Submitted:
20 April 2023
Posted:
21 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
| Compound | P388 | L1210 | Molt 4/C8 | CEM | Human tumor cells |
|---|---|---|---|---|---|
| IIa | 12.7 | 106.0 | 42.7 | 28.9 | 18.6 |
| IIb | 11.8 | 25.0 | 21.3 | 11.4 | 11.2 |
| IIc | 1.6 | 0.34 | 0.47 | 0.35 | 0.27 |
2. Results
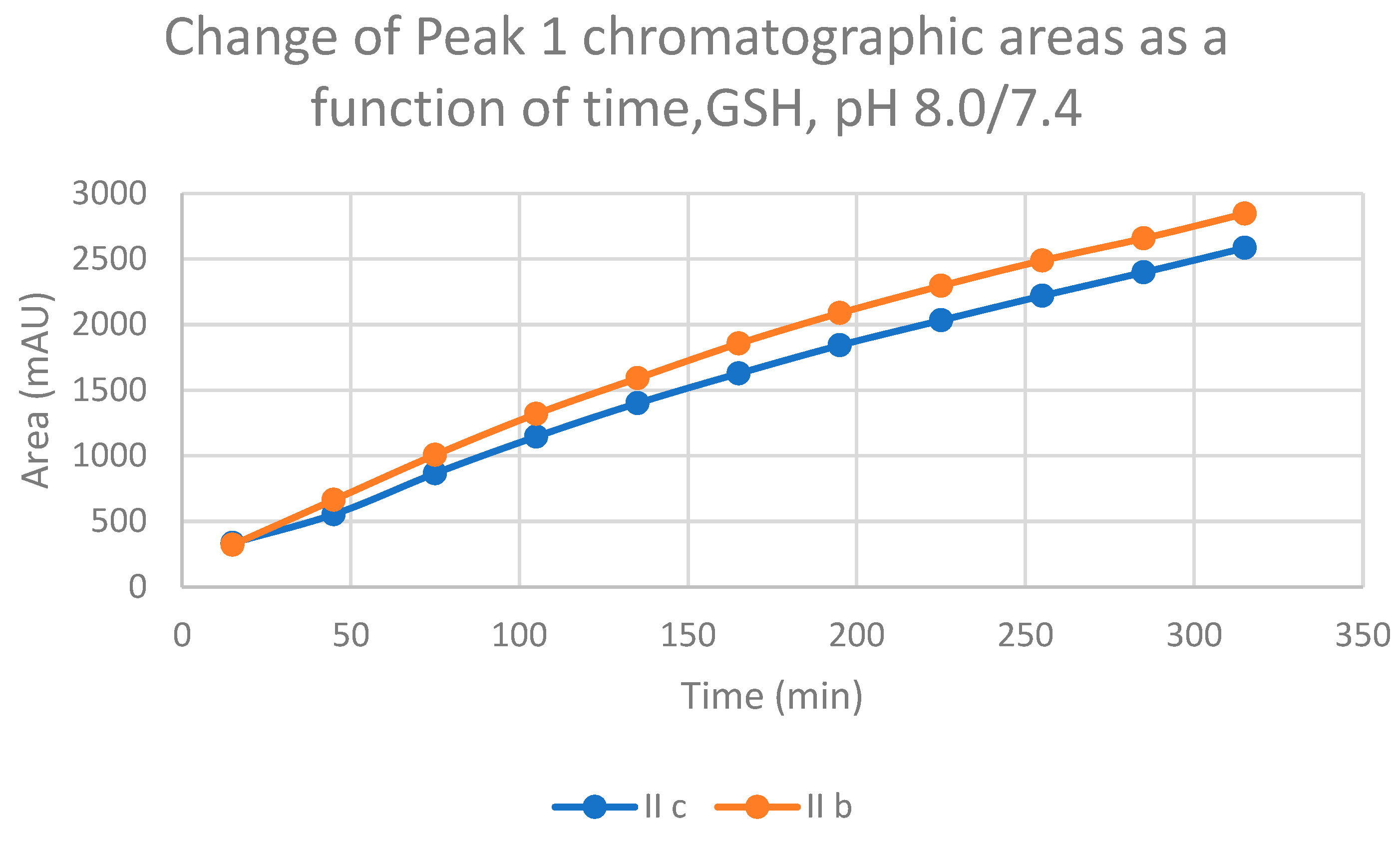
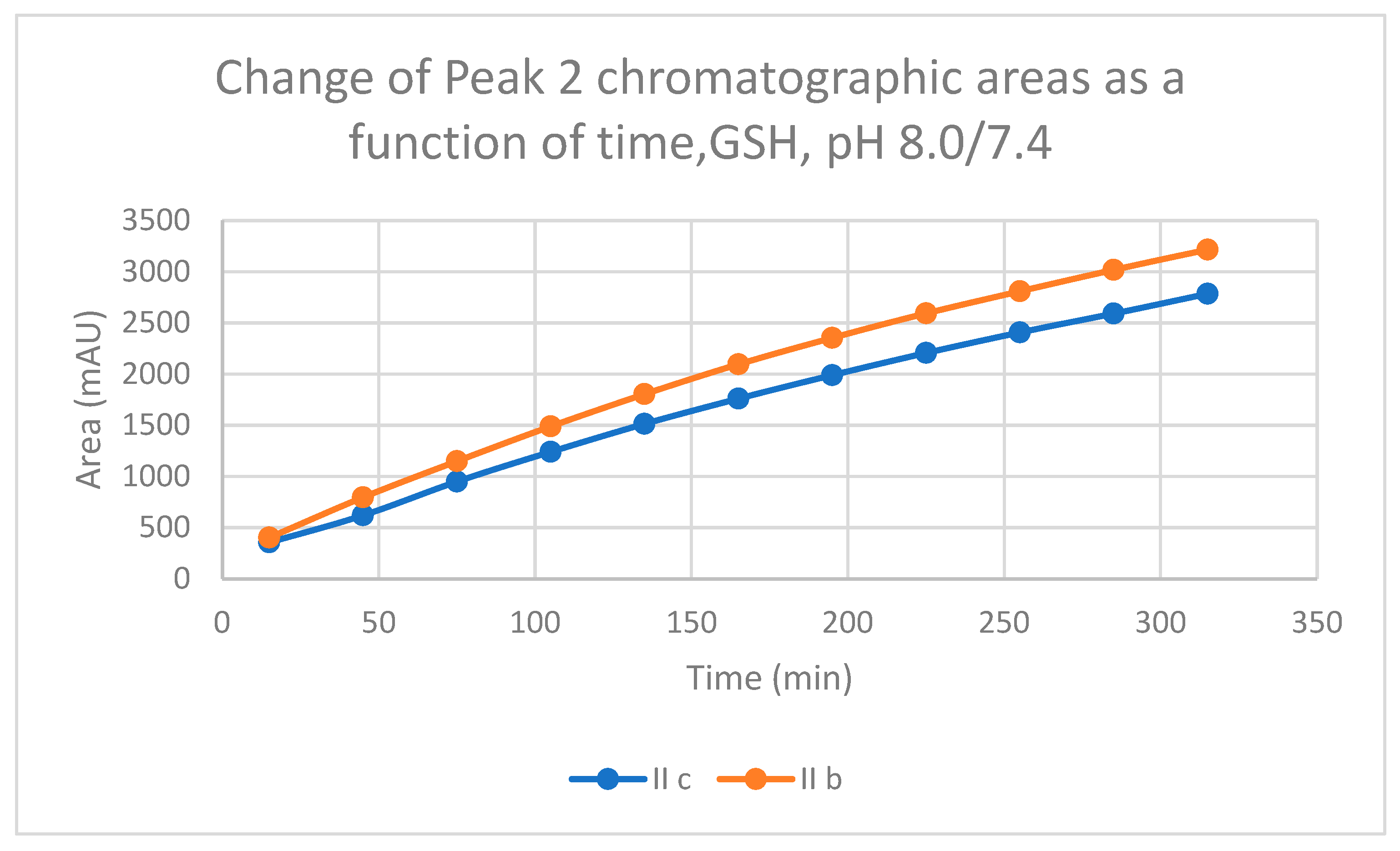
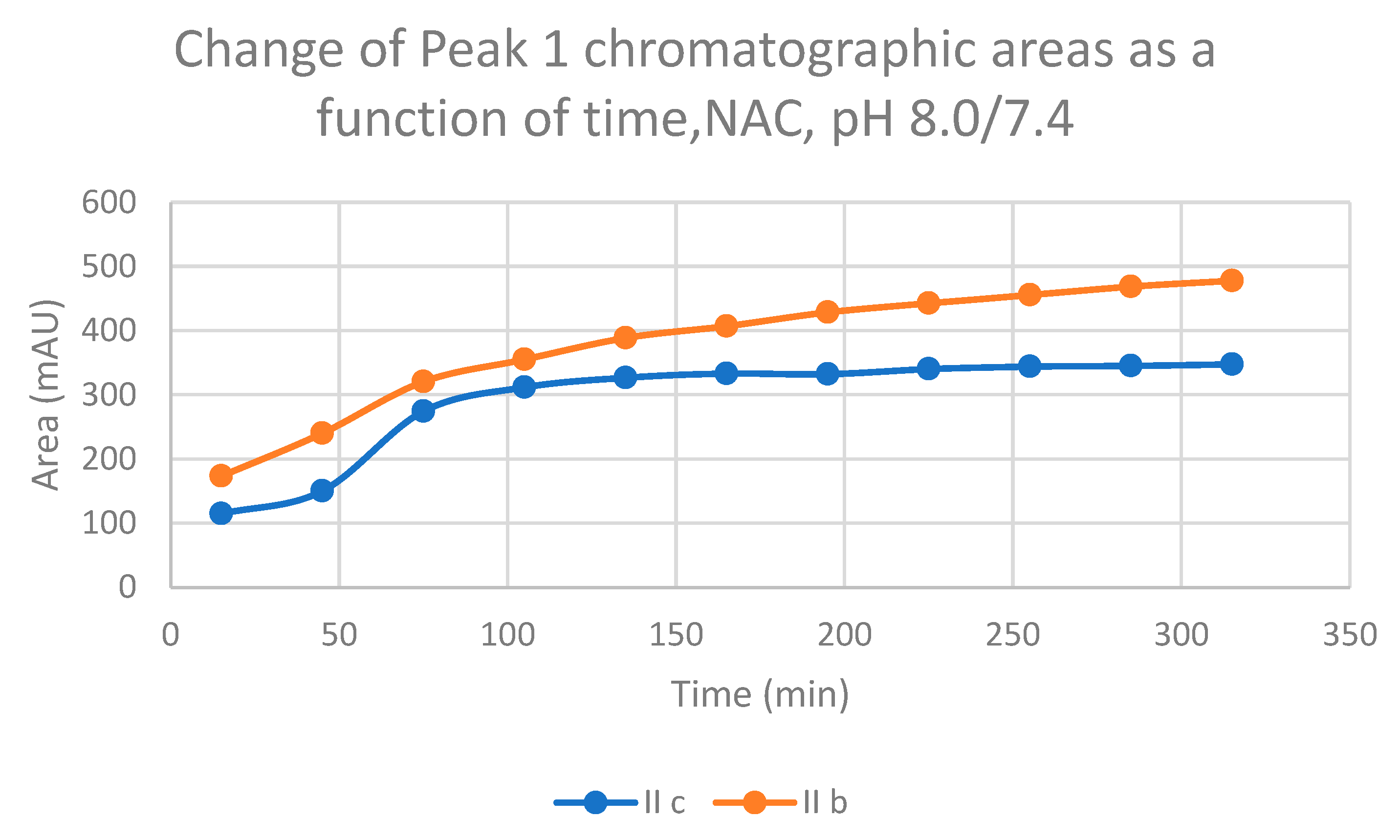
2.1. Reactions under Slightly Basic (pH 8.0/7.4) Conditions
| pH 3 | Compound | tR (E)-Chalcone |
Area Ratio 4 A315/A0 |
tR (Z)-Chalcone |
Area (Z)-Chalcone |
tR GSH–1 |
Area GSH–1 |
tR GSH–2 |
Area GSH–2 |
|---|---|---|---|---|---|---|---|---|---|
| 3.2 | IIb | 17.1 | 0.89 | 16.8 | 55.1 | 14.85 | 74.9 | 15.25 | 111.5 |
| 3.2 | IIc | 16.6 | 0.95 | 16.3 | 136.1 | ND5 | - | ND5 | - |
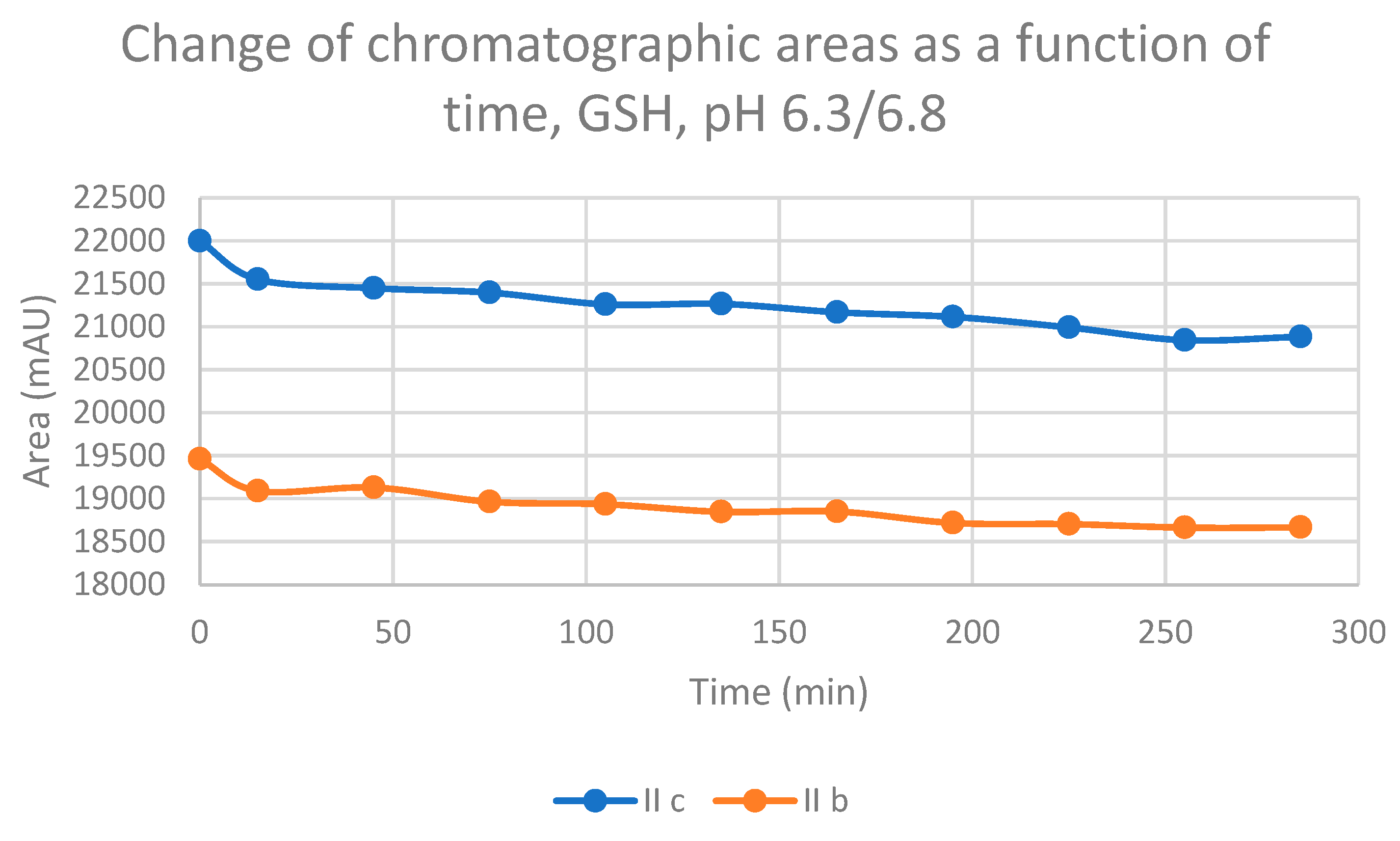
| 6.3 | IIb | 17.0 | 0.84 | 16.7 | 446.6 | 14.6 | 297.2 | 15.1 | 331.8 |
| 6.3 | IIc | 16.9 | 0.91 | 16.7 | 513.4 | 14.2 | 233.6 | 14.8 | 256.4 |
| 8.0 | IIb | 17.4 | 0.57 | 17.1 | 302.8 | 15.0 | 2847.0 | 15.4 | 3216.3 |
| 8.0 | IIc | 16.8 | 0.74 | 16.5 | 412.0 | 13.9 | 2584.9 | 14.6 | 2785.0 |
| pH 3 | Compound | tR (E)-Chalcone |
Area Ratio 4 A315/A0 |
tR (Z)-Chalcone |
Area (Z)-Chalcone |
tR NAC–1 |
Area NAC–1 |
tR NAC–2 |
Area NAC–2 |
|---|---|---|---|---|---|---|---|---|---|
| 3.2 | IIb | 17.1 | 0.76 | 16.8 | 124.1 | N/D5 | - | N/D5 | - |
| 3.2 | IIc | 16.6 | 0.88 | 16.3 | 126.9 | N/D5 | - | N/D5 | - |
| 6.3 | IIb | 17.5 | 0.93 | 17.2 | 118.9 | 16.3 | 60.0 | 16.5 | 513.9 |
| 6.3 | IIc | 16.7 | 0.91 | 16.4 | 184.5 | 15.3 | 61.8 | 15.6 | 392.1 |
| 8.0 | IIb | 17.5 | 0.92 | 17.2 | 467.5 | 16.3 | 477.7 | 16.5 | 913.4 |
| 8.0 | IIc | 17.0 | 0.92 | 16.8 | 541.9 | 15.7 | 347.5 | 15.9 | 624.2 |
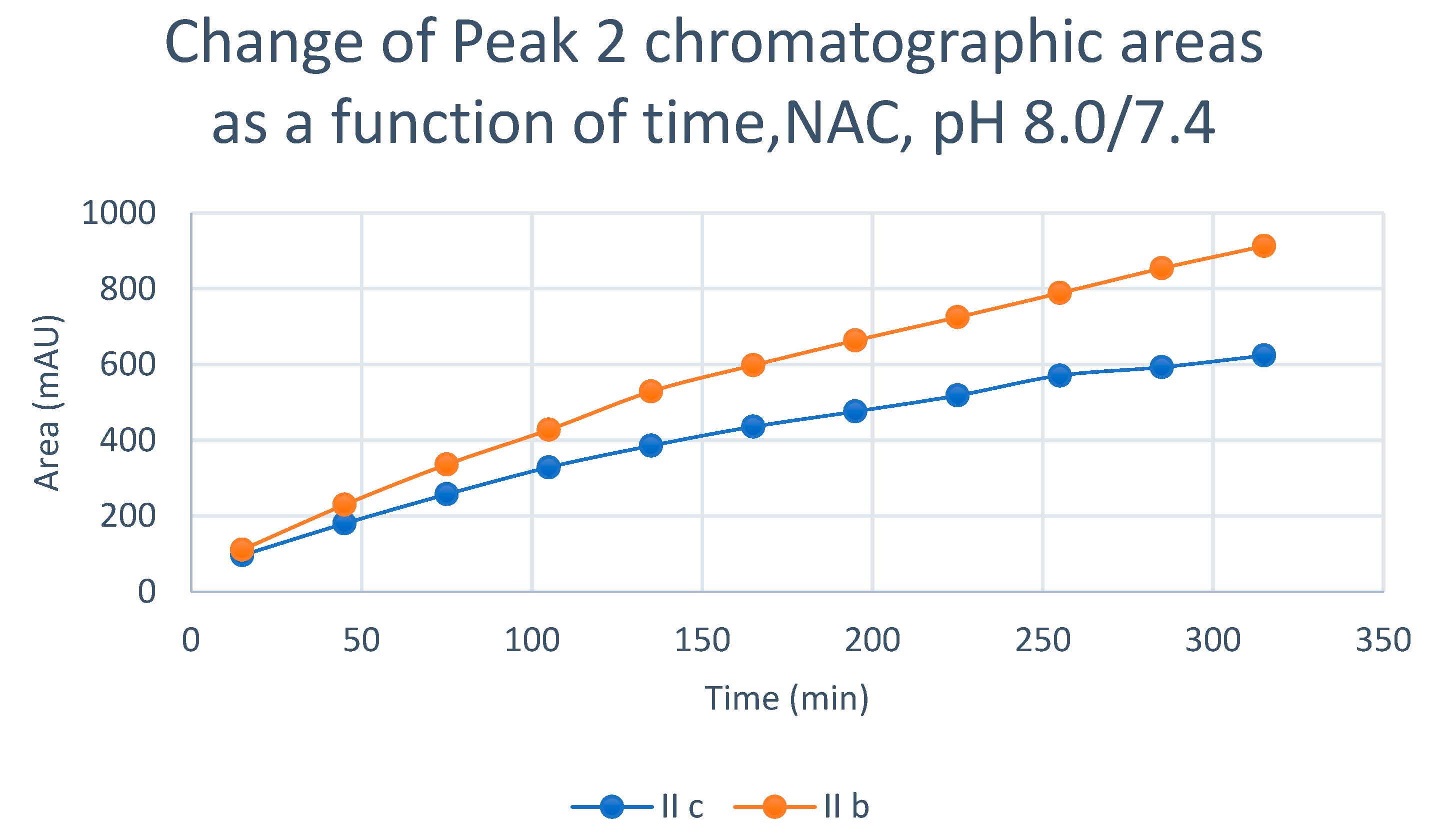
2.2. Reaction under Slightly Acidic (pH 6.3/6.8) Conditions
2.3. Reaction under Acid (pH 3.2/3.8) Conditions
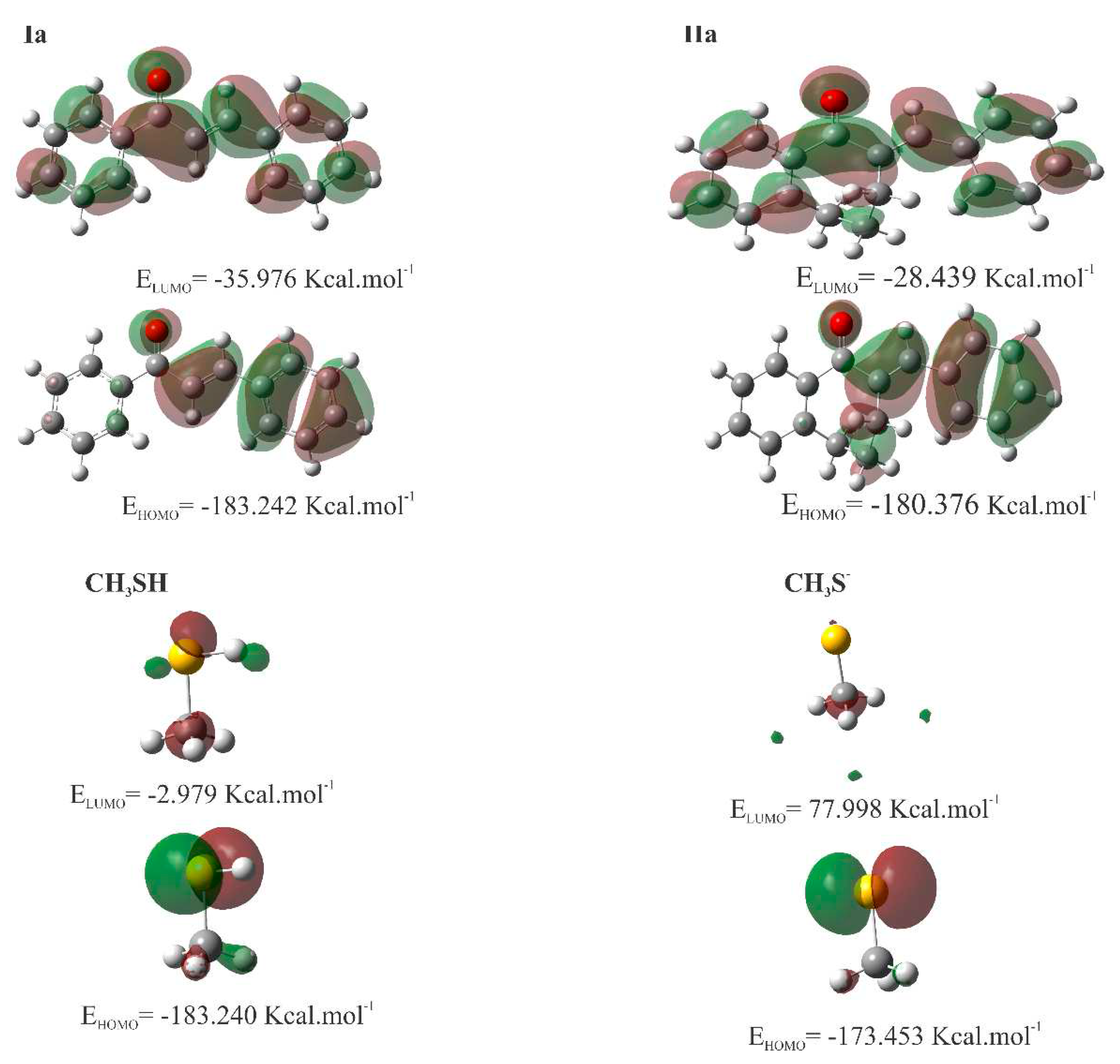
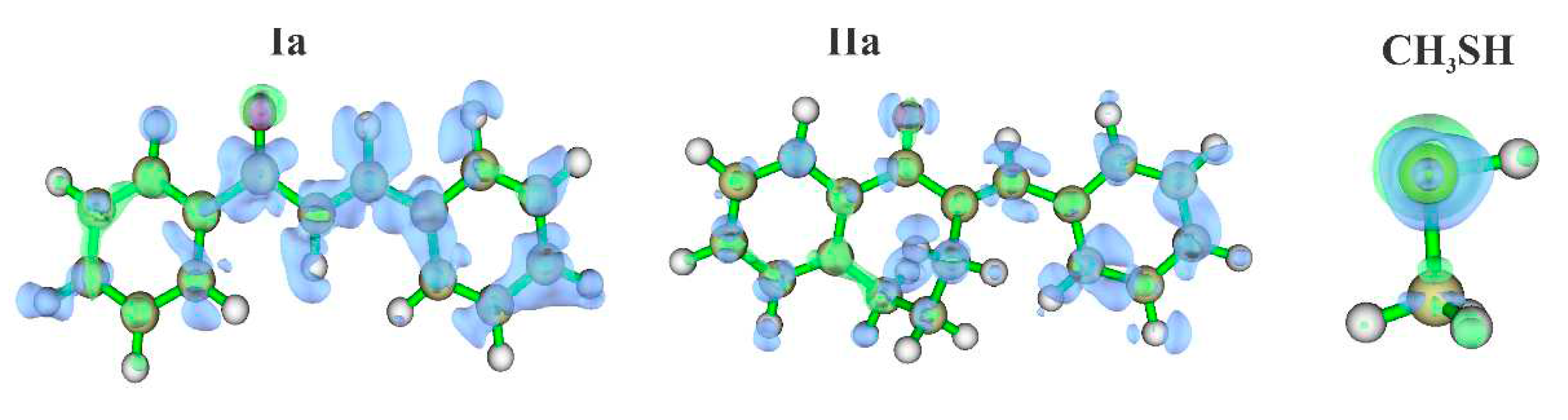
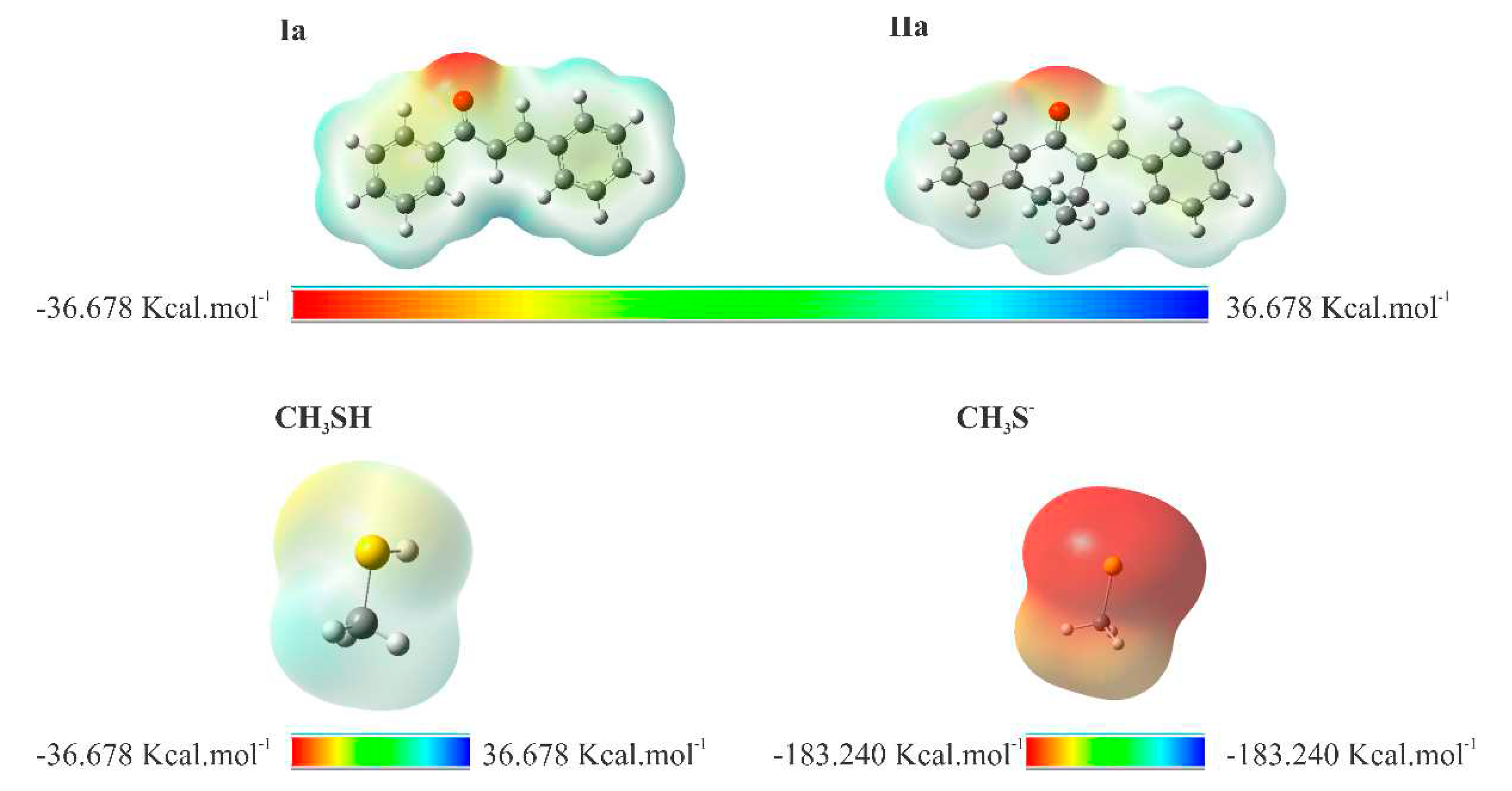
2.4. Molecular modeling analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Solutions
4.3. RP-HPLC-UV-VIS Measurements
4.4. HPLC-MS Measurements
4.5. Molecular modeling analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kenari, F.; Molnár, S.; Pintér, Z.; Bitaraf, S.; Perjési, P. (E)-2-Benzylidenecyclanones: Part XVII. An LC-MS Study of Microsomal Transformation Reactions of (E)-2-[(4’-Methoxyphenyl)Methylene]-Benzosuberon-1-One: A Cyclic Chalcone Analog. J. Pharm. Biopharm. Res. 2023, 4, 326–339. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally Occurring Chalcones and Their Biological Activities. Phytochemistry Reviews 2016, 15, 87–120. [Google Scholar] [CrossRef]
- K. Sahu, N.; S. Balbhadra, S.; Choudhary, J.; V. Kohli, D. Exploring Pharmacological Significance of Chalcone Scaffold: A Review. CMC 2012, 19, 209–225. [CrossRef] [PubMed]
- Singh, P.; Anand, A.; Kumar, V. Recent Developments in Biological Activities of Chalcones: A Mini Review. European Journal of Medicinal Chemistry 2014, 85, 758–777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B. Diverse Molecular Targets for Chalcones with Varied Bioactivities. Med chem 2015. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chemical reviews 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Narayana Moorthy, N.S.H.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, D.; Trivedi, P. Advances in Chalcones with Anticancer Activities. PRA 2014, 10, 97–115. [Google Scholar] [CrossRef]
- Gomes, M.; Muratov, E.; Pereira, M.; Peixoto, J.; Rosseto, L.; Cravo, P.; Andrade, C.; Neves, B. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef]
- Constantinescu, T.; Mihis, A.G. Two Important Anticancer Mechanisms of Natural and Synthetic Chalcones. IJMS 2022, 23, 11595. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Kandepu, N.M.; Nazarali, A.J.; Kowalchuk, T.P.; Motaganahalli, N.; Quail, J.W.; Mykytiuk, P.A.; Audette, G.F.; Prasad, L.; Perjési, P.; et al. Conformational and Quantitative Structure−Activity Relationship Study of Cytotoxic 2-Arylidenebenzocycloalkanones. J. Med. Chem. 1999, 42, 1358–1366. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Zello, G.A.; Oloo, E.O.; Quail, J.W.; Kraatz, H.-B.; Perjési, P.; Aradi, F.; Takács-Novák, K.; Allen, T.M.; Santos, C.L.; et al. Correlations between Cytotoxicity and Topography of Some 2-Arylidenebenzocycloalkanones Determined by X-Ray Crystallography. J. Med. Chem. 2002, 45, 3103–3111. [Google Scholar] [CrossRef]
- Perjési, P.; Das, U.; De Clercq, E.; Balzarini, J.; Kawase, M.; Sakagami, H.; Stables, J.P.; Lorand, T.; Rozmer, Z.; Dimmock, J.R. Design, Synthesis and Antiproliferative Activity of Some 3-Benzylidene-2,3-Dihydro-1-Benzopyran-4-Ones Which Display Selective Toxicity for Malignant Cells. European Journal of Medicinal Chemistry 2008, 43, 839–845. [Google Scholar] [CrossRef]
- Rozmer, Z.; Berki, T.; Perjési, P. Different Effects of Two Cyclic Chalcone Analogues on Cell Cycle of Jurkat T Cells. Toxicology in Vitro 2006, 20, 1354–1362. [Google Scholar] [CrossRef]
- Pilatova, M.; Varinska, L.; Perjesi, P.; Sarissky, M.; Mirossay, L.; Solar, P.; Ostro, A.; Mojzis, J. In Vitro Antiproliferative and Antiangiogenic Effects of Synthetic Chalcone Analogues. Toxicology in Vitro 2010, 24, 1347–1355. [Google Scholar] [CrossRef]
- Perjési, P.; Maász, G.; Reisch, R.; Benkő, A. (E)-2-Benzylidenebenzocyclanones: Part VII. Investigation of the Conjugation Reaction of Two Cytotoxic Cyclic Chalcone Analogues with Glutathione: An HPLC–MS Study. Monatsh Chem 2012, 143, 1107–1114. [Google Scholar] [CrossRef]
- Rozmer, Z.; Berki, T.; Maász, G.; Perjési, P. Different Effects of Two Cyclic Chalcone Analogues on Redox Status of Jurkat T Cells. Toxicology in Vitro 2014, 28, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione Synthesis. Biochimica et Biophysica Acta (BBA) - General Subjects 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione Dysregulation and the Etiology and Progression of Human Diseases. bchm 2009, 390, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Molecular Aspects of Medicine 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Jones, D.P. [11] Redox Potential of GSH/GSSG Couple: Assay and Biological Significance. In Methods in Enzymology; Elsevier, 2002; Volume 348, pp. 93–112. ISBN 978-0-12-182251-4. [Google Scholar]
- Dickinson, D.A.; Forman, H.J. Cellular Glutathione and Thiols Metabolism. Biochemical Pharmacology 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- Moran, L.; Gutteridge, J.; Quinlan, G. Thiols in Cellular Redox Signalling and Control. CMC 2001, 8, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.Y. Cellular Redox: A Modulator of Intestinal Epithelial Cell Proliferation. Physiology 2003, 18, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Kenari, F.; Molnár, S.; Perjési, P. Reaction of Chalcones with Cellular Thiols. The Effect of the 4-Substitution of Chalcones and Protonation State of the Thiols on the Addition Process. Diastereoselective Thiol Addition. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Drutovic, D.; Chripkova, M.; Pilatova, M.; Kruzliak, P.; Perjesi, P.; Sarissky, M.; Lupi, M.; Damia, G.; Broggini, M.; Mojzis, J. Benzylidenetetralones, Cyclic Chalcone Analogues, Induce Cell Cycle Arrest and Apoptosis in HCT116 Colorectal Cancer Cells. Tumor Biol. 2014, 35, 9967–9975. [Google Scholar] [CrossRef] [PubMed]
- Caccuri, A.M.; Antonini, G.; Board, P.G.; Parker, M.W.; Nicotra, M.; Bello, M.L.; Federici, G.; Ricci, G. Proton Release on Binding of Glutathione to Alpha, Mu and Delta Class Glutathione Transferases. Biochemical Journal 1999, 344, 419–425. [Google Scholar] [CrossRef]
- Rohani, N.; Hao, L.; Alexis, M.S.; Joughin, B.A.; Krismer, K.; Moufarrej, M.N.; Soltis, A.R.; Lauffenburger, D.A.; Yaffe, M.B.; Burge, C.B.; et al. Acidification of Tumor at Stromal Boundaries Drives Transcriptome Alterations Associated with Aggressive Phenotypes. Cancer Research 2019, 79, 1952–1966. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why. Free Radical Research 2018, 52, 751–762. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Reactions of Electrophiles with Nucleophilic Thiolate Sites: Relevance to Pathophysiological Mechanisms and Remediation. Free Radical Research 2016, 50, 195–205. [Google Scholar] [CrossRef]
- d’Oliveira, G.D.C.; Custodio, J.M.F.; Moura, A.F.; Napolitano, H.B.; Pérez, C.N.; Moraes, M.O.; Prókai, L.; Perjési, P. Different Reactivity to Glutathione but Similar Tumor Celltoxicity of Chalcones and Their Quinolinone Analogues. Medicinal Chemistry Research 2019, 28, 1448–1460. [Google Scholar] [CrossRef]
- Perjési, P.; Linnanto, J.; Kolehmainen, E.; Ősz, E.; Virtanen, E. E-2-Benzylidenebenzocycloalkanones. IV. Studies on Transmission of Substituent Effects on 13C NMR Chemical Shifts of E-2-(X-Benzylidene)-1-Tetralones, and -Benzosuberones. Comparison with the 13C NMR Data of Chalcones and E-2-(X-Benzylidene)-1-Indanones. Journal of Molecular Structure 2005, 740, 81–89. [Google Scholar] [CrossRef]
- Amslinger, S. The Tunable Functionality of α,β-Unsaturated Carbonyl Compounds Enables Their Differential Application in Biological Systems. ChemMedChem 2010, 5, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Amslinger, S.; Al-Rifai, N.; Winter, K.; Wörmann, K.; Scholz, R.; Baumeister, P.; Wild, M. Reactivity Assessment of Chalcones by a Kinetic Thiol Assay. Org. Biomol. Chem. 2013, 11, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Al-Rifai, N.; Rücker, H.; Amslinger, S. Opening or Closing the Lock? When Reactivity Is the Key to Biological Activity. Chem. Eur. J. 2013, 19, 15384–15395. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.N. Glutathione S-Transferases: Reaction Mechanism, Structure, and Function. Chem. Res. Toxicol. 1991, 4, 131–140. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T.; DeCaprio, A.; Barber, D.S. Application of the Hard and Soft, Acids and Bases (HSAB) Theory to Toxicant–Target Interactions. Chem. Res. Toxicol. 2012, 25, 239–251. [Google Scholar] [CrossRef]
- Perjési, P.; Nusser, T.; Tarczay, Gy.; Sohár, P. E-2-Benzylidenebenzocycloalkanones. Stereostructure and NMR Spectroscopic Investigation. Journal of Molecular Structure 1999, 479, 13–19. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Physical Review 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Physical Review 1965, 140, A1133. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 Revision C. 01. 2016; Gaussian Inc. Wallingford CT 2016, 421. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Non-covalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theoretical Chemistry Accounts 2008, 120, 215–241. [Google Scholar]
- Zhang, G.; Musgrave, C.B. Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations. The Journal of Physical Chemistry A 2007, 111, 1554–1561. [Google Scholar] [CrossRef]
- Weiner, P.K.; Langridge, R.; Blaney, J.M.; Schaefer, R.; Kollman, P.A. Electrostatic Potential Molecular Surfaces. Proc. Natl. Acad. Sci. U.S.A. 1982, 79, 3754–3758. [Google Scholar] [CrossRef] [PubMed]
- Naray-Szabo, G.; Ferenczy, G.G. Molecular Electrostatics. Chemical Reviews 1995, 95, 829–847. [Google Scholar] [CrossRef]
- Fukui, K. The Role of Frontier Orbitals in Chemical Reactions (Nobel Lecture). Angewandte Chemie International Edition in English 1982, 21, 801–809. [Google Scholar] [CrossRef]
- Fukui, K. Role of Frontier Orbitals in Chemical Reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef]
- Lu, T. Multiwfn. Software Manual. 2014. [Google Scholar]
- Sanches-Neto, F.O.; Dias-Silva, J.R.; Keng Queiroz Junior, L.H.; Carvalho-Silva, V.H. "Py SiRC": Machine Learning Combined with Molecular Fingerprints to Predict the Reaction Rate Constant of the Radical-Based Oxidation Processes of Aqueous Organic Contaminants. Environ. Sci. Technol. 2021, 55, 12437–12448. [Google Scholar] [CrossRef]
- Kozurkova, M.; Tomeckova, V. V. Interaction of Chalcone Derivatives with Important Biomacromolecules. In Chalcones and Their Synthetic Analogs; Nova Science Publisher: New York, NY, USA, 2020; pp. 95–133. ISBN 978-1-5361-8709-0. [Google Scholar]
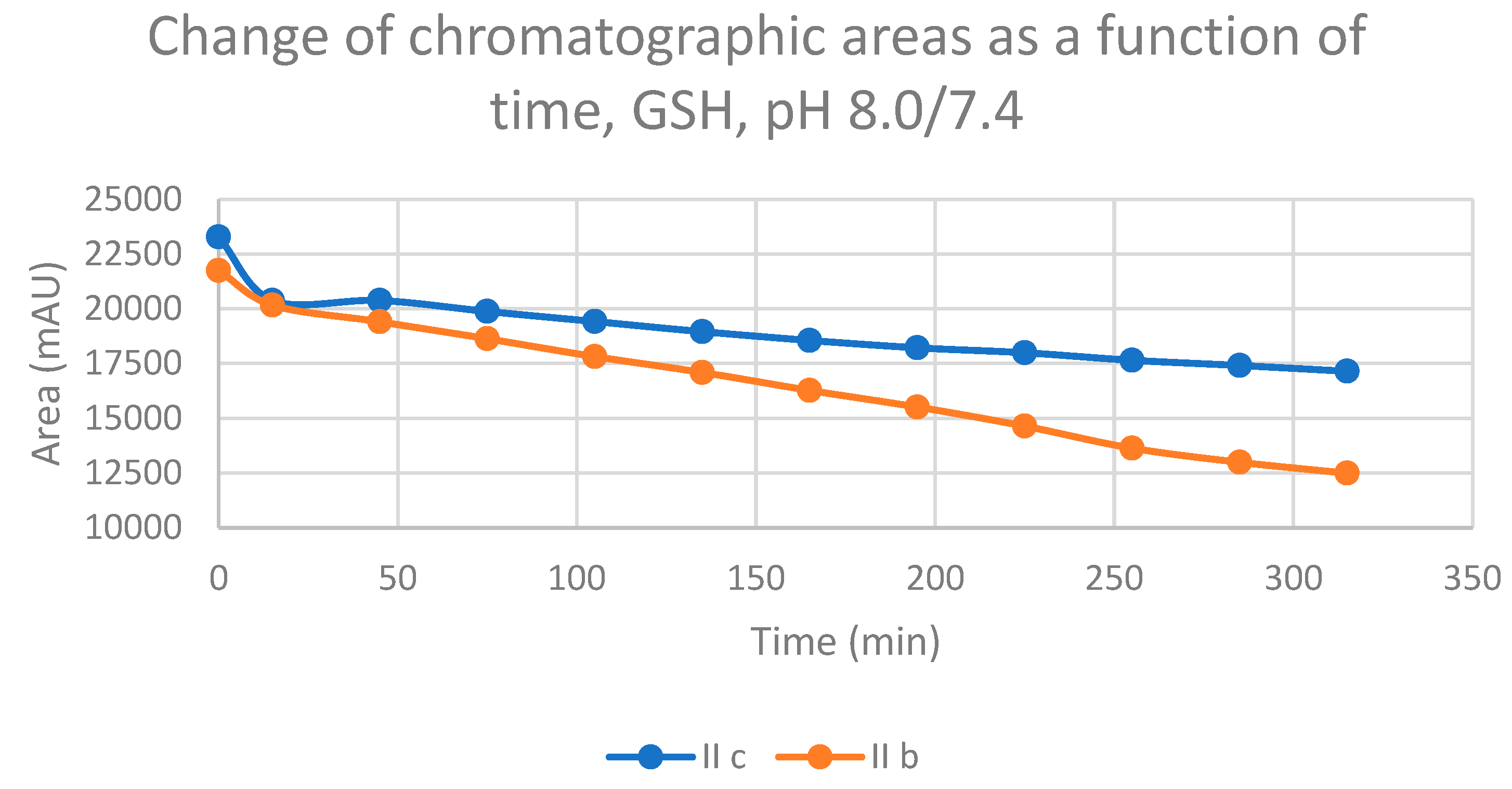
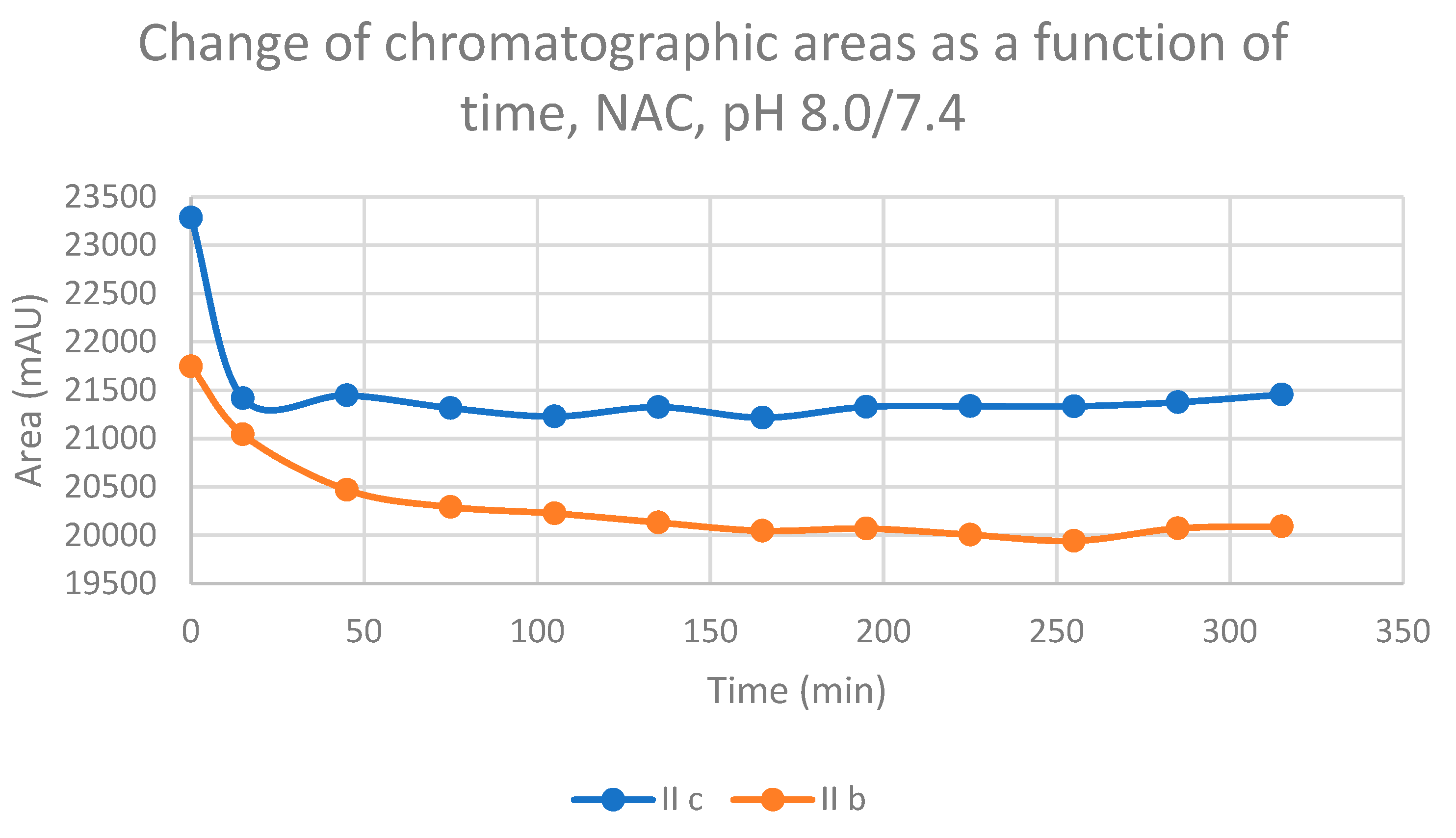

| Compound | Time (minute) |
Area Z-Chalcone |
Area NAC-1 |
Area NAC-2 |
Ratio Area NAC-2/NAC-1 |
|---|---|---|---|---|---|
| IIb | 75 | 91.8 | 35.3 | 224.1 | 6.3 |
| 165 | 107.2 | 52.7 | 349.7 | 6.6 | |
| 255 | 114.9 | 56.8 | 448.3 | 7.9 | |
| 315 | 118.9 | 60.0 | 513.9 | 8.6 | |
| IIc | 75 | 136.5 | 54.0 | 148.9 | 2.8 |
| 165 | 159.4 | 59.5 | 249.3 | 4.2 | |
| 255 | 175.3 | 58.4 | 333.2 | 5.7 | |
| 315 | 184.5 | 61.8 | 392.1 | 6.3 |
| Descriptors | Ia kcal.mol-1 |
IIa kcal.mol-1 |
CH3SH kcal.mol-1 |
CH3S- kcal.mol-1 |
|---|---|---|---|---|
| EHOMO | -183.24 | -180.38 | -183.240 | -173.453 |
| ELUMO | -35.98 | -28.44 | -2.979 | 77.998 |
| ΔEHOMO-LUMO | 147.27 | 151.94 | 180.261 | 251.451 |
| Chemical Potential () | -109.608 | -104.405 | -93.109 | -47.728 |
| Chemical Hardness () | 147.264 | 151.930 | 180.261 | 251.451 |
| Electrophilicity Index () | 40.791 | 35.873 | 24.047 | 4.530 |
| Compound | pH | Reagent thiol | Reduction of initial peak area at the 315 min timepoint (%) | Reagent thiol | Reduction of initial peak area at the 315 min timepoint (%) |
|---|---|---|---|---|---|
| Ib | 8.0/7.4 | GSH | 96.3* | NAC | 94.8* |
| IIb | 8.0/7.4 | GSH | 43.5 | NAC | 7.6 |
| Ic | 8.0/7.4 | GSH | 92.1* | NAC | 90.2* |
| IIc | 8.0/7.4 | GSH | 26.3 | NAC | 7.9 |
| Ib | 6.3/6.7 | GSH | 90.6* | NAC | 75.6* |
| IIb | 6.3/6.7 | GSH | 16.1 | NAC | 7.1 |
| Ic | 6.3/6.7 | GSH | 78.3* | NAC | 53.3* |
| IIc | 6.3/6.7 | GSH | 9.1 | NAC | 9.0 |
| Ib | 3.2/3.7 | GSH | 19.3* | NAC | 10.9* |
| IIb | 3.2/3.7 | GSH | 10.6 | NAC | 23.7 |
| Ic | 3.2/3.7 | GSH | 4.2* | NAC | 1.5* |
| IIc | 3.2/3.7 | GSH | 5.3 | NAC | 12.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





