Submitted:
30 March 2023
Posted:
03 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell lines and Adenoviral vectors
2.2. Flow cytometry
2.3. Viral infection and luciferase assays
2.4. mRNA expression analysis
2.5. Statistical analysis
3. Results
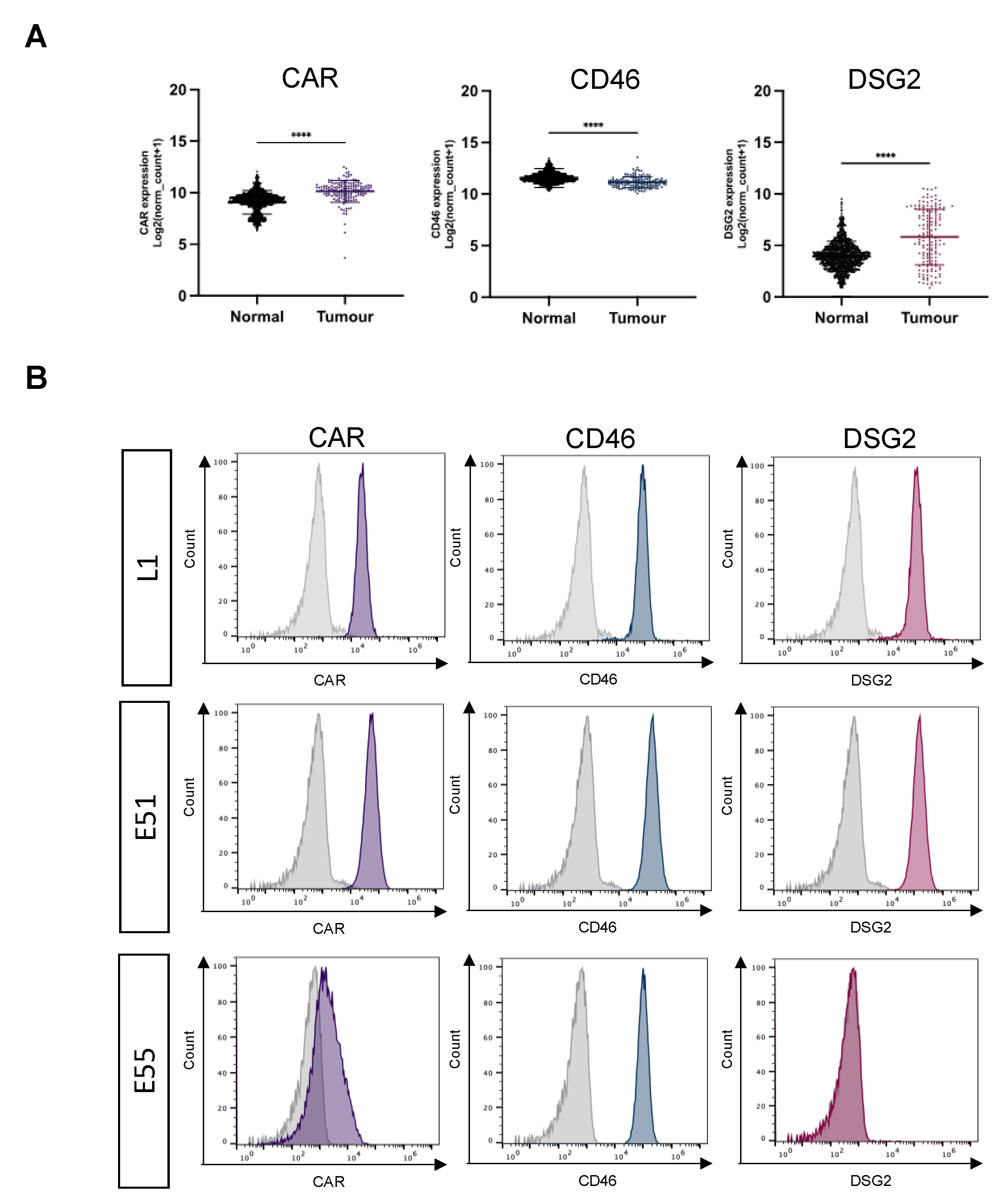
3.1. Expression of the Adenoviral receptors CAR, CD46 and DSG2 in GBM.
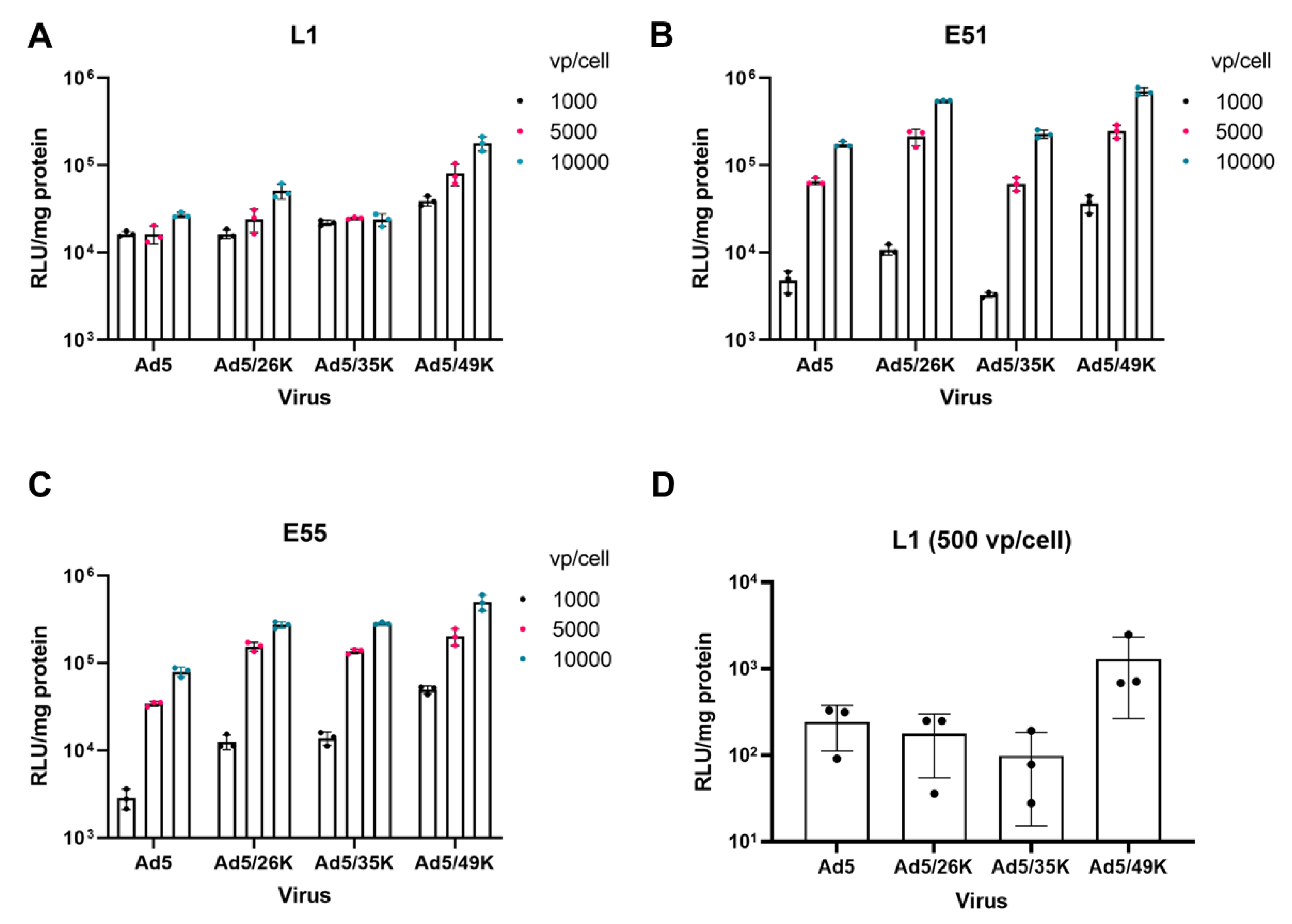
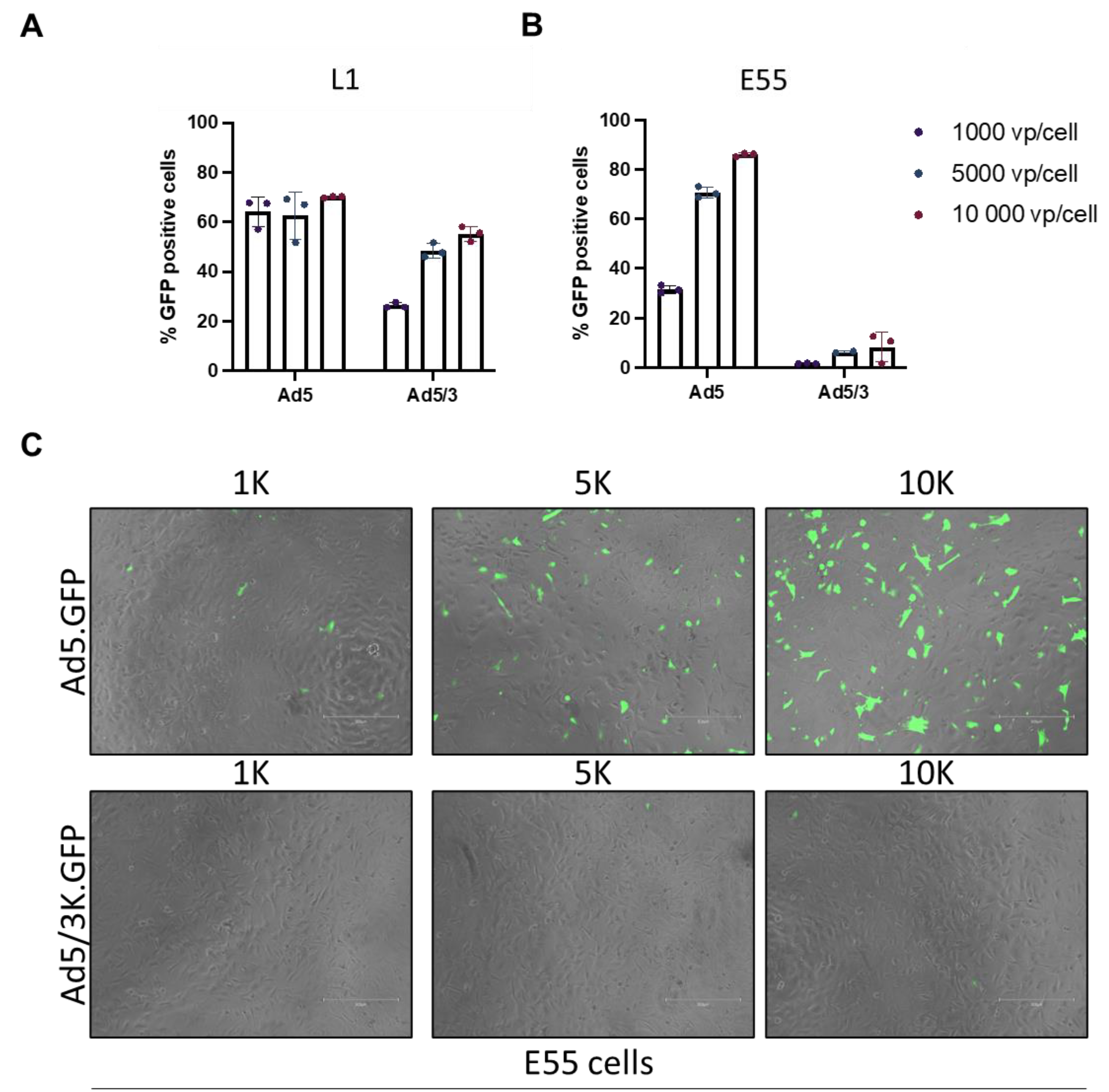
3.2. Transduction of pseudotyped adenoviral vectors in GBM.
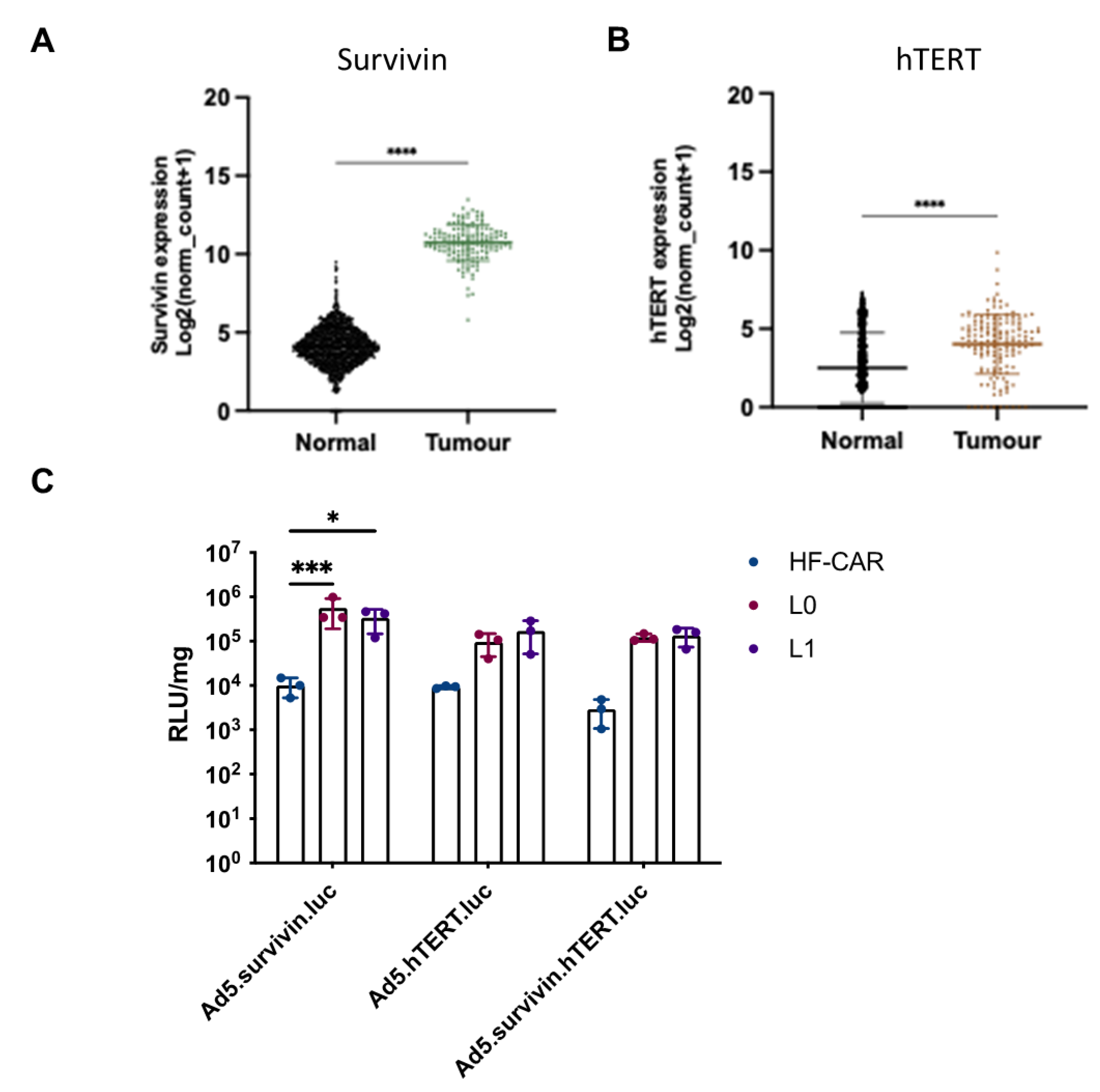
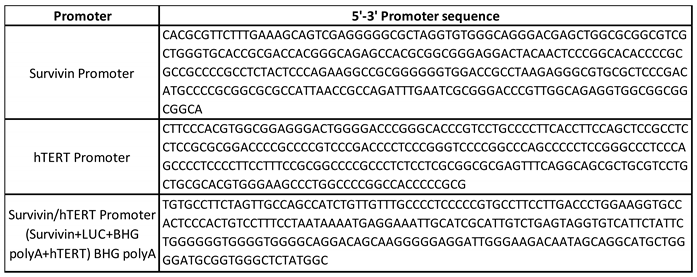
3.3. Tumour specific promoter hTERT and survivin offer benefits in GBM cells over normal cells.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Q. T. Ostrom et al., ‘CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011’, Neuro-Oncol., vol. 16 Suppl 4, no. Suppl 4, pp. iv1-63, Oct. 2014. [CrossRef]
- D. T. Di Carlo, F. Cagnazzo, N. Benedetto, R. Morganti, and P. Perrini, ‘Multiple high-grade gliomas: epidemiology, management, and outcome. A systematic review and meta-analysis’, Neurosurg. Rev., vol. 42, no. 2, pp. 263–275, Jun. 2019. [CrossRef]
- M. Lim, Y. Xia, C. Bettegowda, and M. Weller, ‘Current state of immunotherapy for glioblastoma’, Nat. Rev. Clin. Oncol., vol. 15, no. 7, pp. 422–442, Jul. 2018. [CrossRef]
- N. Macedo, D. M. Miller, R. Haq, and H. L. Kaufman, ‘Clinical landscape of oncolytic virus research in 2020’, J. Immunother. Cancer, vol. 8, no. 2, p. e001486, Oct. 2020. [CrossRef]
- A. J. Bett, L. Prevec, and F. L. Graham, ‘Packaging capacity and stability of human adenovirus type 5 vectors’, J. Virol., vol. 67, no. 10, pp. 5911–5921, Oct. 1993. [CrossRef]
- D. H. Sterman et al., ‘Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma’, Hum. Gene Ther., vol. 9, no. 7, pp. 1083–1092, May 1998. [CrossRef]
- Z. Ram et al., ‘Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells’, Nat. Med., vol. 3, no. 12, pp. 1354–1361, Dec. 1997. [CrossRef]
- ‘HAdV Working Group’. http://hadvwg.gmu.edu/ (accessed Mar. 29, 2023).
- A. L. Parker et al., ‘Effect of neutralizing sera on factor x-mediated adenovirus serotype 5 gene transfer’, J. Virol., vol. 83, no. 1, pp. 479–483, Jan. 2009. [CrossRef]
- T. C. Mast et al., ‘International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials’, Vaccine, vol. 28, no. 4, pp. 950–957, Jan. 2010. [CrossRef]
- E. A. Bates et al., ‘Development of a low-seroprevalence, αvβ6 integrin-selective virotherapy based on human adenovirus type 10’, Mol. Ther. Oncolytics, vol. 25, pp. 43–56, Jun. 2022. [CrossRef]
- J. M. Bergelson et al., ‘Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5′, Science, vol. 275, no. 5304, pp. 1320–1323, Feb. 1997. [CrossRef]
- C. B. Coyne and J. M. Bergelson, ‘CAR: a virus receptor within the tight junction’, Adv. Drug Deliv. Rev., vol. 57, no. 6, pp. 869–882, Apr. 2005. [CrossRef]
- M. Anders et al., ‘Loss of the coxsackie and adenovirus receptor contributes to gastric cancer progression’, Br. J. Cancer, vol. 100, no. 2, pp. 352–359, Jan. 2009. [CrossRef]
- K. Matsumoto, S. F. Shariat, G. E. Ayala, K. A. Rauen, and S. P. Lerner, ‘Loss of coxsackie and adenovirus receptor expression is associated with features of aggressive bladder cancer’, Urology, vol. 66, no. 2, pp. 441–446, Aug. 2005. [CrossRef]
- D. M. Shayakhmetov, A. Gaggar, S. Ni, Z.-Y. Li, and A. Lieber, ‘Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity’, J. Virol., vol. 79, no. 12, pp. 7478–7491, Jun. 2005. [CrossRef]
- S. N. Waddington et al., ‘Adenovirus serotype 5 hexon mediates liver gene transfer’, Cell, vol. 132, no. 3, pp. 397–409, Feb. 2008. [CrossRef]
- A. L. Parker et al., ‘Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes’, Blood, vol. 108, no. 8, pp. 2554–2561, Oct. 2006. [CrossRef]
- R. C. Carlisle et al., ‘Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1’, Blood, vol. 113, no. 9, pp. 1909–1918, Feb. 2009. [CrossRef]
- F. F. Lang et al., ‘Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma’, J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol., vol. 36, no. 14, pp. 1419–1427, May 2018. [CrossRef]
- A. T. Baker, R. M. Mundy, J. A. Davies, P. J. Rizkallah, and A. L. Parker, ‘Human adenovirus type 26 uses sialic acid-bearing glycans as a primary cell entry receptor’, Sci. Adv., vol. 5, no. 9, p. eaax3567, Sep. 2019. [CrossRef]
- A. Gaggar, D. M. Shayakhmetov, and A. Lieber, ‘CD46 is a cellular receptor for group B adenoviruses’, Nat. Med., vol. 9, no. 11, pp. 1408–1412, Nov. 2003. [CrossRef]
- H. Wang et al., ‘Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14′, Nat. Med., vol. 17, no. 1, pp. 96–104, Jan. 2011. [CrossRef]
- P. Rawal and L. Zhao, ‘Sialometabolism in Brain Health and Alzheimer’s Disease’, Front. Neurosci., vol. 15, 2021, Accessed: Mar. 27, 2023. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fnins.2021.648617. [CrossRef]
- L. A. K. Van Aarsen et al., ‘Antibody-mediated blockade of integrin alpha v beta 6 inhibits tumor progression in vivo by a transforming growth factor-beta-regulated mechanism’, Cancer Res., vol. 68, no. 2, pp. 561–570, Jan. 2008. [CrossRef]
- H. Uusi-Kerttula et al., ‘Ad5NULL-A20: A Tropism-Modified, αvβ6 Integrin-Selective Oncolytic Adenovirus for Epithelial Ovarian Cancer Therapies’, Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res., vol. 24, no. 17, pp. 4215–4224, Sep. 2018. [CrossRef]
- J. A. Davies et al., ‘Efficient Intravenous Tumor Targeting Using the αvβ6 Integrin-Selective Precision Virotherapy Ad5NULL-A20’, Viruses, vol. 13, no. 5, p. 864, May 2021. [CrossRef]
- H. Uusi-Kerttula et al., ‘Pseudotyped αvβ6 integrin-targeted adenovirus vectors for ovarian cancer therapies’, Oncotarget, vol. 7, no. 19, pp. 27926–27937, May 2016. [CrossRef]
- S. G. M. Piccirillo et al., ‘Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells’, Nature, vol. 444, no. 7120, pp. 761–765, Dec. 2006. [CrossRef]
- L. B. Hoang-Minh et al., ‘Infiltrative and drug-resistant slow-cycling cells support metabolic heterogeneity in glioblastoma’, EMBO J., vol. 37, no. 23, p. e98772, Dec. 2018. [CrossRef]
- F. A. Siebzehnrubl et al., ‘The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance’, EMBO Mol. Med., vol. 5, no. 8, pp. 1196–1212, Aug. 2013. [CrossRef]
- S. M. Pollard et al., ‘Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens’, Cell Stem Cell, vol. 4, no. 6, pp. 568–580, Jun. 2009. [CrossRef]
- ‘The AdZ adenovirus cloning system | AdZ.cf.ac.uk’. https://adz.cf.ac.uk/ (accessed Mar. 27, 2023).
- H. Uusi-Kerttula et al., ‘Incorporation of Peptides Targeting EGFR and FGFR1 into the Adenoviral Fiber Knob Domain and Their Evaluation as Targeted Cancer Therapies’, Hum. Gene Ther., vol. 26, no. 5, pp. 320–329, May 2015. [CrossRef]
- M. J. Goldman et al., ‘Visualizing and interpreting cancer genomics data via the Xena platform’, Nat. Biotechnol., vol. 38, no. 6, pp. 675–678, Jun. 2020. [CrossRef]
- A. T. Baker et al., ‘The Fiber Knob Protein of Human Adenovirus Type 49 Mediates Highly Efficient and Promiscuous Infection of Cancer Cell Lines Using a Novel Cell Entry Mechanism’, J. Virol., vol. 95, no. 4, pp. e01849-20, Jan. 2021. [CrossRef]
- R. S. Dakin, A. L. Parker, C. Delles, S. A. Nicklin, and A. H. Baker, ‘Efficient transduction of primary vascular cells by the rare adenovirus serotype 49 vector’, Hum. Gene Ther., vol. 26, no. 5, pp. 312–319, May 2015. [CrossRef]
- H. N. Nguyen et al., ‘Human TERT promoter mutation enables survival advantage from MGMT promoter methylation in IDH1 wild-type primary glioblastoma treated by standard chemoradiotherapy’, Neuro-Oncol., vol. 19, no. 3, pp. 394–404, Mar. 2017. [CrossRef]
- A. Das, W.-L. Tan, J. Teo, and D. R. Smith, ‘Expression of survivin in primary glioblastomas’, J. Cancer Res. Clin. Oncol., vol. 128, no. 6, pp. 302–306, Jun. 2002. [CrossRef]
- I. V. Alekseenko et al., ‘Activity of the Upstream Component of Tandem TERT/Survivin Promoters Depends on Features of the Downstream Component’, PLOS ONE, vol. 7, no. 10, p. e46474, Oct. 2012. [CrossRef]
- H. Fukuhara, Y. Ino, and T. Todo, ‘Oncolytic virus therapy: A new era of cancer treatment at dawn’, Cancer Sci., vol. 107, no. 10, pp. 1373–1379, Oct. 2016. [CrossRef]
- E. A. Bates et al., ‘In Vitro and In Vivo Evaluation of Human Adenovirus Type 49 as a Vector for Therapeutic Applications’, Viruses, vol. 13, no. 8, p. 1483, Jul. 2021. [CrossRef]
- A. A. Stepanenko et al., ‘Superior infectivity of the fiber chimeric oncolytic adenoviruses Ad5/35 and Ad5/3 over Ad5-delta-24-RGD in primary glioma cultures’, Mol. Ther. - Oncolytics, vol. 24, pp. 230–248, Mar. 2022. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





