cycle 1 after intravenous heparin was administered for the treatment of a concurrent pulmonary embolism.
Patients in a phase II, single-arm, AVO (Acalabrutinib/Venetoclax/Obinutuzumab) study, also had comparable uMRD rates in peripheral blood (92%) and bone marrow (86%) to those observed with BOVen [48]. However, the novel aspect of the BOVen study described above was the incorporated analysis of early MRD kinetics as a potential predictor for patients who may be more likely to respond to a shorter period of therapy [47]. The reduction of MRD by 400-fold beneath the baseline (MRD400) within the first 4 months of treatment was identified as the optimal threshold to define uMRD. Amongst 21 patients who achieved MRD400, 95% (20/21) required less than 12 cycles of therapy (median eight cycles) to achieve uMRD. On the other hand, amongst the 14 patients who failed to attain MRD400 after four months of BOVen, 50% (7/14) required more than 12 months of therapy (median 13 cycles). It is of interest to note that the recurrence of detectable MRD after one year (with 10−5 sensitivity) was only 5% in those who attained MRD400 and discontinued therapy as a result. In contrast, the 1-year MRD recurrence rate was 75% in patients who did not achieve MRD400 but who discontinued therapy after achieving the MRD endpoint [47]. These findings suggest that MRD400 may be a useful surrogate to recognize a subset of “earlier” responders whose clinical outcome is highly favorable once uMRD is achieved.
4.5. Mechanisms of Zanubrutinib Resistance in CLL
The dominant molecular mechanism associated with clinical resistance and loss of response to ibrutinib is the development of BTK Cys481 codon mutations [49]. Whether a similar mechanism mediates clinical resistance to the next-generation, more selective BTKi zanu is as yet unknown.
Among six patients with zanu resistance who were available for longitudinal, targeted next-generation sequencing (NGS) and the dynamic assessment of clonal evolution, TP53, EGR2, NOTCH1, and SF3B1 were the predominant genes associated with the development of zanu resistance. Two patients developed emergent clones associated with TP53 mutations at the point of progression whilst another two patients showed persistence of TP53 mutated clones. SF3B1, EGR2, and BIRC3 mutated CLL sub-clones were stabile during the development of clinical zanu resistance whilst the BTK Cys481 mutation, as a secondary drug resistance mechanism, evolved during zanu treatment with clonal expansion due to positive clone selection [50].
Recently Blombery et al [51] analyzed the overall genetic landscape of BTK resistance mutations in patients who experienced disease progressed during zanu treatment. The authors noted that BTK Leu528Trp mutations, (also observed in patients with disease progression during pirtobrutinib treatment), occurred more frequently in patients who had been treated with zanu when compared to those treated with ibrutinib (54%; vs 4%; P = .001). The mutational landscape present in the context of BTK inhibitor therapy at loci other than BTK Cys481 remains a poorly studied field that is in need of further investigation [51].
4.6. Zanubrutinib in Patients at Risk of Cardiovascular Complications
In CLL, the current therapeutic approach is influenced by a number of considerations such as the somatic genetic risk profile, comorbidities, concomitant medications, patient adherence, some logistical issues, and most importantly patient preference [52]. Patients with CLL are diagnosed at a median age of 72 years and often have an associated high prevalence of comorbidities which may determine the therapeutic approach and the choice of therapy [53]. As recently reported in a large retrospective cohort of patients mainly treated with BTKis, the three coexisting conditions most relevant for survival outcomes are any cardiovascular disorders, moderate/severe endocrine conditions, and upper gastrointestinal comorbidities [54].
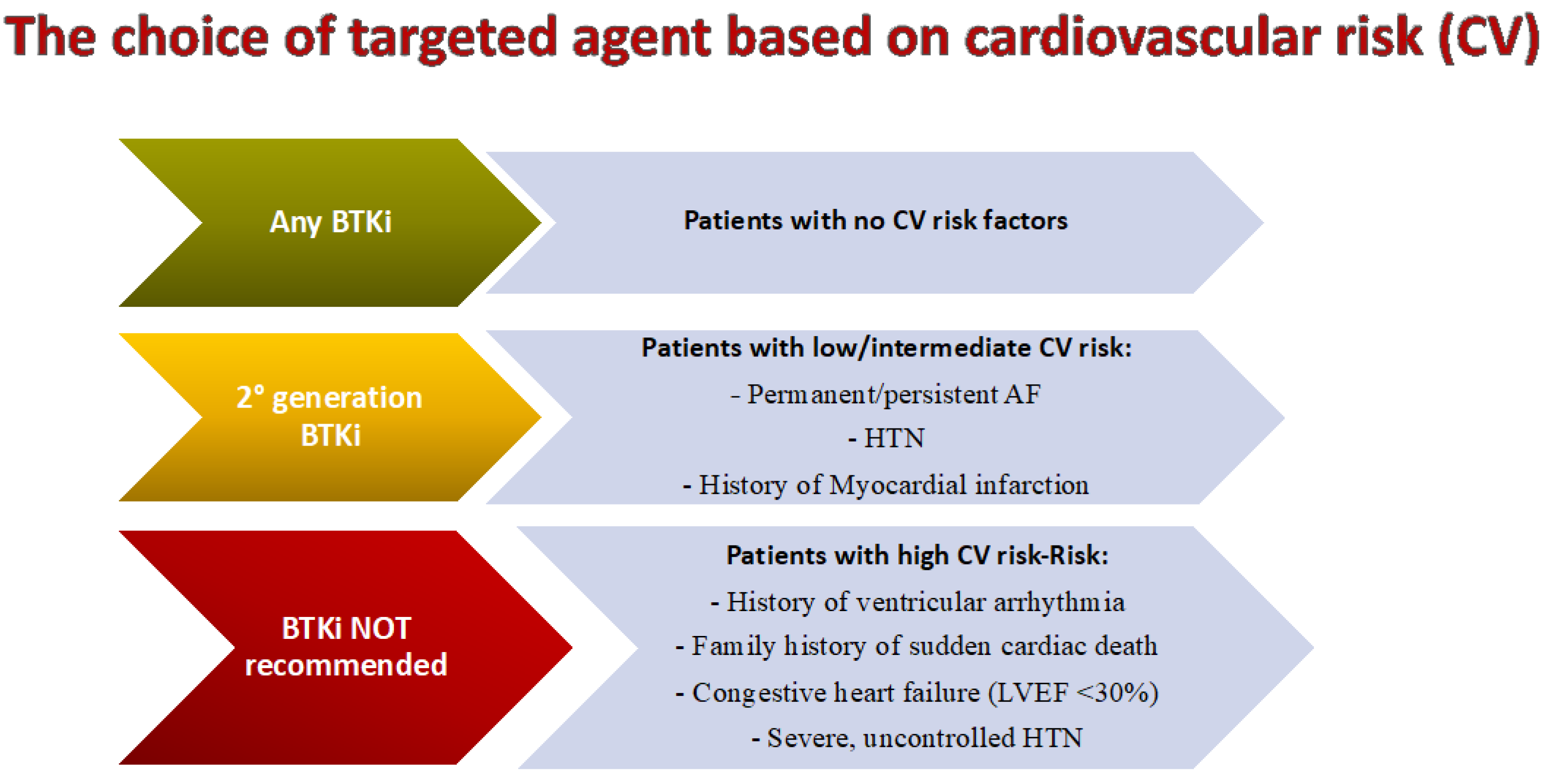
Historically, patients with significant cardiovascular issues have generally been considered poor candidates for ibrutinib, however, with the availability of second-generation BTKi therapies, more patients may be able to take advantage of treatment with BTK inhibition [55]. Overall, a multidisciplinary and holistic approach is most important to assess the eligibility of CLL patients for BTKi therapy. Particularly relevant aspects to consider include a history of valvular heart disease or other disorders that may increase the risk of AF; a history of ventricular arrhythmias, clinical heart failure, or left ventricular dysfunction, and reduced cardiac ejection fraction [56]. As a recent international panel of experts has suggested, the quantification of the cardiovascular (CV) risk posed by BTKis is important as is the interaction of BTKi therapy [57]. BTKi treatment should generally be avoided in patients with clinically significant heart failure (left ventricular ejection fraction of less than 30%). Both ibrutinib and acalabrutinib should not be used in patients with a history of ventricular arrhythmias or in the presence of a family history of sudden cardiac death. It is of interest to note that amongst patients without prior ibrutinib exposure, and in the absence of coronary disease or heart failure, the weighted average incidence of ventricular arrhythmias with acalabrutinib is 394 per 100,000 person-years [58]. In comparison, there are 596 ibrutinib-related ventricular arrhythmias per 100,000 person-years and 48.1 such events per 100,000 person-years amongst similarly aged non-BTKi treated subjects [59]. As yet, the comparable risks for zanu are unknown.
For CLL patients in need of treatment at intermediate- or low-cardiovascular risk, second-generation BTKis such as acalabrutinib and zanu, are preferred. In the ASPEN study patients with WM, treated with ibrutinib, had significantly more AF events of any grade (15%) than those treated with zanu (2%; P =0 .0004), whilst AF events of grade ≥ 3 were also significantly more common in the ibrutinib group (4% vs. 0%; P =0.02). In addition, hypertension was also more common in the ibrutinib-treated group than in the zanu group, but this difference was not statistically significant [18]. In the ALPINE trial the rates of hypertension were similar in the zanu (21.9%) and ibrutinib (19.8%) arms [36]. As yet, it is unclear if these results are due to differences in the sample population or to other factors; however, this is an intriguing observation and patients with CLL who are treated with zanu should be monitored for hypertension.
In conclusion, depending on the severity of cardiac comorbidities, using a second-generation BTKi such as zanu can be challenging (
Figure 2). Nonetheless, clinicians should be aware that effective cardiac screening and monitoring of cardiovascular complications must be undertaken also for patients at risk of cardiovascular toxicities who are treated with second-generation BTKis [57].
5. Conclusions
Updated NCCN (v2.2023) guidelines and the most recent German CLL algorithm both recommend second-generation BTKis, zanu and acalabrutinib, for the treatment of TN and R/R CLL regardless of patient fitness due to their increased selectivity and favorable toxicity profiles [23,24]. A recent comprehensive review and meta-analysis compared treatment-emergent AEs reported in clinical trials of ibrutinib, acalabrutinib and zanu. A total of 61 trials involving 6959 patients treated with ibrutinib, acalabrutinib, and zanu were included. Overall, results from this meta-analysis show an improved AE profile for acalabrutinib and zanu when compared to ibrutinib. Of note, zanu and acalabrutinib have a similar incidence of all grade (RR,1.12) and grade ≥ 3 (RR,0.90) AEs [60]. Therefore, the choice between these two different second-generation BTKis is driven predominantly by specific toxicity profiles and safety in older patients with comorbid conditions and cardiovascular risk factors [61], keeping in mind that certain comorbidities may amplify toxicities related to a given BTKi. For example, in patients with a significant history of headaches, therapy with acalabrutinib may be less preferable [62]. According to recent data, tablet acalabrutinib formulations were similar to capsules, with the added benefit that tablets could be given together with PPIs without affecting pharmacokinetics and pharmacodynamics [63].
We do not yet have robust evidence to suggest that second-generation BTKis may modify the natural course of CLL in specific genetic subgroups, but recent preliminary observations are of interest . A pooled analysis of two clinical studies (ELEVATE-TN and CL-003), designed to compare PFS and OS for acalabrutinib combined with obinutuzumab versus acalabrutinib monotherapy in patients with TN CLL, clearly showed the benefit of adding obinutuzumab to acalabrutinib monotherapy across genomic subgroups, particularly in those with unmutated IGHV or without del(17p)/TP53 mutations or complex karyotype abnormalities [64]. In the ALPINE trial, involving CLL patients with 17p deletion, TP53 mutation or both , zanu showed improved survival outcomes compared to ibrutinib. This observation was not evident in the ELEVATE-RR trial which compared acalabrutinib and ibrutinib [11].
However, the major differences in populations enrolled in the ELEVATE-RR and ALPINE trials does not enable them to be compared. On the other hand, this does not apply to the ASCEND trial that assessed acalabrutinib in a patient population of R/R CLL patients similar to that included in the ALPINE trial [65]. Results of unanchored matching-adjusted indirect comparison (MAIC) comparing the efficacy and safety of acalabrutinib vs zanu ,using individual patient data (IPD) from ASCEND and published aggregate data from ALPINE, have recently been presented. In this indirect comparison, acalabrutinib and zanu were shown to have similar efficacy in patients with RR CLL (PFS at 24 months 76% and 78%, respectively) [66]. However, the recognized limitations of MAIC analyses imply that results should be viewed as hypothesis-generating.
Finally, the CAPTIVATE and GLOW trial results led to the EMA approval of the ibrutinib-venetoclax combination [67,68], while emerging data indicate that second-generation more selective BTKis like zanu ,may well provide a valid alternative to ibrutinib in doublet regimens combining a BTKi with BCL2i. In this respect, preliminary safety data suggests that the combination regimen of ZV Is generally well tolerated in this high-risk population, but no new safety signals were identified [34]. The results of the long-term follow-up of arm D of the SEQUOIA trial are eagerly awaited.
In light of all the above, we feel that zanu represents an important landmark development in the treatment of CLL and is an exciting addition to the clinician's therapeutic armamentarium ,as well as an attractive option for patients with CLL needing therapy.
Declaration of interest
The authors do not declare any conflict of interest.
Author Contributions
Stefano Molica and Aaron Polliack designed the study and wrote the paper. David Allsup and Constantine Tam critically reviewed the paper and contributed to the final version of manuscript. All authors approved the manuscript.
Funding
This paper was not funded.
References
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013, 369, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Brown, J.R.; O'Brien, S.; et al. RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 2014, 371, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; et al. RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E. , et al Ibrutinib- Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A. , et al Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Greil, R.; Demirkan, F.; et al. Ibrutinib plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicenter, randomized, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Munir, T.; Brown, J.R.; O'Brien, S.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef]
- Barr, P.M.; Owen, C.; Robak, T.; et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022, 6, 5641–5654. [Google Scholar] [CrossRef]
- Molica S, Matutes E, Tam C, Polliack A. Ibrutinib in the treatment of chronic lymphocytic leukemia: 5 years on. Hematol. Oncol. 2020, 38, 129–136. [Google Scholar] [CrossRef]
- Lipsky, A.; Lamanna, N. Managing toxicities of Bruton tyrosine kinase inhibitors. Am. Soc. Hematol. Educ. Program. 2020, 2020, 336–345. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Project Orbis: FDA approves acalabrutinib for CLL and SLL. FDA website. Available online: https://bit.ly/35idpJM (accessed on 21 November 2019).
- Calquence approved in the EU for the treatment of chronic lymphocytic leukaemia. News release. AstraZeneca. 2020. Available online: https://bit.ly/3kiCkUT (accessed on 10 November 2019).
- Shirley, D. How does zanubrutinib fare in treatment of B-cell malignancies? Lancet Haematol. 2023, 10, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zanubrutinib-mantle-cell-lymphoma.
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zanubrutinib-marginal-zone-lymphoma.
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-waldenstroms-macroglobulinemia.
- Tam, C.S.; Opat, S.; D'Sa, S.; Jurczak, W. , et al A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood. 2020, 136, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Brown, J.R.; Kahl, B.S. , et al Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): A randomised, controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef] [PubMed]
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma.
- https://www.ema.europa.eu/en/medicines/human/EPAR/brukinsa.
- Wierda, W.G.; Brown, J.; Abramson, J.S.; et al. NCCN Guidelines Insights: Chronic lymphocytic leukemia/small lymphocytic lymphoma, version 1.2023. J Natl Compr Canc Netw. 2022, 20, 622–634. [Google Scholar] [CrossRef]
- https://www.onkopedia.com/de/onkopedia/guidelines/chronische-lymphatische-leukaemie-cll/@@guideline/html/index.html.
- Tam, C.S.; Trotman, J.; Opat, S.; et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019, 134, 851–859. [Google Scholar] [CrossRef]
- Brander, D.M. The use of zanubrutinib in chronic lymphocytic leukemia. Clin. Adv. Hematol. Oncol. 2022, 20, 705–708. [Google Scholar]
- Muñoz, J.; Wang, Y.; Jain, P.; et al. Zanubrutinib in lymphoproliferative disorders: A comprehensive review. Ther. Adv. Hematol. 2022, 13, 20406207221093980. [Google Scholar] [CrossRef]
- Ou, Y.C.; Tang, Z.; Novotny, W.; et al. Evaluation of drug interaction potential of zanubrutinib with cocktail probes representative of CYP3A4, CYP2C9, CYP2C19, P-gp and BCRP. Br. J. Clin. Pharmacol. 2021, 87, 2926–2936. [Google Scholar] [CrossRef]
- Cull, G.; Burger, J.A.; Opat, S.; et al. Zanubrutinib for treatment-naïve and relapsed/refractory chronic lymphocytic leukaemia: Long-term follow-up of the phase I/II AU-003 study. Br. J. Haematol. 2022, 196, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Giannopoulos, K.; Jurczak, W.; et al. SEQUOIA: Results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma [ASH abstract 396]. Blood 2021, 138 (Suppl. 1), 396. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; JurczakW; et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. 2022, 36, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Barnes, G.; Yang, K.; et al. Patient-reported outcomes from phase 3 randomized study of zanubrutinib versus bendamustine and rituximab (BR) in patients with treatment-naïve (TN) CLL/SLL. HemaSphere 2022, 6, 560–561. [Google Scholar] [CrossRef]
- Tam, C.S.; Robak, T.; Ghia, P. , et al Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica 2020, 106, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Ferrant, E.; et al. Zanubrutinib in Combination with Venetoclax for Patients with Treatment-Naïve (TN) Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) with del (17p): Early Results from Arm D of the SEQUOIA (BGB-3111-304) Trial. In Proceedings of the American Society of Hematology Annual Meeting, Atlanta, GA, USA, 11–14 December 2021. [Google Scholar]
- Hillmen, P.; Eichhorst, B.; Brown, J.R.; et al. Zanubrutinib Versus Ibrutinib in Relapsed/Refractory Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: Interim Analysis of a Randomized Phase III Trial. J. Clin. Oncol. 2023, 41, 1035–1045. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2022, 388, 319–332. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; et al. Zanubrutinib demonstrates superior progression-free survival (PFS) compared with ibrutinib for treatment of relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma (R/R CLL/SLL): Results from final analysis of ALPINE randomized phase 3 study [ASH abstract LBA-6]. Blood. [CrossRef]
- Tam, C.S.; Ou, Y.C.; Trotman, J.; Opat, S. Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Rev. Clin. Pharmacol. 2021, 14, 1329–1344. [Google Scholar]
- Hillmen, P.; Brown, J.; Lamanna, N.; et al. Health-related quality of life outcomes associated with zanubrutinib vs ibrutinib monotherapy in patients with relapsed/refractory (RR) CLL/SLL: Results from the randomized phase 3 ALPINE trial. HemaSphere. 2022, 6 (Suppl. 3), P663. [Google Scholar] [CrossRef]
- Ou, Y.C.; Tang, Z.; Novotny, W.; et al. Rationale for once-daily or twice-daily dosing of zanubrutinib in patients with mantle cell lymphoma. Leuk. Lymphoma 2021, 62, 2612–2624. [Google Scholar] [CrossRef]
- Shadman, M.; Flinn, I.W.; Levy, M.Y.; et al. Zanubrutinib in patients with previously treated B-cell malignancies intolerant of previous Bruton tyrosine kinase inhibitors in the USA: A phase 2, open-label, single-arm study. Lancet Haematol. 2023, 10, e35–e45. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Nabhan, C.; Thompson, M.C.; et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: A real-world analysis. Haematologica 2018, 103, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Woyach, J.A.; Furman, R.F.; et al. Acalabrutinib in treatment-naive chronic lymphocytic leukemia. Blood. 2021, 137, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.A.; Thompson, P.A.; Allan, J.N.; et al. Phase II study of acalabrutinib in ibrutinib-intolerant patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica. 2021, 106, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Sivina, M.; Jain, N.; et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood. 2019, 133, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Quach, H.; Nicol, A.; et al. Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv. 2020, 4, 4802–4811. [Google Scholar] [CrossRef] [PubMed]
- Soumerai, J.D.; Mato, A.R.; Dogan, A.; et al. Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: A multicentre, single-arm, phase 2 trial. Lancet Haematol. 2021, 8, e879–e890. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Lampson, B.L.; Tyekucheva, S.; et al. Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: A single-arm, open-label, phase 2 study. Lancet Oncol. 2021, 22, 1391–1402. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Guinn, D.; et al. BTKC481S-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2017, 35, 1437–1443. [Google Scholar] [CrossRef]
- Zhu, H.; Sha, Y.; Miao, Y.; et al. Integrating Multi-Omics to Reveal the Clonal Evolutionary Characteristics in CLL Patients with Zanubrutinib Resistance. [ASH abstract 3104]. Blood. 2022, 140 (Suppl. 1). [CrossRef]
- Blombery, P.; Thompson, E.R.; Lew, T.E.; et al. Enrichment of BTK Leu528Trp mutations in patients with CLL on zanubrutinib: Potential for pirtobrutinib cross-resistance. Blood Adv. 2022, 6, 5589–5592. [Google Scholar] [CrossRef]
- Ahn, I.E.; Brown, J.R. Selecting initial therapy in CLL. Hematology Am. Soc. Hematol. Educ. Program. 2022, 2022, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Shadman, M. Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Review. JAMA. 2023, 329, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.J.; Kaempf, A.; Sitlinger, A.; et al. The Chronic Lymphocytic Leukemia Comorbidity Index (CLL-CI): A Three-Factor Comorbidity Model. Clin. Cancer Res. 2021, 27, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, T.; Wiczer, T.; Waller, A.; et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019, 134, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Parikh, S.A.; Noseworthy, P.A.; et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk. Lymphoma 2017, 58, 1630–1639. [Google Scholar] [CrossRef]
- Awan, F.T.; Addison, D.; Alfraih, F.; et al. International consensus statement on the management of cardiovascular risk of Bruton’s tyrosine kinase inhibitors in CLL. Blood Adv. 2022, 6, 5516–5525. [Google Scholar] [CrossRef]
- Bhat, S.A.; Gambril, J.; Azali, L.; et al. Ventricular arrhythmias and sudden death events following acalabrutinib initiation. Blood 2022, 140, 2142–2145. [Google Scholar] [CrossRef]
- Lampson, B.L.; Yu, L.; Glynn, R.J.; et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017, 129, 2581–2584. [Google Scholar] [CrossRef]
- Hwang, S.; Wang, J.; Tian, Z.; et al. Comparison of treatment-emergent adverse events of acalabrutinib and zanubrutinib in clinical trials in B-cell malignancies: A systematic review and meta-anaysis. 2023. [CrossRef]
- Hampel, P.A.; Parikh, S.A. Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer J. 2022, 12, 161. [Google Scholar] [CrossRef]
- Kuss, B.; Nagarajan, C.; Hsieh, W.S.; et al. Practical management of chronic lymphocytic leukemia with acalabrutinib. Leuk. Lymphoma 2022, 63, 2785–2794. [Google Scholar] [CrossRef]
- Sharma, S.; Pepin, X.; Burri, H.; et al. Bioequivalence and Relative Bioavailability Studies to Assess a New Acalabrutinib Formulation That Enables Coadministration With Proton-Pump Inhibitors. Clin. Pharmacol. Drug Dev. 2022, 11, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Sharman, J.P.; Eyre, T.A.; et al. Contribution of Obinutuzumab to Acalabrutinib therapy in patients with treatment-naive Chronic Lymphocytic Leukemia: Analysis of Survival Outcomes By Genomic Features. [ASH abstract 1815]. Blood. 2022, 140. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; et al. Acalabrutinib Versus Investigator's Choice in Relapsed/Refractory Chronic Lymphocytic Leukemia: Final ASCEND Trial Results. Hemasphere. 2022, 6, e801. [Google Scholar] [CrossRef] [PubMed]
- Skarbnik, A.; Kittai, A.; Miranda, M.; et al. A matching-adjusted indirect comparison of efficacy and safety of acalabrutinib versus zanubrutinib in relapsed or refractory chronic lymphocytic leukemia. EHA 2023. [Google Scholar] [CrossRef]
- Tam, C.S.; Allan, J.N.; Siddiqi, T.; et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: Primary analysis of the CAPTIVATE FD cohort. Blood 2022, 139, 3278–3289. [Google Scholar] [CrossRef]
- Kater, A.P.; Owen, C.; Moreno, C.; et al. Fixed-duration Ibrutinib- Venetoclax in patients with chronic lymphocytic leukemia and comorbidities. N. Eng. J. Med. Evidence. 2022, 1, 2200006. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).