Submitted:
11 April 2023
Posted:
12 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
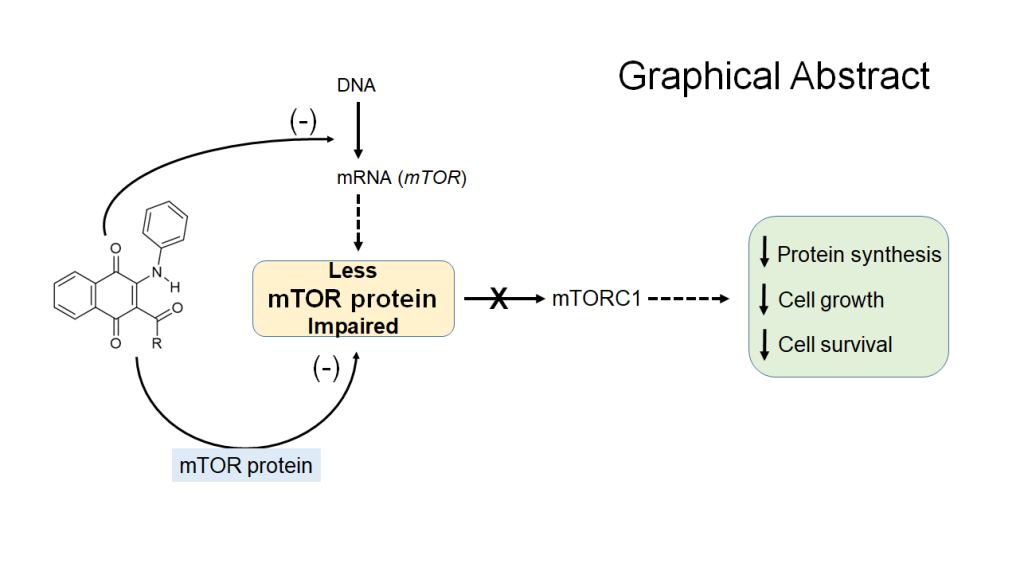
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
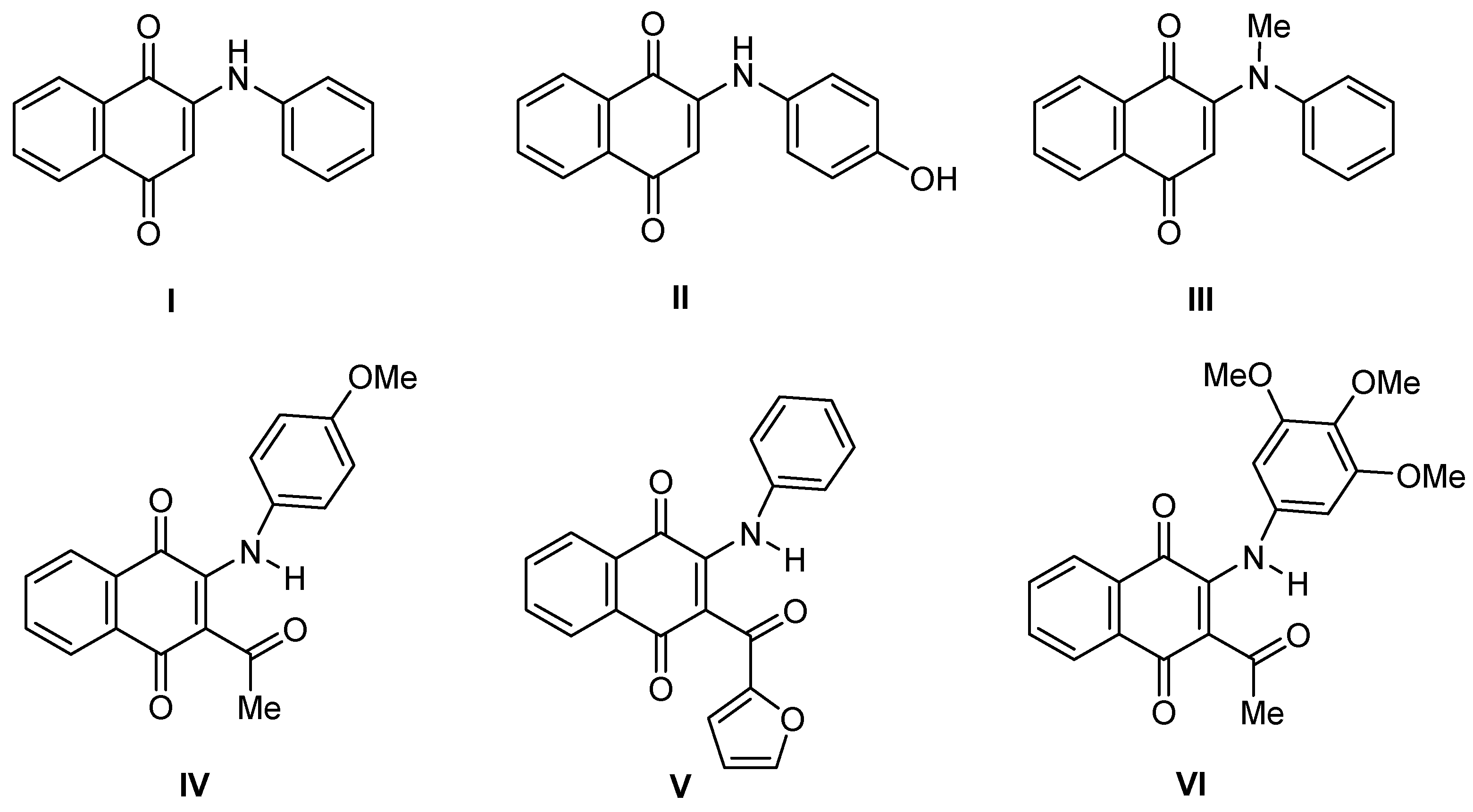
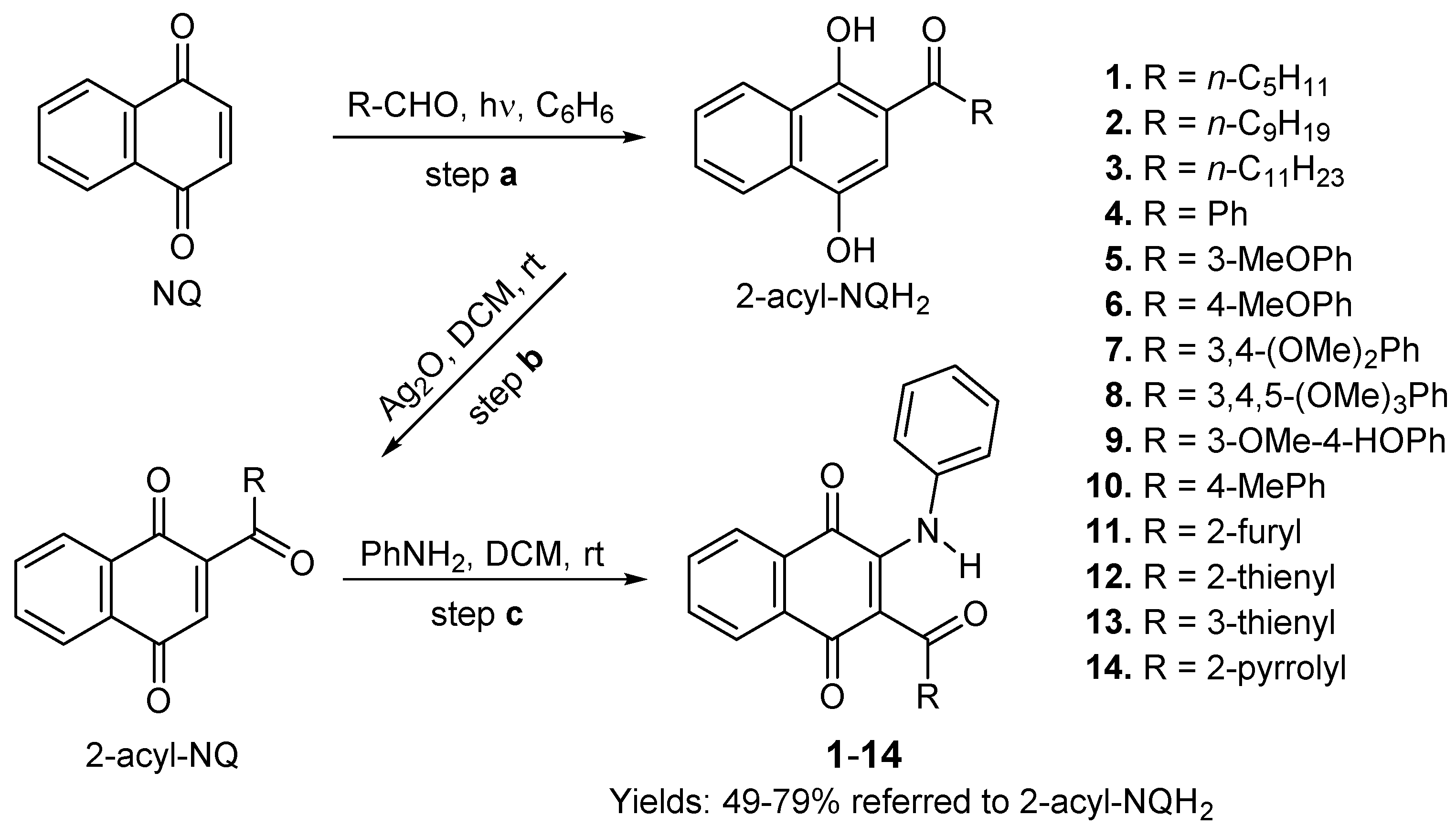
3.1.2. Synthesis of 2-phenylamino-3-acyl-1,4-naphtoquinones 1–14
3.1.3. General Procedure for the preparation of 2-phenylamino-3-acyl-1,4-naphtoquinones 1–10 and 13–14.
3.1.4. Molecular Descriptors
3.2. Cytotoxic assays
3.2.1. Cell lines and cell cultures
3.2.2. Cell survival assays
3.3. Quantitative real-time PCR (qPCR) Assay
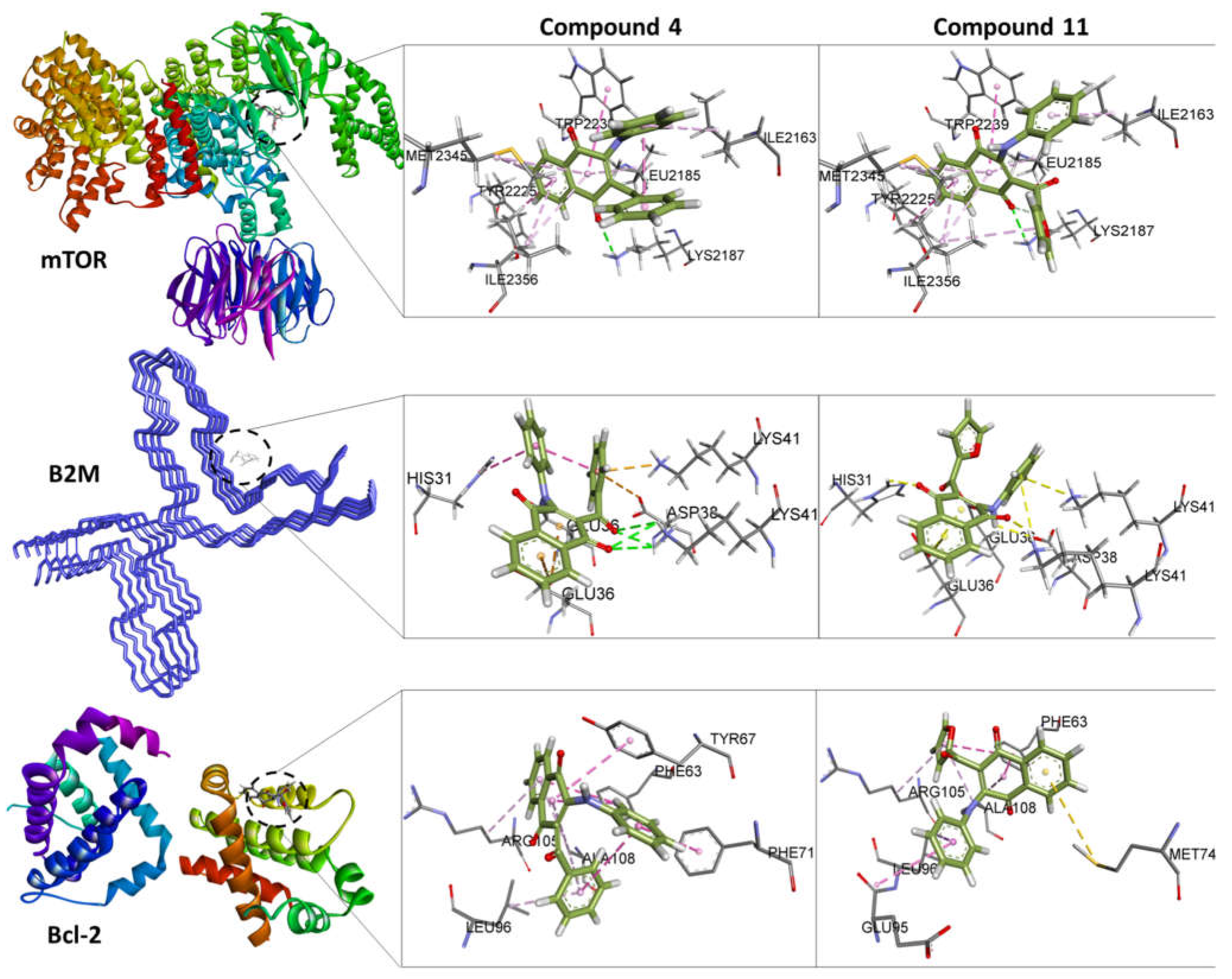
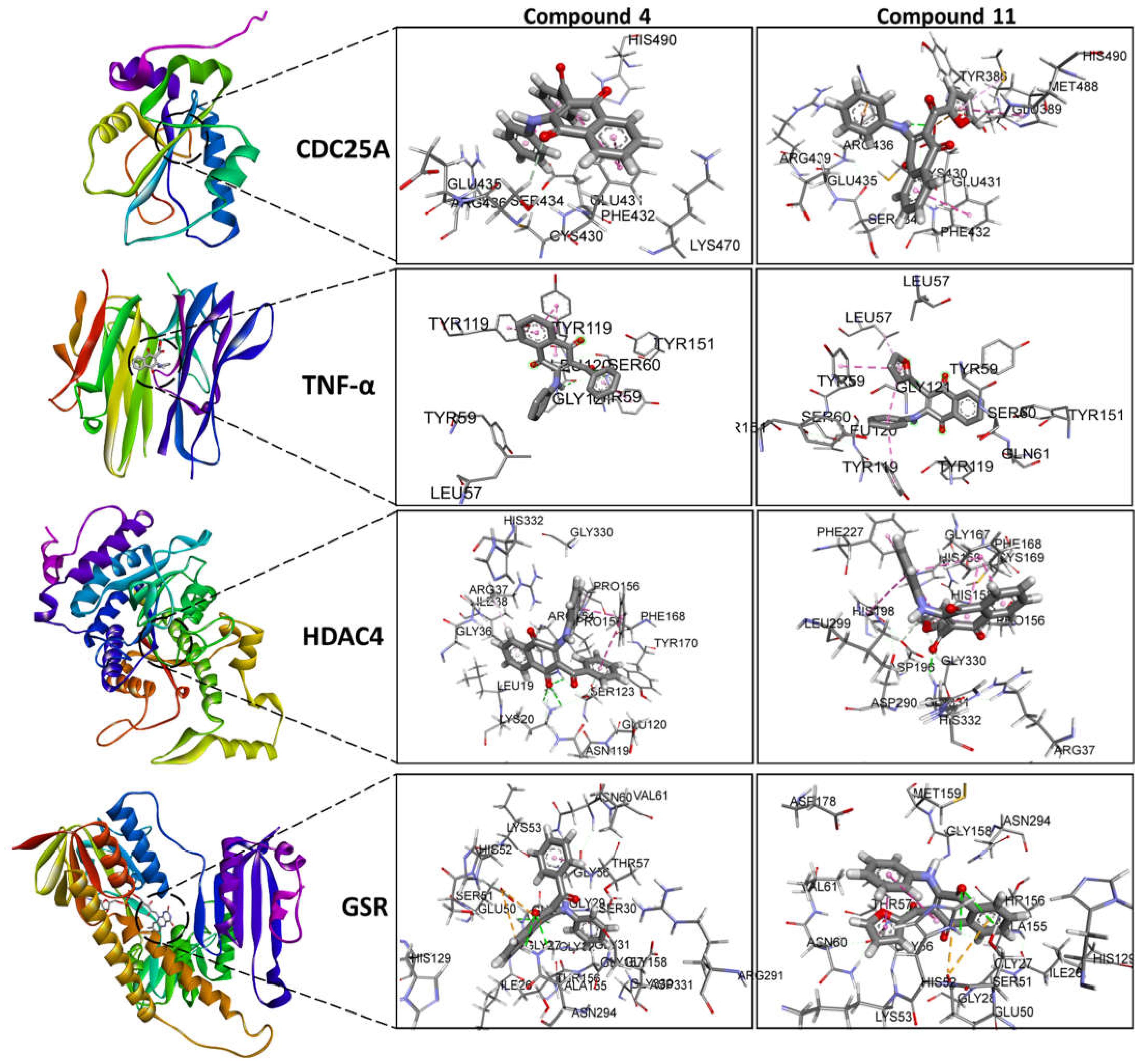
3.4. In Silico Studies
3.4.1. Molecular Docking
3.4.2. Ligand Efficiency
3.5. Physicochemical, Pharmacokinetic, and Druglikeness properties
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, R. Anti-Cancer Activities of 1,4-Naphthoquinones: A QSAR Study. Anticancer Agents Med. Chem. 2006, 6, 489−499. [CrossRef]
- Tandon, V.; Kumar, S. Recent development on naphthoquinone derivatives and their therapeutic applications as anticancer agents. Expert Opin. Ther. Pat. 2013, 23, 1087−1108. [CrossRef]
- Prachayasittikul, V.; Pingaew, R.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Prachayasittikul, V.; Pingaew, R.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, anticancer activity and QSAR study of 1,4-naphthoquinone derivatives. Eur. J. Medl Chem. 2014, 84, 247−263. [CrossRef]
- Pingaew, R.; Prachayasittikul, V.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Novel 1,4-naphthoquinone-based sulfonamides: Synthesis, QSAR, anticancer and antimalarial studies. Eur. J. Med. Chem. 2015, 103, 446−459. [CrossRef]
- Badave, D.K.; Khan, A.A.; Rane, Y.S. Anticancer Vitamin K3 Analogs: A Review. Anticancer Agents Med. Chem. 2016, 16, 1017−1030.
- Benites, J.; Valderrama, J. A.; Bettega, K.; Pedrosa, R. C.; Calderon, P. B.; Verrax, J. Biological evaluation of donor-acceptor aminonaphthoquinones as antitumor agents. Eur. J. Med. Chem. 2010, 45, 6052−6057. [CrossRef]
- Mahalapbutr, P.; Leechaisit, R.; Thongnum, A.; Todsaporn, D.; Prachayasittikul, V.; Rungrotmongkol, T.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V.; Pingaew, R., Discovery of Anilino-1,4-naphthoquinones as Potent EGFR Tyrosine Kinase Inhibitors: Synthesis, Biological Evaluation, and Comprehensive Molecular Modeling. ACS Omega 2022, 7, 17881−17893. [CrossRef]
- Bolton, J.; Dunlap, T. Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects. Chem. Res. Toxicol. 2017, 30, 13−37. [CrossRef]
- Ríos, D.; Benites, J.; Torrejón, F.; Theoduloz, C.; Valderrama, J. Synthesis and in vitro antiproliferative evaluation of 3-acyl-2-arylamino-1,4-naphthoquinones. Med. Chem. Res. 2014, 23, 4149−4155. [CrossRef]
- Rios, D.; Benites, J.; A. Valderrama, J.; Farias, M.; C. Pedrosa, R.; Verrax, J.; Buc Calderon, P. Biological Evaluation of 3-Acyl-2-Arylamino-1,4-Naphthoquinones as Inhibitors of Hsp90 Chaperoning Function. Curr. Top. Med. Chem. 2012, 12, 2094−2102.
- Gorrini, C.; Harris, I.; Mak, T. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931−947. [CrossRef]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dise. 2016, 7, e2253. [CrossRef]
- Locasale, Jason W.; Cantley, Lewis C. Metabolic Flux and the Regulation of Mammalian Cell Growth. Cell Metab. 2011, 14, 443−451. [CrossRef]
- Vafa, O.; Wade, M.; Kern, S.; Beeche, M.; Pandita, T.; Hampton, G.; Wahl, G. c-Myc Can Induce DNA Damage, Increase Reactive Oxygen Species, and Mitigate p53 Function. Mol. Cell 2002, 9, 1031−1044.
- Ghoneum, A.; Abdulfattah, A.; Warren, B.; Shu, J.; Said, N. Redox Homeostasis and Metabolism in Cancer: A Complex Mechanism and Potential Targeted Therapeutics. Int. J. Mol. Sci. 2020, 21, 3100. [CrossRef]
- Sies, H.; Jones, D. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363−383. [CrossRef]
- Xing, F.; Hu, Q.; Qin, Y.; Xu, J.; Zhang, B.; Yu, X.; Wang, W. The Relationship of Redox With Hallmarks of Cancer: The Importance of Homeostasis and Context. Front. Oncol. 2022, 12, 862743. [CrossRef]
- Jamison, J.; Gilloteaux, J.; Taper, H.; Calderon, P.; Summers, J. Autoschizis: a novel cell death. Biochem. Pharmacol. 2002, 63, 1773−1783. [CrossRef]
- Verrax, J.; Cadrobbi, J.; Marques, C.; Taper, H.; Habraken, Y.; Piette, J.; Calderon, P. Ascorbate potentiates the cytotoxicity of menadione leading to an oxidative stress that kills cancer cells by a non-apoptotic caspase-3 independent form of cell death. Apoptosis 2004, 9, 223−233. [CrossRef]
- Zhou, B.; Zhang, J.-Y.; Liu, X.-S.; Chen, H.-Z.; Ai, Y.-L.; Cheng, K.; Sun, R.-Y.; Zhou, D.; Han, J.; Wu, Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018, 28, 1171−1185. [CrossRef]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; Alqudsy, L.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127−136. [CrossRef]
- Kim, S.; Lee, H.; Lim, J.; Kim, H. Astaxanthin induces NADPH oxidase activation and receptor-interacting protein kinase 1-mediated necroptosis in gastric cancer AGS cells. Mol. Med. Report. 2021, 24, 837–849. [CrossRef]
- Valderrama, J.; Ríos, D.; Muccioli, G.; Buc Calderon, P.; Brito, I.; Benites, J. Hetero-annulation reaction between 2-acylnaphthoquinones and 2-aminobenzothiazoles. A new synthetic route to antiproliferative benzo[g]benzothiazolo[2,3-b]quinazoline-7,12-quinones. Tetrahedron Lett. 2015, 56, 5103−5105. [CrossRef]
- Valderrama, J.; Delgado, V.; Sepúlveda, S.; Benites, J.; Theoduloz, C.; Buc Calderon, P.; Muccioli, G. Synthesis and Cytotoxic Activity on Human Cancer Cells of Novel Isoquinolinequinone–Amino Acid Derivatives. Molecules 2016, 21, 1199. [CrossRef]
- Benites, J.; Valderrama, J.; Ríos, D.; Lagos, R.; Monasterio, O.; Calderon, P. Inhibition of cancer cell growth and migration by dihydroxynaphthyl aryl ketones. Mol. Cell. Toxicol. 2016, 12, 237−242. [CrossRef]
- Valderrama, J.; Cabrera, M.; Benites, J.; Ríos, D.; Inostroza-Rivera, R.; Muccioli, G.; Calderon, P. Synthetic approaches and in vitro cytotoxic evaluation of 2-acyl-3-(3,4,5-trimethoxyanilino)-1,4-naphthoquinones. RSC Advances 2017, 7, 24813−24821. [CrossRef]
- Benites, J.; Valderrama, J.; Ramos, M.; Muccioli, G.; Buc Calderon, P. Targeting Akt as strategy to kill cancer cells using 3-substituted 5-anilinobenzo[c]isoxazolequinones: A preliminary study. Biomed. Pharmacother. 2018, 97, 778−783. [CrossRef]
- Benites, J.; Valderrama, J.; Ramos, M.; Valenzuela, M.; Guerrero-Castilla, A.; Muccioli, G.; Buc Calderon, P. Half-Wave Potentials and In Vitro Cytotoxic Evaluation of 3-Acylated 2,5-Bis(phenylamino)-1,4-benzoquinones on Cancer Cells. Molecules 2019, 24, 1780. [CrossRef]
- Ríos, D.; Valderrama, J.; Cautin, M.; Tapia, M.; Salas, F.; Guerrero-Castilla, A.; Muccioli, G.; Buc Calderón, P.; Benites, J. New 2-Acetyl-3-aminophenyl-1,4-naphthoquinones: Synthesis and In Vitro Antiproliferative Activities on Breast and Prostate Human Cancer Cells. Oxid. Med. Cell. Longev. 2020, 2020, 1−11.
- Verma, R.; Hansch, C. Elucidation of structure–activity relationships for 2- or 6-substituted-5,8-dimethoxy-1,4-naphthoquinones. Bioorg. Med. Chem. 2004, 12, 5997−6009.
- Elfaki, M.; Sultan, M.; Mohammed, I. Mechanistic Study of Anticancer Activity of Some Known Aminopyrimidoisoquinolinequinones via QSAR Classification Methodology. Comp. Chem. 2020, 8, 1−13. [CrossRef]
- Veber, D.; Johnson, S.; Cheng, H.-Y.; Smith, B.; Ward, K.; Kopple, K. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615−2623. [CrossRef]
- Angelis, I.; Turco, L. Caco-2 Cells as a Model for Intestinal Absorption. Curr. Protoc. Toxicol. 2011, 47, 20−26.
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099−3105. [CrossRef]
- Nau, R.; Sörgel, F.; Eiffert, H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Revi. 2010, 23, 858−883. [CrossRef]
- Domínguez-Villa, F.; Durán-Iturbide, N.; Ávila-Zárraga, J. Synthesis, molecular docking, and in silico ADME/Tox profiling studies of new 1-aryl-5-(3-azidopropyl)indol-4-ones: Potential inhibitors of SARS CoV-2 main protease. Bioorg. Chem. 2021, 106, 104497. [CrossRef]
- Vaux, D.; Cory, S.; Adams, J. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988, 335, 440−442. [CrossRef]
- Gross, A.; McDonnell, J.; Korsmeyer, S. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899−1911. [CrossRef]
- Hardwick, J.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [CrossRef]
- Adams, J.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27−36. [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 1926, 18, 1926−1945. [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471−484. [CrossRef]
- Xu, K.; Liu, P.; Wei, W. mTOR signaling in tumorigenesis. Biochim. Biophys. Acta 2014, 1846, 638−654. [CrossRef]
- Guertin, D.; Sabatini, D. An expanding role for mTOR in cancer. Trends Mol. Med. 2005, 11, 353−361. [CrossRef]
- Rafalski, V.; Brunet, A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 2011, 93, 182-203. [CrossRef]
- King, D.; Yeomanson, D.; Bryant, H. PI3King the lock: targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J. Ped. Hematol. Oncol. 2015, 37, 245–251.
- Mossmann, D.; Park, S.; Hall, M. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744−757. [CrossRef]
- Magaway, C.; Kim, E.; Jacinto, E. Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells 2019, 8, 1584−1584. [CrossRef]
- Huang, S. mTOR Signaling in Metabolism and Cancer. Cells 2020, 9, 2278−2278. [CrossRef]
- Alzahrani, A. S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125−132. [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [CrossRef]
- Popova, N.; Jücker, M. The Role of mTOR Signaling as a Therapeutic Target in Cancer. Int. J. Mol. Sci. 2021, 22, 1743. [CrossRef]
- Qiu, H.-Y.; Wang, P.-F.; Zhang, M. A patent review of mTOR inhibitors for cancer therapy (2011–2020). Expert Opin. Ther. Pat. 2011, 31, 965−975. [CrossRef]
- Sun, S.-Y. mTOR-targeted cancer therapy: great target but disappointing clinical outcomes, why? Front. Med. 2020, 15, 221−231.
- Bendavit, G.; Aboulkassim, T.; Hilmi, K.; Shah, S.; Batist, G. Nrf2 Transcription Factor Can Directly Regulate mTOR: Linking cytoprotective gene expression to a major metabolic regulator that generates redox activity. J. Biol. Chem. 2016, 291, 25476–25488.
- Benites, J.; Rios, D.; Díaz, P.; Valderrama, J. The solar-chemical photo-Friedel–Crafts heteroacylation of 1,4-quinones. Tetrahedron Lett. 2011, 52, 609−611.
- Prieto, Y.; Muñoz, M.; Arancibia, V.; Valderrama, M.; Lahoz, F.; Luisa Martín, M. Synthesis, structure and properties of ruthenium(II) complexes with quinolinedione derivatives as chelate ligands: Crystal structure of [Ru(CO)2Cl2(6-methoxybenzo[g]quinoline-5,10-dione)]. Polyhedron 2007, 26, 5527−5532. [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55−63. [CrossRef]
- Guerrero-Castilla, A.; Olivero-Verbel, J.; Sandoval, I.; Jones, D. Toxic effects of a methanolic coal dust extract on fish early life stage. Chemosphere 2019, 227, 100−108. [CrossRef]
- Fauman, E.; Cogswell, J.; Lovejoy, B.; Rocque, W.; Holmes, W.; Montana, V.; Piwnica-Worms, H.; Rink, M.; Saper, M. Crystal Structure of the Catalytic Domain of the Human Cell Cycle Control Phosphatase, Cdc25A. Cell 1998, 93, 617−625. [CrossRef]
- Smith, A.; Oslob, J.; Flanagan, W.; Braisted, A.; Whitty, A.; Cancilla, M.; Wang, J.; Lugovskoy, A.; Yoburn, J.; Fung, A.; Farrington, G.; Eldredge, J.; Day, E.; Cruz, L.; Cachero, T.; Miller, S.; Friedman, J.; Choong, I.; Cunningham, B. Small-molecule inhibition of TNF-alpha. Science 2005, 11, 1022–1025. [CrossRef]
- Bottomley, M.; Lo Surdo, P.; Di Giovine, P.; Cirillo, A.; Scarpelli, R.; Ferrigno, F.; Jones, P.; Neddermann, P.; De Francesco, R.; Steinkühler, C.; Gallinari, P.; Carfí, A. Structural and Functional Analysis of the Human HDAC4 Catalytic Domain Reveals a Regulatory Structural Zinc-binding Domain. J, Biol. Chem. 2008, 283, 26694−26704. [CrossRef]
- Berkholz, D.; Faber, H.; Savvides, S.; Karplus, P. Catalytic Cycle of Human Glutathione Reductase Near 1 Å Resolution. J. Mol. Biol. 2008, 382, 371−384. [CrossRef]
- Yang, H.; Rudge, D.; Koos, J.; Vaidialingam, B.; Yang, H.; Pavletich, N. mTOR kinase structure, mechanism and regulation. Nature 2013, 497, 217−223.
- Iadanza, M.; Silvers, R.; Boardman, J.; Smith, H.; Karamanos, T.; Debelouchina, G.; Su, Y.; Griffin, R.; Ranson, N.; Radford, S. The structure of a β2-microglobulin fibril suggests a molecular basis for its amyloid polymorphism. Nature Comm. 2018, 9, 4517. [CrossRef]
- Porter, J.; Payne, A.; De Candole, B.; Ford, D.; Hutchinson, B.; Trevitt, G.; Turner, J.; Edwards, C.; Watkins, C.; Whitcombe, I.; Davis, J.; Stubberfield, C. Tetrahydroisoquinoline amide substituted phenyl pyrazoles as selective Bcl-2 inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 230−233. [CrossRef]
- Stewart, J. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and appli-cation to 70 elements. J. Mol. Model. 2007, 13, 1173–1213.
- Řezáč, J.; Hobza, P. Advanced Corrections of Hydrogen Bonding and Dispersion for Semiempirical Quantum Mechanical Methods. J. Chem. Theory Comput. 2012, 8, 141−151.
- Sanner, M. Using the Python programming language for bioinformatics. John Wiley & Sons, Ltd.: 1999; Vol. 17, pp 55–84.
- Berman, H.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.; Weissig, H.; Ilya, N., The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235−242.
- Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W., Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [CrossRef]
- Morris, G.; Goodsell, D.; Halliday, R.; Huey, R.; Hart, W.; Belew, R. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662.
- rott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 455−461. [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891−3898. [CrossRef]
- Lauria, A.; Martorana, A.; La Monica, G.; Mannino, S.; Mannino, G.; Peri, D.; Gentile, C. In Silico Identification of Small Molecules as New Cdc25 Inhibitors through the Correlation between Chemosensitivity and Protein Expression Pattern. Int. J. Mol. Sci. 2021, 22, 3714−3714. [CrossRef]
- Khan, M.; Nasr, F.; Baabbad, A.; Alqahtani, A.; Wadaan, M. Investigating the Anticancer Activity and Characterization of Bioactive Constituents of Moricandia sinaica (Boiss.) Boiss through In Vitro and In Silico Approaches in Triple-Negative Breast Cancer Cell Line. Appl. Sci. 2021, 1244.
- Deokar, S.; Shaikh, K. Exploring Cytotoxic Potential of Ciclopirox on Colorectal Cancer Cells by In-Silico Methodology. Biointerface Res. Appl. Chem. 2021 12, 7287–7310.
- Bhat, S.; Nayek, U.; Bhattacharjee, H.; Abdul Ajees, A. In-silico studies on the protein-protein interactions between human Cdc25 and glutaredoxin. J. Comput. Methods Sci. Eng. 2017, 17, 235−242. [CrossRef]
- Tao, Y.; Hao, X.; Jing, L.; Sun, L.; Cherukupalli, S.; Liu, S.; Wu, G.; Xu, S.; Zhang, X.; Shi, X.; Song, Y.; Liu, X.; Zhan, P. Discovery of potent and selective Cdc25 phosphatase inhibitors via rapid assembly and in situ screening of Quinonoid-focused libraries. Bioorg. Chem. 2021, 115, 105254. [CrossRef]
- Kellenberger, E.; Hofmann, A.; Quinn, R. Similar interactions of natural products with biosynthetic enzymes and therapeutic targets could explain why nature produces such a large proportion of existing drugs. Nat. Prod. Rep. 2011, 28, 1483. [CrossRef]
- Evain-Bana, E.; Schiavo, L.; Bour, C.; Lanfranchi, D.; Berardozzi, S.; Ghirga, F.; Bagrel, D.; Botta, B.; Hanquet, G.; Mori, M. Synthesis, biological evaluation and molecular modeling studies on novel quinonoid inhibitors of CDC25 phosphatases. J. Enzyme Inhib. Med. Chem. 2017, 32, 113−118. [CrossRef]
- Sarkis, M.; Miteva, M.; Dasso Lang, M.; Jaouen, M.; Sari, M.-A.; Galcéra, M.-O.; Ethève-Quelquejeu, M.; Garbay, C.; Bertho, G.; Braud, E. Insights into the interaction of high potency inhibitor IRC-083864 with phosphatase CDC25. Proteins 2017, 85, 593−601. [CrossRef]
- Altowyan, M.; Barakat, A.; Al-Majid, A.; Al-Ghulikah, H. Spiroindolone analogues bearing benzofuran moiety as a selective cyclooxygenase COX-1 with TNF-α and IL-6 inhibitors. Saudi J. Biol. Sci. 2020, 27, 1208−1216. [CrossRef]
- Xu, S.; Peng, H.; Wang, N.; Zhao, M. Inhibition of TNF-α and IL-1 by compounds from selected plants for rheumatoid arthritis therapy: In vivo and in silico studies. Trop. J. Pharm. Res. 2018, 17, 277. [CrossRef]
- Kumar, P.; Krishnaswamy, G.; Desai, N.; Sreenivasa, S.; Kumar, D. Design, synthesis, PASS prediction, in-silico ADME and molecular docking studies of substituted-(Z)-3-benzylidine-5-aza-2-oxindole derivatives (Part-1). Chem. Data Collec. 2021, 31, 100617. [CrossRef]
- Upadhyay, N.; Tilekar, K.; Jänsch, N.; Schweipert, M.; Hess, J.; Henze Macias, L.; Mrowka, P.; Aguilera, R.; Choe, J.-Y.; Meyer-Almes, F.-J.; Ramaa, C. Discovery of novel N-substituted thiazolidinediones (TZDs) as HDAC8 inhibitors: in-silico studies, synthesis, and biological evaluation. Bioorg. Chem. 2020, 100, 103934. [CrossRef]
- Schweipert, M.; Jänsch, N.; Upadhyay, N.; Tilekar, K.; Wozny, E.; Basheer, S.; Wurster, E.; Müller, M.; Ramaa, C. S.; Meyer-Almes, F.-J. Mechanistic Insights into Binding of Ligands with Thiazolidinedione Warhead to Human Histone Deacetylase 4. Pharmaceuticals 2021, 14, 1032. [CrossRef]
- Tilekar, K.; Hess, J.; Upadhyay, N.; Schweipert, M.; Flath, F.; Gutierrez, D.; Loiodice, F.; Lavecchia, A.; Meyer-Almes, F.J.; Aguilera, R.; Ramaa, C. HDAC4 Inhibitors with Cyclic Linker and Non-hydroxamate Zinc Binding Group: Design, Synthesis, HDAC Screening and in vitro Cytotoxicity evaluation. ChemistrySelect 2021, 6, 6748−6763.
- Cetin, A.; Bursal, E.; Türkan, F. 2-methylindole analogs as cholinesterases and glutathione S-transferase inhibitors: Synthesis, biological evaluation, molecular docking, and pharmacokinetic studies. Arab. J. Chem. 2021, 14, 103449. [CrossRef]
- Karaman, M. Molecular Insight of the Possible Inhibition Mechanism of Therapeutic Cephalosporin Derivatives against Human Glutathione Reductase Enzyme. Cumhuriyet Sci. J. 2020, 41, 747−755. [CrossRef]
- Beaufils, F.; Cmiljanovic, N.; Cmiljanovic, V.; Bohnacker, T.; Melone, A.; Marone, R.; Jackson, E.; Zhang, X.; Sele, A.; Borsari, C.; Mestan, J.; Hebeisen, P.; Hillmann, P.; Giese, B.; Zvelebil, M.; Fabbro, D.; Williams, R.; Rageot, D.; Wymann, M. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (PQR309), a Potent, Brain-Penetrant, Orally Bioavailable, Pan-Class I PI3K/mTOR Inhibitor as Clinical Candidate in Oncology. J. Med. Chem. 2017, 60, 7524−7538. [CrossRef]
- Ruiz-Torres, V.; Losada-Echeberría, M.; Herranz-López, M.; Barrajón-Catalán, E.; Galiano, V.; Micol, V.; Encinar, J. New Mammalian Target of Rapamycin (mTOR) Modulators Derived from Natural Product Databases and Marine Extracts by Using Molecular Docking Techniques. Mar. Drugs 2018, 16, 385−385. [CrossRef]
- Li, D.-D.; Meng, X.-F.; Wang, Q.; Yu, P.; Zhao, L.-G.; Zhang, Z.-P.; Wang, Z.-Z.; Xiao, W. Consensus scoring model for the molecular docking study of mTOR kinase inhibitor. J. Mol. Graphics Modell. 2018, 79, 81−87. [CrossRef]
- Kashyap, B.; Barge, S.; Bharadwaj, S.; Deka, B.; Rahman, S.; Ghosh, A.; Manna, P.; Dutta, P.; Sheikh, Y.; Kandimalla, R.; Samanta, S.; Boruwa, J.; Saikia, S.; Swargiary, D.; Kamboj, P.; Tuli, D.; Pal, U.; Borah, J.; Banerjee, S.; Talukdar, N. Evaluation of therapeutic effect of Premna herbacea in diabetic rat and isoverbascoside against insulin resistance in L6 muscle cells through bioenergetics and stimulation of JNK and AKT/mTOR signaling cascade. Phytomedicine 2021, 93, 153761. [CrossRef]
- Gomha, S.; Riyadh, S.; Huwaimel, B.; Zayed, M.; Abdellattif, M. Synthesis, Molecular Docking Study, and Cytotoxic Activity against MCF Cells of New Thiazole–Thiophene Scaffolds. Molecules 2022, 27, 4639. [CrossRef]
- Suganya, V.; Anuradha, V. In silico molecular docking of astaxanthin and sorafenib with different apoptotic proteins involved in hepatocellular carcinoma. Biocatal. Agric. Biotechnol. 2019, 19, 101076. [CrossRef]
- El-Sawy, E.; Mandour, A.; Yahya, S.; Abo-Salem, H.; Ebaid, M. Synthesis, cytotoxic, proapoptotic evaluation, and molecular docking study of some new N-substituted sulfonyl-3-indolyl heterocycles. Egypt. Pharm. J. 2015, 14, 15. [CrossRef]
- Gowtham, H.; Ahmed, F.; Anandan, S.; Shivakumara, C.; Bilagi, A.; Pradeep, S.; Shivamallu, C.; Shati, A.; Alfaifi, M.; Elbehairi, S.; Achar, R.; Silina, E.; Stupin, V.; Murali, M.; Kollur, S. In Silico Computational Studies of Bioactive Secondary Metabolites from Wedelia trilobata against Anti-Apoptotic B-Cell Lymphoma-2 (Bcl-2) Protein Associated with Cancer Cell Survival and Resistance. Molecules 2023, 28, 1588. [CrossRef]
- Lewkowicz, E.; Jayaraman, S.; Gursky, O., Protein Amyloid Cofactors: Charged Side-Chain Arrays Meet Their Match? Trends Biochem. Sci. 2021, 46, 626.
- Abad-Zapatero, C., Ligand efficiency indices for effective drug discovery. Expert Opin. Drug Discov. 2007, 2, 469−488. [CrossRef]
- Abad-Zapatero, C.; Perišić, O.; Wass, J.; Bento, A.; Overington, J.; Al-Lazikani, B.; Johnson, M. Ligand efficiency indices for an effective mapping of chemico-biological space: the concept of an atlas-like representation. Drug Discov. Today 2010, 15, 804–811.
- Reynolds, C.; Tounge, B.; Bembenek, S. Ligand Binding Efficiency: Trends, Physical Basis, and Implications. J. Med. Chem. 2008, 51, 2432−2438. [CrossRef]
- Cavalluzzi, M.; Mangiatordi, G.; Nicolotti, O.; Lentini, G. Ligand efficiency metrics in drug discovery: the pros and cons from a practical perspective. Expert Opin. Drug Discov. 2017, 12, 1087−1104. [CrossRef]
- Pan, Y.; Huang, N.; Cho, S.; Mackerell, A. Consideration of Molecular Weight during Compound Selection in Virtual Target-Based Database Screening. J. Chem. Inf. Comput. Sci. 2003, 43, 267−272. [CrossRef]
- Bakchi, B.; Krishna, A.; Sreecharan, E.; Ganesh, V.; Niharika, M.; Maharshi, S.; Puttagunta, S.; Sigalapalli, D.; Bhandare, R.; Shaik, A. An overview on applications of SwissADME web tool in the design and development of anticancer, antitubercular and antimicrobial agents: A medicinal chemist’s perspective. J. Mol. Struct. 1259, 1259, 132712. [CrossRef]
- Pires, D.; Blundell, T.; Ascher, D. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066−4072. [CrossRef]
 | ||||||
|---|---|---|---|---|---|---|
| Compound | R | DU-145 | MCF-7 | T24 | HEK-293 | MSI |
| 1 | C5H11 | 60.89 ± 0.34 | 62.68 ± 0.53 | 86.69 ± 2.52 | 77.32 ± 3.66 | 1.1 |
| 2 | C9H19 | > 100 | > 100 | > 100 | 79.76 ± 9.74 | 0.8 |
| 3 | C11H23 | > 100 | > 100 | > 100 | > 100 | 1.0 |
| 4 | Ph | 0.82 ± 0.10 | 5.16 ± 0.16 | 15.84 ± 0.90 | 45.18 ± 6.59 | 6.2 |
| 5 | 3-MeOPh | 10.22 ± 0.95 | 6.63 ± 0.13 | 11.96 ± 0.37 | 30.77 ± 4.07 | 3.2 |
| 6 | 4-MeOPh | 19.52 ± 0.95 | 11.84 ± 0.54 | 13.28 ± 0.44 | 38.93 ± 3.30 | 2.6 |
| 7 | 3,4-(OMe)2Ph | 11.90 ± 0.22 | 10.61 ± 0.27 | 15.15 ± 0.88 | 79.92 ± 10.41 | 6.4 |
| 8 | 3,4,5-(OMe)3Ph | 11.68 ± 0.29 | 4.88 ± 0.52 | 7.14 ± 0.20 | 37.41 ± 1.70 | 4.7 |
| 9 | 3-OMe-4-OHPh | 12.48 ± 0.55 | 5.81 ± 0.71 | 13.80 ± 1.87 | 47.73 ± 3.29 | 4.5 |
| 10 | 4-MePh | 11.11 ± 0.57 | 14.19 ± 0.53 | 21.48 ± 2.26 | 78.83 ± 5.53 | 5.0 |
| 11 | 2-Furyl | 5.45 ± 0.21 | 4.64 ± 0.23 | 11.71 ± 0.84 | > 100 | 13.7 |
| 12 | 2-Thienyl | 6.97 ± 0.60 | 4.20 ± 0.06 | 11.72 ± 0.89 | 24.80 ± 1.41 | 3.3 |
| 13 | 3-Thienyl | 8.91 ± 0.82 | 6.37 ± 0.23 | 13.77 ± 0.58 | 20.38 ± 2.77 | 2.1 |
| 14 | 2-Pyrrolyl | 14.45 ± 0.25 | 10.71 ± 0.35 | 21.66 ± 0.22 | 13.66 ± 1.36 | 0.9 |
| DOX | 0.93 ± 0.06 | 0.33 ± 0.05 | 0.46 ± 0.08 | 4.27 ± 0.34 | 7.5 | |
 |
||||
|---|---|---|---|---|
| Product No | R | -EI½ (mV) a | ClogP b | CMR(cm3/mol) b |
| 1 | C5H11 | 475 | 2.73 | 9.96 |
| 2 | C9H19 | 480 | 4.40 | 11.81 |
| 3 | C11H23 | 484 | 5.23 | 12.74 |
| 4 | Ph | 695 | 2.62 | 10.15 |
| 5 | 3-MeOPh | 500 | 2.50 | 10.77 |
| 6 | 4-MeOPh | 800 | 2.50 | 10.77 |
| 7 | 3,4-(OMe)2Ph | 604 | 2.37 | 11.38 |
| 8 | 3,4,5-(OMe)3Ph | 690 | 2.25 | 12.00 |
| 9 | 3-OMe-4-OH-Ph | 595 | 2.11 | 10.92 |
| 10 | 4-MePh | 770 | 3.11 | 10.61 |
| 11 | 2-Furyl | 578 | 1.24 | 9.36 |
| 12 | 2-Thienyl | 552 | 2.61 | 9.96 |
| 13 | 3-Thienyl | 635 | 2.55 | 9.96 |
| 14 | 2-Pyrrolyl | 685 | 1.17 | 9.58 |
| Compound | ||||
|---|---|---|---|---|
| N° | 4 | 11 | ||
| Structure | 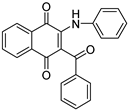 |
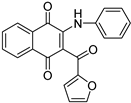 |
||
| Physicochemical parameters | MW(g/mol) | 353.37 | 343.33 | |
| ᵑRot | 4 | 4 | ||
| HBA | 3 | 4 | ||
| HBD | 1 | 1 | ||
| TPSA | 63.24 | 76.38 | ||
| Pharmacokinetic parameters | Absorption | Caco2 permeability (log Papp in 10-6 cm/s) | 0.483 | 0.939 |
| Intestinal absorption (human) (%Absorbed) | 94.39 | 94.324 | ||
| Skin Permeability (log Kp) | -2.774 | -2.798 | ||
| Distribution | VDss (human) (log L/Kg) | -0.105 | 0.013 | |
| BBB permeability (log BB) | 0.059 | -0.027 | ||
| CNS permeability (log PS) | -1.738 | -1.864 | ||
| Metabolism | CYP2D6 inhibitor | No | No | |
| CYP3A4 inhibitor | Yes | Yes | ||
| Excretion | Total Clearance (log mL/min/Kg) | 0.138 | 0.138 | |
| Renal OCT2 substrate | No | No | ||
| Toxicity | Oral Rat Acute Toxicity (LD50) (mol/Kg) | 2.704 | 2.779 | |
| Oral Rat Chronic Toxicity (log mg/Kg_bw/day) | 1.762 | 1.44 | ||
| Hepatotoxicity | Yes | No | ||
| Druglikeness properties | Lipinski rule | Yes; 0 violation | Yes; 0 violation | |
| Bioavailability | 0.55 | 0.55 | ||
| Gene name | Gene symbol | Relative expression levels (mRNA levels) in DU145 cells | ||
|---|---|---|---|---|
| Vehicle | Compound 4 | Compound 11 | ||
| Apoptosis regulator (Bcl2), transcript variant α | Bcl-2 | 1.00 ± 0.08 | 0.81 ± 0.05 | 0.78 ± 0.04 |
| Cell division cycle 25A | CDC25A | 1.00 ± 0.07 | 1.09 ± 0.10 | 0.90 ± 0.05 |
| Cellular communication network factor 2 | CCN2 | 1.00 ± 0.05 | 0.95 ± 0.03 | 0.95 ± 0.09 |
| Glutathione-disulfide reductase | GSR | 1.00 ± 0.07 | 0.95 ± 0.05 | 0.94 ± 0.06 |
| Histone deacetylase 4 | HDAC4 | 1.00 ± 0.07 | 0.91 ± 0.05 | 0.81 ± 0.07 |
| Mechanistic target of rapamycin kinase | mTOR | 1.00 ± 0.09 | 0.84 ± 0.06 | 0.72 ± 0.05* |
| Tumor necrosis factor | TNF | 1.00 ± 0.10 | 1.20 ± 0.08 | 0.93 ± 0.08 |
| Tumor protein p53 | TP53 | 1.00 ± 0.07 | 0.94 ± 0.04 | 0.87 ± 0.05 |
| Docking and Ligand Efficiency Analysis | ||||||
|---|---|---|---|---|---|---|
| Protein | PDBID | Molecule | ΔEbind (kcal/mol) |
Kd | LE (kcal/mol) |
ΔEMW (kcal/mol) |
| CDC25A | 1C25 | 4 | -5.2 | 10-4 | 0.19 | -1.0 |
| 11 | -5.9 | 10-5 | 0.23 | -1.2 | ||
| TNF-α | 2AZ5 | 4 | -9.1 | 10-7 | 0.34 | -1.8 |
| 11 | -8.4 | 10-7 | 0.32 | -1.6 | ||
| HDAC4 | 2VQJ | 4 | -8.6 | 10-7 | 0.32 | -1.7 |
| 11 | -8.2 | 10-7 | 0.32 | -1.6 | ||
| GSR | 3DK9 | 4 | -8.7 | 10-7 | 0.32 | -1.7 |
| 11 | -8.6 | 10-7 | 0.33 | -1.7 | ||
| mTOR | 4JSN | 4 | -7.7 | 10-6 | 0.29 | -1.5 |
| 11 | -8.0 | 10-6 | 0.31 | -1.6 | ||
| B2M | 6GK3 | 4 | -3.4 | 10-3 | 0.13 | -0.7 |
| 11 | -2.3 | 10-2 | 0.09 | -0.5 | ||
| Bcl-2 | 6ZX7 | 4 | -6.9 | 10-6 | 0.26 | -1.3 |
| 11 | -6.7 | 10-5 | 0.26 | -1.3 | ||
| Protein | PDBID | Center grid box | Binding site reference | ||
|---|---|---|---|---|---|
| x | y | z | |||
| CDC25A | 1C25 | 7.26 | 38.60 | 64.43 | [75,76,77,78,79,80,81,82] |
| TNF-α | 2AZ5 | -19.40 | 74.65 | 33.84 | [61,83,84,85] [36,58,59,60] |
| HDAC4 | 2VQJ | 19.33 | 77.30 | 37.91 | [co-crystallized ligand TFG, [86,87,88] |
| GSR | 3DK9 | 6.80 | 17.30 | 20.70 | [co-crystallized ligand FAD,[89,90] |
| mTOR | 4JSN | 55.48 | 2.38 | -46.53 | [91,92,93,94] |
| Bcl-2 | 2W3L | 39.80 | 26.93 | -12.41 | [co-crystallized ligand DRO,[95,96,97,98] |
| B2M | 6GK3 | 167.59 | 179.49 | 157.08 | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





