1. Introduction
Aspartame (E951, CAS# 22839-47-0) is a widely used artificial sweetener in low-calorie beverages, prepared foods, and tabletop sweeteners that is approved for use in over 90 countries, and concerns about its safety and potential health risks have led to calls for further information and research. In 1981, the WHO/FAO Joint Expert Committee on Food Additives (JECFA) evaluated its health effects as part of a programme to assess the risks of food additives and chemicals [
1]. However, the WHO International Agency for Research on Cancer (IARC)
Monographs programme, which identifies potential carcinogenic hazards, has not evaluated aspartame to date. According to a recent IARC priorities advisory group report [
2,
3], epidemiologic studies have generally not demonstrated an association between aspartame intake and cancer risk. However, a prospective study on hematologic malignancies found a positive association for multiple myeloma and non-Hodgkin lymphoma in men [
4]. Also, a case-control study of exocrine pancreatic adenocarcinoma found a positive association with low-calorie soft drink consumption in men [
5], and another case-control study showed a positive association of regular use of artificial sweeteners with urinary tract tumors [
6]. More recently, a large cohort study of 102,865 French adults showed that aspartame intake was associated with increased breast and obesity-related cancer risks [
4]. Concerns have also been raised by a few studies in experimental animals showing dose-related increased risks of certain cancers at exposure levels previously considered safe for human consumption [
5]. Mechanistic studies relevant to the key characteristics of carcinogens have also been conducted (e.g. [
6,
7,
8]). For these reasons, the IARC priorities advisory group recommended aspartame as a high priority for evaluation [
2,
3]. In addition to IARC, JECFA has also recommended aspartame as a high-priority substance for re-evaluation [
9].
According to the proposals, both the
IARC Monographs programme and the JECFA will conduct complementary evaluations in 2023: IARC will investigate whether aspartame has any potential carcinogenic effects (hazard identification), while JECFA will update its risk assessment by reviewing the acceptable daily intake and dietary exposure assessment of aspartame [
10]. The sequence of these evaluations will allow for a comprehensive evaluation of the health effects of aspartame consumption based on the most recent available evidence.
Both IARC and JECFA have published calls for data [
10,
11] that include data relevant to the dietary exposure assessment, such as the level of use of the additive in foods. To properly characterize the exposure of the general population and specific subgroups to aspartame, detailed data on its content in a variety of food groups are needed. However, although aspartame is one of the most widely used artificial sweeteners, there is a paucity of data in the scientific literature on its occurrence in various foods other than artificially sweetened beverages. To provide the agencies with such data from Germany to inform their exposure assessments, this article examines the results of a recent request for information on aspartame, providing a summary of the data and an analysis of their implications.
2. Materials and Methods
2.1. Data Request under the Consumer Information Act
The samples in this dataset were collected and analyzed between 2000 and 2022 by official food control laboratories in Germany as part of the governmental food control using validated and externally accredited methods according to ISO/IEC 17025 [
12]. The methodology for sample selection in the study is a combination of convenience sampling and systematic testing such as annual food monitoring projects. Samples were collected using a risk-based sampling approach, which means that samples are not selected at random, but rather based on a perceived risk of contamination or non-compliance [
13]. Samples were collected from a variety of sources, including retailers, importers and manufacturers, which may introduce some bias into the results, such as the focus of food control on the “bottleneck”, i.e., primarily at the manufacturer or importer. The data did not include information on consumption patterns of the products, which limits the ability to estimate actual exposure levels.
The data were then collected and consolidated by the German Federal Office of Consumer Protection and Food Safety (BVL) in March 2023 and published on the website FragDenStaat.de in response to a consumer request from the last author (D.W.L.) [
14]. The consumer request is based on the Consumer Information Act, which requires food control authorities to provide consumers with information on food, feed, consumer products, and cosmetics. The obligation to provide information includes, for example, unauthorized deviations from requirements, and measures and decisions taken in connection with the deviations, hazards, and risks to health and safety posed by products, monitoring activities, and measures for consumer protection [
15].
The original consumer request, including the complete dataset, can be accessed via the internet portal FragDenStaat.de of the non-profit association Open Knowledge Foundation Deutschland e.V. (Berlin, Germany) [
14].
2.2. Data Description and Analysis Methods
The available data include information such as the year and type of shop where the sample was collected, the country of origin, the product group and matrix of the sample, the aspartame content, the analytical methods, and the limit of detection (LOD) and the limit of quantification (LOQ) of the method used. In total, this dataset contained 53116 analytical results for aspartame in different food products. The dataset included separate tables with qualitative data (n=720) and quantitative data (n=52396). In this study, only the quantitative data were further evaluated.
The analytical methods used to determine the aspartame content in the major food groups were primarily test methods according to the official collection of paragraph 64 of the German Food, Commodities and Feed Code (Lebensmittel-, Bedarfsgegenstände- und Futtermittelgesetzbuch) for food control authorities and testing institutions. This ensures consistent quality of testing and comparability of results [
16]. Generally, the high-performance liquid chromatography (HPLC) method BVL L 00.00-28 is used, which is an adoption of the standard DIN EN 12856 with identical wording [
17]. Some laboratories have used other validated and accredited methods, based on HPLC coupled with different detectors, or based on nuclear magnetic resonance (NMR) spectroscopy (e.g., [
18]). The method used for each analytical result is indicated in the raw data Excel table called “Anlage 5” of the dataset [
14].
2.3. Data Analysis and Selection of Food Items for Inclusion: Regrouping and Prioritizing Foods
The analysis of the dataset presented in this study, including all statistical calculations, was performed using Microsoft Excel version 2016 (Microsoft, Redmond, WA, USA).
During the evaluation of the data, it quickly became apparent that most of the samples (73%) were negative for aspartame, i.e., the food was analyzed but aspartame was not detected. This finding can be explained by the fact that multiparameter methods, which analyze several sweeteners with the same assay, are commonly used for food control. This means that aspartame could have been measured even if the original intention was to measure another sweetener. Therefore, it is important to note that the dataset is heavily biased toward foods that were suspected of containing aspartame or other compounds that were measured with the same assay. This means that the sample of foods in Germany is not representative, and that the mean values should be interpreted with caution.
The original BVL grouping of the raw data was less informative (see the Excel table called “Anlage 7” of the original dataset [
14]) because only subgroups of the major food groups appeared to contain aspartame. For example, in the dairy category, aspartame was found only in certain subgroups such as ready-to-drink buttermilk beverages. It was decided to re-analyze the data and categorize them into more meaningful groups using the standardized FoodEx2 food classification and description system of the European Food Safety Authority (EFSA) [
19]. Only food groups in which at least 20 samples were analyzed and at least 40% of the samples were found to contain aspartame were retained in the analysis.
3. Results
Nine major food groups were included in this analysis based on minimum detection frequency and number of samples. In relation to the total values provided, this represents a percentage of 11% that were finally included in this study (5703 samples). Samples that did not belong to the selected food groups were not included in the assessment of aspartame occurrence.
Food items were grouped according to their respective product groups (flavored milk drinks, soft drinks, diet soft drinks, mixed beer drinks, candies, chewing gum, powdered drink bases, sports foods and fiber supplements). The product groups and the corresponding individual products included in each product group are listed in
Table A1 in the
appendix A.
Table 1 shows these food groups with the calculated percentage of positive samples.
Most of the samples were collected from German retailers. Additionally, samples were collected from German kiosks, restaurants, grocery stores, drugstores, canteens, breweries, butchers, bakeries, fitness centers, and all other food outlets or directly from importers. The main country of origin of the samples was Germany (79%). A high number of samples had no information on the country of origin (9.5%). If known, the most common other countries were Poland (1.0%), the Netherlands (0.8%), France (0.4%), Turkey (0.4%), Denmark (0.3%), China (0.2%), Belgium (0.2%), and the United Kingdom (0.2%) (
Supplementary Materials,
Table S1).
The main descriptive statistical parameters, including mean, standard deviation, median, maximum, and 95th percentiles, were calculated from the positive samples and are presented in
Table 2 (
Supplementary Materials,
Table S2). The highest mean levels of aspartame were found in sports foods and chewing gum. The data also showed that the levels of aspartame varied widely within food categories, with some products containing much higher levels than others.
Finally, the data were re-evaluated against the EU maximum levels. Generally, the levels of aspartame found were within the legal limits. Apart from the isolated outliers, there were only two exceedances (diet soft drinks: 970 mg/l, 636 mg/l) which were not excluded. In addition, the isolated outliers (see footnotes in
Table 2) were considered to be data entry errors due to their technologically unlikely high levels.
4. Discussion
According to market data published by the German Federal Institute for Risk Assessment, between 2015 and 2018, 989 foods and 1055 beverages with added non-nutritive sweeteners (NNS) were launched in Germany, of which 16% contained aspartame [
21]. Despite this widespread use, a lack of occurrence and exposure data in Germany was noted. The last major European assessment by EFSA in 2013 [
22] did not include data from Germany.
The only comprehensive study on aspartame exposure in Germany was published by Bär and Biermann in 1992 [
23]. In this study, the occurrence data of aspartame in different food and beverage products were evaluated by different methods. First, the researchers collected data from food manufacturers by requesting information on the sweetener content of their products. Second, package labels were examined to determine the presence and amount of aspartame. Finally, chemical analysis was used in cases where information was not readily available. These multiple complementary approaches provided a comprehensive understanding of the prevalence of aspartame in the marketplace and allowed for a comprehensive exposure assessment when the data were combined with food frequency questionnaires, but the authors did not publish the occurrence data, so that no comparison with this survey is possible. Currently, quantitative labeling of aspartame is not required, so only the qualitative presence of the compound is indicated in the ingredient list. Therefore, it is not possible to assess quantitative occurrence by evaluating package labels.
Some data from previous surveys have been published, mostly in government reports. As part of the German National Surveillance Plan 2006, fruit, vegetable, and mushroom products were analyzed for additives. Aspartame was not detected in any of the 237 samples analyzed [
24]. According to the German National Surveillance Plan 2007, the occurrence of aspartame was determined in different types of beverages. Of the 170 samples of fruit juice drink samples analyzed, 29 contained aspartame at levels ranging from 11 to 381 mg/kg, with a mean of 85 mg/kg. Of the 51 nectar samples tested, only one was positive for aspartame at 0.1 mg/kg. In other types of beverages, 21 of 124 samples were positive for aspartame, with levels ranging from 18 to 444 mg/kg and an average of 115 mg/kg. None of the samples tested in any category exceeded the legal limit [
25]. According to the German National Surveillance Plan 2008, sweeteners in confectionery without added sugar (including hard and soft candies and confectionery for diabetics) were analyzed. Of a total of 353 samples, 185 were found to contain aspartame. The mean amount of aspartame was 404 mg/kg, with a maximum of 1203 mg/kg. The 90th percentile value was 774 mg/kg. The maximum limit for aspartame of 1000 mg/kg was exceeded in one sample, which was an unfilled hard candy [
26]. During the German National Food Monitoring 2015, mineral waters, including raw waters, were analyzed for selected sweeteners. Aspartame was not detected in any of the 19 samples [
27].
Van Vliet et al. reported aspartame analyses of seven soft drinks from Germany. Aspartame was detected in five samples with a mean of 119 mg/l (range 53–330 mg/l) [
28]. Maes et al. detected aspartame in two brands of diet coke at 138 and 149 mg/l [
18].
Recently, the German Federal Institute for Risk Assessment (BfR) published data from an analytical survey of 92 energy-reduced or sugar-free non-alcoholic beverages. For energy-reduced beverages, the study found that of three aspartame-positive samples, the average level of aspartame was 20 mg/kg, with a range of 0.05–45 mg/kg. For sugar-free non-alcoholic beverages, the study found 64 positive samples and found that the average level of aspartame was 75 mg/kg, with a range of 11–492 mg/kg [
29].
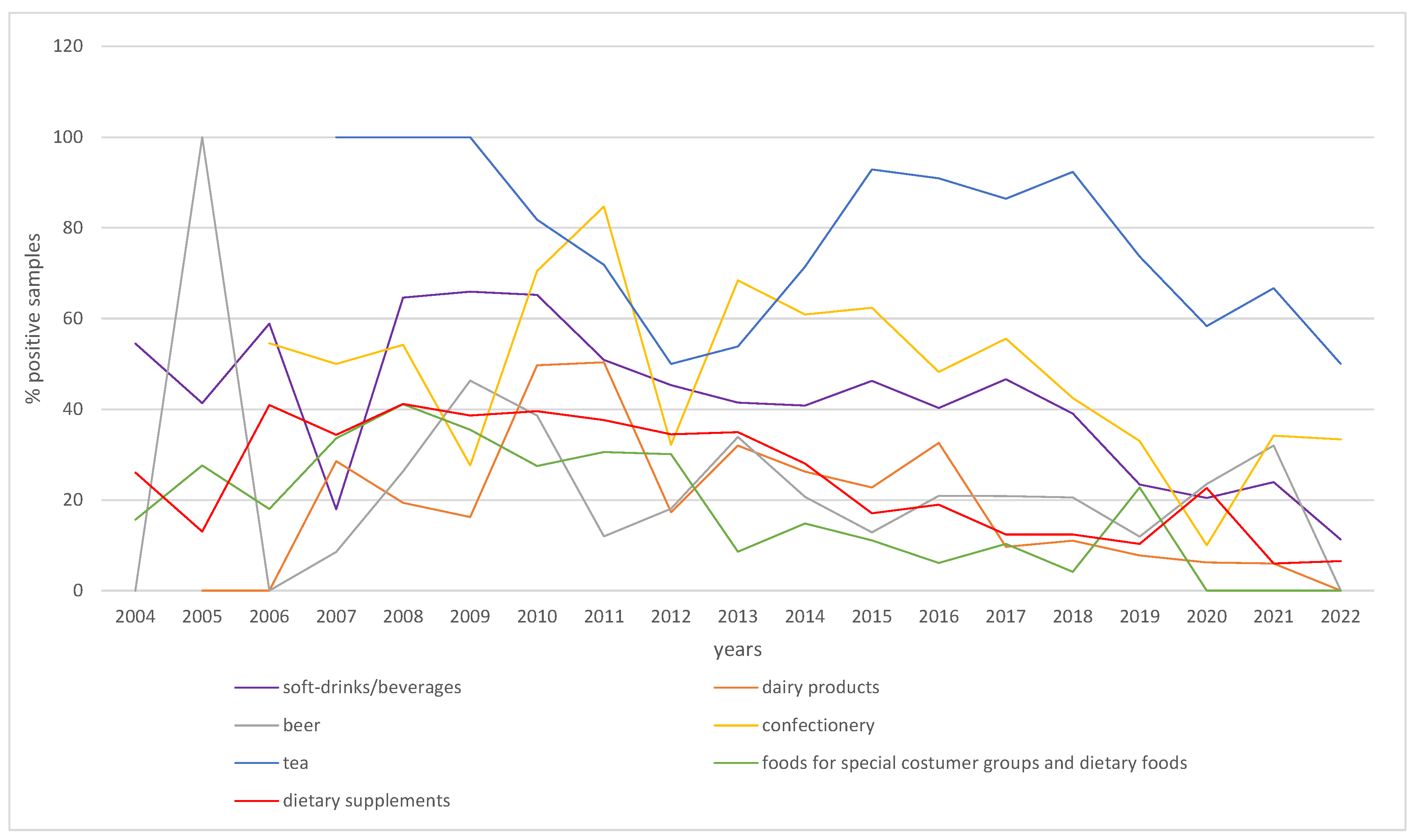
The results of this study confirm that aspartame is widely used in foods in Germany and that some individuals may be consuming high levels of the sweetener through certain products. Given the wide variation in aspartame content in foods and beverages, the survey data in this study are in reasonable agreement with the results of the previous studies. However, this study is based on a much larger number of samples and should therefore provide more stable statistics. The data analysis showed no obvious time trends or changes in content (Appendix 1,
Figure A1), even when compared to previous literature. However, a statistical evaluation and time trend analysis was not possible due to the lack of data from several years depending on the product group.
The survey of the occurrence of aspartame in Germany based on official food controls has some limitations. The data may not be representative of the general population's consumption of foods containing aspartame, because the sampling was a combination of systematic and convenience sampling and a threshold of at least 20 samples was applied in this analysis. However, the sample size is comparatively large, which may indicate that representativeness was achieved for the food groups containing aspartame. This means that the data may still be suitable for exposure analysis of consumers of food groups containing aspartame, even if they are not fully representative of the general population. Despite the large sample size, it is important to consider that there may be other factors that influence aspartame consumption, such as individual preferences and purchasing habits, including regular consumption of a product containing aspartame. Therefore, the data should be interpreted with caution and further research, such as combining occurrence data with dietary survey data, may be needed to fully understand the extent of aspartame consumption in the population. Second, the samples were collected only in Germany and may not be representative of other countries or regions. Studies reporting the occurrence of aspartame in foods and beverages in other countries are mainly from Europe and focus primarily on beverages. Comprehensive data on the occurrence of aspartame are available from only four European countries [
22].
Given this lack of comprehensive data, the main purpose of obtaining the data through the Consumer Information Act was to make the German data on aspartame publicly available so that the conditions of the IARC preamble are met, which require that the data be made publicly available to meet transparency requirements and to provide protection against preliminary or non-peer-reviewed information [
30]. It is hoped that the data can now be used in the work of the IARC and JECFA working groups. Because this study is the first to provide significant data on food groups such as dietary fiber supplements, certain dairy products, and powders used to make beverages, it is considered critical to providing a comprehensive understanding of aspartame exposure, despite the limitations noted above.
5. Conclusions
These data on the occurrence and levels of aspartame in various foods in Germany provide new and updated information on the use of aspartame in the food industry that may help researchers better understand its prevalence and potential health effects. Based on the data provided, it appears that aspartame is present at varying levels in a wide variety of foods. In terms of the levels of aspartame detected, the data show that the highest concentrations were found in sports foods and chewing gum. However, the levels of aspartame found were generally within the legal limits set by the European Union.
Overall, the data suggest that aspartame is a commonly used sweetener in a wide variety of foods and that consumers concerned about their intake of this additive should carefully read the labels of the foods they consume. However, based on the levels found in this dataset, it does not appear that there is cause for alarm in terms of exceeding the intake levels that are currently considered safe [
22].
Nevertheless, these new data can contribute to a better understanding of the presence and levels of aspartame in different foods and help ensure the safety and health of consumers, especially when considering the cumulative exposure from different food groups, such as sports foods and some dietary supplements, which may have been underestimated.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Evaluation of product origin, Table S2: Evaluation of qualitative and quantitative data.
Author Contributions
Conceptualization, D.W.L., S.G.W. and R.W..; methodology, D.W.L.; software, D.W.L.; validation, S.S., K.G.; formal analysis, S.S., K.G.; investigation, S.S., K.G.; resources, D.W.L., S.G.W.; data curation, D.W.L.; writing—original draft preparation, S.S., K.G., D.W.L.; writing—review and editing, S.G.W., R.W., E.S., M.K., and A.K.K.; visualization, S.,S., K.G.; supervision, D.W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here:
https://fragdenstaat.de/en/request/aspartam/ (accessed on 28 March 2023). The data were provided by the German authority Federal Office of Consumer Protection and Food Safety (BVL) in the area of food safety and consumer protection, survey on aspartame, 2000-2022. The own derivative calculations presented in this study are available in the
Supplementary Materials.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Acknowledgments
The authors would like to thank the German Federal Office of Consumer Protection and Food Safety (BVL) for providing the data. Manuscript editing, data textualization and summarization, and German-English translation were performed using ChatGPT, an artificial intelligence (AI) language model developed by OpenAI (
https://chat.openai.com/). ChatGPT provided helpful insights and suggestions throughout the writing process, and its advanced language processing capabilities improved the clarity and accuracy of the manuscript. The final manuscript was also edited using DeepL Write (
https://www.deepl.com/write) and Trinka (
https://www.trinka.ai/) These tools utilize AI technology to suggest text changes, suggest synonyms, and improve overall writing quality.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Table A1.
Product subgroups included in the product groups.
Table A1.
Product subgroups included in the product groups.
| Product group |
Product subgroups contained in the product group |
| Flavored milk drinks |
Whey with other added food |
| Whey blend products from whey with fruit/fruit preparation |
| Buttermilk products with fruit preparation |
| Soft drink with added dairy products |
| Soft drinks |
Apple juice beverage |
| Orange juice beverage |
| Orange lemonade |
| Citrus lemonade |
| Cola lemonade |
| Bitter Lemon |
| Tonic-Water |
| Effervescent cold drink with flavor (raspberry, cherry, woodruff, citrus) |
| Diet soft drinks |
Fruit juice reduced in calorific value |
| Lemonade reduced in calorific value |
| Cola lemonade reduced in calorific value |
| Lemonade containing quinine and/or containing bitter substances, reduced in calorific value |
| Bitter Lemon reduced in calorific value |
| Effervescent cold drink reduced in calorific value |
| Energy drink reduced in calorific value |
| Non-alcoholic drinks for diabetics excl. fruit nectar |
| Mixed beer drinks |
Full beer without wheat beer with clear lemonade (1:1) "Alsterwasser” |
| Beer with sugars |
| Candies |
Hard caramel unfilled |
| Hard caramel unfilled reduced in calorific value |
| Pressings |
| Chewing gum |
Chewing gum |
| Chewing gum coated |
| Powdered drink bases |
Tea extract with lemon extracts or lemon flavoring |
| Other Tea extracts |
| Beverage powder with tea extract |
| Sports foods (with protein and amino acids) |
Food for intense muscular effort, especially for athletes |
| Protein concentrate incl. proteins/protein hydrolysates/amino acid mixture |
| Dietary supplements containing carnitine |
| Fiber supplements |
Fiber concentrates |
Figure A1.
Samples with detectable levels of aspartame in the major food groups analyzed between 2004 and 2022.
Figure A1.
Samples with detectable levels of aspartame in the major food groups analyzed between 2004 and 2022.
References
- WHO. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) - Aspartame. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/62 (accessed on 3 April 2023).
- International Agency for Research on Cancer. Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024; 25-27 March, 2019. France: Monographs on the Evaluation of Carcinogenic Risks to Humans, 2019. Available online: https://monographs.iarc.who.int/wp-content/uploads/2019/10/IARCMonographs-AGReport-Priorities_2020-2024.pdf (accessed on 3 April 2023).
- IARC Monographs Priorities Group. Advisory Group recommendations on priorities for the IARC Monographs. Lancet Oncol. 2019, 20, 763–764. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaësse, C.; Sa, A. de; Lutchia, R.; Gigandet, S.; et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022, 19, e1003950. [Google Scholar] [CrossRef] [PubMed]
- Soffritti, M.; Padovani, M.; Tibaldi, E.; Falcioni, L.; Manservisi, F.; Belpoggi, F. The carcinogenic effects of aspartame: The urgent need for regulatory re-evaluation. Am. J. Ind. Med. 2014, 57, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Kamenickova, A.; Pecova, M.; Bachleda, P.; Dvorak, Z. Effects of artificial sweeteners on the AhR- and GR-dependent CYP1A1 expression in primary human hepatocytes and human cancer cells. Toxicol. In Vitro 2013, 27, 2283–2288. [Google Scholar] [CrossRef]
- Saunders, F.J.; Pautsch, W.F.; Nutting, E.F. The biological properties of aspartame. III. Examination for endocrine-like activities. J. Environ. Pathol. Toxicol. 1980, 3, 363–373. [Google Scholar]
- Çadirci, K.; Özdemir Tozlu, Ö.; Türkez, H.; Mardinoğlu, A. The in vitro cytotoxic, genotoxic, and oxidative damage potentials of the oral artificial sweetener aspartame on cultured human blood cells. Turk. J. Med. Sci. 2020, 50, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Request for information and comments on the priority list of substances proposed for evaluation by JECFA. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/fr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FCircular%252520Letters%252FCL%2525202021-81%252Fcl21_81e.pdf (accessed on 3 April 2023).
- International Agency for Research on Cancer. IARC Monographs – Volume 134 – Call for Data. Available online: https://monographs.iarc.who.int/iarc-monographs-volume-134-call-for-data/ (accessed on 3 April 2023).
- Joint FAO/WHO expert committee on food additives (JECFA). Food Additives List of Substances scheduled for evaluation and request for data. Available online: https://cdn.who.int/media/docs/default-source/food-safety/jecfa/call-for-data/jecfa96-call-for-data.pdf?sfvrsn=41389585_3 (accessed on 3 April 2023).
- International Organization for Standardization. ISO/IEC 17025:2017 - General requirements for the competence of testing and calibration laboratories. International Organization for Standardization (ISO), Geneva, Switzerland. Available online: https://www.iso.org/standard/66912.html (accessed on 3 April 2023).
- Roth, M. , Hartmann S., Renner R., Hörtig W. Risikoorientiertes Probenmanagement in Baden-Württemberg. Deut. Lebensm. Rundsch. 2007, 103, 45–52. [Google Scholar] [CrossRef]
- Open Knowledge Foundation Deutschland e.V. Aspartam. Available online: https://fragdenstaat.de/anfrage/aspartam/ (accessed on 28 March 2023).
- Verbraucherinformationsgesetz in der Fassung der Bekanntmachung vom 17. Oktober 2012 (BGBl. I S. 2166, 2725), das zuletzt durch Artikel 8 des Gesetzes vom 27. Juli 2021 (BGBl. I S. 3146) geändert worden ist. Available online: https://www.gesetze-im-internet.de/vig/BJNR255810007.html (accessed on 28 March 2023).
- Lebensmittel- und Futtermittelgesetzbuch in der Fassung der Bekanntmachung vom 15. September 2021 (BGBl. I S. 4253. Available online: https://www.gesetze-im-internet.de/lfgb/BJNR261810005.html (accessed on 3 April 2023).
-
DIN EN 12856:1999-07; Lebensmittel - Bestimmung von Acesulfam-K, Aspartam und Saccharin - Hochleistungs-flüssigchromatographisches Verfahren; Deutsche Fassung EN_12856:1999. Beuth Verlag GmbH: Berlin, Germany, 1999. [CrossRef]
- Maes, P.; Monakhova, Y.B.; Kuballa, T.; Reusch, H.; Lachenmeier, D.W. Qualitative and quantitative control of carbonated cola beverages using ¹H NMR spectroscopy. J. Agric. Food Chem. 2012, 60, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; Nikolic, M.; Ioannidou, S. FoodEx2 maintenance 2020. EFSA Supp. Publ. 2021, 18, EN-6507. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Union. 2008, L354, 16–33. [Google Scholar]
- Bundesinstitut für Risikobewertung. Süßungsmittel: Mehrheit der Studien bestätigt keine Gesundheitsbeeinträchtigung - allerdings ist die Studienlage unzureichend: Stellungnahme Nr. 004/2023 des BfR vom 07. Februar 2023 (Bewertungsstand 23. September 2019) 2023, Bundesinstitut für Risikobewertung, Berlin, Germany. 20 September. [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA J. 2013, 11, 3496. [Google Scholar] [CrossRef]
- Bär, A.; Biermann, C. Intake of intense sweeteners in Germany. Z. Ernahrungswiss. 1992, 31, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Brandt, P. Bundesweiter Überwachungsplan. In Berichte zur Lebensmittelsicherheit 2006. BVL-Reporte, vol 2,3; Birkhäuser: Basel, Switzerland, 2008. [Google Scholar] [CrossRef]
- Hartmann, F. Zusatzstoffe in Getränken. In Berichte zur Lebensmittelsicherheit 2007. BVL-Reporte, vol 3,3; Birkhäuser: Basel, Switzerland, 2007. [Google Scholar] [CrossRef]
- Hanewinkel-Meshkini, S. Süßstoffe in Süßwaren ohne Zuckerzusatz. In Berichte zur Lebensmittelsicherheit 2008. BVL-Reporte, vol 4,5; Brandt, P., Ed.; Birkhäuser: Basel, Switzerland, 2009. [Google Scholar]
- BVL. Monitoring 2015. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/01_Lebensmittel/01_lm_mon_dokumente/01_Monitoring_Berichte/2015_lm_monitoring_bericht.pdf?__blob=publicationFile&v=6 (accessed on 3 April 2023).
- van Vliet, K.; Melis, E.S.; Blaauw, P. de; van Dam, E.; Maatman, Ronald G.H. J.; Abeln, D.; van Spronsen, F.J.; Heiner-Fokkema, M.R. Aspartame and Phe-Containing Degradation Products in Soft Drinks across Europe. Nutrients 2020, 12, 1887. [Google Scholar] [CrossRef] [PubMed]
- Bundesinstitut für Risikobewertung. Alternativen zu Zucker: Wie viel Süßungsmittel steckt in Erfrischungsgetränken? Stellungnahme Nr. 006/2023 des BfR vom 07. Februar 2023, 2023, Bundesinstitut für Risikobewertung, Berlin, Germany. [CrossRef]
- International Agency for Research on Cancer. IARC Monographs Preamble. Available online: https://monographs.iarc.who.int/wp-content/uploads/2019/07/Preamble-2019.pdf (accessed on 3 April 2023).
Table 1.
Overview of the different product groups analyzed for the presence of aspartame.
Table 1.
Overview of the different product groups analyzed for the presence of aspartame.
| Product Group |
Total number of samples |
Frequency of aspartame-positive samples [%] |
| Diet soft drinks |
2783 |
72.7 |
| Soft drinks |
1167 |
49.2 |
| Candies |
603 |
56.2 |
| Chewing gum |
312 |
77.2 |
| Sports foods (with protein and amino acids) |
297 |
42.1 |
| Flavored milk drinks |
268 |
78.0 |
| Powdered drink bases |
195 |
83.6 |
| Mixed beer drinks |
58 |
69.0 |
| Fiber supplements |
20 |
55.0 |
Table 2.
Aspartame content in various product groups analyzed 2000 to 2022.
Table 2.
Aspartame content in various product groups analyzed 2000 to 2022.
| Product Group |
Number of Quantifiable Samples |
Unit |
Mean |
Median |
Maximum |
Standard deviation |
95th Percentile |
EU maximum level [20] |
| Chewing gum |
241 |
mg/kg |
1543 |
1369 |
4617 |
1042 |
3649 |
2500d
5500e
|
| Sports foods (with protein and amino acids) |
125 |
mg/kg |
1453 |
1030 |
6615 |
1461 |
5002 |
2000f
5000f,g
|
| Fiber supplements |
11 |
mg/kg |
1248 |
1276 |
1469 |
175 |
1460 |
2000f
5000f,g
|
| Powdered drink basesa
|
162 |
mg/kg |
1068 |
1133 |
4861 |
672 |
1600 |
600h
|
| Candies |
339 |
mg/kg |
473 |
440 |
3096 |
332 |
890 |
2000i
6000j
|
| Diet soft drinks |
2021 |
mg/l |
91 |
60 |
970 |
101 |
335 |
600 |
| Soft drinksb
|
574 |
mg/l |
59 |
34 |
531 |
74 |
203 |
600 |
| Flavored milk drinksc
|
207 |
mg/kg |
48 |
47 |
90 |
17 |
79 |
1000 |
| Mixed beer drinks |
40 |
mg/l |
24 |
26 |
55 |
15 |
42 |
600 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).