Submitted:
20 April 2023
Posted:
20 April 2023
You are already at the latest version
Abstract
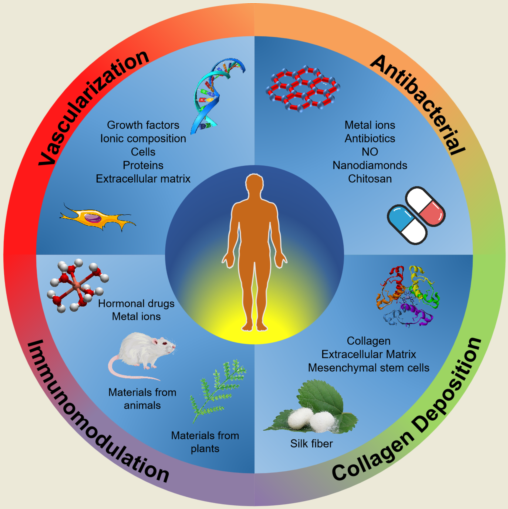
Keywords:
1. Introduction
2. Vascularization
2.1. Growth Factors
2.1.1. VEGF
2.1.2. FGF-2
2.1.3. PDGF
2.2. Heparin and Its Derivatives
2.3. Ionic Composition
2.3.1. Silicon Ions
2.3.2. Magnesium Ions
2.3.3. Copper Ions
2.4. Cell
2.4.1. Human Umbilical Vein Endothelial Cells
2.4.2. Mesenchymal Stem Cells
2.5. Proteins
2.5.1. Silk Fiber
2.5.2. Angiogenic Peptides
2.6. Other Materials
2.6.1. Desferrioxamine
2.6.2. Catalase
2.6.3. Decellularized Extracellular Matrix
3. Antibacterial
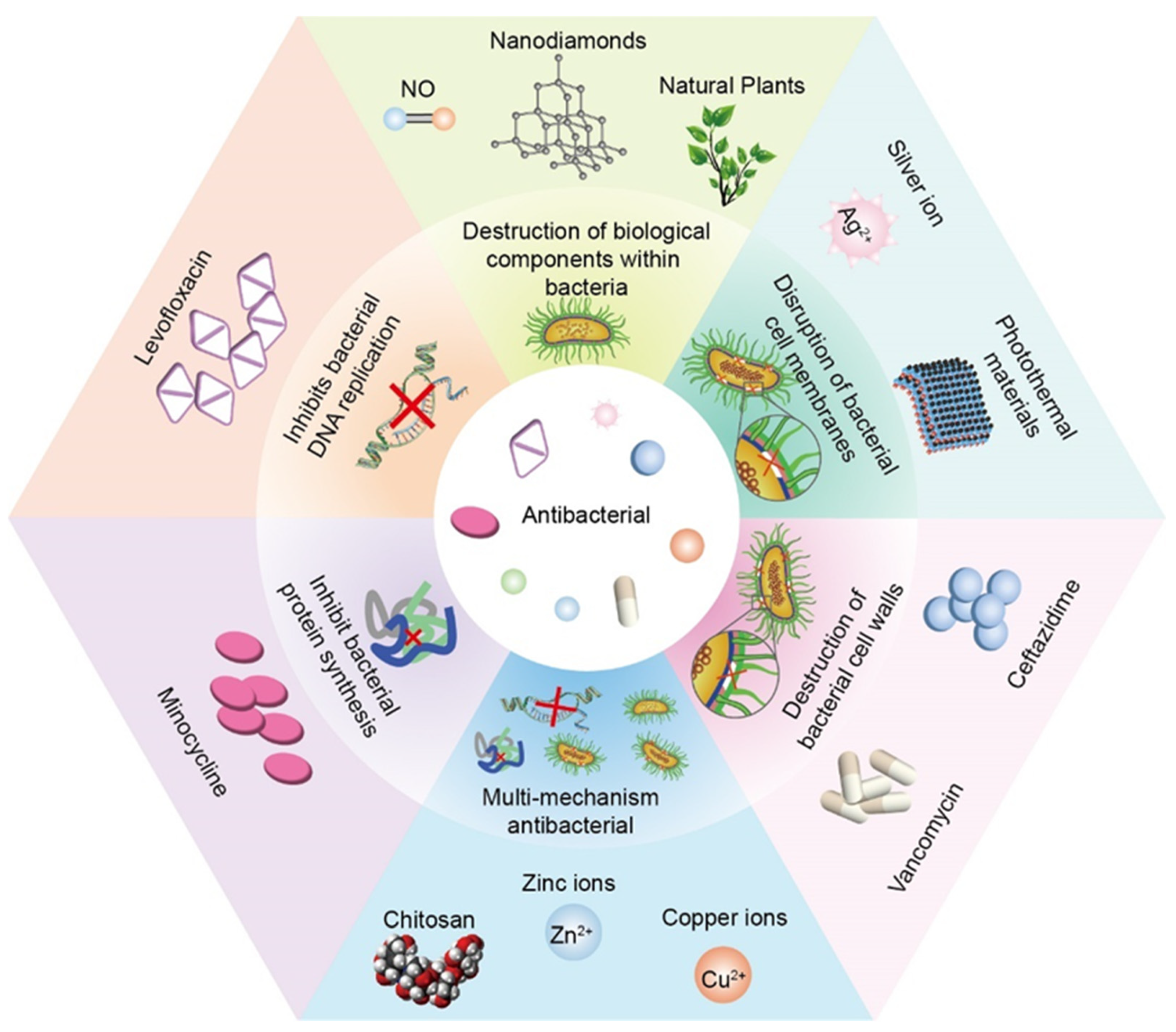
3.1. Disruption of Bacterial Cell Membranes
3.1.1. Silver Ion
3.1.2. Photothermal Materials
3.2. Destruction of Bacterial Cell Walls
3.2.1. Vancomycin + Ceftazidime
3.3. Inhibits Bacterial DNA Replication
3.3.1. Levofloxacin
3.4. Inhibit Bacterial Protein Synthesis
3.4.1. Minocycline
3.5. Destruction of Biological Components within Bacteria
3.5.1. NO
3.5.2. Natural Plants
3.5.3. Nanodiamonds
3.6. Multi-Mechanism Antibacterial
3.6.1. Chitosan
3.6.2. Zinc Ions
3.6.3. Copper Ions
4. Immunomodulation
4.1. Clinical Drugs
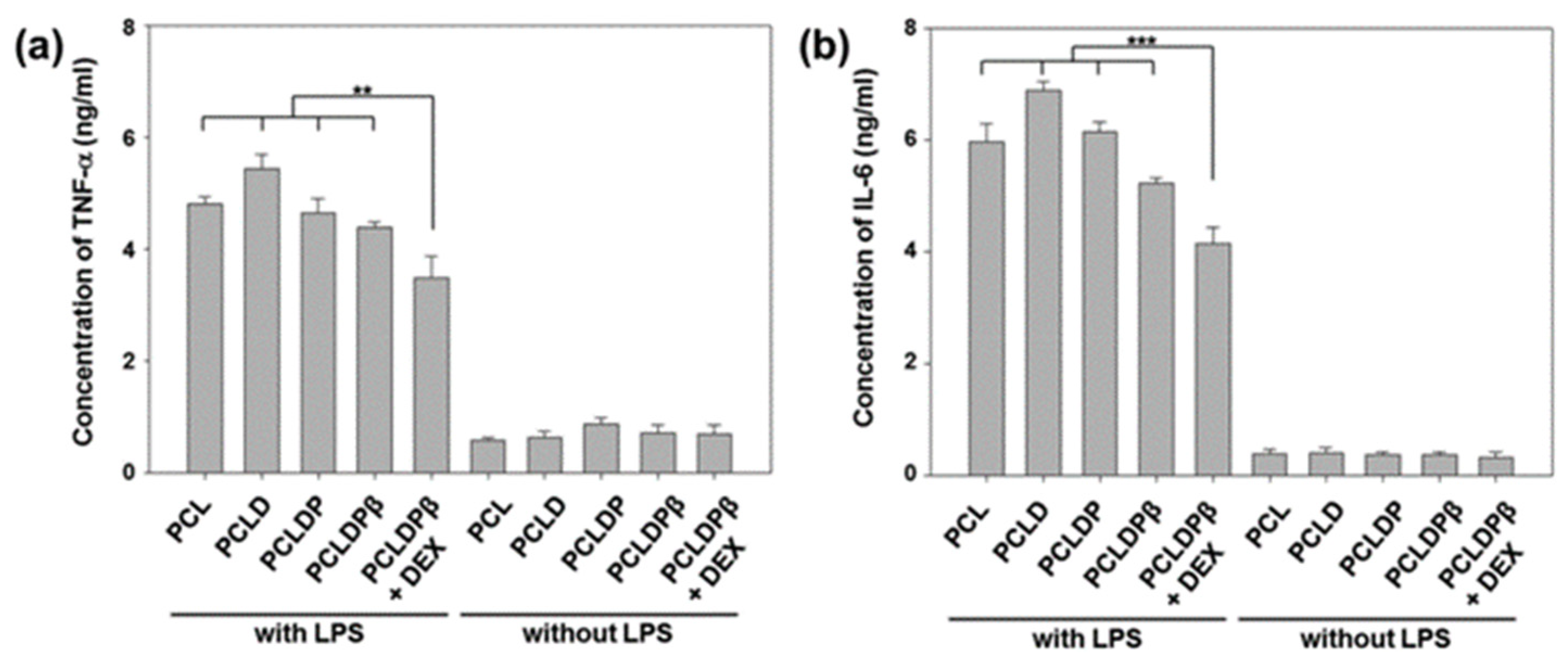
4.1.1. Dexamethasone
4.1.2. Prednisolone
4.2. Metal Ions
4.2.1. Copper Ions
4.2.2. Magnesium Ions
4.3. Animal Sources
4.3.1. Interleukin-4
4.3.2. Mesenchymal Stem Cells
4.3.3. Ac2-26 Peptide
4.4. Plant Sources
4.4.1. Curcumin
4.4.2. Isoflavones
4.4.3. Quercetin
5. Regulation of Collagen Deposition
5.1. Direct Regulation
5.1.1. Collagen
5.1.2. Extracellular Matrix
5.2. Indirect Regulation
5.2.1. Copper Ion
5.2.2. Silk Fiber
5.2.3. Curcumin
5.2.4. Mesenchymal Stem Cells
5.2.5. Granulocyte Colony-Stimulating Factor
5.2.6. Piezoelectric Effect
6. Future Perspective and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Hosseini V, Maroufi N F, Saghati S; et al., 2019, Current progress in hepatic tissue regeneration by tissue engineering. Journal of translational medicine, 17(1): 383. [CrossRef]
- Ozbolat I T, Peng W and Ozbolat V, 2016, Application areas of 3D bioprinting. Drug Discov Today, 21(8): 1257-1271. [CrossRef]
- Beheshtizadeh N, Lotfibakhshaiesh N, Pazhouhnia Z; et al., 2020, A review of 3D bio-printing for bone and skin tissue engineering: A commercial approach. Journal of Materials Science, 55(9): 3729-3749. [CrossRef]
- Hassan M, Dave K, Chandrawati R; et al., 2019, 3D printing of biopolymer nanocomposites for tissue engineering: Nanomaterials, processing and structure-function relation. European Polymer Journal, 121(109340. [CrossRef]
- Aljohani W, Ullah M W, Zhang X; et al., 2018, Bioprinting and its applications in tissue engineering and regenerative medicine. International journal of biological macromolecules, 107(Pt A): 261-275. [CrossRef]
- Groll J, Burdick J A, Cho D W; et al., 2018, A definition of bioinks and their distinction from biomaterial inks. Biofabrication, 11(1): 013001. [CrossRef]
- Kabirian F and Mozafari M, 2020, Decellularized ECM-derived bioinks: Prospects for the future. Methods, 171(108-118. [CrossRef]
- Hospodiuk M, Dey M, Sosnoski D; et al., 2017, The bioink: A comprehensive review on bioprintable materials. Biotechnol Adv, 35(2): 217-239. [CrossRef]
- Gungor-Ozkerim P S, Inci I, Zhang Y S; et al., 2018, Bioinks for 3D bioprinting: An overview. Biomaterials science, 6(5): 915-946. [CrossRef]
- A S A E, B B B, D T O; et al., 2008, Regulation of angiogenesis: Wound healing as a model. Progress in Histochemistry, 42(3): 115-170. [CrossRef]
- Xie H, Cui Z, Wang L; et al., 2014, PDGF-BB secreted by preosteoclasts induces CD31hiEmcnhi vessel subtype in coupling osteogenesis. Nature Medicine, 20(11): 1270-1278. [CrossRef]
- Fahimipour F, Rasoulianboroujeni M, Dashtimoghadam E; et al., 2017, 3D printed TCP-based scaffold incorporating VEGF-loaded PLGA microspheres for craniofacial tissue engineering. 33(11): 1205-1216. [CrossRef]
- Bianco P, 2015, Stem cells and bone: A historical perspective. Bone, 70(2-9. [CrossRef]
- Martino M M, Briquez P S, Maruyama K; et al., 2015, Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Advanced Drug Delivery Reviews, 41-52. [CrossRef]
- Henning and Robert J, 2016, Therapeutic angiogenesis: Angiogenic growth factors for ischemic heart disease. Future Cardiology, 585-599. [CrossRef]
- Ribatti D, Vacca A, Nico B; et al., 2001, Postnatal vasculogenesis. Mechanisms of Development, 100(2): 157-163. [CrossRef]
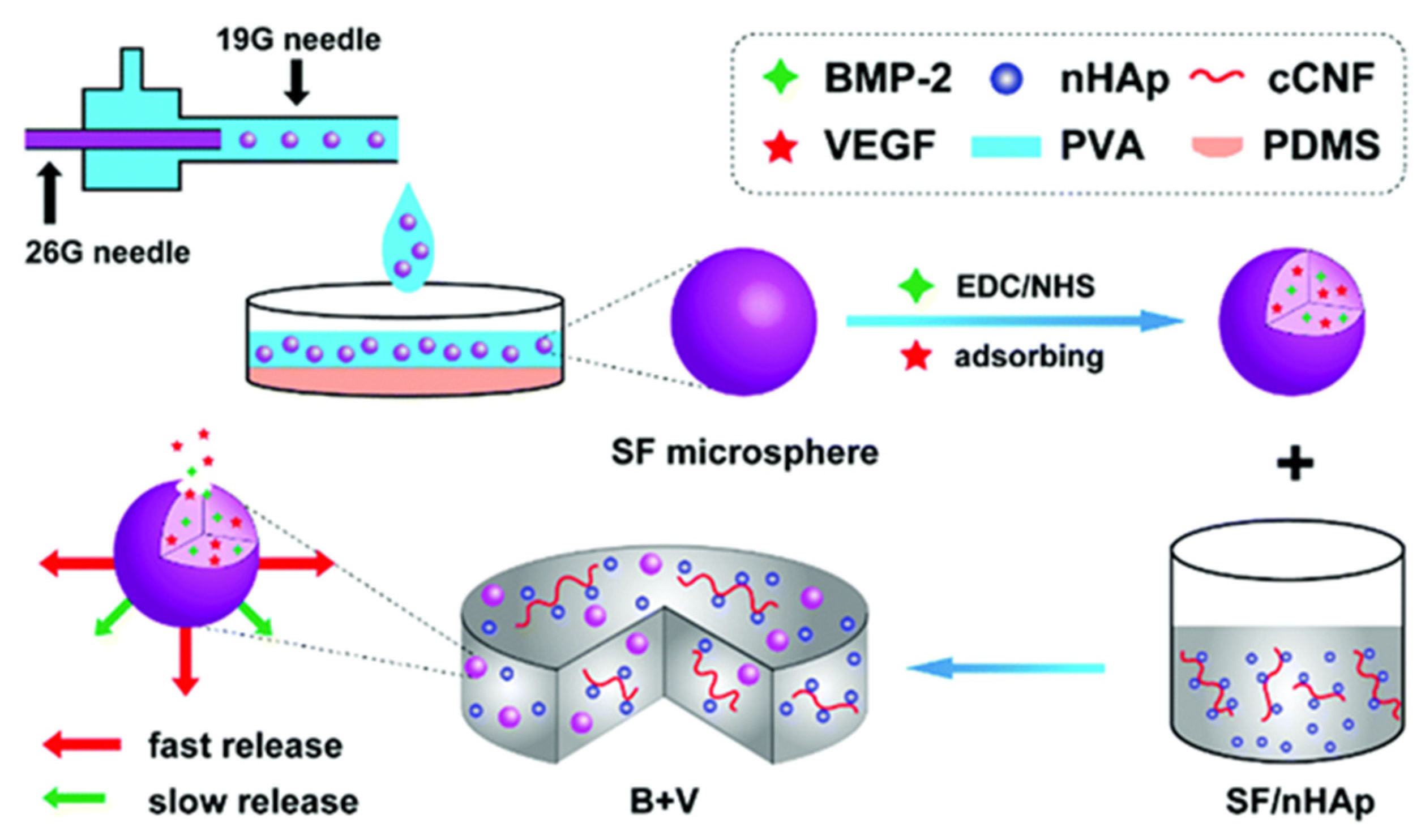
- Shen X, Zhang Y, Gu Y; et al., 2016, Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials 205-216. [CrossRef]
- Wenk E, Wandrey A J, Merkle H P; et al., 2008, Silk fibroin spheres as a platform for controlled drug delivery. Journal of Controlled Release, 132(1): 26-34. [CrossRef]
- Qiang W, Zhang Y, Li B; et al., 2017, Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin–nanohydroxyapatite scaffold for vascularized bone regeneration. 5(. [CrossRef]
- Zelzer E, Mclean W, Ng Y S; et al., 2002, Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development, 129(8): 1893. [CrossRef]
- Gerber H P, Vu T H, Ryan A M; et al., VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Medicine,. [CrossRef]
- Tiede S, Ernst N, Bayat A; et al., 2009, Basic fibroblast growth factor: A potential new therapeutic tool for the treatment of hypertrophic and keloid scars. 191(1): 33-44. [CrossRef]
- Wu J, Ye J, Zhu J; et al., 2016, Heparin-Based Coacervate of FGF2 Improves Dermal Regeneration by Asserting a Synergistic Role with Cell Proliferation and Endogenous Facilitated VEGF for Cutaneous Wound Healing. 2168. [CrossRef]
- Claffey K P, Abrams K, Shih S C; et al., 2001, Fibroblast Growth Factor 2 Activation of Stromal Cell Vascular Endothelial Growth Factor Expression and Angiogenesis. 81(1): 61. [CrossRef]
- Xiong S, Zhang X, Lu P; et al., 2017, A Gelatin-sulfonated Silk Composite Scaffold based on 3D Printing Technology Enhances Skin Regeneration by Stimulating Epidermal Growth and Dermal Neovascularization. 7(1): 4288. [CrossRef]
- Wang Y, Kim H J, Vunjak-Novakovic G; et al., 2006, Stem cell-based tissue engineering with silk biomaterials. Biomaterials, 27(36): 6064-6082. [CrossRef]
- Wang X, Gu Y, Xiong Z; et al., 2014, Silk-molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Advanced materials (Deerfield Beach, Fla.), 26(9): 1336-1342. [CrossRef]
- Shen W, Chen X, Chen J; et al., 2010, The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials, 31(28): 7239-7249. [CrossRef]
- Benjamin L E, I. H I and Keshet E, 1998, A plasticity window for blood vessel remodeling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development, 125(9): 1591-1598. [CrossRef]
- Nih L R, Gojgini S, Carmichael S T; et al., 2018, Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nature Materials, 17(7): 642-651. [CrossRef]
- Nie, Jing-Jun, Qiao; et al., 2018, Unlockable Nanocomplexes with Self-Accelerating Nucleic Acid Release for Effective Staged Gene Therapy of Cardiovascular Diseases. Advanced Materials,. [CrossRef]
- Liu B, Lee B W, Nakanishi K; et al., 2018, Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nature biomedical engineering, 2(5): 293-303. [CrossRef]
- Hudalla G A, Koepsel J T and Murphy W L, 2011, Surfaces that sequester serum-borne heparin amplify growth factor activity. Advanced Materials, 23(45): 5415-5418. [CrossRef]
- An G, Guo F, Liu X; et al., 2020, Functional reconstruction of injured corpus cavernosa using 3D-printed hydrogel scaffolds seeded with HIF-1α-expressing stem cells. Nature communications, 11(1): 2687. [CrossRef]
- You J, Shin D S, Patel D; et al., 2014, Multilayered heparin hydrogel microwells for cultivation of primary hepatocytes. Advanced healthcare materials, 3(1): 126-132. [CrossRef]
- Zhao B, Katagiri T, Toyoda H; et al., 2006, Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. The Journal of biological chemistry, 281(32): 23246-23253. [CrossRef]
- Jiao X, Billings P C, O’connell M P; et al., 2007, Heparan sulfate proteoglycans (HSPGs) modulate BMP2 osteogenic bioactivity in C2C12 cells. The Journal of biological chemistry, 282(2): 1080-1086. [CrossRef]
- Silva C, Carretero A, Soares Da Costa D; et al., 2017, Design of protein delivery systems by mimicking extracellular mechanisms for protection of growth factors. Acta biomaterialia, 63(283-293. [CrossRef]
- Jiang J, Liu X, Chen H; et al., 2020, 3D printing collagen/heparin sulfate scaffolds boost neural network reconstruction and motor function recovery after traumatic brain injury in canine. Biomaterials science, 8(22): 6362-6374. [CrossRef]
- Zhang W, Feng C, Yang G; et al., 2017, 3D-printed scaffolds with synergistic effect of hollow-pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials, 135(85-95. [CrossRef]
- Yin S, Zhang W, Zhang Z; et al., 2019, Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration. Advanced healthcare materials, 8(10): e1801433. [CrossRef]
- Li H, Xue K, Kong N; et al., 2014, Silicate bioceramics enhanced vascularization and osteogenesis through stimulating interactions between endothelia cells and bone marrow stromal cells. Biomaterials, 35(12): 3803-3818. [CrossRef]
- Schwarz K, Ricci B A, Punsar S; et al., 1977, Inverse relation of silicon in drinking water and atherosclerosis in Finland. Lancet (London, England), 1(8010): 538-539. [CrossRef]
- Pietak A M, Reid J W, Stott M J; et al., 2007, Silicon substitution in the calcium phosphate bioceramics. Biomaterials, 28(28): 4023-4032. [CrossRef]
- Shi M, Zhou Y, Shao J; et al., 2015, Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta biomaterialia, 21(178-189. [CrossRef]
- Gorustovich A A, Roether J A and Boccaccini A R, 2010, Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue engineering. Part B, Reviews, 16(2): 199-207. [CrossRef]
- Gerhardt L C, Widdows K L, Erol M M; et al., 2011, The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials, 32(17): 4096-4108. [CrossRef]
- Li C, Jiang C, Deng Y; et al., 2017, RhBMP-2 loaded 3D-printed mesoporous silica/calcium phosphate cement porous scaffolds with enhanced vascularization and osteogenesis properties. Scientific reports, 7(41331. [CrossRef]
- Nieves J W, 2014, Bone. Maximizing bone health--magnesium, BMD and fractures. Nature reviews. Endocrinology, 10(5): 255-256. [CrossRef]
- De Baaij J H, Hoenderop J G and Bindels R J, 2015, Magnesium in man: Implications for health and disease. Physiological reviews, 95(1): 1-46. [CrossRef]
- Maier J A, Bernardini D, Rayssiguier Y; et al., 2004, High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochimica et biophysica acta, 1689(1): 6-12. [CrossRef]
- Bernardini D, Nasulewic A, Mazur A; et al., 2005, Magnesium and microvascular endothelial cells: A role in inflammation and angiogenesis. Frontiers in bioscience : A journal and virtual library, 10(1177-1182. [CrossRef]
- Romani A M P, 2018, Beneficial Role of Mg(2+) in Prevention and Treatment of Hypertension. International journal of hypertension, 2018(9013721. [CrossRef]
- Wang W and Yeung K W K, 2017, Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioactive materials, 2(4): 224-247. [CrossRef]
- Tarafder S, Dernell W S, Bandyopadhyay A; et al., 2015, SrO- and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. Journal of biomedical materials research. Part B, Applied biomaterials, 103(3): 679-690. [CrossRef]
- Gu Y, Zhang J, Zhang X; et al., 2019, Three-dimensional Printed Mg-Doped β-TCP Bone Tissue Engineering Scaffolds: Effects of Magnesium Ion Concentration on Osteogenesis and Angiogenesis In Vitro. Tissue engineering and regenerative medicine, 16(4): 415-429. [CrossRef]
- Zou F, Zhao N, Fu X; et al., 2016, Enhanced osteogenic differentiation and biomineralization in mouse mesenchymal stromal cells on a β-TCP robocast scaffold modified with collagen nanofibers. Rsc Advances, 6(28): 23588-23598. [CrossRef]
- Tao W, Zeng X, Wu J; et al., 2016, Polydopamine-Based Surface Modification of Novel Nanoparticle-Aptamer Bioconjugates for In Vivo Breast Cancer Targeting and Enhanced Therapeutic Effects. Theranostics, 6(4): 470-484. [CrossRef]
- Li H, Peng F, Wang D; et al., 2018, Layered double hydroxide/poly-dopamine composite coating with surface heparinization on Mg alloys: Improved anticorrosion, endothelialization and hemocompatibility. Biomaterials science, 6(7): 1846-1858. [CrossRef]
- Ma L, Cheng S, Ji X; et al., 2020, Immobilizing magnesium ions on 3D printed porous tantalum scaffolds with polydopamine for improved vascularization and osteogenesis. Materials science & engineering. C, Materials for biological applications, 117(111303. [CrossRef]
- Dang W, Ma B, Li B; et al., 2020, 3D printing of metal-organic framework nanosheets-structured scaffolds with tumor therapy and bone construction. Biofabrication, 12(2): 025005. [CrossRef]
- Shi M, Chen Z, Farnaghi S; et al., 2016, Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta biomaterialia, 30(334-344. [CrossRef]
- Sen C K, Khanna S, Venojarvi M; et al., 2019, copper-induced vascular endo-thelial growth factor expression and wound healing. [CrossRef]
- Kornblatt A P, Nicoletti V G and Travaglia A, 2016, The neglected role of copper ions in wound healing. Journal of inorganic biochemistry, 161(1-8. [CrossRef]
- Wu M X and Yang Y W, 2017, Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Advanced materials (Deerfield Beach, Fla.), 29(23). [CrossRef]
- Wang D, Zhou J, Shi R; et al., 2017, Biodegradable Core-shell Dual-Metal-Organic-Frameworks Nanotheranostic Agent for Multiple Imaging Guided Combination Cancer Therapy. Theranostics, 7(18): 4605-4617. [CrossRef]
- Piard C, Baker H, Kamalitdinov T; et al., 2019, Bioprinted osteon-like scaffolds enhance in vivo neovascularization. Biofabrication, 11(2): 025013. [CrossRef]
- Melero-Martin J M, Khan Z A, Picard A; et al., 2007, In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood, 109(11): 4761-4768. [CrossRef]
- Staton C A, Reed M W and Brown N J, 2009, A critical analysis of current in vitro and in vivo angiogenesis assays. International journal of experimental pathology, 90(3): 195-221. [CrossRef]
- Cidonio G, Glinka M, Kim Y H; et al., 2020, Nanoclay-based 3D printed scaffolds promote vascular ingrowth ex vivo and generate bone mineral tissue in vitro and in vivo. Biofabrication, 12(3): 035010. [CrossRef]
- Farahani R D, Dubé M and Therriault D, 2016, Three-Dimensional Printing of Multifunctional Nanocomposites: Manufacturing Techniques and Applications. Advanced materials (Deerfield Beach, Fla.), 28(28): 5794-5821. [CrossRef]
- Bates D O, 2010, Vascular endothelial growth factors and vascular permeability. Cardiovascular research, 87(2): 262-271. [CrossRef]
- Park S S, 2016, Cell Therapy Applications for Retinal Vascular Diseases: Diabetic Retinopathy and Retinal Vein Occlusion. Investigative ophthalmology & visual science, 57(5): ORSFj1-ORSFj10. [CrossRef]
- Pittenger M F and Martin B J, 2004, Mesenchymal stem cells and their potential as cardiac therapeutics. Circulation research, 95(1): 9-20. [CrossRef]
- Pittenger M F, Mackay A M, Beck S C; et al., 1999, Multilineage potential of adult human mesenchymal stem cells. Science (New York, N.Y.), 284(5411): 143-147. [CrossRef]
- Sheehy E J, Mesallati T, Kelly L; et al., 2015, Tissue Engineering Whole Bones Through Endochondral Ossification: Regenerating the Distal Phalanx. BioResearch open access, 4(1): 229-241. [CrossRef]
- Yan Y, Chen H, Zhang H; et al., 2019, Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials, 190-191(97-110. [CrossRef]
- Kanczler J M and Oreffo R O, 2008, Osteogenesis and angiogenesis: The potential for engineering bone. European cells & materials, 15(100-114. [CrossRef]
- Deng Y, Jiang C, Li C; et al., 2017, 3D printed scaffolds of calcium silicate-doped β-TCP synergize with co-cultured endothelial and stromal cells to promote vascularization and bone formation. Scientific reports, 7(1): 5588. [CrossRef]
- Rong Q, Li S, Zhou Y; et al., 2020, A novel method to improve the osteogenesis capacity of hUCMSCs with dual-directional pre-induction under screened co-culture conditions. Cell proliferation, 53(2): e12740. [CrossRef]
- Li J, Mao Q, He J; et al., 2017, Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem cell research & therapy, 8(1): 55. [CrossRef]
- Zhang C, Zhang Y, Shao H; et al., 2016, Hybrid Silk Fibers Dry-Spun from Regenerated Silk Fibroin/Graphene Oxide Aqueous Solutions. ACS applied materials & interfaces, 8(5): 3349-3358. [CrossRef]
- Melke J, Midha S, Ghosh S; et al., 2016, Silk fibroin as biomaterial for bone tissue engineering. Acta biomaterialia, 31(1-16. [CrossRef]
- Ribeiro V P, Silva-Correia J, Nascimento A I; et al., 2017, Silk-based anisotropical 3D biotextiles for bone regeneration. Biomaterials, 123(92-106. [CrossRef]
- Kumar V A, Liu Q, Wickremasinghe N C; et al., 2016, Treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials, 98(113-119. [CrossRef]
- Wang C, Lai J, Li K; et al., 2021, Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioactive materials, 6(1): 137-145. [CrossRef]
- Wang Y, Wan C, Deng L; et al., 2007, The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. The Journal of clinical investigation, 117(6): 1616-1626. [CrossRef]
- Wan C, Gilbert S R, Wang Y; et al., 2008, Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proceedings of the National Academy of Sciences of the United States of America, 105(2): 686-691. [CrossRef]
- Kusumbe A P, Ramasamy S K and Adams R H, 2014, Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature, 507(7492): 323-328. [CrossRef]
- Shen X, Wan C, Ramaswamy G; et al., 2009, Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. Journal of orthopaedic research : Official publication of the Orthopaedic Research Society, 27(10): 1298-1305. [CrossRef]
- Farberg A S, Sarhaddi D, Donneys A; et al., 2014, Deferoxamine enhances bone regeneration in mandibular distraction osteogenesis. Plastic and reconstructive surgery, 133(3): 666-671. [CrossRef]
- Donneys A, Weiss D M, Deshpande S S; et al., 2013, Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone, 52(1): 318-325. [CrossRef]
- V S S and P V M, 2018, Degradation of Poly(ε-caprolactone) and bio-interactions with mouse bone marrow mesenchymal stem cells. Colloids and surfaces. B, Biointerfaces, 163(107-118. [CrossRef]
- Orozco-Castellanos L, Marcos-Fernández A and Martínez-Richa A, 2011, Hydrolytic degradation of poly(ε-caprolactone) with different end groups and poly(ε-caprolactone-co-γ-butyrolactone): Characterization and kinetics of hydrocortisone delivery. Polymers for Advanced Technologies, 22(. [CrossRef]
- Ragu S, Faye G, Iraqui I; et al., 2007, Oxygen metabolism and reactive oxygen species cause chromosomal rearrangements and cell death. Proceedings of the National Academy of Sciences of the United States of America, 104(23): 9747-9752. [CrossRef]
- Poole K M, Nelson C E, Joshi R V; et al., 2015, ROS-responsive microspheres for on demand antioxidant therapy in a model of diabetic peripheral arterial disease. Biomaterials, 41(166-175. [CrossRef]
- Fraisl P, Mazzone M, Schmidt T; et al., 2009, Regulation of angiogenesis by oxygen and metabolism. Developmental cell, 16(2): 167-179. [CrossRef]
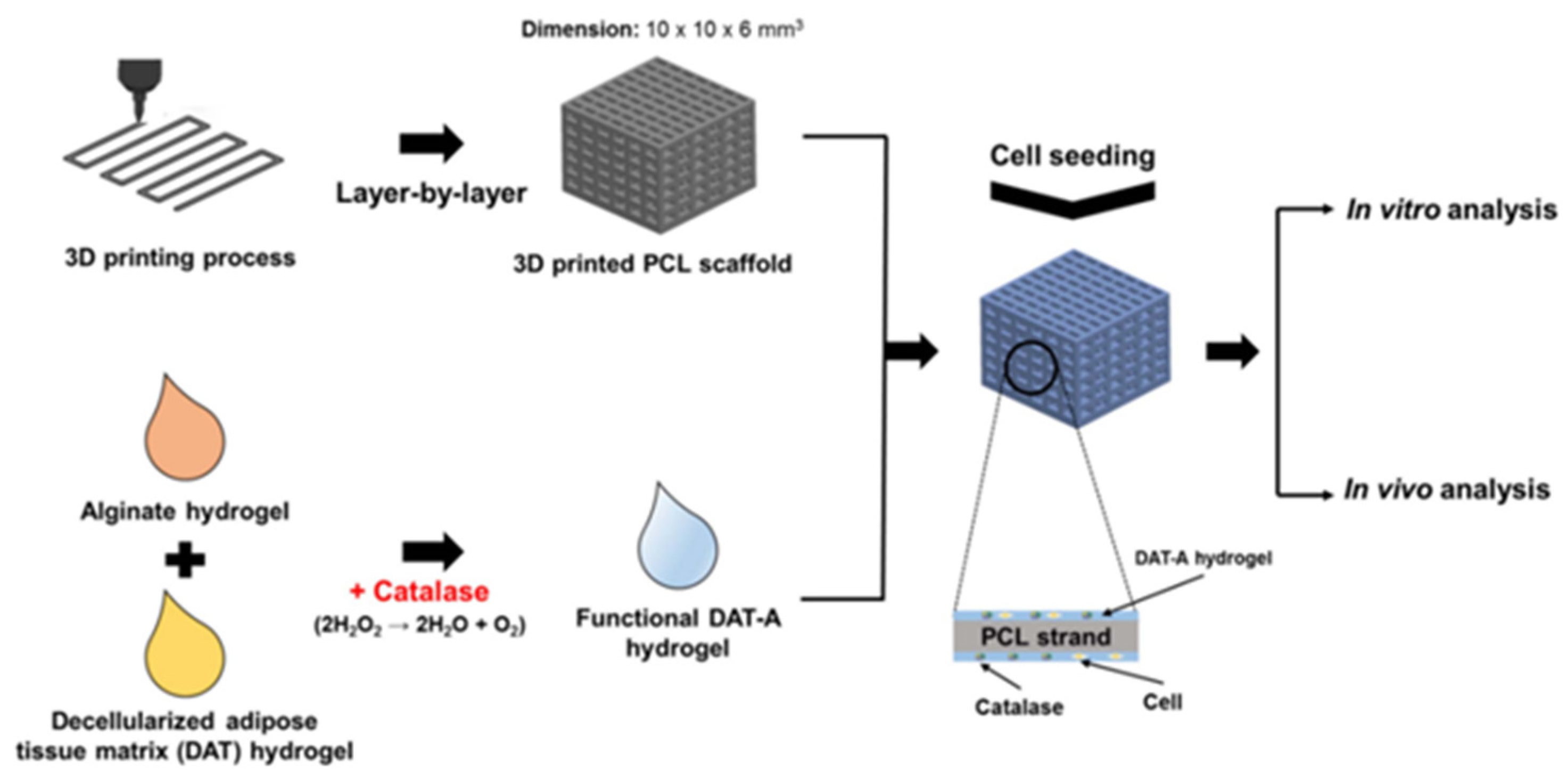
- Rijal G, Kim B S, Pati F; et al., 2017, Robust tissue growth and angiogenesis in large-sized scaffold by reducing H(2)O(2)-mediated oxidative stress. Biofabrication, 9(1): 015013. [CrossRef]
- Pati F, Jang J, Ha D H; et al., 2014, Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nature communications, 5(3935. [CrossRef]
- Choi J S, Kim B S, Kim J Y; et al., 2011, Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering. Journal of biomedical materials research. Part A, 97(3): 292-299. [CrossRef]
- Brown C F, Yan J, Han T T; et al., 2015, Effect of decellularized adipose tissue particle size and cell density on adipose-derived stem cell proliferation and adipogenic differentiation in composite methacrylated chondroitin sulphate hydrogels. Biomedical materials (Bristol, England), 10(4): 045010. [CrossRef]
- De Santis M M, Alsafadi H N, Tas S; et al., 2021, Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Advanced materials (Deerfield Beach, Fla.), 33(3): e2005476. [CrossRef]
- Ross E A, Williams M J, Hamazaki T; et al., 2009, Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. Journal of the American Society of Nephrology : JASN, 20(11): 2338-2347. [CrossRef]
- Cortiella J, Niles J, Cantu A; et al., 2010, Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue engineering. Part A, 16(8): 2565-2580. [CrossRef]
- Lee H, Han W, Kim H; et al., 2017, Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules, 18(4): 1229-1237. [CrossRef]
- Choi Y J, Kim T G, Jeong J; et al., 2016, 3D Cell Printing of Functional Skeletal Muscle Constructs Using Skeletal Muscle-Derived Bioink. Advanced healthcare materials, 5(20): 2636-2645. [CrossRef]
- Kim B S, Kwon Y W, Kong J S; et al., 2018, 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials, 168(38-53. [CrossRef]
- French K M, Boopathy A V, Dequach J A; et al., 2012, A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta biomaterialia, 8(12): 4357-4364. [CrossRef]
- Bejleri D, Streeter B W, Nachlas A L Y; et al., 2018, A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Advanced healthcare materials, 7(23): e1800672. [CrossRef]
- Rahim K, Saleha S, Zhu X; et al., 2017, Bacterial Contribution in Chronicity of Wounds. Microbial ecology, 73(3): 710-721. [CrossRef]
- Bessa L J, Fazii P, Di Giulio M; et al., 2015, Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. International wound journal, 12(1): 47-52. [CrossRef]
- Davies D, 2003, Understanding biofilm resistance to antibacterial agents. Nature reviews. Drug discovery, 2(2): 114-122. [CrossRef]
- Veerachamy S, Yarlagadda T, Manivasagam G; et al., 2014, Bacterial adherence and biofilm formation on medical implants: A review. Proceedings of the Institution of Mechanical Engineers. Part H, Journal of engineering in medicine, 228(10): 1083-1099. [CrossRef]
- Cyphert E L, Zhang N, Learn G D; et al., 2021, Recent Advances in the Evaluation of Antimicrobial Materials for Resolution of Orthopedic Implant-Associated Infections In Vivo. ACS infectious diseases, 7(12): 3125-3160. [CrossRef]
- Vanepps J S and Younger J G, 2016, Implantable Device-Related Infection. Shock (Augusta, Ga.), 46(6): 597-608. [CrossRef]
- Schierholz J M and Beuth J, 2001, Implant infections: A haven for opportunistic bacteria. The Journal of hospital infection, 49(2): 87-93. [CrossRef]
- Kim J S, Kuk E, Yu K N; et al., 2007, Antimicrobial effects of silver nanoparticles. Nanomedicine Nanotechnology Biology Medicine, 3(1): 95-101. [CrossRef]
- Guzman M, Dille J and Godet S, 2012, Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomedicine : Nanotechnology, biology, and medicine, 8(1): 37-45. [CrossRef]
- Qing Y, Cheng L, Li R; et al., 2018, Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. International journal of nanomedicine, 13(3311-3327. [CrossRef]
- García-Astrain C, Chen C, Burón M; et al., 2015, Biocompatible hydrogel nanocomposite with covalently embedded silver nanoparticles. Biomacromolecules, 16(4): 1301-1310. [CrossRef]
- Dubey P, Matai I, Kumar S U; et al., 2015, Perturbation of cellular mechanistic system by silver nanoparticle toxicity: Cytotoxic, genotoxic and epigenetic potentials. Advances in colloid and interface science, 221(4-21. [CrossRef]
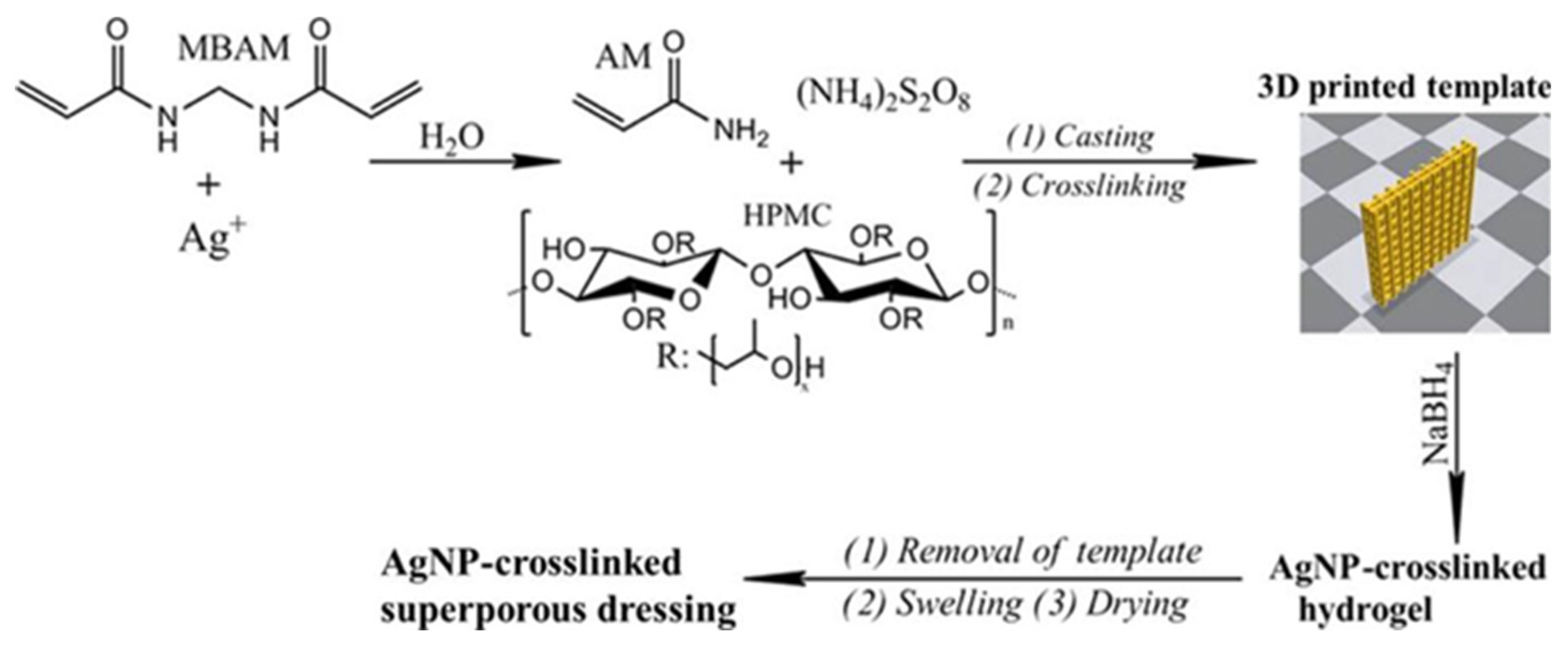
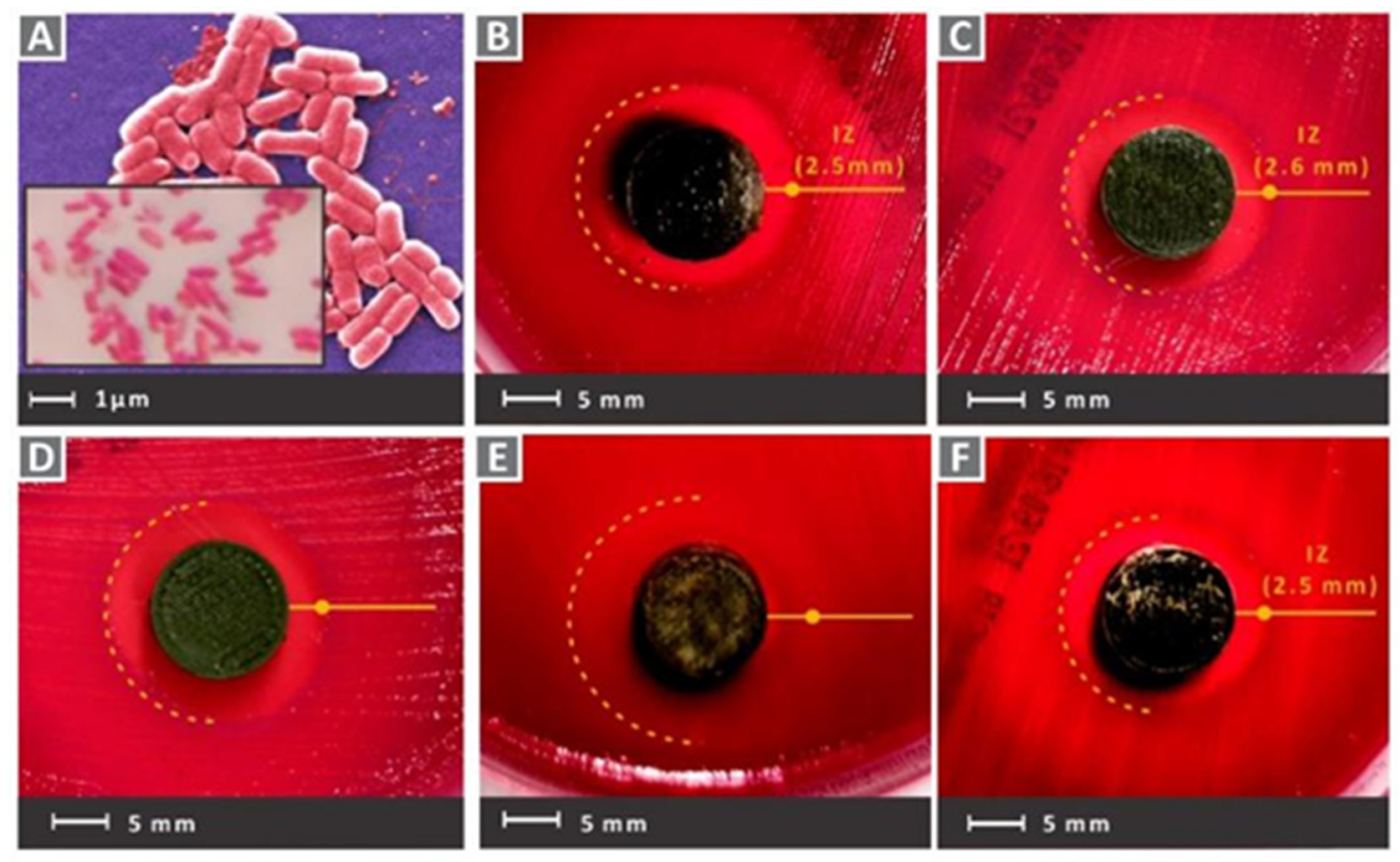
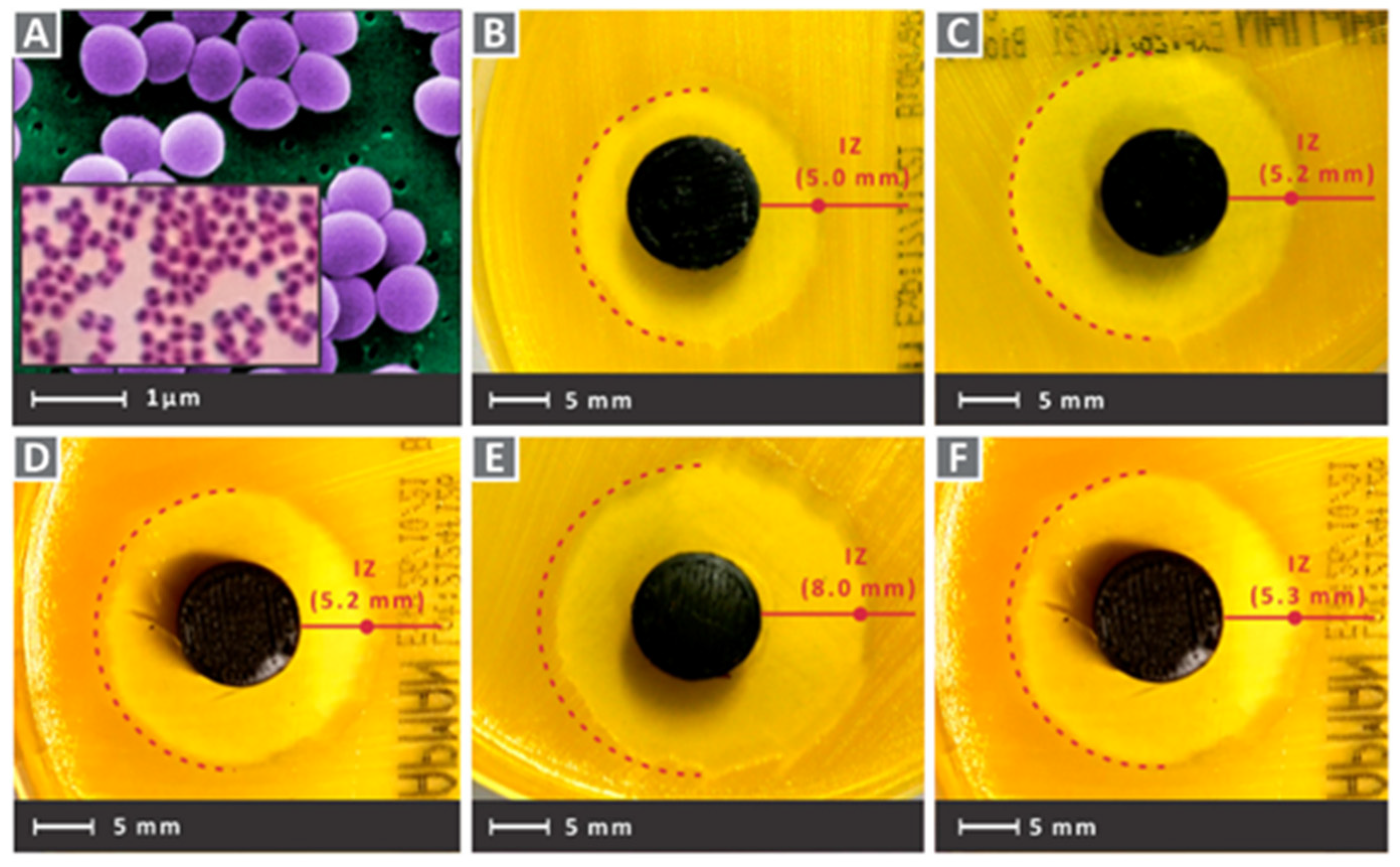
- Wu Z and Hong Y, 2019, Combination of the Silver-Ethylene Interaction and 3D Printing To Develop Antibacterial Superporous Hydrogels for Wound Management. ACS applied materials & interfaces, 11(37): 33734-33747. [CrossRef]
- Kangari H, Abedini-Talemi A, Akbarzadeh-Bagheban A; et al., 2015, Comparison of Two Marketed Hydroxypropyl Methylcellulose Based Artificial Tear Drops in Young Patients with Dry Eye Syndrome. Novelty in Biomedicine.
- Powers J G, Morton L M and Phillips T J, 2013, Dressings for chronic wounds. Dermatologic Therapy, 26(3): 197-206. [CrossRef]
- Li Y, Liu X, Li B; et al., 2020, Near-Infrared Light Triggered Phototherapy and Immunotherapy for Elimination of Methicillin-Resistant Staphylococcus aureus Biofilm Infection on Bone Implant. ACS nano, 14(7): 8157-8170. [CrossRef]
- Nie R, Sun Y, Lv H; et al., 2022, 3D printing of MXene composite hydrogel scaffolds for photothermal antibacterial activity and bone regeneration in infected bone defect models. Nanoscale, 14(22): 8112-8129. [CrossRef]
- Soleymaniha M, Shahbazi M A, Rafieerad A R; et al., 2019, Promoting Role of MXene Nanosheets in Biomedical Sciences: Therapeutic and Biosensing Innovations. Advanced healthcare materials, 8(1): e1801137. [CrossRef]
- Parajuli D, Murali N, K C D; et al., 2022, Advancements in MXene-Polymer Nanocomposites in Energy Storage and Biomedical Applications. Polymers, 14(16). [CrossRef]
- Pan S, Yin J, Yu L; et al., 2020, 2D MXene-Integrated 3D-Printing Scaffolds for Augmented Osteosarcoma Phototherapy and Accelerated Tissue Reconstruction. Advanced science (Weinheim, Baden-Wurttemberg, Germany), 7(2): 1901511. [CrossRef]
- Rasool K, Helal M, Ali A; et al., 2016, Antibacterial Activity of Ti₃C₂Tx MXene. ACS nano, 10(3): 3674-3684. [CrossRef]
- Han X, Huang J, Lin H; et al., 2018, 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Advanced healthcare materials, 7(9): e1701394. [CrossRef]
- Yang C, Luo Y, Lin H; et al., 2021, Niobium Carbide MXene Augmented Medical Implant Elicits Bacterial Infection Elimination and Tissue Regeneration. ACS nano, 15(1): 1086-1099. [CrossRef]
- Zada S, Lu H, Yang F; et al., 2021, V(2)C Nanosheets as Dual-Functional Antibacterial Agents. ACS applied bio materials, 4(5): 4215-4223. [CrossRef]
- Rao J, Lahiri J, Isaacs L; et al., 1998, A trivalent system from vancomycin.D-ala-D-Ala with higher affinity than avidin.biotin. Science (New York, N.Y.), 280(5364): 708-711. [CrossRef]
- He W, Bai J, Chen X; et al., 2022, Reversible dougong structured receptor-ligand recognition for building dynamic extracellular matrix mimics. Proceedings of the National Academy of Sciences of the United States of America, 119(8). [CrossRef]
- Andrei S, Valeanu L, Chirvasuta R; et al., 2018, New FDA approved antibacterial drugs: 2015-2017. Discoveries (Craiova, Romania), 6(1): e81. [CrossRef]
- Yu Y H, Lee D, Hsu Y H; et al., 2020, A Three-Dimensional Printed Polycaprolactone Scaffold Combined with Co-Axially Electrospun Vancomycin/Ceftazidime/Bone Morphological Protein-2 Sheath-Core Nanofibers for the Repair of Segmental Bone Defects During the Masquelet Procedure. International journal of nanomedicine, 15(913-925. [CrossRef]
- Cicuéndez M, Doadrio J C, Hernández A; et al., 2018, Multifunctional pH sensitive 3D scaffolds for treatment and prevention of bone infection. Acta biomaterialia, 65(450-461. [CrossRef]
- Sadaba N, Larrañaga A, Orpella-Aceret G; et al., 2020, Benefits of Polydopamine as Particle/Matrix Interface in Polylactide/PD-BaSO(4) Scaffolds. International journal of molecular sciences, 21(15). [CrossRef]
- Zuza E, Meaurio E and Sarasua J R, 2016, Biodegradable Polylactide-Based Composites.
- Garrido-Mesa N, Zarzuelo A and Gálvez J, 2013, Minocycline: Far beyond an antibiotic. British journal of pharmacology, 169(2): 337-352. [CrossRef]
- Silva T, Grenho L, Barros J; et al., 2017, A minocycline-releasing PMMA system as a space maintainer for staged bone reconstructions-in vitro antibacterial, cytocompatibility and anti-inflammatory characterization. Biomedical materials (Bristol, England), 12(3): 035009. [CrossRef]
- Ferreira M, Rzhepishevska O, Grenho L; et al., 2017, Levofloxacin-loaded bone cement delivery system: Highly effective against intracellular bacteria and Staphylococcus aureus biofilms. International journal of pharmaceutics, 532(1): 241-248. [CrossRef]
- Martin V, Ribeiro I A, Alves M M; et al., 2019, Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Materials science & engineering. C, Materials for biological applications, 101(15-26. [CrossRef]
- Dou X C, Zhu X P, Zhou J; et al., 2011, Minocycline-released hydroxyapatite-gelatin nanocomposite and its cytocompatibility in vitro. Biomedical materials (Bristol, England), 6(2): 025002. [CrossRef]
- Luo J D and Chen A F, 2005, Nitric oxide: A newly discovered function on wound healing. Acta pharmacologica Sinica, 26(3): 259-264. [CrossRef]
- Fang F C, 1997, Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. The Journal of clinical investigation, 99(12): 2818-2825. [CrossRef]
- Kabirian F, Brouki Milan P, Zamanian A; et al., 2019, Nitric oxide-releasing vascular grafts: A therapeutic strategy to promote angiogenic activity and endothelium regeneration. Acta biomaterialia, 92(82-91. [CrossRef]
- Moreno-Jiménez I, Hulsart-Billstrom G, Lanham S A; et al., 2016, The chorioallantoic membrane (CAM) assay for the study of human bone regeneration: A refinement animal model for tissue engineering. Scientific reports, 6(32168. [CrossRef]
- Sharifi-Rad J, Sharifi-Rad M, Hoseini-Alfatemi S M; et al., 2015, Composition, Cytotoxic and Antimicrobial Activities of Satureja intermedia C.A.Mey Essential Oil. International journal of molecular sciences, 16(8): 17812-17825. [CrossRef]
- Skocibusić M and Bezić N, 2004, Phytochemical analysis and in vitro antimicrobial activity of two Satureja species essential oils. Phytotherapy research : PTR, 18(12): 967-970. [CrossRef]
- Jafari F, Ghavidel F and Zarshenas M M, 2016, A Critical Overview on the Pharmacological and Clinical Aspects of Popular Satureja Species. Journal of acupuncture and meridian studies, 9(3): 118-127. [CrossRef]
- Sait U, Uca N, Kartal M U; et al., 2014, GC-MS Analysis and Antibacterial Activity of Cultivated Satureja cuneifolia Ten. Essential Oil. Turkish Journal of Chemistry, 30(2):.
- Ilhan E, Cesur S, Guler E; et al., 2020, Development of Satureja cuneifolia-loaded sodium alginate/polyethylene glycol scaffolds produced by 3D-printing technology as a diabetic wound dressing material. International journal of biological macromolecules, 161(1040-1054. [CrossRef]
- Pt A, Ek B, Acgc D; et al., 2020, Anti-Alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. 13(3): 4528-4537. [CrossRef]
- Mochalin V N, Shenderova O, Ho D; et al., 2011, The properties and applications of nanodiamonds. Nature nanotechnology, 7(1): 11-23. [CrossRef]
- Amaral M, Dias A G, Gomes P S; et al., 2008, Nanocrystalline diamond: In vitro biocompatibility assessment by MG63 and human bone marrow cells cultures. Journal of biomedical materials research. Part A, 87(1): 91-99. [CrossRef]
- Wehling J, Dringen R, Zare R N; et al., 2014, Bactericidal activity of partially oxidized nanodiamonds. ACS nano, 8(6): 6475-6483. [CrossRef]
- Papo M J, Catledge S A, Vohra Y K; et al., 2004, Mechanical wear behavior of nanocrystalline and multilayer diamond coatings on temporomandibular joint implants. Journal of materials science. Materials in medicine, 15(7): 773-777. [CrossRef]
- Catledge S A, Thomas V and Vohra Y K, 2013, Nanostructured diamond coatings for orthopaedic applications. Woodhead publishing series in biomaterials, 2013(105-150. [CrossRef]
- Rifai A, Tran N, Reineck P; et al., 2019, Engineering the Interface: Nanodiamond Coating on 3D-Printed Titanium Promotes Mammalian Cell Growth and Inhibits Staphylococcus aureus Colonization. ACS applied materials & interfaces, 11(27): 24588-24597. [CrossRef]
- Ueno H, Mori T and Fujinaga T, 2001, Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev, 52(2): 105-115. [CrossRef]
- Azad A K, Sermsintham N, Chandrkrachang S; et al., 2004, Chitosan membrane as a wound-healing dressing: Characterization and clinical application. Journal of biomedical materials research. Part B, Applied biomaterials, 69(2): 216-222. [CrossRef]
- Ahmed S and Ikram S, 2016, Chitosan Based Scaffolds and Their Applications in Wound Healing. Achievements in the Life Sciences, 27-37. 165. [CrossRef]
- Intini C, Elviri L, Cabral J; et al., 2018, 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydrate polymers, 199(593-602. [CrossRef]
- Thian E S, Konishi T, Kawanobe Y; et al., 2013, Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. Journal of materials science. Materials in medicine, 24(2): 437-445. [CrossRef]
- Voicu G, Miu D, Ghitulica C D; et al., 2019, Co doped ZnO thin films deposited by spin coating as antibacterial coating for metallic implants. Ceramics International, 46(3):. [CrossRef]
- Liu Y, He L, Mustapha A; et al., 2009, Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. Journal of applied microbiology, 107(4): 1193-1201. [CrossRef]
- Chhabra H, Deshpande R, Kanitkar M; et al., 2015, A nano zinc oxide doped electrospun scaffold improves wound healing in a rodent model. Rsc Advances, 6(. [CrossRef]
- Jin S E, Hwang W, Lee H J; et al., 2017, Dual UV irradiation-based metal oxide nanoparticles for enhanced antimicrobial activity in Escherichia coli and M13 bacteriophage. International journal of nanomedicine, 12(8057-8070. [CrossRef]
- Liu J, Rojas-Andrade M D, Chata G; et al., 2017, Photo-enhanced antibacterial activity of ZnO/graphene quantum dot nanocomposites. Nanoscale, 10(1): 158-166. [CrossRef]
- Raja A, Ashokkumar S, Pavithra Marthandam R; et al., 2018, Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. Journal of photochemistry and photobiology. B, Biology, 181(53-58. [CrossRef]
- Bhande R M, Khobragade C N, Mane R S; et al., 2013, Enhanced synergism of antibiotics with zinc oxide nanoparticles against extended spectrum β-lactamase producers implicated in urinary tract infections. Journal of Nanoparticle Research, 15(1): 1413. [CrossRef]
- Li M, Lin D and Zhu L, 2013, Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli. Environmental pollution (Barking, Essex : 1987), 173(97-102. [CrossRef]
- Li C, Ai F, Miao X; et al., 2018, “The return of ceramic implants”: Rose stem inspired dual layered modification of ceramic scaffolds with improved mechanical and anti-infective properties. Materials science & engineering. C, Materials for biological applications, 93(873-879. [CrossRef]
- Liao H, Miao X, Ye J; et al., 2017, Falling Leaves Inspired ZnO Nanorods-Nanoslices Hierarchical Structure for Implant Surface Modification with Two Stage Releasing Features. ACS applied materials & interfaces, 9(15): 13009-13015. [CrossRef]
- Lutsenko S, 2010, Human copper homeostasis: A network of interconnected pathways. Current opinion in chemical biology, 14(2): 211-217. [CrossRef]
- Hasan N M and Lutsenko S, 2012, Regulation of copper transporters in human cells. Current topics in membranes, 69(137-161. [CrossRef]
- Noyce J O, Michels H and Keevil C W, 2006, Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. The Journal of hospital infection, 63(3): 289-297. [CrossRef]
- Kadiiska M B and Mason R P, 2002, In vivo copper-mediated free radical production: An ESR spin-trapping study. Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 58(6): 1227-1239. [CrossRef]
- Jiménez Del Río M and Vélez-Pardo C, 2004, Transition metal-induced apoptosis in lymphocytes via hydroxyl radical generation, mitochondria dysfunction, and caspase-3 activation: An in vitro model for neurodegeneration. Archives of medical research, 35(3): 185-193. [CrossRef]
- Warnes S L, Caves V and Keevil C W, 2012, Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environmental microbiology, 14(7): 1730-1743. [CrossRef]
- Hodgkinson V and Petris M J, 2012, Copper homeostasis at the host-pathogen interface. The Journal of biological chemistry, 287(17): 13549-13555. [CrossRef]
- Vidakis N, Petousis M, Michailidis N; et al., 2022, Development and Optimization of Medical-Grade Multi-Functional Polyamide 12-Cuprous Oxide Nanocomposites with Superior Mechanical and Antibacterial Properties for Cost-Effective 3D Printing. Nanomaterials (Basel, Switzerland), 12(3). [CrossRef]
- Dilberoglu U M, Gharehpapagh B, Yaman U; et al., 2017, The Role of Additive Manufacturing in the Era of Industry 4.0. Procedia Manufacturing, 11(545-554. [CrossRef]
- Annamaria, Gisario, Michele; et al., 2019, Metal additive manufacturing in the commercial aviation industry: A review - ScienceDirect. Journal of Manufacturing Systems, 53(124-149. [CrossRef]
- Vidakis N, Petousis M, Maniadi A; et al., 2020, Mechanical Properties of 3D-Printed Acrylonitrile-Butadiene-Styrene TiO(2) and ATO Nanocomposites. Polymers, 12(7). [CrossRef]
- Ford S and Despeisse M, 2016, Additive manufacturing and sustainability: An exploratory study of the advantages and challenges. Journal of Cleaner Production, 137(nov.20): 1573-1587. [CrossRef]
- Turner R D, Wingham J R, Paterson T E; et al., 2020, Use of silver-based additives for the development of antibacterial functionality in Laser Sintered polyamide 12 parts. Scientific reports, 10(1): 892. [CrossRef]
- Li Y D, Guan J P, Tang R C; et al., 2019, Application of Natural Flavonoids to Impart Antioxidant and Antibacterial Activities to Polyamide Fiber for Health Care Applications. Antioxidants (Basel, Switzerland), 8(8). [CrossRef]
- Wencke Y L, Kutlu Y, Seefeldt M; et al., Additive manufacturing of PA12 carbon nanotube composites with a novel laser polymer deposition process. Journal of Applied Polymer Science,. [CrossRef]
- Espera A H, Valino A D, Palaganas J O; et al., 2019, 3D Printing of a Robust Polyamide-12-Carbon Black Composite via Selective Laser Sintering: Thermal and Electrical Conductivity. Macromolecular Materials Engineering,. [CrossRef]
- Liu Y C, Zou X B, Chai Y F; et al., 2014, Macrophage polarization in inflammatory diseases. International journal of biological sciences, 10(5): 520-529. [CrossRef]
- Sridharan R, Cameron A R, Kelly D J; et al., 2015, Biomaterial based modulation of macrophage polarization: A review and suggested design principles. 18(6): 313-325. [CrossRef]
- Fernandes T L, Gomoll A H, Lattermann C; et al., 2020, Macrophage: A Potential Target on Cartilage Regeneration. Frontiers in immunology, 11(111. [CrossRef]
- Stout R D, Watkins S K and Suttles J, 2009, Functional plasticity of macrophages: In situ reprogramming of tumor-associated macrophages. Journal of leukocyte biology, 86(5): 1105-1109. [CrossRef]
- Arnold L, Henry A, Poron F; et al., 2007, Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of experimental medicine, 204(5): 1057-1069. [CrossRef]
- Mantovani A, Sica A, Allavena P; et al., 2009, Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Human immunology, 70(5): 325-330. [CrossRef]
- Troidl C, Möllmann H, Nef H; et al., 2009, Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. Journal of cellular and molecular medicine, 13(9b): 3485-3496. [CrossRef]
- Yunna C, Mengru H, Lei W; et al., 2020, Macrophage M1/M2 polarization. European journal of pharmacology, 877(173090. [CrossRef]
- Viola A, Munari F, Sánchez-Rodríguez R; et al., 2019, The Metabolic Signature of Macrophage Responses. Frontiers in immunology, 10(1462. [CrossRef]
- Saklatvala J, 2002, Glucocorticoids: Do we know how they work? Arthritis research, 4(3): 146-150. [CrossRef]
- Lasa M, Brook M, Saklatvala J; et al., 2001, Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Molecular and cellular biology, 21(3): 771-780. [CrossRef]
- Oray M, Abu Samra K, Ebrahimiadib N; et al., 2016, Long-term side effects of glucocorticoids. Expert opinion on drug safety, 15(4): 457-465. [CrossRef]
- Kim D H and Martin D C, 2006, Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials, 27(15): 3031-3037. [CrossRef]
- Rodrigues P O, Becker A C, Dellagnelo V A; et al., 2014, Pharmacodynamic and Pharmacokinetic Studies of β-Cyclodextrin:Dexamethasone Acetate Complexes in Mice. Brazilian Archives of Biology and Technology, 57(6): 887-894. [CrossRef]
- Loftsson T and Stefánsson E, 2007, Cyclodextrins in ocular drug delivery: Theoretical basis with dexamethasone as a sample drug. Journal of Drug Delivery Science Technology, 17(1): 3-9. [CrossRef]
- Lee S J, Choi J S, Eom M R; et al., 2020, Dexamethasone loaded bilayered 3D tubular scaffold reduces restenosis at the anastomotic site of tracheal replacement: In vitro and in vivo assessments. Nanoscale, 12(8): 4846-4858. [CrossRef]
- Lopez R F, Collett J H and Bentley M V, 2000, Influence of cyclodextrin complexation on the in vitro permeation and skin metabolism of dexamethasone. International journal of pharmaceutics, 200(1): 127-132. [CrossRef]
- Loftsson T, Fririksdóttir H, Thórisdóttir S; et al., 1994, The effect of hydroxypropyl methylcellulose on the release of dexamethasone from aqueous 2-hydroxypropyl-β-cyclodextrin formulations. International Journal of Pharmaceutics. [CrossRef]
- 、, 104(2): 181-184. [CrossRef]
- Doile M M, Fortunato K A, Schmücker I C; et al., 2008, Physicochemical properties and dissolution studies of dexamethasone acetate-beta-cyclodextrin inclusion complexes produced by different methods. AAPS PharmSciTech, 9(1): 314-321. [CrossRef]
- Zhao C, Zuo F, Liao Z; et al., 2015, Mussel-inspired one-pot synthesis of a fluorescent and water-soluble polydopamine-polyethyleneimine copolymer. Macromolecular rapid communications, 36(10): 909-915. [CrossRef]
- Sang J L, Jo H H, Lim K S; et al., 2019, Heparin coating on 3D printed poly (l-lactic acid) biodegradable cardiovascular stent via mild surface modification approach for coronary artery implantation. Chemical Engineering Journal, 378(122116-. [CrossRef]
- Loftsson T, Fririksdóttir H, Thórisdóttir S; et al., 1994, The effect of hydroxypropyl methylcellulose on the release of dexamethasone from aqueous 2-hydroxypropyl-β-cyclodextrin formulations. International journal of pharmaceutics, 104(2): 181-184. [CrossRef]
- Farto-Vaamonde X, Auriemma G, Aquino R P; et al., 2019, Post-manufacture loading of filaments and 3D printed PLA scaffolds with prednisolone and dexamethasone for tissue regeneration applications. European journal of pharmaceutics and biopharmaceutics : Official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 141(100-110. [CrossRef]
- Bost M, Houdart S, Oberli M; et al., 2016, Dietary copper and human health: Current evidence and unresolved issues. Journal of trace elements in medicine and biology : Organ of the Society for Minerals and Trace Elements (GMS), 35(107-115. [CrossRef]
- Medeiros D M, 2016, Copper, iron, and selenium dietary deficiencies negatively impact skeletal integrity: A review. Experimental biology and medicine (Maywood, N.J.), 241(12): 1316-1322. [CrossRef]
- Goggs R, Vaughan-Thomas A, Clegg P D; et al., 2005, Nutraceutical therapies for degenerative joint diseases: A critical review. Critical reviews in food science and nutrition, 45(3): 145-164. [CrossRef]
- Lin R, Deng C, Li X; et al., 2019, Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics, 9(21): 6300-6313. [CrossRef]
- Pham P C, Pham P A, Pham S V; et al., 2014, Hypomagnesemia: A clinical perspective. International journal of nephrology and renovascular disease, 7(219-230. [CrossRef]
- Mclean R M, 1994, Magnesium and its therapeutic uses: A review. The American journal of medicine, 96(1): 63-76. [CrossRef]
- Rice N R and Ernst M K, 1993, In vivo control of NF-kappa B activation by I kappa B alpha. The EMBO journal, 12(12): 4685-4695. [CrossRef]
- Rechsteiner M and Rogers S W, 1996, PEST sequences and regulation by proteolysis. Trends in biochemical sciences, 21(7): 267-271. [CrossRef]
- Didonato J A, Mercurio F and Karin M, 1995, Phosphorylation of IκB α precedes but is not sufficient for its dissociation from NF-κB. Molecular Cellular Biology, 15(3): 1302-1311. [CrossRef]
- Nakano T, Katsuki S, Chen M; et al., 2019, Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation, 139(1): 78-96. [CrossRef]
- Kim M J, Park J S, Lee S J; et al., 2015, Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. Journal of controlled release : Official journal of the Controlled Release Society, 216(140-148. [CrossRef]
- Wang W, Xiong Y, Zhao R; et al., 2022, A novel hierarchical biofunctionalized 3D-printed porous Ti6Al4V scaffold with enhanced osteoporotic osseointegration through osteoimmunomodulation. Journal of nanobiotechnology, 20(1): 68. [CrossRef]
- Xu C Q, Liu B J, Wu J F; et al., 2010, Icariin attenuates LPS-induced acute inflammatory responses: Involvement of PI3K/Akt and NF-kappaB signaling pathway. European journal of pharmacology, 642(1-3): 146-153. [CrossRef]
- Sun X, Deng X, Cai W; et al., 2018, Icariin inhibits LPS-induced cell inflammatory response by promoting GRα nuclear translocation and upregulating GRα expression. Life sciences, 195(33-43. [CrossRef]
- Horcajada P, Gref R, Baati T; et al., 2012, Metal-organic frameworks in biomedicine. Chemical reviews, 112(2): 1232-1268. [CrossRef]
- Hu Q, Yu J, Liu M; et al., 2014, A low cytotoxic cationic metal-organic framework carrier for controllable drug release. Journal of medicinal chemistry, 57(13): 5679-5685. [CrossRef]
- Frazer K A, Ueda Y, Zhu Y; et al., 1997, Computational and biological analysis of 680 kb of DNA sequence from the human 5q31 cytokine gene cluster region. Genome research, 7(5): 495-512. [CrossRef]
- Bao K and Reinhardt R L, 2015, The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine, 75(1): 25-37. [CrossRef]
- Hershey G K, 2003, IL-13 receptors and signaling pathways: An evolving web. The Journal of allergy and clinical immunology, 111(4): 677-690; quiz 691. [CrossRef]
- Brombacher F, 2000, The role of interleukin-13 in infectious diseases and allergy. BioEssays : News and reviews in molecular, cellular and developmental biology, 22(7): 646-656. [CrossRef]
- Wang M, Li W, Luo Z; et al., 2022, A multifunctional micropore-forming bioink with enhanced anti-bacterial and anti-inflammatory properties. Biofabrication, 14(2). [CrossRef]
- Nauta A J and Fibbe W E, 2007, Immunomodulatory properties of mesenchymal stromal cells. Blood, 110(10): 3499-3506. [CrossRef]
- Lo Sicco C, Reverberi D, Balbi C; et al., 2017, Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem cells translational medicine, 6(3): 1018-1028. [CrossRef]
- Dimarino A M, Caplan A I and Bonfield T L, 2013, Mesenchymal stem cells in tissue repair. Frontiers in immunology, 4(201. [CrossRef]
- Zhao J, Li X, Hu J; et al., 2019, Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovascular research, 115(7): 1205-1216. [CrossRef]
- Song N, Scholtemeijer M and Shah K, 2020, Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends in pharmacological sciences, 41(9): 653-664. [CrossRef]
- Paul K, Darzi S, Mcphee G; et al., 2019, 3D bioprinted endometrial stem cells on melt electrospun poly ε-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta biomaterialia, 97(162-176. [CrossRef]
- Reischl S, Lee J H, Miltschitzky J R E; et al., 2021, Ac2-26-Nanoparticles Induce Resolution of Intestinal Inflammation and Anastomotic Healing via Inhibition of NF-κB Signaling in a Model of Perioperative Colitis. Inflammatory bowel diseases, 27(9): 1379-1393. [CrossRef]
- Pupjalis D, Goetsch J, Kottas D J; et al., 2011, Annexin A1 released from apoptotic cells acts through formyl peptide receptors to dampen inflammatory monocyte activation via JAK/STAT/SOCS signalling. EMBO molecular medicine, 3(2): 102-114. [CrossRef]
- Huang J J, Xia C J, Wei Y; et al., 2020, Annexin A1-derived peptide Ac2-26 facilitates wound healing in diabetic mice. Wound repair and regeneration : Official publication of the Wound Healing Society [and] the European Tissue Repair Society, 28(6): 772-779. [CrossRef]
- Xu B, Ye J, Fan B S; et al., 2023, Protein-spatiotemporal partition releasing gradient porous scaffolds and anti-inflammatory and antioxidant regulation remodel tissue engineered anisotropic meniscus. Bioactive materials, 20(194-207. [CrossRef]
- Tsekova P B, Spasova M G, Manolova N E; et al., 2017, Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Materials science & engineering. C, Materials for biological applications, 73(206-214. [CrossRef]
- Ahsan H, Parveen N, Khan N U; et al., 1999, Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chemico-biological interactions, 121(2): 161-175. [CrossRef]
- Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C; et al., 2016, Curcumin and Health. Molecules (Basel, Switzerland), 21(3): 264. [CrossRef]
- Karuppagounder V, Arumugam S, Thandavarayan R A; et al., 2016, Curcumin alleviates renal dysfunction and suppresses inflammation by shifting from M1 to M2 macrophage polarization in daunorubicin induced nephrotoxicity in rats. Cytokine, 84(1-9. [CrossRef]
- Kim S J, 2011, Curcumin suppresses the production of interleukin-6 in Prevotella intermedia lipopolysaccharide-activated RAW 264.7 cells. Journal of periodontal & implant science, 41(3): 157-163. [CrossRef]
- Lee K H, Chow Y L, Sharmili V; et al., 2012, BDMC33, A curcumin derivative suppresses inflammatory responses in macrophage-like cellular system: Role of inhibition in NF-κB and MAPK signaling pathways. International journal of molecular sciences, 13(3): 2985-3008. [CrossRef]
- Chen Y C, Shie M Y, Wu Y A; et al., 2017, Anti-inflammation performance of curcumin-loaded mesoporous calcium silicate cement. Journal of the Formosan Medical Association = Taiwan yi zhi, 116(9): 679-688. [CrossRef]
- Huang C Y, Huang T H, Kao C T; et al., 2017, Mesoporous Calcium Silicate Nanoparticles with Drug Delivery and Odontogenesis Properties. Journal of endodontics, 43(1): 69-76. [CrossRef]
- Moustapha A, Pérétout P A, Rainey N E; et al., 2015, Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell death discovery, 1(15017. [CrossRef]
- Lemos D R, Babaeijandaghi F, Low M; et al., 2015, Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med, 21(7): 786-794. [CrossRef]
- Kim D C, Ku S K and Bae J S, 2012, Anticoagulant activities of curcumin and its derivative. BMB reports, 45(4): 221-226. [CrossRef]
- Zhang D W, Fu M, Gao S H; et al., 2013, Curcumin and diabetes: A systematic review. Evidence-based complementary and alternative medicine : eCAM, 2013(636053. [CrossRef]
- Setchell K D, 1998, Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. The American journal of clinical nutrition, 68(6 Suppl): 1333s-1346s. [CrossRef]
- Banerjee S, Li Y, Wang Z; et al., 2008, Multi-targeted therapy of cancer by genistein. Cancer letters, 269(2): 226-242. [CrossRef]
- Jia T L, Wang H Z, Xie L P; et al., 2003, Daidzein enhances osteoblast growth that may be mediated by increased bone morphogenetic protein (BMP) production. Biochemical pharmacology, 65(5): 709-715. [CrossRef]
- Li H Y, Pan L, Ke Y S; et al., 2014, Daidzein suppresses pro-inflammatory chemokine Cxcl2 transcription in TNF-α-stimulated murine lung epithelial cells via depressing PARP-1 activity. Acta pharmacologica Sinica, 35(4): 496-503. [CrossRef]
- Smith B N and Dilger R N, 2018, Immunomodulatory potential of dietary soybean-derived isoflavones and saponins in pigs. Journal of animal science, 96(4): 1288-1304. [CrossRef]
- Lai H H and Yen G C, 2002, Inhibitory effect of isoflavones on peroxynitrite-mediated low-density lipoprotein oxidation. Bioscience, biotechnology, and biochemistry, 66(1): 22-28. [CrossRef]
- García-Lafuente A, Guillamón E, Villares A; et al., 2009, Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflammation research : Official journal of the European Histamine Research Society ... [et al.], 58(9): 537-552. [CrossRef]
- Eo H, Ann J Y and Lim Y, 2015, Dietary Supplementation of Genistein Attenuates Inflammatory Responses and Oxidative Stress during Cutaneous Wound Healing in Diabetic Mice. Journal of Agricultural Science, 7(2):. [CrossRef]
- Barnes P J and Karin M, 1997, Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. The New England journal of medicine, 336(15): 1066-1071. [CrossRef]
- Sarkar N and Bose S, 2020, Controlled release of soy isoflavones from multifunctional 3D printed bone tissue engineering scaffolds. Acta biomaterialia, 114(407-420. [CrossRef]
- Russo M, Spagnuolo C, Tedesco I; et al., 2012, The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochemical pharmacology, 83(1): 6-15. [CrossRef]
- Zhou Y, Wu Y, Ma W; et al., 2017, The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. Journal of materials chemistry. B, 5(3): 612-625. [CrossRef]
- Wang Y, Li C, Wan Y; et al., 2021, Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation. Small (Weinheim an der Bergstrasse, Germany), 17(41): e2101505. [CrossRef]
- Hu Y, Gui Z, Zhou Y; et al., 2019, Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free radical biology & medicine, 145(146-160. [CrossRef]
- Tan R Z, Wang C, Deng C; et al., 2020, Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytotherapy research : PTR, 34(1): 139-152. [CrossRef]
- Liu N, Wang H, Fu Z; et al., 2022, Quercetin-Coating Promotes Osteogenic Differentiation, Osseointegration and Anti-Inflammatory Properties of Nano-Topographic Modificated 3D-Printed Ti6Al4V Implant. Frontiers in bioengineering and biotechnology, 10(933135. [CrossRef]
- Sun S, Chen W, Cao W; et al., 2008, Research on the chelation between quercetin and Cr(III) ion by Density Functional Theory (DFT) method. Journal of Molecular Structure: THEOCHEM,. [CrossRef]
- Ricard-Blum S, 2011, The collagen family. Cold Spring Harbor perspectives in biology, 3(1): a004978. [CrossRef]
- Theocharis A D, Skandalis S S, Gialeli C; et al., 2016, Extracellular matrix structure. Adv Drug Deliv Rev, 97(4-27. [CrossRef]
- Friedman S L, 2010, Evolving challenges in hepatic fibrosis. Nature reviews. Gastroenterology & hepatology, 7(8): 425-436. [CrossRef]
- Coelho N M and Mcculloch C A, 2016, Contribution of collagen adhesion receptors to tissue fibrosis. Cell and tissue research, 365(3): 521-538. [CrossRef]
- Zhang Y P, Li W B, Wang W L; et al., 2012, siRNA against plasminogen activator inhibitor-1 ameliorates bleomycin-induced lung fibrosis in rats. Acta pharmacologica Sinica, 33(7): 897-908. [CrossRef]
- Sefat F, Denyer M C and Youseffi M, 2014, Effects of different transforming growth factor beta (TGF-β) isomers on wound closure of bone cell monolayers. Cytokine, 69(1): 75-86. [CrossRef]
- Mueller S, Phillips J, Onar-Thomas A; et al., 2012, PTEN promoter methylation and activation of the PI3K/Akt/mTOR pathway in pediatric gliomas and influence on clinical outcome. Neuro-oncology, 14(9): 1146-1152. [CrossRef]
- Tsai P C, Zhang Z, Florek C; et al., 2016, Constructing Human Skin Equivalents on Porcine Acellular Peritoneum Extracellular Matrix for In Vitro Irritation Testing. Tissue engineering. Part A, 22(1-2): 111-122. [CrossRef]
- Stark H J, Boehnke K, Mirancea N; et al., 2006, Epidermal homeostasis in long-term scaffold-enforced skin equivalents. The journal of investigative dermatology. Symposium proceedings, 11(1): 93-105. [CrossRef]
- Karsdal M A, Detlefsen S, Daniels S J; et al., 2020, Is the Total Amount as Important as Localization and Type of Collagen in Liver Fibrosis Attributable to Steatohepatitis? Hepatology (Baltimore, Md.), 71(1): 346-351. [CrossRef]
- Feng W, Ye F, Xue W; et al., 2009, Copper regulation of hypoxia-inducible factor-1 activity. Molecular pharmacology, 75(1): 174-182. [CrossRef]
- Duval E, Leclercq S, Elissalde J M; et al., 2009, Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: Hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis and rheumatism, 60(10): 3038-3048. [CrossRef]
- Agarwal S, Loder S, Brownley C; et al., 2016, Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proceedings of the National Academy of Sciences of the United States of America, 113(3): E338-347. [CrossRef]
- Bandyopadhyay A and Mandal B B, 2019, A three-dimensional printed silk-based biomimetic tri-layered meniscus for potential patient-specific implantation. Biofabrication, 12(1): 015003. [CrossRef]
- Bose S, Sarkar N and Banerjee D, 2018, Effects of PCL, PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration. Materials today. Chemistry, 8(110-120. [CrossRef]
- Wynn T A and Ramalingam T R, 2012, Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med, 18(7): 1028-1040. [CrossRef]
- Uccelli A, Moretta L and Pistoia V, 2008, Mesenchymal stem cells in health and disease. Nature reviews. Immunology, 8(9): 726-736. [CrossRef]
- Wu M, Ji S, Xiao S; et al., 2015, JAM-A promotes wound healing by enhancing both homing and secretory activities of mesenchymal stem cells. Clinical science (London, England : 1979), 129(7): 575-588. [CrossRef]
- Zhang J, Guan J, Niu X; et al., 2015, Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. Journal of translational medicine, 13(49. [CrossRef]
- Wang X, Ma Y, Gao Z; et al., 2018, Human adipose-derived stem cells inhibit bioactivity of keloid fibroblasts. Stem cell research & therapy, 9(1): 40. [CrossRef]
- Aoki M, Miyake K, Ogawa R; et al., 2014, siRNA knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type I. The Journal of investigative dermatology, 134(3): 818-826. [CrossRef]
- Zhang B, Wang M, Gong A; et al., 2015, HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem cells (Dayton, Ohio), 33(7): 2158-2168. [CrossRef]
- Sun Q, Nakata H, Yamamoto M; et al., 2019, Comparison of gingiva-derived and bone marrow mesenchymal stem cells for osteogenesis. Journal of cellular and molecular medicine, 23(11): 7592-7601. [CrossRef]
- Moshaverinia A, Xu X, Chen C; et al., 2014, Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials, 35(9): 2642-2650. [CrossRef]
- Chaussain Miller C, Septier D, Bonnefoix M; et al., 2002, Human dermal and gingival fibroblasts in a three-dimensional culture: A comparative study on matrix remodeling. Clinical oral investigations, 6(1): 39-50. [CrossRef]
- Shafiee A, Cavalcanti A S, Saidy N T; et al., 2021, Convergence of 3D printed biomimetic wound dressings and adult stem cell therapy. Biomaterials, 268(120558. [CrossRef]
- Cavalcante M B, Costa Fda S, Barini R; et al., 2015, Granulocyte colony-stimulating factor and reproductive medicine: A review. Iranian journal of reproductive medicine, 13(4): 195-202.
- Malashchenko V V, Meniailo M E, Shmarov V A; et al., 2018, Direct anti-inflammatory effects of granulocyte colony-stimulating factor (G-CSF) on activation and functional properties of human T cell subpopulations in vitro. Cellular immunology, 325(23-32. [CrossRef]
- Lin X, Zhang Y, Pan Y; et al., 2018, Endometrial stem cell-derived granulocyte-colony stimulating factor attenuates endometrial fibrosis via sonic hedgehog transcriptional activator Gli2. Biology of reproduction, 98(4): 480-490. [CrossRef]
- Zhao S, Zhang Y, Tian H; et al., 2013, Extending the serum half-life of G-CSF via fusion with the domain III of human serum albumin. BioMed research international, 2013(107238. [CrossRef]
- Choi S H and Park T G, 2006, G-CSF loaded biodegradable PLGA nanoparticles prepared by a single oil-in-water emulsion method. International journal of pharmaceutics, 311(1-2): 223-228. [CrossRef]
- Wen J, Hou B, Lin W; et al., 2022, 3D-printed hydrogel scaffold-loaded granulocyte colony-stimulating factor sustained-release microspheres and their effect on endometrial regeneration. Biomaterials science, 10(12): 3346-3358. [CrossRef]
- Zhao M, Song B, Pu J; et al., 2006, Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature, 442(7101): 457-460. [CrossRef]
- Sorg H, Tilkorn D J, Hager S; et al., 2017, Skin Wound Healing: An Update on the Current Knowledge and Concepts. European surgical research. Europaische chirurgische Forschung. Recherches chirurgicales europeennes, 58(1-2): 81-94. [CrossRef]
- Rhett J M, Ghatnekar G S, Palatinus J A; et al., 2008, Novel therapies for scar reduction and regenerative healing of skin wounds. Trends in biotechnology, 26(4): 173-180. [CrossRef]
- Liang J, Zeng H, Qiao L; et al., 2022, 3D Printed Piezoelectric Wound Dressing with Dual Piezoelectric Response Models for Scar-Prevention Wound Healing. ACS applied materials & interfaces, 14(27): 30507-30522. [CrossRef]
- Kang G-d and Cao Y-m, 2014, Application and modification of poly(vinylidene fluoride) (PVDF) membranes – A review. Journal of Membrane Science, 463(145-165. [CrossRef]
- Efome J E, Rana D, Matsuura T; et al., 2016, Enhanced performance of PVDF nanocomposite membrane by nanofiber coating: A membrane for sustainable desalination through MD. Water research, 89(39-49. [CrossRef]
- Devi P I and Ramachandran K, 2011, Dielectric studies on hybridised PVDF–ZnO nanocomposites. Journal of Experimental Nanoscience, 6(3): 281-293. [CrossRef]
- Xu Q, Guo L, A S; et al., 2018, Injectable hyperbranched poly(β-amino ester) hydrogels with on-demand degradation profiles to match wound healing processes. Chemical science, 9(8): 2179-2187. [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



