Submitted:
17 April 2023
Posted:
18 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
| Study | Target | CAR T cell/lymphodepletion | Number of Patients | Response | Safety | Reference |
|---|---|---|---|---|---|---|
| Phase I/II | CLL-1 | 0.35-1x10^6.kg/anti-CLL-1-CD8-41BB/ Cy+Flu |
8 | 4/8 morphological leukemia-free, MRD (-) 1 morphological leukemia-free, MRD+, 1 CR with incomplete hematologic recovery MRD(+), 1 PR, 1 SD |
CRS: 5 grade 1, 3 grade 2 | 150 |
| Phase I | CD123 | Dose escalation 50x10^6-200x10^6/anti-CD123-IgG4-CD28/Cy+Flu | 6 | 4/6 CR 2 reduced blast |
CRS: 4 grade 1, 1 grade 2; 1 adenoviral pneumonia requiring intubation; and 1 grade 3 rash due to drug hypersensitivity | 151 |
| Phase I | CD33 | CD33-4-1BB/0.3x10^6/kg | 3 | 0/3 response | 2 CRS; 1 ICANS. A grade 3 tumor lysis syndrome-acute kidney injury, grade 2 mucositis, grade 1 tachycardia for 1 patient; and a second patient experienced grade 2 intermittent orthostatic hypotension, grade 2 increased bilirubin and grade 3 increased ALT and AST | 152 |
| Case report | CD33 | CD33-4-1BB/ 1.12x10^9 | 1 | Disease progression at week 9 | Grade 4 chills and a high fever, pancytopenia | 153 |
| Phase I | CD38 | NA | 6 | 66.7% of patients (4/6) CR (including 1 with CR and 3 with CR with incomplete count recovery (CRi)) and full donor chimerism) | Five patients presented mild CRS (Grade I–II), and only one experienced grade III hepatotoxicity with elevated serum transaminase and bilirubin levels | 154 |
| Phase I | LeY | Anti-LeY-CD28/Flu-Cy | 4 | In the patient with active leukemia, a temporary reduction in peripheral blood blast cells was observed. One other patient achieved a cytogenetic remission, while the other two patients had SD | One patient (patient 2) had a transient grade 2 neutropenia | 155 |
2. Challenges in Adoptive T cell Therapy for Acute Myeloid Leukemia
2.1. AML is highly heterogenous:
2.2. There is no ideal surface antigen to target:
| Target Antigen | Function | Expression on normal cells | Expression on HSCs | Expression on LSCs |
|---|---|---|---|---|
| CLL-1 | Glycoprotein, Transmembrane receptor | Myeloid, lung, epithelial cells | - | + |
| CD 33 | SIGLEC family protein, Transmembrane receptor | Progenitor, myeloid, kuppffer cells | + | + |
| CD 7 | Ig superfamily/Glycoprotein, B and T cell lymphoid development, transmembrane protein | T, NK cells and myeloid progenitor | - | + |
| FLT3 | Type III cytokine receptor, Tyrosine kinase receptor | Neurons, testis | + | + |
| CD 38 | Glycoprotein, Cyclic ADP ribose hydroxylase | B, T, NK cells | - | + |
| CD 123 | Type I cytokine receptor of IL-3, IL3 receptor a subunit | Myeloid progenitors, DC and basophils | + | + |
| CD 44v6 | Glycoprotein, Transmembrane receptor | Keratinocytes | - | + |
| LeY | Glycosphingolipid, Blood group Ag | Intestinal epithelial cells | + | + |
| NKG2D | C-type lectin-like receptor protein, Activator receptor | NK, NKT, Tαδ, Th, and CTL | - | + |
| CD 70 | Glycoprotein from the TNF family, Transmembrane receptor | T and B cells | - | + |
| CD 96 | Member of immunoglobulin superfamily, adhesion of activated T and NK cells | T cells and NK cells | - | + |
2.3. Interactions in the Tumor Microenvironment:
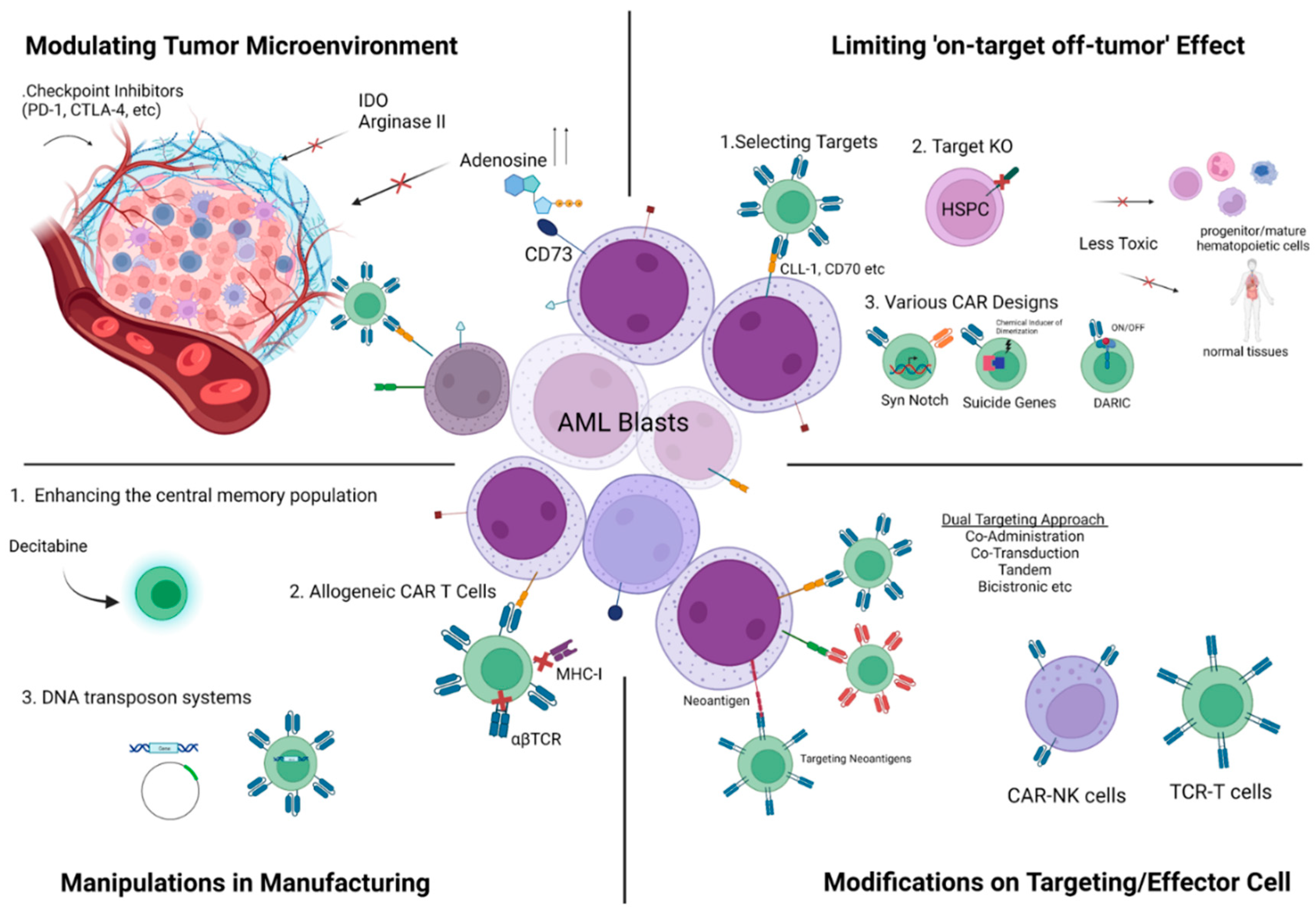
3. Promising Strategies to Overcome Challenges
3.1. Safer targets with less ‘on-target off-tumor’ effect:
3.2. Limiting the ‘on target-off tumor’ effect:
3.3. Combinatorial Antigen Targeting for Heterogeneity:
3.4. Neoantigens:
3.5. T cell receptor (TCR) T cells for treatment of AML:
| Study | TCR-T Therapy | Study Phase/Number of Patients | Study Outcomes | Adverse Events |
|---|---|---|---|---|
| NCT02550535 | Autologous WT1 TCR-T cells | Phase I/II, 10 patients (6 AML, 3 MDS and 1 TKI-resistant CML) | All 6 AML patients were alive at last follow up (median 12 months; range 7-12.8 months). The 3 patients with MDS had a median survival of 3 months (range 2.1-3.96 months).2 died from progressive disease and one from other causes. 2 patients had disease progression. |
1 CRS |
| UMIN00001159 | Autologous WT1 siTCR-T cells | Unknown | 2 patients showed transient decrease in blast counts. | None |
| NCT01640301 | Allogeneic WT1 TCR-T cells | Phase I/II, 12 patients | With a median follow-up of 44 months (range 21–57 months) following infusion all 12 patients did not have evidence of disease. | None |
| NCT03503958 | Autologous PRAME TCR-T cells | Phase I | Not posted | Not posted |
| NCT01621724 | Autologous WT1 TCR-T cells | Phase I/II, 7 patients | Not posted | Not posted |
3.6. CAR NK cells:
3.7. Manipulations in Manufacturing:
3.8. Strategies to Overcome the Negative Effects of Microenvironment:
3.9. Allogeneic Hematopoietic Stem Cell Transplantation with CAR T cells:
4. Summary and Conclusion:
Acknowledgments
Conflicts of Interest
References
- Vishwasrao P, Li G, Boucher JC, Smith DL, Hui SK. Emerging CAR T Cell Strategies for the Treatment of AML. Cancers (Basel). 2022 Feb 27;14(5):1241. [CrossRef]
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. New England Journal of Medicine, 2015 373(12), 1136-1152.
- Hofmann S, Schubert ML, Wang L, He B, Neuber B, Dreger P, Müller-Tidow C, Schmitt M. Chimeric Antigen Receptor (CAR) T Cell Therapy in Acute Myeloid Leukemia (AML). J Clin Med. 2019 Feb 6;8(2):200. [CrossRef]
- Marofi F, Rahman HS, Al-Obaidi ZMJ, Jalil AT, Abdelbasset WK, Suksatan W, Dorofeev AE, Shomali N, Chartrand MS, Pathak Y, Hassanzadeh A, Baradaran B, Ahmadi M, Saeedi H, Tahmasebi S, Jarahian M. Novel CAR T therapy is a ray of hope in the treatment of seriously ill AML patients. Stem Cell Res Ther. 2021 Aug 20;12(1):465. [CrossRef]
- Karamitros D, Stoilova B, Aboukhalil Z, Hamey F, Reinisch A, Samitsch M, Quek L, Otto G, Repapi E, Doondeea J, Usukhbayar B, Calvo J, Taylor S, Goardon N, Six E, Pflumio F, Porcher C, Majeti R, Göttgens B, Vyas P. Single-cell analysis reveals the continuum of human lympho-myeloid progenitor cells. Nat Immunol. 2018 Jan;19(1):85-97. [CrossRef]
- Pollyea DA, Jordan CT. Therapeutic targeting of acute myeloid leukemia stem cells. Blood. 2017 Mar 23;129(12):1627-1635. [CrossRef]
- Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017 Jan 26;129(4):424-447. [CrossRef]
- Cancer Genome Atlas Research Network; Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Hoadley K, Triche TJ Jr, Laird PW, Baty JD, Fulton LL, Fulton R, Heath SE, Kalicki-Veizer J, Kandoth C, Klco JM, Koboldt DC, Kanchi KL, Kulkarni S, Lamprecht TL, Larson DE, Lin L, Lu C, McLellan MD, McMichael JF, Payton J, Schmidt H, Spencer DH, Tomasson MH, Wallis JW, Wartman LD, Watson MA, Welch J, Wendl MC, Ally A, Balasundaram M, Birol I, Butterfield Y, Chiu R, Chu A, Chuah E, Chun HJ, Corbett R, Dhalla N, Guin R, He A, Hirst C, Hirst M, Holt RA, Jones S, Karsan A, Lee D, Li HI, Marra MA, Mayo M, Moore RA, Mungall K, Parker J, Pleasance E, Plettner P, Schein J, Stoll D, Swanson L, Tam A, Thiessen N, Varhol R, Wye N, Zhao Y, Gabriel S, Getz G, Sougnez C, Zou L, Leiserson MD, Vandin F, Wu HT, Applebaum F, Baylin SB, Akbani R, Broom BM, Chen K, Motter TC, Nguyen K, Weinstein JN, Zhang N, Ferguson ML, Adams C, Black A, Bowen J, Gastier-Foster J, Grossman T, Lichtenberg T, Wise L, Davidsen T, Demchok JA, Shaw KR, Sheth M, Sofia HJ, Yang L, Downing JR, Eley G. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013 May 30;368(22):2059-74.
- DiNardo CD, Cortes JE. Mutations in AML: prognostic and therapeutic implications. Hematology Am Soc Hematol Educ Program. 2016;2016(1):348–55. [CrossRef]
- Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, Quake SR, Majeti R. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med. 2012 Aug 29;4(149):149ra118.
- Corces MR, Buenrostro JD, Wu B, Greenside PG, Chan SM, Koenig JL, Snyder MP, Pritchard JK, Kundaje A, Greenleaf WJ, Majeti R, Chang HY. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat Genet. 2016 Oct;48(10):1193-203. [CrossRef]
- Stengel A, Shahswar R, Haferlach T, Walter W, Hutter S, Meggendorfer M, Kern W, Haferlach C. Whole transcriptome sequencing detects a large number of novel fusion transcripts in patients with AML and MDS. Blood Adv. 2020 Nov 10;4(21):5393-5401. doi: 10.1182/bloodadvances.2020003007. [CrossRef]
- Chakraborty, S., Park, C.Y. Pathogenic Mechanisms in Acute Myeloid Leukemia. Curr. Treat. Options in Oncol. 23, 1522–1534 (2022). [CrossRef]
- Li S, Garrett-Bakelman FE, Chung SS, Sanders MA, Hricik T, Rapaport F, Patel J, Dillon R, Vijay P, Brown AL, Perl AE, Cannon J, Bullinger L, Luger S, Becker M, Lewis ID, To LB, Delwel R, Löwenberg B, Döhner H, Döhner K, Guzman ML, Hassane DC, Roboz GJ, Grimwade D, Valk PJ, D'Andrea RJ, Carroll M, Park CY, Neuberg D, Levine R, Melnick AM, Mason CE. Distinct evolution and dynamics of epigenetic and genetic heterogeneity in acute myeloid leukemia. Nat Med. 2016 Jul;22(7):792-9. doi: 10.1038/nm.4125. [CrossRef]
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–91. [CrossRef]
- Thomas D, Majeti R. Biology and relevance of human acute myeloid leukemia stem cells. Blood. 2017;129:1577–85. [CrossRef]
- Zeng AGX, Bansal S, Jin L, Mitchell A, Chen WC, Abbas HA, Chan-Seng-Yue M, Voisin V, van Galen P, Tierens A, Cheok M, Preudhomme C, Dombret H, Daver N, Futreal PA, Minden MD, Kennedy JA, Wang JCY, Dick JE. A cellular hierarchy framework for understanding heterogeneity and predicting drug response in acute myeloid leukemia. Nat Med. 2022 Jun;28(6):1212-1223. [CrossRef]
- Jiang, X.-P. Li, Y.-T. Dai, B. Chen, X.-Q. Weng, S.-M. Xiong, M. Zhang, J.- Y. Huang, Z. Chen, S.-J. Chen, Multidimensional study of the heterogeneity of leukemia cells in t(8;21) acute myelogenous leukemia identifies the subtype with poor outcome, Proc. Natl. Acad. Sci. U. S. A. 117 (2020) 20117–20126. [CrossRef]
- Li K, Du Y, Cai Y, Liu W, Lv Y, Huang B, Zhang L, Wang Z, Liu P, Sun Q, Li N, Zhu M, Bosco B, Li L, Wu W, Wu L, Li J, Wang Q, Hong M, Qian S. Single-cell analysis reveals the chemotherapy-induced cellular reprogramming and novel therapeutic targets in relapsed/refractory acute myeloid leukemia. Leukemia. 2023 Feb;37(2):308-325. [CrossRef]
- Stratmann S, Vesterlund M, Umer HM, Eshtad S, Skaftason A, Herlin MK, Sundström C, Eriksson A, Höglund M, Palle J, Abrahamsson J, Jahnukainen K, Munthe-Kaas MC, Zeller B, Tamm KP, Lindskog C, Cavelier L, Lehtiö J, Holmfeldt L. Proteogenomic analysis of acute myeloid leukemia associates relapsed disease with reprogrammed energy metabolism both in adults and children. Leukemia. 2023 Mar;37(3):550-559.
- Sadelain M (2016). Chimeric antigen receptors: driving immunology towards synthetic biology. Curr Opin Immunol 41, 68–76. [CrossRef]
- LeBien TW, and Tedder TF (2008). B lymphocytes: how they develop and function. Blood 112, 1570– 1580. [CrossRef]
- Bakker AB, van den Oudenrijn S, Bakker AQ, Feller N, van Meijer M, Bia JA, Jongeneelen MA, Visser TJ, Bijl N, Geuijen CA, et al. (2004). C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res 64, 8443–8450.
- Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter DL, Carroll M, et al. (2015). CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia 29, 1637–1647. [CrossRef]
- Bendall LJ, Bradstock KF, and Gottlieb DJ (2000). Expression of CD44 variant exons in acute myeloid leukemia is more common and more complex than that observed in normal blood, bone marrow or CD34+ cells. Leukemia 14, 1239–1246. [CrossRef]
- Perna F, Berman SH, Soni RK, Mansilla-Soto J, Eyquem J, Hamieh M, Hendrickson RC, Brennan CW, Sadelain M. Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell. 2017 Oct 9;32(4):506-519. [CrossRef]
- Melao, A. (2017). FDA Suspends UCART123 Trials after Patient Death (Immuno-Oncology News).
- Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, Wang LL, Han WD. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015 Jan;23(1):184-91. [CrossRef]
- Laszlo GS, Estey EH, Walter RB. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev. 2014;28(4):143–53. [CrossRef]
- Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, Wang LL, Han WD. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015 Jan;23(1):184-91. [CrossRef]
- Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, Gentner B, Gullotta F, Ponzoni M, Bernardi M, Marcatti M, Saudemont A, Bordignon C, Savoldo B, Ciceri F, Naldini L, Dotti G, Bonini C, Bondanza A. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013 Nov 14;122(20):3461-72. [CrossRef]
- Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, Luger SM, Phillips GL. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000 Oct;14(10):1777-84. [CrossRef]
- Geiger M, Stubenrauch KG, Sam J, Richter WF, Jordan G, Eckmann J, Hage C, Nicolini V, Freimoser-Grundschober A, Ritter M, Lauer ME, Stahlberg H, Ringler P, Patel J, Sullivan E, Grau-Richards S, Endres S, Kobold S, Umaña P, Brünker P, Klein C. Protease-activation using anti-idiotypic masks enables tumor specificity of a folate receptor 1-T cell bispecific antibody. Nat Commun. 2020 Jun 24;11(1):3196. [CrossRef]
- Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5(4):311–21. [CrossRef]
- Dias S, Hattori K, Heissig B, Zhu Z, Wu Y, Witte L, Hicklin DJ, Tateno M, Bohlen P, Moore MA, Rafii S. Inhibition of both paracrine and autocrine VEGF/ VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10857-62. [CrossRef]
- Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95(1):309–13.
- Mussai F, De Santo C, Abu-Dayyeh I, Booth S, Quek L, McEwen-Smith RM, Qureshi A, Dazzi F, Vyas P, Cerundolo V. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013 Aug 1;122(5):749-58. [CrossRef]
- Ben-Batalla I, Schultze A, Wroblewski M, Erdmann R, Heuser M, Waizenegger JS, Riecken K, Binder M, Schewe D, Sawall S, Witzke V, Cubas-Cordova M, Janning M, Wellbrock J, Fehse B, Hagel C, Krauter J, Ganser A, Lorens JB, Fiedler W, Carmeliet P, Pantel K, Bokemeyer C, Loges S. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood. 2013 Oct 3;122(14):2443-52. doi: 10.1182/blood-2013-03-491431. [CrossRef]
- Austin R, Smyth MJ, Lane SW. Harnessing the immune system in acute myeloid leukaemia. Crit Rev Oncol Hematol. 2016 Jul;103:62-77. [CrossRef]
- Epperly R, Gottschalk S, Velasquez MP. A Bump in the Road: How the Hostile AML Microenvironment Affects CAR T Cell Therapy. Front Oncol. 2020 Feb 28;10:262. [CrossRef]
- Baragaño Raneros A, López-Larrea C, Suárez-Á lvarez B. Acute Myeloid Leukemia and NK Cells: Two Warriors Confront Each Other. OncoImmunology (2019) 8(2):e1539617.
- Al-Kaabneh B, Frisch B, Aljitawi OS. The Potential Role of 3D In Vitro Acute Myeloid Leukemia Culture Models in Understanding Drug Resistance in Leukemia Stem Cells. Cancers (Basel). 2022 Oct 26;14(21):5252. [CrossRef]
- Mbongue JC, Nicholas DA, Torrez TW, Kim NS, Firek AF, Langridge WH. The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines (Basel). 2015 Sep 10;3(3):703-29. [CrossRef]
- Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, Lacombe J, Armstrong SA, Dührsen U, Frenette PS. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014 Sep 4;15(3):365-375. [CrossRef]
- Lane SW, Wang YJ, Lo Celso C, Ragu C, Bullinger L, Sykes SM, Ferraro F, Shterental S, Lin CP, Gilliland DG, Scadden DT, Armstrong SA, Williams DA. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011 Sep 8;118(10):2849-56. [CrossRef]
- Rickmann M, Macke L, Sundarasetty BS, Stamer K, Figueiredo C, Blasczyk R, Heuser M, Krauter J, Ganser A, Stripecke R. Monitoring dendritic cell and cytokine biomarkers during remission prior to relapse in patients with FLT3-ITD acute myeloid leukemia. Ann Hematol. 2013 Aug;92(8):1079-90. [CrossRef]
- van Galen P, Hovestadt V, Wadsworth Ii MH, Hughes TK, Griffin GK, Battaglia S, Verga JA, Stephansky J, Pastika TJ, Lombardi Story J, Pinkus GS, Pozdnyakova O, Galinsky I, Stone RM, Graubert TA, Shalek AK, Aster JC, Lane AA, Bernstein BE. Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell. 2019 Mar 7;176(6):1265-1281.e24. [CrossRef]
- Kueberuwa, G., Zheng, W., Kalaitsidou, M., Gilham, D.E., Hawkins, R.E. A Syngeneic Mouse B-Cell Lymphoma Model for Pre-Clinical Evaluation of CD19 CAR T Cells. J. Vis. Exp. (140), e58492, doi:10.3791/58492 (2018). [CrossRef]
- Minnie SA, Waltner OG, Ensbey KS, Nemychenkov NS, Schmidt CR, Bhise SS, Legg SRW, Campoy G, Samson LD, Kuns RD, Zhou T, Huck JD, Vuckovic S, Zamora D, Yeh A, Spencer A, Koyama M, Markey KA, Lane SW, Boeckh M, Ring AM, Furlan SN, Hill GR. Depletion of exhausted alloreactive T cells enables targeting of stem-like memory T cells to generate tumor-specific immunity. Sci Immunol. 2022 Oct 21;7(76):eabo3420. [CrossRef]
- Tashiro H, Sauer T, Shum T, Parikh K, Mamonkin M, Omer B, et al. Treatment of acute myeloid leukemia with T cells expressing chimeric antigen receptors directed to c-type lectin-like molecule 1. Mol Ther (2017) 25(9):2202–13. [CrossRef]
- Ataca Atilla P, McKenna MK, Tashiro H, Srinivasan M, Mo F, Watanabe N, Simons BW, McLean Stevens A, Redell MS, Heslop HE, Mamonkin M, Brenner MK, Atilla E. Modulating TNFα activity allows transgenic IL15-Expressing CLL-1 CAR T cells to safely eliminate acute myeloid leukemia. J Immunother Cancer. 2020 Sep;8(2):e001229. [CrossRef]
- Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci U S A. 2019 May 14;116(20):9714-9722. [CrossRef]
- Sauer T, Parikh K, Sharma S, Omer B, Sedloev D, Chen Q, Angenendt L, Schliemann C, Schmitt M, Müller-Tidow C, Gottschalk S, Rooney CM. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood. 2021 Jul 29;138(4):318-330. [CrossRef]
- Leick MB, Silva H, Scarfò I, Larson R, Choi BD, Bouffard AA, Gallagher K, Schmidts A, Bailey SR, Kann MC, Jan M, Wehrli M, Grauwet K, Horick N, Frigault MJ, Maus MV. Non-cleavable hinge enhances avidity and expansion of CAR-T cells for acute myeloid leukemia. Cancer Cell. 2022 May 9;40(5):494-508.e5. [CrossRef]
- Saxena A, Sheridan DP, Card RT, McPeek AM, Mewdell CC, Skinnider LF. Biologic and clinical significance of CD7 expression in acute myeloid leukemia. Am J Hematol. 1998;58(4):278–84.
- Gomes-Silva D, Atilla E, Atilla PA, Mo F, Tashiro H, Srinivasan M, Lulla P, Rouce RH, Cabral JMS, Ramos CA, Brenner MK, Mamonkin M. CD7 CAR T Cells for the Therapy of Acute Myeloid Leukemia. Mol Ther. 2019 Jan 2;27(1):272-280. [CrossRef]
- Trad R, Warda W, Alcazer V, Neto da Rocha M, Berceanu A, Nicod C, Haderbache R, Roussel X, Desbrosses Y, Daguindau E, Renosi F, Roumier C, Bouquet L, Biichle S, Guiot M, Seffar E, Caillot D, Depil S, Robinet E, Salma Y, Deconinck E, Deschamps M, Ferrand C. Chimeric antigen receptor T-cells targeting IL-1RAP: a promising new cellular immunotherapy to treat acute myeloid leukemia. J Immunother Cancer. 2022 Jul;10(7):e004222. [CrossRef]
- Kirkey DC, Loeb AM, Castro S, McKay CN, Perkins L, Pardo L, Leonti AR, Tang TT, Loken MR, Brodersen LE, Loeb KR, Scheinberg DA, Le Q, Meshinchi S. Therapeutic targeting of PRAME with mTCRCAR T cells in acute myeloid leukemia. Blood Adv. 2023 Apr 11;7(7):1178-1189. [CrossRef]
- Jetani H, Navarro-Bailon A, Maucher M, Frenz S, Verbruggen CM, Yeguas A, et al. Siglec-6 Is a Novel Target for CAR T-Cell Therapy in Acute Myeloid Leukemia (AML). Blood (2021) 138(19):1830–42.
- Hofmann A, Gerrits B, Schmidt A, Bock T, Bausch-Fluck D, Aebersold R, Wollscheid B. Proteomic cell surface phenotyping of differentiating acute myeloid leukemia cells. Blood. 2010 Sep 30;116(13):e26-34.
- Köhnke, T., Liu, X., Haubner, S. et al. Integrated multiomic approach for identification of novel immunotherapeutic targets in AML. Biomark Res 10, 43 (2022). [CrossRef]
- Gottschlich, A., Thomas, M., Grünmeier, R. et al. Single-cell transcriptomic atlas-guided development of CAR-T cells for the treatment of acute myeloid leukemia. Nat Biotechnol (2023). [CrossRef]
- Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005. Jun 1;105(11):4247–54. [CrossRef]
- Warda W, Da Rocha MN, Trad R, Haderbache R, Salma Y, Bouquet L, Roussel X, Nicod C, Deschamps M, Ferrand C. Overcoming target epitope masking resistance that can occur on low-antigen-expresser AML blasts after IL-1RAP chimeric antigen receptor T cell therapy using the inducible caspase 9 suicide gene safety switch. Cancer Gene Ther. 2021 Dec;28(12):1365-1375. [CrossRef]
- Tasian SK, Kenderian SS, Shen F, Ruella M, Shestova O, Kozlowski M, et al. Optimized depletion of chimeric antigen receptor T cells in murine xenograft models of human acute myeloid leukemia. Blood (2017) 129(17):2395–407. [CrossRef]
- Simon S, Bugos G, Salter AI, Riddell SR. Synthetic receptors for logic gated T cell recognition and function. Curr Opin Immunol. 2022 Feb;74:9-17. [CrossRef]
- Arcangeli S, Rotiroti MC, Bardelli M, Simonelli L, Magnani CF, Biondi A, et al. Balance of anti-Cd123 chimeric antigen receptor binding affinity and density for the targeting of acute myeloid leukemia. Mol Ther (2017) 25(8):1933–45. [CrossRef]
- Loff S, Dietrich J, Meyer JE, Riewaldt J, Spehr J, von Bonin M, et al. Rapidly switchable universal car-T cells for treatment of Cd123-positive leukemia. Mol Ther Oncol (2020) 17:408–20. [CrossRef]
- Wermke M, Kraus S, Ehninger A, Bargou RC, Goebeler M-E, Middeke JM, et al. Proof of concept for a rapidly switchable universal car-T platform with unicar-T-Cd123 in Relapsed/Refractory aml. Blood (2021) 137(22):3145–8.
- Benmebarek, MR., Cadilha, B.L., Herrmann, M. et al. A modular and controllable T cell therapy platform for acute myeloid leukemia. Leukemia 35, 2243–2257 (2021). [CrossRef]
- Fernández L, Fernández A, Mirones I, Escudero A, Cardoso L, Vela M, Lanzarot D, de Paz R, Leivas A, Gallardo M, Marcos A, Romero AB, Martínez-López J, Pérez-Martínez A. GMP-Compliant Manufacturing of NKG2D CAR Memory T Cells Using CliniMACS Prodigy. Front Immunol. 2019 Oct 10;10:2361. [CrossRef]
- Todd Michael Cooper, Vicky Wu, Ashley Wilson, Jacob Appelbaum, Jordan Jarjour, Josh Gustafson, Stephanie Mgebroff, Catherine Lindgren, Christopher Brown, Michael C. Jensen, Julie R. Park, and Rebecca Alice Gardner. Pediatric and young adult leukemia adoptive therapy (PLAT)-08: A phase 1 study of SC-DARIC33 in pediatric and young adults with relapsed or refractory CD33+AML. Journal of Clinical Oncology 2022 40:16_suppl, TPS7078-TPS7078. [CrossRef]
- Kim MY, Yu KR, Kenderian SS, et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell. 2018;173:1439-53.e19. [CrossRef]
- Liu Y, Wang S, Schubert ML, Lauk A, Yao H, Blank MF, Cui C, Janssen M, Schmidt C, Göllner S, Kleist C, Zhou F, Rahfeld JU, Sauer T, Schmitt M, Müller-Tidow C. CD33-directed immunotherapy with third-generation chimeric antigen receptor T cells and gemtuzumab ozogamicin in intact and CD33-edited acute myeloid leukemia and hematopoietic stem and progenitor cells. Int J Cancer. 2022 Apr 1;150(7):1141-1155. [CrossRef]
- Kailayangiri S, Altvater B, Wiebel M, Jamitzky S, Rossig C. Overcoming Heterogeneity of Antigen Expression for Effective CAR T Cell Targeting of Cancers. Cancers (Basel). 2020 Apr 26;12(5):1075. [CrossRef]
- Schneider D, Xiong Y, Wu D, Hu P, Alabanza L, Steimle B, Mahmud H, Anthony-Gonda K, Krueger W, Zhu Z, Dimitrov DS, Orentas RJ, Dropulić B. Trispecific CD19-CD20-CD22-targeting duoCAR-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models. Sci Transl Med. 2021 Mar 24;13(586):eabc6401.
- Atilla PA, McKenna MK, Watanabe N, Mamonkin M, Brenner MK, Atilla E. Combinatorial antigen targeting strategies for acute leukemia: application in myeloid malignancy. Cytotherapy. 2022 Mar;24(3):282-290. [CrossRef]
- Damiani D, Tiribelli M. Present and Future Role of Immune Targets in Acute Myeloid Leukemia. Cancers (Basel). 2022 Dec 30;15(1):253.
- Xiong W, Chen Y, Kang X, Chen Z, Zheng P, Hsu YH, et al. Immunological Synapse Predicts Effectiveness of Chimeric Antigen Receptor Cells. Mol Ther 2018;26 (4):963–75. [CrossRef]
- Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, et al. Treatment of CD33- directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther 2015;23(1):184– 91. [CrossRef]
- Petrov JC, Wada M, Pinz KG, Yan LE, Chen KH, Shuai X, et al. Compound CAR Tcells as a double-pronged approach for treating acute myeloid leukemia. Leukemia 2018;32(6):1317–26. [CrossRef]
- Liu, Fang & Cao, Yuanzhen & Pinz, Kevin & Ma, Yu & Wada, Masayuki & Chen, Kevin & Ma, Gina & Shen, Jiaqi & Tse, Charlotte & Su, Yi & Xiong, Yisong & He, Guangcui & Li, Yecheng & Ma, Yupo. (2018). First-in-Human CLL1-CD33 Compound CAR T Cell Therapy Induces Complete Remission in Patients with Refractory Acute Myeloid Leukemia: Update on Phase 1 Clinical Trial. Blood. 132. 901-901. 10.1182/blood-2018-99-110579. [CrossRef]
- Zhang H, Bu C, Pen Z, et al. The efficacy and safety of anti-CLL1 based CAR-T cells in children with relapsed or refractory acute myeloid leukemia: A multicenter interim analysis. J Clin Oncol. 2021;39(suppl 15):10000.
- Cartellieri M, Feldmann A, Koristka S, Arndt C, Loff S, Ehninger AV, et al. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6:e458. [CrossRef]
- Sascha Haubner, Jorge Mansilla-Soto, Sarah Nataraj, Friederike Kogel, Qing Chang, Elisa De Stanchina, Kathryn Fraser, Jae H Park, Xiuyan Wang, Isabelle Rivière, Michel Sadelain; Target Densities in Malignant and Normal Cells Determine CAR T Cell Efficacy and Off-Target Hematotoxicity. Blood 2022; 140 (Supplement 1): 869–870.
- Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18:215–29. [CrossRef]
- Blankenstein T, Leisegang M, Uckert W, Schreiber H. Targeting cancerspecifc mutations by T cell receptor gene therapy. Curr Opin Immunol. 2015;33:112–9.
- Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, Kriley IR, Rosenberg SA. T-Cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375:2255–62. [CrossRef]
- Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, Pigneux A, Wetzler M, Stuart RK, Erba HP, Damon LE, Powell BL, Lindeman N, Steensma DP, Wadleigh M, DeAngelo DJ, Neuberg D, Stone RM, Ebert BL. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015 Feb 26;125(9):1367-76. [CrossRef]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, Utikal J, Hassel JC, Weide B, Kaehler KC, Loquai C, Mohr P, Gutzmer R, Dummer R, Gabriel S, Wu CJ, Schadendorf D, Garraway LA. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015 Oct 9;350(6257):207-211. [CrossRef]
- Blankenstein T, Leisegang M, Uckert W, Schreiber H. Targeting cancerspecific mutations by T cell receptor gene therapy. Curr Opin Immunol (2015) 33:112–9. [CrossRef]
- Ehx G, Larouche JD, Durette C, Laverdure JP, Hesnard L, Vincent K, Hardy MP, Theriault C, Rulleau C, Lanoix J, Bonneil E, Feghaly A, Apavaloaei A, Noronha N, Laumont CM, Delisle JS, Vago L, Hebert J, Sauvageau G, Lemieux S, Thibault P, Perreault C. Atypical acute myeloid leukemiaspecifc transcripts generate shared and immunogenic MHC class-Iassociated epitopes. Immunity. 2021;54:737–52.
- Biernacki MA, Foster KA, Woodward KB, Coon ME, Cummings C, Cunningham TM, Dossa RG, Brault M, Stokke J, Olsen TM, Gardner K, Estey E, Meshinchi S, Rongvaux A, Bleakley M. CBFB-MYH11 fusion neoantigen enables T cell recognition and killing of acute myeloid leukemia. J Clin Invest. 2020;130:5127–41. [CrossRef]
- Cohen CJ, Gartner JJ, Horovitz-Fried M, Shamalov K, Trebska-McGowan K, Bliskovsky VV, Parkhurst MR, Ankri C, Prickett TD, Crystal JS, Li YF, El-Gamil M, Rosenberg SA, Robbins PF. Isolation of neoantigenspecifc T cells from tumor and peripheral lymphocytes. J Clin Invest. 2015;125:3981–91.
- van der Lee DI, Reijmers RM, Honders MW, Hagedoorn RS, de Jong RC, Kester MG, van der Steen DM, de Ru AH, Kweekel C, Bijen HM, Jedema I, Veelken H, van Veelen PA, Heemskerk MH, Falkenburg J, Grifoen M. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J Clin Invest. 2019;129:774–85. [CrossRef]
- Graf C, Heidel F, Tenzer S, Radsak MP, Solem FK, Britten CM, Huber C, Fischer T, Wolfel T. A neoepitope generated by an FLT3 internal tandem duplication (FLT3-ITD) is recognized by leukemia-reactive autologous CD8+ T cells. Blood. 2007;109:2985–8. [CrossRef]
- Harris DT, Kranz DM. Adoptive T Cell Therapies: A Comparison of T Cell Receptors and Chimeric Antigen Receptors. Trends Pharmacol Sci (2016) 37:220–30. [CrossRef]
- Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–46. [CrossRef]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62. [CrossRef]
- Kang S, Li Y, Qiao J, Meng X, He Z, Gao X, Yu L. Antigen-Specific TCR-T Cells for Acute Myeloid Leukemia: State of the Art and Challenges. Front Oncol. 2022 Mar 9;12:787108. [CrossRef]
- Xue S, Gillmore R, Downs A, et al. Exploiting T cell receptor genes for cancer immunotherapy. Clin Exp Immunol 2005;139:167–72. [CrossRef]
- Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–86. [CrossRef]
- Van Loenen MM, de Boer R, Hagedoorn RS, van Egmond EH, Falkenburg JH, Heemskerk MH. Optimization of the HA-1-specific T-cell receptor for gene therapy of hematologic malignancies. Haematologica. (2011) 96:477–81. [CrossRef]
- Poorebrahim, M.; Mohammadkhani, N.; Mahmoudi, R.; Gholizadeh, M.; Fakhr, E.; Cid-Arregui, A. TCR-like CARs and TCR-CARs targeting neoepitopes: An emerging potential. Cancer Gene Ther. 2021, 28, 581–589. [CrossRef]
- Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG, Voillet V, Gottardo R, Ragnarsson GB, Bleakley M, Yeung CC, Muhlhauser P, Nguyen HN, Kropp LA, Castelli L, Wagener F, Hunter D, Lindberg M, Cohen K, Seese A, McElrath MJ, Duerkopp N, Gooley TA, Greenberg PD. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019;25:1064–72. [CrossRef]
- Tawara I, Kageyama S, Miyahara Y, Fujiwara H, Nishida T, Akatsuka Y, Ikeda H, Tanimoto K, Terakura S, Murata M, Inaguma Y, Masuya M, Inoue N, Kidokoro T, Okamoto S, Tomura D, Chono H, Nukaya I, Mineno J, Naoe T, Emi N, Yasukawa M, Katayama N, Shiku H. Safety and persistence of WT1-specifc T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood. 2017;130:1985–94.
- Amir AL, van der Steen DM, van Loenen MM, Hagedoorn RS, de Boer R, Kester MD, de Ru AH, Lugthart GJ, van Kooten C, Hiemstra PS, Jedema I, Grifoen M, van Veelen PA, Falkenburg JH, Heemskerk MH. PRAMEspecifc Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin Cancer Res. 2011;17:5615–25.
- Marijt WA, Heemskerk MH, Kloosterboer FM, Goulmy E, Kester MG, van der Hoorn MA, van Luxemburg-Heys SA, Hoogeboom M, Mutis T, Drijfhout JW, van Rood JJ, Willemze R, Falkenburg JH. Hematopoiesisrestricted minor histocompatibility antigens HA-1- or HA-2-specifc T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci U S A. 2003;100:2742.
- Tawara I, Kageyama S, Miyahara Y, Fujiwara H, Nishida T, Akatsuka Y, Ikeda H, Tanimoto K, Terakura S, Murata M, Inaguma Y, Masuya M, Inoue N, Kidokoro T, Okamoto S, Tomura D, Chono H, Nukaya I, Mineno J, Naoe T, Emi N, Yasukawa M, Katayama N, Shiku H. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood. 2017 Nov 2;130(18):1985-1994. [CrossRef]
- Morris EC, Tendeiro-Rego R, Richardson R, Fox TA, Sillito F, Holler A, et al. A Phase I Study Evaluating the Safety and Persistence of Allorestricted WT1- TCR Gene Modified Autologous T Cells in Patients With High-Risk Myeloid Malignancies Unsuitable for Allogeneic Stem Cell Transplantation. Blood (2019) 134:1367–7. Doi: 10.1182/blood-2019-128044). [CrossRef]
- van Balen P, Jedema I, van Loenen MM, de Boer R, van Egmond HM, Hagedoorn RS, Hoogstaten C, Veld SAJ, Hageman L, van Liempt PAG, Zwaginga JJ, Meij P, Veelken H, Falkenburg JHF, Heemskerk MHM. HA-1H T-Cell Receptor Gene Transfer to Redirect Virus-Specific T Cells for Treatment of Hematological Malignancies After Allogeneic Stem Cell Transplantation: A Phase 1 Clinical Study. Front Immunol. 2020 Aug 20;11:1804.
- T. Bald, M. F. Krummel, M. J. Smyth, K. C. Barry, The NK cell-cancer cycle: Advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 21, 835–847 (2020). [CrossRef]
- S. Sivori et al., NK cells and ILCs in tumor immunotherapy. Mol. Aspects Med. 80, 100870 (2021). [CrossRef]
- Jennifer A. Foltz, Melissa M. Berrien-Elliott, David A. Russler-Germain, Carly C. Neal, Jennifer Tran, Margery Gang, Pamela Wong, Matthew Mosior, Jeffrey J. Bednarski, Clare Zimmerman, Celia C. Cubitt, Nancy D. Marin, Alice Y. Zhou, Miriam T. Jacobs, Mark Foster, Timothy Schappe, Ethan McClain, Sweta Desai, Patrick Pence, Michelle Becker-Hapak, Lynne Marsala, Obi L. Griffith, Malachi Griffith, Saad M. Khan, Bryan Fisk, Amanda F. Cashen, Allegra A. Petti, Todd A. Fehniger; Cytokine-Induced Memory-like NK Cells Have a Distinct Single Cell Transcriptional Profile and Persist for Months in Adult and Pediatric Leukemia Patients after Adoptive Transfer. Blood 2021; 138 (Supplement 1): 3825.
- R. Romee et al., Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 8, 357ra123 (2016). [CrossRef]
- Dong H, Ham JD, Hu G, Xie G, Vergara J, Liang Y, Ali A, Tarannum M, Donner H, Baginska J, Abdulhamid Y, Dinh K, Soiffer RJ, Ritz J, Glimcher LH, Chen J, Romee R. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc Natl Acad Sci U S A. 2022 Jun 21;119(25):e2122379119. [CrossRef]
- Pomeroy, E.J.; Hunzeker, J.T.; Kluesner, M.G.; Lahr, W.S.; Smeester, B.A.; Crosby, M.R.; Lonetree, C.-l.; Yamamoto, K.; Bendzick, L.; Miller, J.S.; et al. A Genetically Engineered Primary Human Natural Killer Cell Platform for Cancer Immunotherapy. Mol. Ther. 2020, 28, 52–63). [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [CrossRef]
- Albinger, N., Pfeifer, R., Nitsche, M. et al. Primary CD33-targeting CAR-NK cells for the treatment of acute myeloid leukemia. Blood Cancer J. 12, 61 (2022). [CrossRef]
- Christodoulou I, Ho WJ, Marple A, et al Engineering CAR-NK cells to secrete IL-15 sustains their anti-AML functionality but is associated with systemic toxicities Journal for ImmunoTherapy of Cancer 2021;9:e003894. ).
- Anthony G. Mansour, Kun-Yu Teng, Zhiyao Li, Zheng Zu, Hanyu Chen, Aliya Ali, Jianying Zhang, Ting Lu, Shoubao Ma, Michael A. Caligiuri, Jianhua Yu; Abstract LB102: Off-the-shelf cord blood FLT3 CAR-NK cells for immunotherapy of acute myeloid leukemia. Cancer Res 15 June 2022; 82 (12_Supplement): LB102.
- Ruihao Huang, Qin Wen, Xiaoqi Wang, Hongju Yan, Yingying Ma, Wang Mai-Hong, Xiao Han, Li Gao, Lei Gao, Cheng Zhang, Xi Zhang; Off-the-Shelf CD33 CAR-NK Cell Therapy for Relapse/Refractory AML: First-in-Human, Phase I Trial. Blood 2022; 140 (Supplement 1): 7450–7451.).
- You, L.; Han, Q.; Zhu, L.; Zhu, Y.; Bao, C.; Yang, C.; Lei, W.; Qian, W. Decitabine-Mediated Epigenetic Reprograming Enhances Anti-leukemia Efficacy of CD123-Targeted Chimeric Antigen Receptor T-Cells. Front. Immunol. 2020, 11, 1787. [CrossRef]
- Guzman ML, Sugita M, Zong H, Ewing-Crystal N, Trujillo-Alonso V, et al. Allogeneic Tcra/b Deficient CAR T-Cells Targeting CD123 Prolong Overall Survival of AML Patient-Derived Xenografts. Blood (2016) 128 (22):765.
- Cui, Q., Qian, C., Xu, N. et al. CD38-directed CAR-T cell therapy: a novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol 14, 82 (2021). [CrossRef]
- Sugita, M., Galetto, R., Zong, H. et al. Allogeneic TCRαβ deficient CAR T-cells targeting CD123 in acute myeloid leukemia. Nat Commun 13, 2227 (2022). [CrossRef]
- Celectis Reports Clinical Hold of UCART 123 Studies, published on September 04, 2017, New York (N.Y.), https://www.cellectis.com/en/press/cellectis-reports-clinical-hold-of-ucart123-studies.
- Hudecek M, Ivics Z. Non-viral therapeutic cell engineering with the Sleeping Beauty transposon system. Curr Opin Genet Dev 2018;52:100–8. https://doi.org/ 10.1016/j.gde.2018.06.003. [CrossRef]
- Costello CL, Gregory TK, Ali SA, Berdeja JG, Patel KK, Shah ND, et al. Phase 2 Study of the Response and Safety of P-Bcma-101 CAR-T Cells in Patients with Relapsed/ Refractory (r/r) Multiple Myeloma (MM) (PRIME). Blood 2019;134:3184. https:// doi.org/10.1182/blood-2019-129562.
- Magnani CF, Gaipa G, Lussana F, Belotti D, Gritti G, Napolitano S, et al. Sleeping Beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J Clin Invest 2020;130:6021–33. [CrossRef]
- Prommersberger S, Reiser M, Beckmann J, Danhof S, Amberger M, Quade-Lyssy P, et al. CARAMBA: a first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma. Gene Ther 2021;28:560. [CrossRef]
- Gurney M, O’Reilly E, Corcoran S, Brophy S, Krawczyk J, Otto NM, Hermanson DL, Childs RW, Szegezdi E, O’Dwyer ME. Concurrent transposon engineering and CRISPR/Cas9 genome editing of primary CLL-1 chimeric antigen receptor-natural killer cells. Cytotherapy. 2022 Nov;24(11):1087-1094. Doi: 10.1016/j.jcyt.2022.07.008. [CrossRef]
- Daver N, Basu S, Garcia-Manero G, Cortes J, Ravandi E, Jabbour E, et al. Phase IB/II study of nivolumab in combination wth azacytidine in patients with relapsed acute myeloid leukemia. Blood. (2016) 22:763. doi: 10.1182/blood.V128.22.763.763 39. [CrossRef]
- Davids M, Kim H, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. (2016) 375:143–53. doi: 10.1056/NEJMoa1601202. [CrossRef]
- Kadia T, Cortes J, Ghorab A, Ravandi F, Jabbour E, Daver N, et al. Nivolumab maintenance in high-risk acute myeloid leukemia patients. J Clin Oncol. (2018) 36:15. doi: 10.1200/JCO.2018.36.15_suppl.7014. [CrossRef]
- Epperly R, Gottschalk S, Velasquez MP. A Bump in the Road: How the Hostile AML Microenvironment Affects CAR T Cell Therapy. Front Oncol. 2020 Feb 28;10:262.). [CrossRef]
- Jitschin R, Saul D, Braun M, Tohumeken S, Volkl S, Kischel R, et al. CD33/CD3-bispecific T-cell engaging (BiTE R ) antibody construct targets monocytic AML myeloid-derived suppressor cells. J Immunother Cancer. (2018) 6:116. doi: 10.1186/s40425-018-0432-9). [CrossRef]
- Zhou Q, Bucher C, Munger M, Highfill S, Tolar J, Munn D, et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. (2009) 114:3793–802. doi: 10.1182/blood-2009-03-208181. [CrossRef]
- Suryadevara C, Desai R, Farber S, Choi B, Swartz A, Shen S, et al. Preventing Lck activation in CAR T cells confers Treg resistance but requires 4-1BB signaling for them to persist and treat solid tumors in nonlymphodepleted hosts. Clin Cancer Res. (2019) 25:358– 68. doi: 10.1158/1078-0432.CCR-18-1211. [CrossRef]
- Ninomiya S, Narala N, Huye L, Yagyu S, Savoldo B, Dotti G, Heslop HE, Brenner MK, Rooney CM, Ramos CA. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015 Jun 18;125(25):3905-16. [CrossRef]
- Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, Courtois R, Déjou C, Jecko D, Becquart O, Rispaud-Blanc H, Gauthier L, Rossi B, Chanteux S, Gourdin N, Amigues B, Roussel A, Bensussan A, Eliaou JF, Bastid J, Romagné F, Morel Y, Narni-Mancinelli E, Vivier E, Paturel C, Bonnefoy N. Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell Rep. 2019 May 21;27(8):2411-2425.e9.
- Minnie SA, Waltner OG, Ensbey KS, Nemychenkov NS, Schmidt CR, Bhise SS, Legg SRW, Campoy G, Samson LD, Kuns RD, Zhou T, Huck JD, Vuckovic S, Zamora D, Yeh A, Spencer A, Koyama M, Markey KA, Lane SW, Boeckh M, Ring AM, Furlan SN, Hill GR. Depletion of exhausted alloreactive T cells enables targeting of stem-like memory T cells to generate tumor-specific immunity. Sci Immunol. 2022 Oct 21;7(76):eabo3420. doi: 10.1126/sciimmunol.abo3420. [CrossRef]
- Antonio Pierini, Loredana Ruggeri, Alessandra Carotti, Franca Falzetti, Simonetta Saldi, Adelmo Terenzi, Claudio Zucchetti, Gianluca Ingrosso, Tiziana Zei, Roberta Iacucci Ostini, Sara Piccinelli, Samanta Bonato, Sara Tricarico, Antonella Mancusi, Sara Ciardelli, Roberto Limongello, Mara Merluzzi, Mauro Di Ianni, Rita Tognellini, Olivia Minelli, Cristina Mecucci, Maria Paola Martelli, Brunangelo Falini, Massimo Fabrizio Martelli, Cynthia Aristei, Andrea Velardi; Haploidentical age-adapted myeloablative transplant and regulatory and effector T cells for acute myeloid leukemia. Blood Adv 2021; 5 (5): 1199–1208.
- Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X, Lin YH, et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 2017;31:2587–93. [CrossRef]
- Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood 2019;133:1652–63. [CrossRef]
- Carlos A. Ramos. Should CD19 CAR-T Cells for ALL be Followed by Allogeneic Stem Cell Transplant? Transplantation and Cellular Therapy, Volume 28, Issue 1, 2022, Pages 1-2, ISSN 2666-6367.
- Summers C,Wu QV, Annesley C, et al.Hematopoietic Cell Transplantation after CD19 Chimeric Antigen Receptor T Cell-Induced Acute Lymphoblastic Lymphoma Remission Confers a Leukemia-Free Survival Advantage.Transplant Cell Ther. 2021; 28: 19-27.
- Zhang, H., Bu, C., Peng, Z. et al. Characteristics of anti-CLL1 based CAR-T therapy for children with relapsed or refractory acute myeloid leukemia: the multi-center efficacy and safety interim analysis. Leukemia 36, 2596–2604 (2022). [CrossRef]
- Yao S, Jianlin C, Yarong L, Botao L, Qinghan W, Hongliang F, Lu Z, Hongmei N, Pin W, Hu C, Liangding H, Bin Z. Donor-Derived CD123-Targeted CAR T Cell Serves as a RIC Regimen for Haploidentical Transplantation in a Patient With FUS-ERG+ AML. Front Oncol. 2019 Dec 3;9:1358. [CrossRef]
- Zhang, H., Bu, C., Peng, Z. et al. Characteristics of anti-CLL1 based CAR-T therapy for children with relapsed or refractory acute myeloid leukemia: the multi-center efficacy and safety interim analysis. Leukemia 36, 2596–2604 (2022). [CrossRef]
- Budde L, Song JY, Kim Y, Blanchard S, Wagner J, Stein AS, et al. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasmfollowing treatmentwith Cd123- specific car T cells: A first-in-Human clinical trial. Blood (2017) 130(Suppl_1):811–. Doi: 10.1182/ blood.V130.Suppl_1.811.811. [CrossRef]
- Tambaro FP, Singh H, Jones E, Rytting M, Mahadeo KM, Thompson P, Daver N, DiNardo C, Kadia T, Garcia-Manero G, Chan T, Shah RR, Wierda WG. Autologous CD33-CAR-T cells for treatment of relapsed/refractory acute myelogenous leukemia. Leukemia. 2021 Nov;35(11):3282-3286. [CrossRef]
- Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, et al. Treatment of CD33-Directed Chimeric Antigen Receptor-Modified T Cells in One Patient With Relapsed and Refractory Acute Myeloid Leukemia. Mol Ther (2015) 23 (1):184–91. [CrossRef]
- Cui, Q., Qian, C., Xu, N. et al. CD38-directed CAR-T cell therapy: a novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol 14, 82 (2021). [CrossRef]
- Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, et al. Persistence and Efficacy of Second Generation CAR T Cell Against the LeY Antigen in Acute Myeloid Leukemia. Mol Ther (2013) 21(11):2122–9. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





