Submitted:
17 April 2023
Posted:
17 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of bacterial cultures
2.2. Preparation of nutrient solution
2.3. Inoculation of nutrient solution
2.4. Sample collection and bacterial quantification
2.5. Data analysis
3. Results
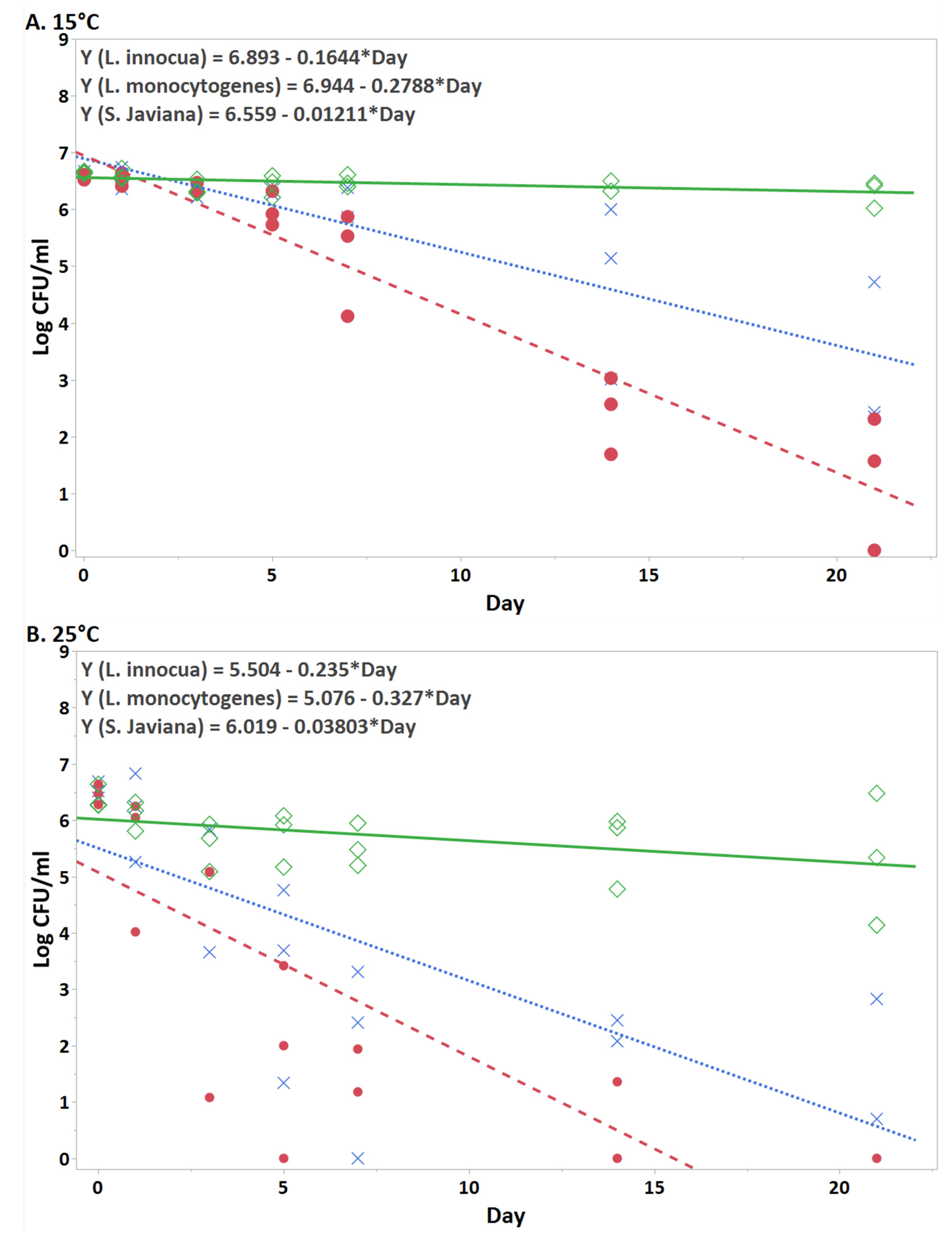
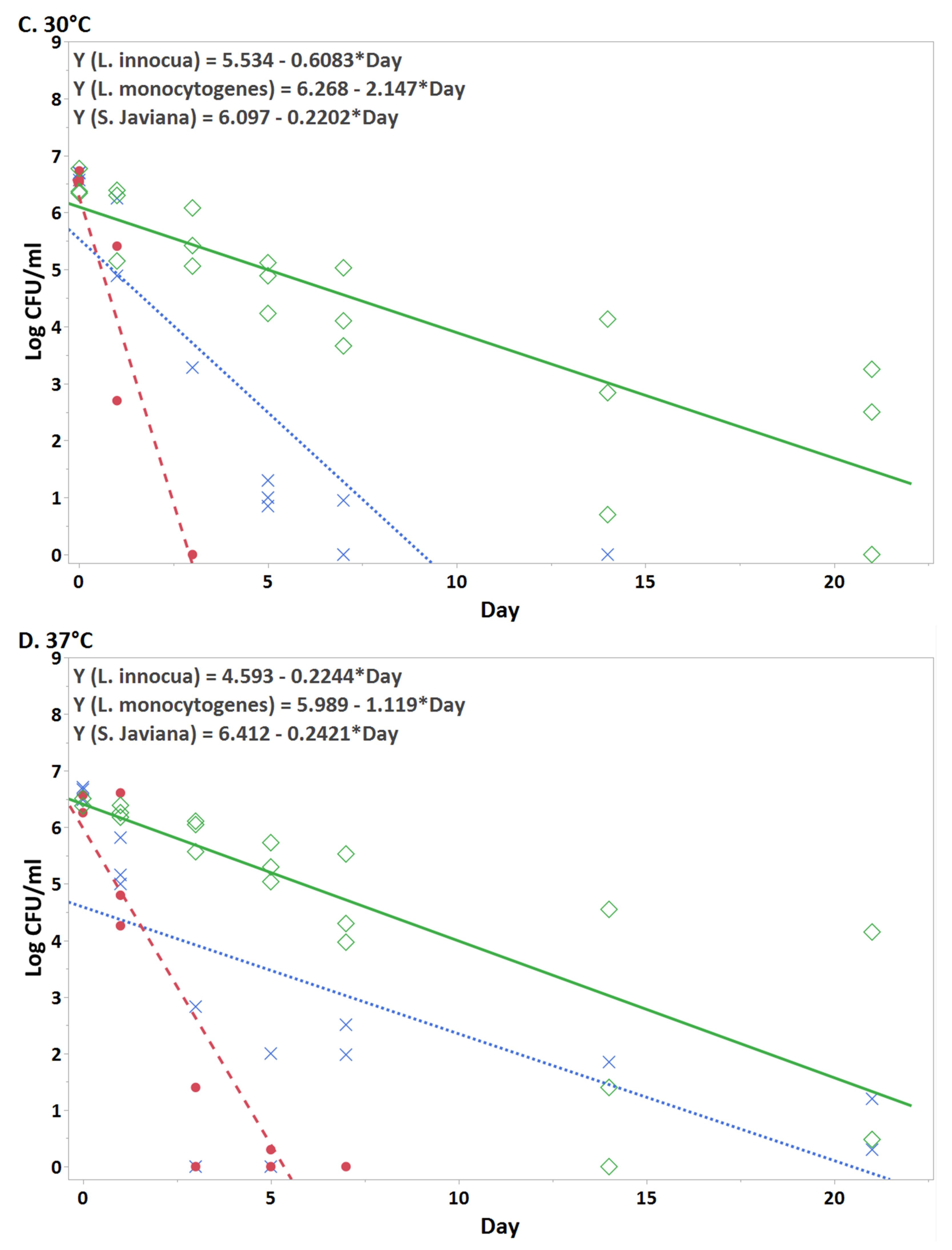
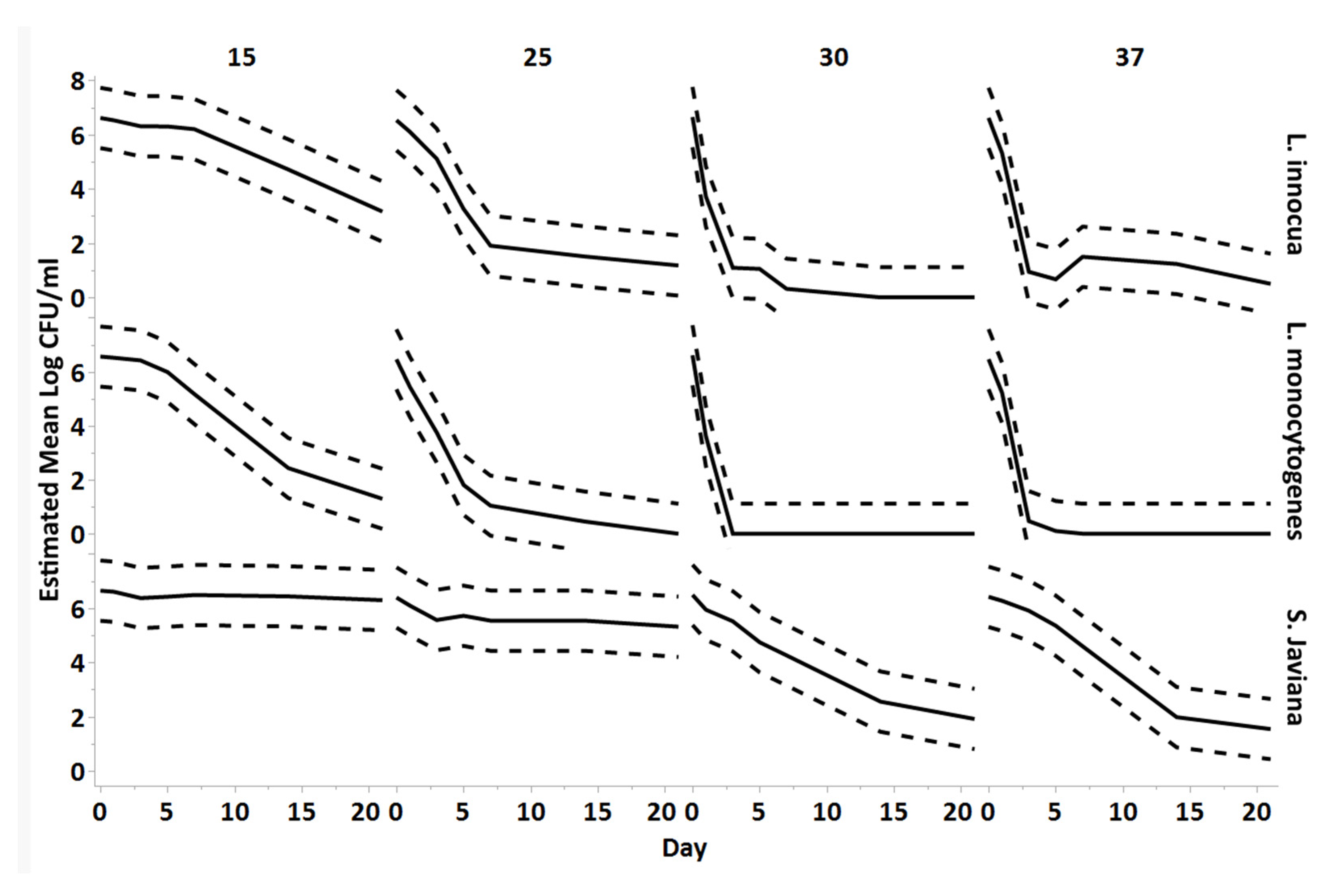
3.1. Persistence of bacteria in hydroponic nutient solution at each temperature
3.2. Estimated decimal reduction values of bacteria in hydroponic nutrient solution by temperature (in days)
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture, Sustainability: Science, Practice and Policy 2017, vol. 13, no. 1, pp. 13-26. [CrossRef]
- Wang, S.; Adekunle, A.; Raghavan, V. Exploring the integration of bioelectrochemical systems and hydroponics: Possibilities, challenges, and innovations, Journal of Cleaner Production 2022, vol. 366, p. 132855. [CrossRef]
- U.S. Centers for Disease Control and Prevention (CDC). Salmonella outbreak linked to BrightFarms packaged salad greens. https://www.cdc.gov/salmonella/typhimurium-07-21/index.html (accessed 17 November 2022).
- Lenzi, A.; Marvasi, M.; Baldi, A. Agronomic practices to limit pre- and post-harvest contamination and proliferation of human pathogenic Enterobacteriaceae in vegetable produce, Food Control 2021 vol. 119, p. 107486. [CrossRef]
- Franz, E.; Visser, A.; Vandiepeningen, A.; Klerks, M.; Termorshuizen, A.; Vanbruggen, A. Quantification of contamination of lettuce by GFP-expressing Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium, Food Microbiology 2007, vol. 24, no. 1, pp. 106-112. [CrossRef]
- Warriner, K.; Huber, A.; Namvar, A.; Fan, W.; Dunfield, K. Chapter 4 Recent advances in the microbial safety of fresh fruits and vegetables, Advances in Food and Nutrition Research 2009 vol. 57: Academic Press, pp. 155-208. [CrossRef]
- Alegbeleye, O. O.; Singleton, I.; Sant'Ana, A. S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review, Food Microbiol 2018, vol. 73, pp. 177-208. [CrossRef]
- Yang, Z. H.; Chambers, H.; DiCaprio, E.; Gao, G.; Li, J. R. Internalization and dissemination of human norovirus and Tulane virus in fresh produce is plant dependent, Food Microbiology 2018, vol. 69, pp. 25-32. [CrossRef]
- Jablasone, J.; Warriner, K.; Griffiths, M. Interactions of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes plants cultivated in a gnotobiotic system, Int J Food Microbiol 2005, vol. 99, no. 1, pp. 7-18. [CrossRef]
- Erickson,M. C. Internalization of fresh produce by foodborne pathogens, Annual Review of Food Science and Technology 2012, vol. 3, no. 1, pp. 283-310. [CrossRef]
- Li, D.; Uyttendaele, M. Potential of Human Norovirus surrogates and Salmonella enterica contamination of pre-harvest Basil (Ocimum basilicum) via leaf surface and plant substrate, Frontiers in Microbiology 2018, vol. 9, Art no. 1728. [CrossRef]
- Standing, T.-A.; Du Plessis, E.; Duvenage, S.; Korsten, L. Internalization potential of Escherichia coli O157:H7, Listeria monocytogenes, Salmonella enterica subsp. enterica serovar Typhimurium and Staphylococcus aureus in lettuce seedlings and mature plants, Journal of Water and Health 2013, vol. 11, no. 2, pp. 210-223. [CrossRef]
- Xylia, P.; Chrysargyris, A.; Botsaris, G.; Skandamis, P.; Tzortzakis, N. Salmonella Enteritidis survival in different temperatures and nutrient solution pH levels in hydroponically grown lettuce, Food Microbiology 2022, vol. 102, p. 103898. [CrossRef]
- Settanni, L.; Miceli, A.; Francesca, N.; Cruciata, M.; Moschetti, G. Microbiological investigation of Raphanus sativus L. grown hydroponically in nutrient solutions contaminated with spoilage and pathogenic bacteria, International Journal of Food Microbiology 2013, vol. 160, no. 3, pp. 344-352. [CrossRef]
- Lopez-Galvez, F.; Allende, A.; Pedrero-Salcedo, F.; Alarcon, J. J.; Gil, M. I. Safety assessment of greenhouse hydroponic tomatoes irrigated with reclaimed and surface water," International Journal of Food Microbiology 2014, vol. 191, pp. 97-102. [CrossRef]
- Lopez-Galvez, F.; Gil, M. I.; Pedrero-Salcedo, F. Alarcón, J. J.; Allende, A. Monitoring generic Escherichia coli in reclaimed and surface water used in hydroponically cultivated greenhouse peppers and the influence of fertilizer solutions, Food Control 2016, vol. 67, pp. 90-95. [CrossRef]
- Harrand, A. S.; Kovac, J.; Carroll, L. M.; Guariglia-Oropeza, V.; Kent, D. J.; Wiedmann, M. Assembly and characterization of a pathogen strain collection for produce safety applications: Pre-growth conditions have a larger effect on peroxyacetic acid tolerance than strain diversity. Frontiers in Microbiology, 2019, vol. 10, 1223. [CrossRef]
- Pu, S. H.; Beaulieu, J. C.; Prinyawiwatkul, W.; Ge, B. L. Effects of plant maturity and growth media bacterial inoculum level on the surface contamination and internalization of Escherichia coli O157:H7 in growing spinach leaves, Journal of Food Protection 2009, vol. 72, no. 11, pp. 2313-2320. [CrossRef]
- Hirneisen K. A.; Kniel, K. E.; Comparative uptake of enteric viruses into spinach and green onions," Food and Environmental Virology 2013, vol. 5, no. 1, pp. 24-34. [CrossRef]
- Resh, H. M. Hydroponic food production: A definitive guidebook for the advanced home gardener and the commercial hydroponic grower, 7th ed. Boca Raton, Fla: CRC Press, 2013.
- Stokes, J. L. Nutrition of microorganisms, Annu Rev Microbiol. 1952, vol. 6, pp. 29-48. [CrossRef]
- Shaw, A.; Helterbran, K.; Evans, M. R.; Currey, C. Growth of Escherichia coli O157:H7, Non-O157 Shiga Toxin-Producing Escherichia coli , and Salmonella in water and hydroponic fertilizer solutions, J Food Prot 2016, vol. 79, no. 12, pp. 2179-2183. [CrossRef]
- Dong, M.; Feng, H.; Microbial community analysis and food safety practice survey-based hazard identification and risk assessment for controlled environment hydroponic/aquaponic farming systems, Front Microbiol 2022, vol. 13, p. 879260. [CrossRef]
- Cooley, M.; Chao, B. D.; Mandrell, R. E. Escherichia coli O157:H7 survival and growth on lettuce is altered by the presence of epiphytic bacteria, Journal of Food Protection 2006, vol. 69, no. 10, pp. 2329-2335. [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J. M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms, FEMS Microbiology Reviews 2013, vol. 37, no. 5, pp. 634-663. [CrossRef]
- U.S. Food and Drug Administration (FDA). Revolution Farms, LLC Announces Expanded Recall of Lettuce Due to Possible Health Risk. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/revolution-farms-llc-announces-expanded-recall-lettuce-due-possible-health-risk?permalink=4B15E7436E5517838E7BDA8780FDD85D1CA25C867076E853C93222EE111AC0F2 (Accessed 15 April 2023).
| Stock Solution | Fertilizer salts | Conc. (grams/L) | |
| Macronutrients* | A |
Calcium nitrate Potassium nitrate Mono potassium phosphate Magnesium sulfate Potassium chloride |
208.75 65.00 28.49 100.63 27.50 |
| B | |||
| Micronutrients† | C | Fe-EDTA Mn-EDTA Boric acid Copper sulfate Zinc sulfate Sodium ammonium molybdate |
3.00 1.50 0.50 0.38 0.28 0.05 |
| Day | L. innocua | L. monocytogenes | S. Javiana |
| 0 | 6.62 ± 0.04 | 6.56 ± 0.05 | 6.65 ± 0.02 |
| 1 | 6.53 ± 0.19 | 6.52 ± 0.12 | 6.61 ± 0.10 |
| 3 | 6.31 ± 0.09 | 6.42 ± 0.09 | 6.38 ± 0.13 |
| 5 | 6.31 ± 0.06 | 5.99 ± 0.30 | 6.43 ± 0.20 |
| 7 | 6.21 ± 0.30 | 5.17 ± 0.93 | 6.49 ± 0.11 |
| 14 | 4.72 ± 1.54 | 2.30 ± 0.90 | 6.44 ± 0.10 |
| 21 | 2.24 ± 2.37 | 0.77 ± 1.33 | 6.30 ± 0.24 |
| Day | L. innocua | L. monocytogenes | S. Javiana |
| 0 | 6.53 ± 0.15 | 6.46 ± 0.18 | 6.40 ± 0.21 |
| 1 | 6.11 ± 0.79 | 5.44 ± 1.23 | 6.10 ± 0.26 |
| 3 | 5.11 ± 1.26 | 3.39 ± 2.94 | 5.57 ±0.43 |
| 5 | 3.21± 1.84 | 1.81 ± 1.72 | 5.72 ± 0.49 |
| 7 | 1.91 ± 1.71 | 0.53 ± 0.92 | 5.55 ± 0.38 |
| 14 | 1.51 ± 1.32 | ND | 5.54 ± 0.66 |
| 21 | 0.94 ± 1.63 | ND | 5.32 ± 1.17 |
| Day | L. innocua | L. monocytogenes | S. Javiana |
| 0 | 6.65 ± 0.07 | 6.62 ± 0.10 | 6.49 ± 0.24 |
| 1 | 3.71 ± 3.29 | 3.60 ± 1.56 | 6.35 ± 0.06 |
| 3 | 1.09 ± 1.89 | ND | 5.75 ± 0.47 |
| 5 | 0.43 ± 0.75 | ND | 5.00 ± 0.16 |
| 7 | ND | ND | 4.56 ± 0.66 |
| 14 | ND | ND | 2.42 ± 2.43 |
| 21 | ND | ND | 1.62 ± 2.30 |
| Day | L. innocua | L. monocytogenes | S. Javiana |
| 0 | 6.62 ± 0.12 | 6.46 ± 0.23 | 6.42 ± 0.08 |
| 1 | 5.33 ± 0.44 | 5.22 ± 0.38 | 6.28 ± 0.11 |
| 3 | 0.94 ± 1.64 | 0.47 ± 0.99 | 5.91 ± 0.30 |
| 5 | 0.67 ± 1.15 | ND | 5.36 ± 0.35 |
| 7 | 1.50 ± 1.32 | ND | 4.60 ± 0.82 |
| 14 | 1.23 ± 1.07 | ND | 1.98 ± 2.33 |
| 21 | ND | ND | 1.38 ± 2.40 |
| Decimal Reduction Values (in Days) | |||
| Temperature °C | L. innocua | L. monocytogenes | S. Javiana |
| 15 | 6.08 | 3.59 | 82.60 |
| 25 | 4.26 | 3.06 | 26.30 |
| 30 | 1.64 | 0.47 | 4.54 |
| 37 | 4.47 | 0.89 | 4.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




