Submitted:
28 March 2023
Posted:
30 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Alterations in Cellular Structures and Organelles due to SARS-CoV-2 Infection
2.1. Cytopathic Effects on Mitochondria
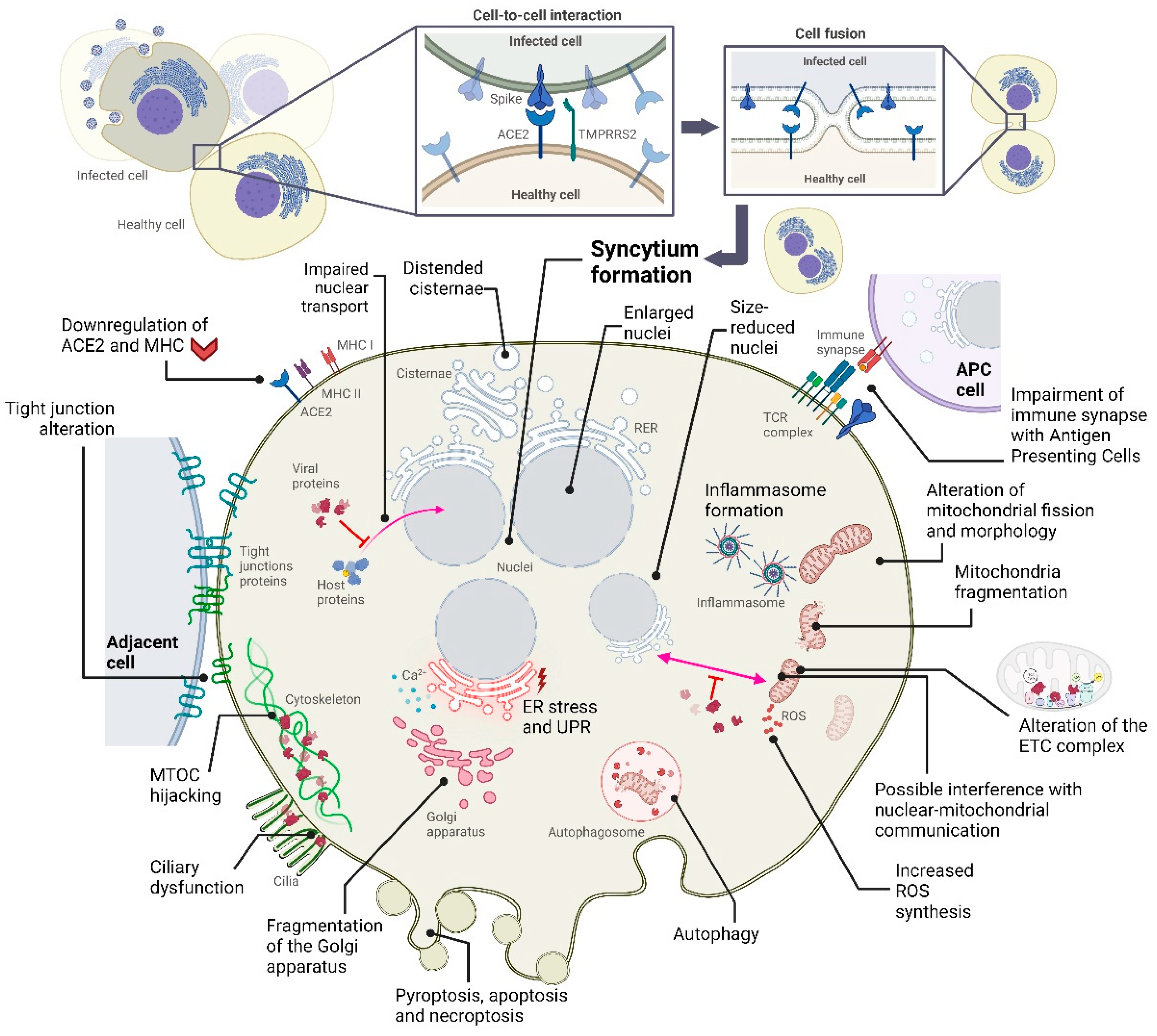
2.2. Cytopathic Effects on the Endoplasmic Reticulum
2.3. Cytopathic Effects on the Golgi Apparatus
2.4. Cytopathic Effects on the Cytoskeleton and Plasma Membrane
2.5. Cytopathic Effects on the Nucleus
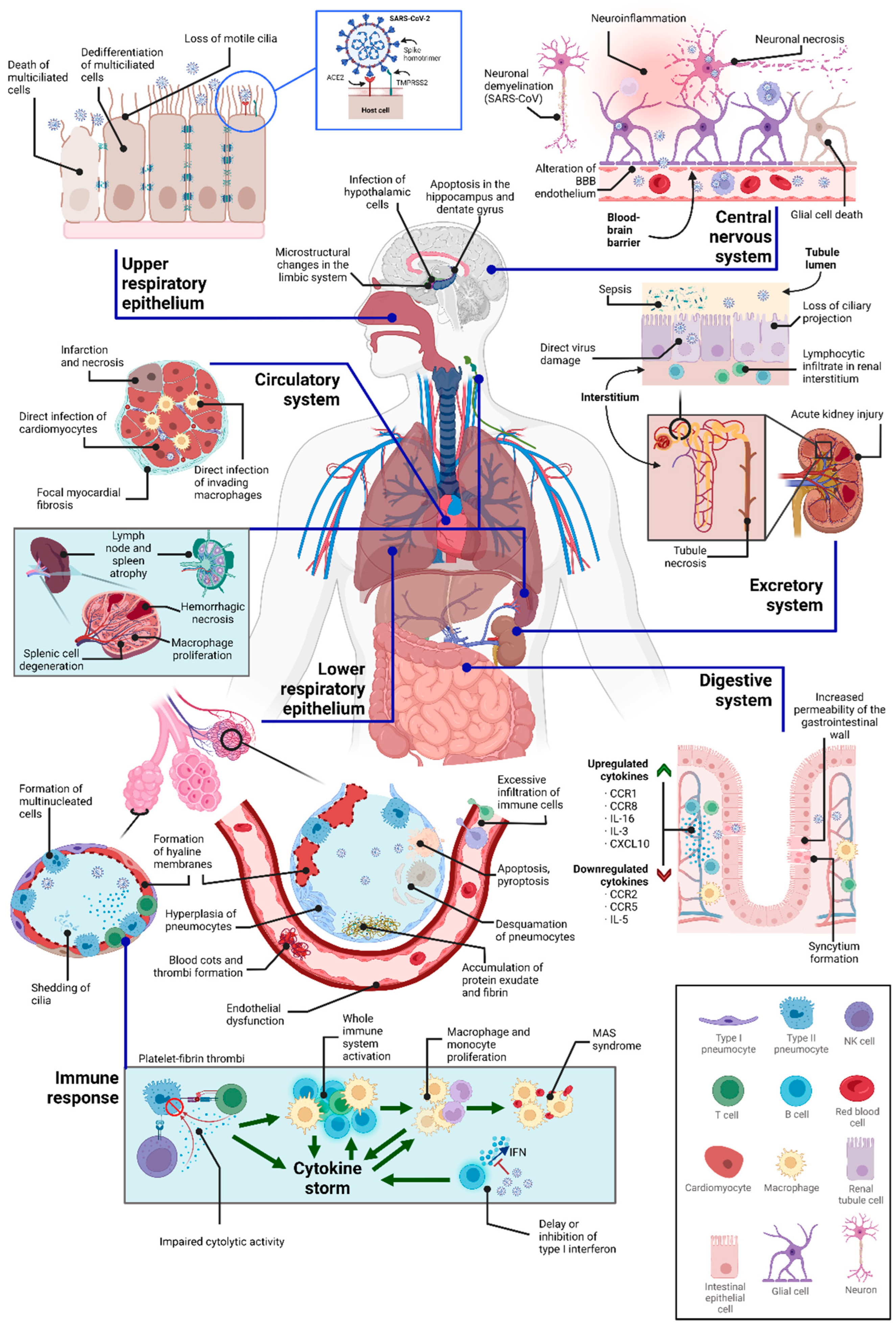
3. Direct Cytopathic Effects in Various Tissues
3.1. Cytopathic Effects on the Respiratory System
3.2. Cytopathic Effects on the Circulatory System
3.3. Cytopathic Effects on the Kidney
3.4. Cytopathic Effects on the Digestive System
3.5. Cytopathic Effects on the Central Nervous System
3.6. Cytopathic Effects on the Immune System
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [CrossRef]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [CrossRef]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020, 295, 12910–12934. [CrossRef]
- Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The Architecture of SARS-CoV-2 Transcriptome. Cell. 2020 May;181(4):914-921.e10.
- Marzi, A.; Gramberg, T.; Simmons, G.; Möller, P.; Rennekamp, A.J.; Krumbiegel, M.; Geier, M.; Eisemann, J.; Turza, N.; Saunier, B.; et al. DC-SIGN and DC-SIGNR Interact with the Glycoprotein of Marburg Virus and the S Protein of Severe Acute Respiratory Syndrome Coronavirus. J. Virol. 2004, 78, 12090–12095. [CrossRef]
- Wong, A.H.M.; Zhou, D.; Rini, J.M. The X-ray Crystal Structure of Human Aminopeptidase N Reveals a Novel Dimer and the Basis for Peptide Processing. J. Biol. Chem. 2012, 287, 36804–36813. [CrossRef]
- Zhang S, Zhou P, Wang P, Li Y, Jiang L, Jia W, et al. Structural Definition of a Unique Neutralization Epitope on the Receptor-Binding Domain of MERS-CoV Spike Glycoprotein. Cell Reports. 2018 Jul;24(2):441–52. [CrossRef]
- Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020 Nov 13;370(6518):856–60. [CrossRef]
- Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Antón-Plágaro C, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020 Nov 13;370(6518):861–5. [CrossRef]
- Zelus, B.D.; Schickli, J.H.; Blau, D.M.; Weiss, S.R.; Holmes, K.V. Conformational Changes in the Spike Glycoprotein of Murine Coronavirus Are Induced at 37°C either by Soluble Murine CEACAM1 Receptors or by pH 8. J. Virol. 2003, 77, 830–840. [CrossRef]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr;181(2):271-280.e8. [CrossRef]
- Momtazi-Borojeni AA, Banach M, Reiner Ž, Pirro M, Bianconi V, Al-Rasadi K, et al. Interaction Between Coronavirus S-Protein and Human ACE2: Hints for Exploring Efficient Therapeutic Targets to Treat COVID-19. Angiology. 2021 Feb;72(2):122–30. [CrossRef]
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020 May 29;368(6494):1016–20. [CrossRef]
- Glowacka, I.; Bertram, S.; Müller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response. J. Virol. 2011, 85, 4122–4134. [CrossRef]
- Bayati A, Kumar R, Francis V, McPherson PS. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. Journal of Biological Chemistry. 2021 Jan;296:100306. [CrossRef]
- Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022 Jan;23(1):3–20. [CrossRef]
- Boson B, Legros V, Zhou B, Siret E, Mathieu C, Cosset FL, et al. The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. Journal of Biological Chemistry. 2021 Jan;296:100111. [CrossRef]
- Khan MT, Irfan M, Ahsan H, Ahmed A, Kaushik AC, Khan AS, et al. Structures of SARS-CoV-2 RNA-Binding Proteins and Therapeutic Targets. Intervirology. 2021;64(2):55–68. [CrossRef]
- Wu, H.-Y.; Brian, D.A. Subgenomic messenger RNA amplification in coronaviruses. Proc. Natl. Acad. Sci. 2010, 107, 12257–12262. [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [CrossRef]
- Delorey TM, Ziegler CGK, Heimberg G, Normand R, Yang Y, Segerstolpe Å, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021 Jul;595(7865):107–13. [CrossRef]
- Wang XM, Mannan R, Xiao L, Abdulfatah E, Qiao Y, Farver C, et al. Characterization of SARS-CoV-2 and host entry factors distribution in a COVID-19 autopsy series. Commun Med (Lond). 2021;1:24. [CrossRef]
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol [Internet]. 2023 Jan 13 [cited 2023 Mar 2]; Available from: https://www.nature.com/articles/s41579-022-00846-2. [CrossRef]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021 Apr;27(4):601–15. [CrossRef]
- Javadov, S.; Kozlov, A.V.; Camara, A.K.S. Mitochondria in Health and Diseases. Cells 2020, 9, 1177. [CrossRef]
- Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 Jul;583(7816):459–68. [CrossRef]
- Morita, M.; Ler, L.W.; Fabian, M.R.; Siddiqui, N.; Mullin, M.; Henderson, V.C.; Alain, T.; Fonseca, B.D.; Karashchuk, G.; Bennett, C.F.; et al. A Novel 4EHP-GIGYF2 Translational Repressor Complex Is Essential for Mammalian Development. Mol. Cell. Biol. 2012, 32, 3585–3593. [CrossRef]
- Zhao, G.; Shi, S.-Q.; Yang, Y.; Peng, J.-P. M and N proteins of SARS coronavirus induce apoptosis in HPF cells. Cell Biol. Toxicol. 2006, 22, 313–322. [CrossRef]
- Gao S, Zhang L. ACE2 partially dictates the host range and tropism of SARS-CoV-2. Comput Struct Biotechnol J. 2020;18:4040–7. [CrossRef]
- Archer SL, Dasgupta A, Chen KH, Wu D, Baid K, Mamatis JE, et al. SARS-CoV-2 mitochondriopathy in COVID-19 pneumonia exacerbates hypoxemia. Redox Biol. 2022 Dec;58:102508. [CrossRef]
- Du, J.; Zhou, Y.; Su, X.; Yu, J.J.; Khan, S.; Jiang, H.; Kim, J.; Woo, J.; Kim, J.H.; Choi, B.H.; et al. Sirt5 Is a NAD-Dependent Protein Lysine Demalonylase and Desuccinylase. Science 2011, 334, 806–809. [CrossRef]
- Walter M, Chen IP, Vallejo-Gracia A, Kim IJ, Bielska O, Lam VL, et al. SIRT5 is a proviral factor that interacts with SARS-CoV-2 Nsp14 protein. Dittmann M, editor. PLoS Pathog. 2022 Sep 12;18(9):e1010811. [CrossRef]
- Batra N, De Souza C, Batra J, Raetz AG, Yu AM. The HMOX1 Pathway as a Promising Target for the Treatment and Prevention of SARS-CoV-2 of 2019 (COVID-19). Int J Mol Sci. 2020 Sep 3;21(17):6412. [CrossRef]
- Zhang S, Wang J, Wang L, Aliyari S, Cheng G. SARS-CoV-2 virus NSP14 Impairs NRF2/HMOX1 activation by targeting Sirtuin 1. Cell Mol Immunol. 2022 Jun 23;19(8):872–82. [CrossRef]
- Feng Y, Tang K, Lai Q, Liang J, Feng M, Zhou ZW, et al. The Landscape of Aminoacyl-tRNA Synthetases Involved in Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Front Physiol. 2021;12:818297. [CrossRef]
- Ghosh N, Saha I, Sharma N. Interactome of human and SARS-CoV-2 proteins to identify human hub proteins associated with comorbidities. Comput Biol Med. 2021 Nov;138:104889. [CrossRef]
- Wang, T.; Cao, Y.; Zhang, H.; Wang, Z.; Man, C.H.; Yang, Y.; Chen, L.; Xu, S.; Yan, X.; Zheng, Q.; et al. COVID-19 metabolism: Mechanisms and therapeutic targets. Medcomm 2022, 3, e157. [CrossRef]
- Neupane, N.; Rajendran, J.; Kvist, J.; Harjuhaahto, S.; Hu, B.; Kinnunen, V.; Yang, Y.; Nieminen, A.I.; Tyynismaa, H. Inter-organellar and systemic responses to impaired mitochondrial matrix protein import in skeletal muscle. Commun. Biol. 2022, 5, 1–12. [CrossRef]
- Jiang HW, Zhang HN, Meng QF, Xie J, Li Y, Chen H, et al. SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell Mol Immunol. 2020 Sep;17(9):998–1000. [CrossRef]
- Liu Q, Chang CE, Wooldredge AC, Fong B, Kennedy BK, Zhou C. Tom70-based transcriptional regulation of mitochondrial biogenesis and aging. eLife. 2022 Mar 2;11:e75658. [CrossRef]
- Miller, K.; McGrath, M.E.; Hu, Z.; Ariannejad, S.; Weston, S.; Frieman, M.; Jackson, W.T. Coronavirus interactions with the cellular autophagy machinery. Autophagy 2020, 16, 2131–2139. [CrossRef]
- Shi CS, Qi HY, Boularan C, Huang NN, Abu-Asab M, Shelhamer JH, et al. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol. 2014 Sep 15;193(6):3080–9. [CrossRef]
- Wang, T.; Cao, Y.; Zhang, H.; Wang, Z.; Man, C.H.; Yang, Y.; Chen, L.; Xu, S.; Yan, X.; Zheng, Q.; et al. COVID-19 metabolism: Mechanisms and therapeutic targets. Medcomm 2022, 3, e157. [CrossRef]
- Du, C.; Liu, W.-J.; Yang, J.; Zhao, S.-S.; Liu, H.-X. The Role of Branched-Chain Amino Acids and Branched-Chain α-Keto Acid Dehydrogenase Kinase in Metabolic Disorders. Front. Nutr. 2022, 9, 932670. [CrossRef]
- Nunn AVW, Guy GW, Brysch W, Bell JD. Understanding Long COVID; Mitochondrial Health and Adaptation—Old Pathways, New Problems. Biomedicines. 2022 Dec 2;10(12):3113. [CrossRef]
- Stefano, G.B.; Büttiker, P.; Weissenberger, S.; Martin, A.; Ptacek, R.; Kream, R.M. Editorial: The Pathogenesis of Long-Term Neuropsychiatric COVID-19 and the Role of Microglia, Mitochondria, and Persistent Neuroinflammation: A Hypothesis. Med Sci. Monit. 2021, 27, e933015. [CrossRef]
- Paul, B.D.; Lemle, M.D.; Komaroff, A.L.; Snyder, S.H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2021, 118. [CrossRef]
- Wood E, Hall KH, Tate W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 ‘long-haulers’? Chronic Diseases and Translational Medicine. 2021 Mar;7(1):14–26.
- Rosa-Fernandes L, Lazari LC, da Silva JM, de Morais Gomes V, Machado RRG, dos Santos AF, et al. SARS-CoV-2 activates ER stress and Unfolded protein response [Internet]. Biochemistry; 2021 Jun [cited 2023 Mar 2]. Available from: http://biorxiv.org/lookup/doi/10.1101/2021.06.21. [CrossRef]
- Aoe, T. Pathological Aspects of COVID-19 as a Conformational Disease and the Use of Pharmacological Chaperones as a Potential Therapeutic Strategy. Front. Pharmacol. 2020, 11, 1095. [CrossRef]
- Sureda A, Alizadeh J, Nabavi SF, Berindan-Neagoe I, Cismaru CA, Jeandet P, et al. Endoplasmic reticulum as a potential therapeutic target for covid-19 infection management? European Journal of Pharmacology. 2020 Sep;882:173288. [CrossRef]
- Upadhyay M, Gupta S. Endoplasmic reticulum secretory pathway: Potential target against SARS-CoV-2. Virus Research. 2022 Oct;320:198897. [CrossRef]
- Zhang Z, Nomura N, Muramoto Y, Ekimoto T, Uemura T, Liu K, et al. Structure of SARS-CoV-2 membrane protein essential for virus assembly. Nat Commun. 2022 Aug 5;13(1):4399. [CrossRef]
- Rashid F, Dzakah EE, Wang H, Tang S. The ORF8 protein of SARS-CoV-2 induced endoplasmic reticulum stress and mediated immune evasion by antagonizing production of interferon beta. Virus Research. 2021 Apr;296:198350. [CrossRef]
- Yiang, G.-T.; Wu, C.-C.; Lu, C.-L.; Hu, W.-C.; Tsai, Y.-J.; Huang, Y.-M.; Su, W.-L.; Lu, K.-C. Endoplasmic Reticulum Stress in Elderly Patients with COVID-19: Potential of Melatonin Treatment. Viruses 2023, 15, 156. [CrossRef]
- Wang S, Tukachinsky H, Romano FB, Rapoport TA. Cooperation of the ER-shaping proteins atlastin, lunapark, and reticulons to generate a tubular membrane network. Elife. 2016 Sep 13;5:e18605. [CrossRef]
- Yao, L.; Xie, D.; Geng, L.; Shi, D.; Huang, J.; Wu, Y.; Lv, F.; Liang, D.; Li, L.; Liu, Y.; et al. REEP5 (Receptor Accessory Protein 5) Acts as a Sarcoplasmic Reticulum Membrane Sculptor to Modulate Cardiac Function. J. Am. Hear. Assoc. 2018, 7. [CrossRef]
- Björk S, Hurt CM, Ho VK, Angelotti T. REEPs Are Membrane Shaping Adapter Proteins That Modulate Specific G Protein-Coupled Receptor Trafficking by Affecting ER Cargo Capacity. Behrens M, editor. PLoS ONE. 2013 Oct 2;8(10):e76366. [CrossRef]
- Son, Y.; Choi, C.; Saha, A.; Park, J.-H.; Im, H.; Cho, Y.K.; Seong, J.K.; Burl, R.B.; Rondini, E.A.; Granneman, J.G.; et al. REEP6 knockout leads to defective β-adrenergic signaling in adipocytes and promotes obesity-related metabolic dysfunction.. Metabolism 2022, 130, 155159. [CrossRef]
- Feng L, Yin YY, Liu CH, Xu KR, Li QR, Wu JR, et al. Proteome-wide data analysis reveals tissue-specific network associated with SARS-CoV-2 infection. Chen L, editor. Journal of Molecular Cell Biology. 2021 Mar 10;12(12):946–57. [CrossRef]
- Park, C.R.; You, D.-J.; Park, S.; Mander, S.; Jang, D.-E.; Yeom, S.-C.; Oh, S.-H.; Ahn, C.; Lee, S.H.; Seong, J.Y.; et al. The accessory proteins REEP5 and REEP6 refine CXCR1-mediated cellular responses and lung cancer progression. Sci. Rep. 2016, 6, 39041. [CrossRef]
- Hayashi, T.; Su, T.-P. Sigma-1 Receptor Chaperones at the ER- Mitochondrion Interface Regulate Ca2+ Signaling and Cell Survival. Cell 2007, 131, 596–610. [CrossRef]
- van Waarde, A.; Rybczynska, A.A.; Ramakrishnan, N.K.; Ishiwata, K.; Elsinga, P.H.; Dierckx, R.A. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. Biochim. et Biophys. Acta (BBA)—Biomembr. 2015, 1848, 2703–2714. [CrossRef]
- Huang YS, Lu HL, Zhang LJ, Wu Z. Sigma-2 Receptor Ligands and Their Perspectives in Cancer Diagnosis and Therapy: SIGMA-2 RECEPTOR LIGANDS. Med Res Rev. 2014 May;34(3):532–66. [CrossRef]
- Rosen, D.A.; Seki, S.M.; Fernández-Castañeda, A.; Beiter, R.M.; Eccles, J.D.; Woodfolk, J.A.; Gaultier, A. Modulation of the sigma-1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 2019, 11. [CrossRef]
- Alon A, Schmidt HR, Wood MD, Sahn JJ, Martin SF, Kruse AC. Identification of the gene that codes for the σ 2 receptor. Proc Natl Acad Sci USA. 2017 Jul 3;114(27):7160–5. [CrossRef]
- Ahmed ISA, Chamberlain C, Craven RJ. S2R Pgrmc1 : the cytochrome-related sigma-2 receptor that regulates lipid and drug metabolism and hormone signaling. Expert Opinion on Drug Metabolism & Toxicology. 2012 Mar;8(3):361–70. [CrossRef]
- Skuza, G. Potential antidepressant activity of sigma ligands. Pol J Pharmacol. 2003, 55, 923–34.
- Tang SW, Leonard BE, Helmeste DM. Long COVID, neuropsychiatric disorders, psychotropics, present and future. Acta Neuropsychiatr. 2022 Jun;34(3):109–26. [CrossRef]
- Hashimoto, K. Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 249–258. [CrossRef]
- Martin-Montalvo, A.; Sun, Y.; Diaz-Ruiz, A.; Ali, A.; Gutierrez, V.; Palacios, H.H.; Curtis, J.; Siendones, E.; Ariza, J.; A Abulwerdi, G.; et al. Cytochrome b5 reductase and the control of lipid metabolism and healthspan. npj Aging Mech. Dis. 2016, 2, 16006. [CrossRef]
- Nagasawa, M.; Kanzaki, M.; Iino, Y.; Morishita, Y.; Kojima, I. Identification of a Novel Chloride Channel Expressed in the Endoplasmic Reticulum, Golgi Apparatus, and Nucleus. J. Biol. Chem. 2001, 276, 20413–20418. [CrossRef]
- Wang, C.; Yoo, Y.; Fan, H.; Kim, E.; Guan, K.-L.; Guan, J.-L. Regulation of Integrin β1 Recycling to Lipid Rafts by Rab1a to Promote Cell Migration. J. Biol. Chem. 2010, 285, 29398–29405. [CrossRef]
- Reggiori, F.; Monastyrska, I.; Verheije, M.H.; Calì, T.; Ulasli, M.; Bianchi, S.; Bernasconi, R.; de Haan, C.A.; Molinari, M. Coronaviruses Hijack the LC3-I-Positive EDEMosomes, ER-Derived Vesicles Exporting Short-Lived ERAD Regulators, for Replication. Cell Host Microbe 2010, 7, 500–508. [CrossRef]
- Sicari D, Chatziioannou A, Koutsandreas T, Sitia R, Chevet E. Role of the early secretory pathway in SARS-CoV-2 infection. Journal of Cell Biology. 2020 Sep 7;219(9):e202006005.
- Cortese M, Lee JY, Cerikan B, Neufeldt CJ, Oorschot VMJ, Köhrer S, et al. Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies. Cell Host Microbe. 2020 Dec 9;28(6):853-866.e5. [CrossRef]
- Zhang J, Kennedy A, Xing L, Bui S, Reid W, Joppich J, et al. SARS-CoV-2 triggers Golgi fragmentation via down-regulation of GRASP55 to facilitate viral trafficking [Internet]. Cell Biology; 2022 Mar [cited 2023 Mar 3]. Available from: http://biorxiv.org/lookup/doi/10.1101/2022.03.04.483074. [CrossRef]
- Liu, J.; Huang, Y.; Li, T.; Jiang, Z.; Zeng, L.; Hu, Z. The role of the Golgi apparatus in disease (Review). Int. J. Mol. Med. 2021, 47, 1–1. [CrossRef]
- Paces, J.; Strizova, Z.; Smrz, D.; Cerny, J. COVID-19 and the Immune System. Physiol. Res. 2020, 69, 379–388. [CrossRef]
- Wang Y, Gandy S. The Golgi apparatus: Site for convergence of COVID-19 brain fog and Alzheimer’s disease? Mol Neurodegeneration. 2022 Oct 21;17(1):67. [CrossRef]
- Devergnas S, Chimienti F, Naud N, Pennequin A, Coquerel Y, Chantegrel J, et al. Differential regulation of zinc efflux transporters ZnT-1, ZnT-5 and ZnT-7 gene expression by zinc levels: a real-time RT-PCR study. Biochem Pharmacol. 2004 Aug 15;68(4):699–709. [CrossRef]
- Kirschke, C.P.; Huang, L. ZnT7, a Novel Mammalian Zinc Transporter, Accumulates Zinc in the Golgi Apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [CrossRef]
- Matern, H.; Yang, X.; Andrulis, E.; Sternglanz, R.; Trepte, H.; Gallwitz, D. A novel Golgi membrane protein is part of a GTPase-binding protein complex involved in vesicle targeting. EMBO J. 2000, 19, 4485–4492. [CrossRef]
- Schulz, J.; Avci, D.; Queisser, M.A.; Gutschmidt, A.; Dreher, L.-S.; Fenech, E.J.; Volkmar, N.; Hayashi, Y.; Hoppe, T.; Christianson, J.C. Conserved cytoplasmic domains promote Hrd1 ubiquitin ligase complex formation for ER-associated degradation (ERAD). J. Cell Sci. 2017, 130, 3322–3335. [CrossRef]
- van de Weijer ML, Krshnan L, Liberatori S, Guerrero EN, Robson-Tull J, Hahn L, et al. Quality Control of ER Membrane Proteins by the RNF185/Membralin Ubiquitin Ligase Complex. Mol Cell. 2020 Sep 3;79(5):768-781.e7. [CrossRef]
- Jin, C.; Zhang, Y.; Zhu, H.; Ahmed, K.; Fu, C.; Yao, X. Human Yip1A specifies the localization of Yif1 to the Golgi apparatus. Biochem. Biophys. Res. Commun. 2005, 334, 16–22. [CrossRef]
- Adelino, J.; Addobbati, C.; Pontillo, A.; Fragoso, T.; Duarte, .; Crovella, S.; Silva, J.D.A.; Sandrin-Garcia, P. A genetic variant within SLC30A6 has a protective role in the severity of rheumatoid arthritis. Scand. J. Rheumatol. 2017, 46, 326–327. [CrossRef]
- Fukunaka, A.; Suzuki, T.; Kurokawa, Y.; Yamazaki, T.; Fujiwara, N.; Ishihara, K.; Migaki, H.; Okumura, K.; Masuda, S.; Yamaguchi-Iwai, Y.; et al. Demonstration and Characterization of the Heterodimerization of ZnT5 and ZnT6 in the Early Secretory Pathway. J. Biol. Chem. 2009, 284, 30798–30806. [CrossRef]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [CrossRef]
- Mahmoud, M.M.; Abuohashish, H.M.; A Khairy, D.; Bugshan, A.S.; Khan, A.M.; Moothedath, M.M. Pathogenesis of dysgeusia in COVID-19 patients: a scoping review. European Review for Medical and Pharmacological Sciences. 2021, 25, 1114–1134. [CrossRef]
- Larocca, M.C.; Shanks, R.A.; Tian, L.; Nelson, D.L.; Stewart, D.M.; Goldenring, J.R. AKAP350 Interaction with cdc42 Interacting Protein 4 at the Golgi Apparatus. Mol. Biol. Cell 2004, 15, 2771–2781. [CrossRef]
- Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006 Mar;8(3):238–48. [CrossRef]
- Witczak, O.; Skålhegg, B.S.; Keryer, G.; Bornens, M.; Taskén, K.; Jahnsen, T.; Ørstavik, S. Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450. EMBO J. 1999, 18, 1858–1868. [CrossRef]
- Wu, J.; de Heus, C.; Liu, Q.; Bouchet, B.P.; Noordstra, I.; Jiang, K.; Hua, S.; Martin, M.; Yang, C.; Grigoriev, I.; et al. Molecular Pathway of Microtubule Organization at the Golgi Apparatus. Dev. Cell 2016, 39, 44–60. [CrossRef]
- Munro, S. The Golgin Coiled-Coil Proteins of the Golgi Apparatus. Cold Spring Harb. Perspect. Biol. 2011, 3, a005256–a005256. [CrossRef]
- Lowe, M. The Physiological Functions of the Golgin Vesicle Tethering Proteins. Front. Cell Dev. Biol. 2019, 7, 94. [CrossRef]
- Weiss, R.J.; Spahn, P.N.; Toledo, A.G.; Chiang, A.W.T.; Kellman, B.P.; Li, J.; Benner, C.; Glass, C.K.; Gordts, P.L.S.M.; Lewis, N.E.; et al. ZNF263 is a transcriptional regulator of heparin and heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. 2020, 117, 9311–9317. [CrossRef]
- Kloc M, Uosef A, Wosik J, Kubiak JZ, Ghobrial RM. Virus interactions with the actin cytoskeleton—what we know and do not know about SARS-CoV-2. Arch Virol. 2022 Mar;167(3):737–49. [CrossRef]
- Aminpour, M.; Hameroff, S.; Tuszynski, J.A. How COVID-19 Hijacks the Cytoskeleton: Therapeutic Implications. Life 2022, 12, 814. [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Oldridge, D.A.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals patient heterogeneity and distinct immunotypes with implications for therapeutic interventions. [Internet]. Immunology; 2020 May [cited 2023 Mar 3]. Available from: http://bioRxiv.org/lookup/doi/10.1101/2020.05.20.106401 2020, 369. [CrossRef]
- Michie, K.A.; Bermeister, A.; Robertson, N.O.; Goodchild, S.C.; Curmi, P.M.G. Two Sides of the Coin: Ezrin/Radixin/Moesin and Merlin Control Membrane Structure and Contact Inhibition. Int. J. Mol. Sci. 2019, 20, 1996. [CrossRef]
- Pasapera, A.M.; Heissler, S.M.; Eto, M.; Nishimura, Y.; Fischer, R.S.; Thiam, H.R.; Waterman, C.M. MARK2 regulates directed cell migration through modulation of myosin II contractility and focal adhesion organization. Curr. Biol. 2022, 32, 2704–2718.e6. [CrossRef]
- Thies E, Mandelkow EM. Missorting of Tau in Neurons Causes Degeneration of Synapses That Can Be Rescued by the Kinase MARK2/Par-1. J Neurosci. 2007 Mar 14;27(11):2896–907. [CrossRef]
- Matenia, D.; Hempp, C.; Timm, T.; Eikhof, A.; Mandelkow, E.-M. Microtubule Affinity-regulating Kinase 2 (MARK2) Turns on Phosphatase and Tensin Homolog (PTEN)-induced Kinase 1 (PINK1) at Thr-313, a Mutation Site in Parkinson Disease. J. Biol. Chem. 2012, 287, 8174–8186. [CrossRef]
- Pera, T.; Tompkins, E.; Katz, M.; Wang, B.; Deshpande, D.A.; Weinman, E.J.; Penn, R.B. Specificity of NHERF1 regulation of GPCR signaling and function in human airway smooth muscle. FASEB J. 2019, 33, 9008–9016. [CrossRef]
- Youn JY, Dunham WH, Hong SJ, Knight JDR, Bashkurov M, Chen GI, et al. High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies. Mol Cell. 2018 Feb 1;69(3):517-532.e11. [CrossRef]
- Szymanski, D. Tubulin Folding Cofactors: Half a Dozen for a Dimer. Curr. Biol. 2002, 12, R767–R769. [CrossRef]
- Wang, Y.; Zhan, Q. Cell Cycle-dependent Expression of Centrosomal Ninein-like Protein in Human Cells Is Regulated by the Anaphase-promoting Complex. J. Biol. Chem. 2007, 282, 17712–17719. [CrossRef]
- Bachmann-Gagescu, R.; Dona, M.; Hetterschijt, L.; Tonnaer, E.; Peters, T.; de Vrieze, E.; Mans, D.A.; van Beersum, S.E.C.; Phelps, I.G.; Arts, H.H.; et al. The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking. PLOS Genet. 2015, 11, e1005575. [CrossRef]
- Dona, M.; Bachmann-Gagescu, R.; Texier, Y.; Toedt, G.; Hetterschijt, L.; Tonnaer, E.L.; Peters, T.A.; van Beersum, S.E.C.; Bergboer, J.G.M.; Horn, N.; et al. NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish. PLOS Genet. 2015, 11, e1005574. [CrossRef]
- van Wijk E, Kersten FFJ, Kartono A, Mans DA, Brandwijk K, Letteboer SJF, et al. Usher syndrome and Leber congenital amaurosis are molecularly linked via a novel isoform of the centrosomal ninein-like protein. Hum Mol Genet. 2009 Jan 1;18(1):51–64. [CrossRef]
- Wang L, Liu C, Yang B, Zhang H, Jiao J, Zhang R, et al. SARS-CoV-2 ORF10 impairs cilia by enhancing CUL2ZYG11B activity. Journal of Cell Biology. 2022 Jul 4;221(7):e202108015.
- Waters, A.M.; Asfahani, R.; Carroll, P.; Bicknell, L.; Lescai, F.; Bright, A.; Chanudet, E.; Brooks, A.; Christou-Savina, S.; Osman, G.; et al. The kinetochore protein,CENPF, is mutated in human ciliopathy and microcephaly phenotypes. J. Med Genet. 2015, 52, 147–156. [CrossRef]
- Whitsett, J.A. Airway Epithelial Differentiation and Mucociliary Clearance. Ann. Am. Thorac. Soc. 2018, 15, S143–S148. [CrossRef]
- Christie, D.A.; Mitsopoulos, P.; Blagih, J.; Dunn, S.D.; St-Pierre, J.; Jones, R.G.; Hatch, G.M.; Madrenas, J. Stomatin-like Protein 2 Deficiency in T Cells Is Associated with Altered Mitochondrial Respiration and Defective CD4+ T Cell Responses. J. Immunol. 2012, 189, 4349–4360. [CrossRef]
- Onnis A, Andreano E, Cassioli C, Finetti F, Della Bella C, Staufer O, et al. SARS-CoV-2 Spike protein suppresses CTL-mediated killing by inhibiting immune synapse assembly. Journal of Experimental Medicine. 2023 Feb 6;220(2):e20220906. [CrossRef]
- Fackler OT, Alcover A, Schwartz O. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat Rev Immunol. 2007 Apr;7(4):310–7. [CrossRef]
- Abdel Hameid R, Cormet-Boyaka E, Kuebler WM, Uddin M, Berdiev BK. SARS-CoV-2 may hijack GPCR signaling pathways to dysregulate lung ion and fluid transport. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2021 Mar 1;320(3):L430–5. [CrossRef]
- Motley, A.; Bright, N.A.; Seaman, M.N.; Robinson, M.S. Clathrin-mediated endocytosis in AP-2–depleted cells. J. Cell Biol. 2003, 162, 909–918. [CrossRef]
- Liu, Q.; Bautista-Gomez, J.; Higgins, D.A.; Yu, J.; Xiong, Y. Dysregulation of the AP2M1 phosphorylation cycle by LRRK2 impairs endocytosis and leads to dopaminergic neurodegeneration. Sci. Signal. 2021, 14. [CrossRef]
- Karim M, Saul S, Ghita L, Sahoo MK, Ye C, Bhalla N, et al. Numb-associated kinases are required for SARS-CoV-2 infection and are cellular targets for antiviral strategies. Antiviral Research. 2022 Aug;204:105367. [CrossRef]
- Puray-Chavez M, LaPak KM, Schrank TP, Elliott JL, Bhatt DP, Agajanian MJ, et al. Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell. Cell Reports. 2021 Jul;36(2):109364. [CrossRef]
- Schreiner T, Allnoch L, Beythien G, Marek K, Becker K, Schaudien D, et al. SARS-CoV-2 Infection Dysregulates Cilia and Basal Cell Homeostasis in the Respiratory Epithelium of Hamsters. IJMS. 2022 ;23(9):5124. [CrossRef]
- Wang W, Zhou Z, Xiao X, Tian Z, Dong X, Wang C, et al. SARS-CoV-2 nsp12 attenuates type I interferon production by inhibiting IRF3 nuclear translocation. Cell Mol Immunol. 2021 Apr;18(4):945–53. [CrossRef]
- Kato K, Ikliptikawati DK, Kobayashi A, Kondo H, Lim K, Hazawa M, et al. Overexpression of SARS-CoV-2 protein ORF6 dislocates RAE1 and NUP98 from the nuclear pore complex. Biochem Biophys Res Commun. 2021 Jan 15;536:59–66. [CrossRef]
- Miorin L, Kehrer T, Sanchez-Aparicio MT, Zhang K, Cohen P, Patel RS, et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc Natl Acad Sci U S A. 2020 Nov 10;117(45):28344–54. [CrossRef]
- Mu J, Fang Y, Yang Q, Shu T, Wang A, Huang M, et al. SARS-CoV-2 N protein antagonizes type I interferon signaling by suppressing phosphorylation and nuclear translocation of STAT1 and STAT2. Cell Discov. 2020;6:65. [CrossRef]
- Collins, S.E.; Noyce, R.S.; Mossman, K.L. Innate Cellular Response to Virus Particle Entry Requires IRF3 but Not Virus Replication. J. Virol. 2004, 78, 1706–17. [CrossRef]
- Zhang K, Miorin L, Makio T, Dehghan I, Gao S, Xie Y, et al. Nsp1 protein of SARS-CoV-2 disrupts the mRNA export machinery to inhibit host gene expression. Sci Adv. 2021 Feb;7(6):eabe7386. [CrossRef]
- Matuck BF, Dolhnikoff M, Duarte-Neto AN, Maia G, Gomes SC, Sendyk DI, et al. Salivary glands are a target for SARS-CoV-2: a source for saliva contamination. J Pathol. 2021 Jul;254(3):239–43. [CrossRef]
- Nardacci R, Colavita F, Castilletti C, Lapa D, Matusali G, Meschi S, et al. Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis. Cell Death Dis. 2021 Mar 12;12(3):263. [CrossRef]
- Buchrieser J, Dufloo J, Hubert M, Monel B, Planas D, Rajah MM, et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J. 2021 Feb 1;40(3):e107405. [CrossRef]
- Bussani, R.; Schneider, E.; Zentilin, L.; Collesi, C.; Ali, H.; Braga, L.; Volpe, M.C.; Colliva, A.; Zanconati, F.; Berlot, G.; et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine 2020, 61, 103104. [CrossRef]
- Hayden, M.R.; Tyagi, S.C. Impaired Folate-Mediated One-Carbon Metabolism in Type 2 Diabetes, Late-Onset Alzheimer’s Disease and Long COVID. Medicina 2021, 58, 16. [CrossRef]
- LeGros HL, Halim AB, Geller AM, Kotb M. Cloning, expression, and functional characterization of the beta regulatory subunit of human methionine adenosyltransferase (MAT II). J Biol Chem. 2000 Jan 28;275(4):2359–66. [CrossRef]
- Wang F, Kream RM, Stefano GB. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med Sci Monit [Internet]. 2020 Nov 1 [cited 2023 Jan 31];26. Available from: https://www.medscimonit.com/abstract/index/idArt/928996. [CrossRef]
- Gallo, O.; Locatello, L.G.; Mazzoni, A.; Novelli, L.; Annunziato, F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2020, 14, 305–316. [CrossRef]
- Zhu N, Wang W, Liu Z, Liang C, Wang W, Ye F, et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat Commun. 2020 Aug 6;11(1):3910. [CrossRef]
- Bridges JP, Vladar EK, Huang H, Mason RJ. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax. 2022 Feb;77(2):203–9. [CrossRef]
- Morrison CB, Edwards CE, Shaffer KM, Araba KC, Wykoff JA, Williams DR, et al. SARS-CoV-2 infection of airway cells causes intense viral and cell shedding, two spreading mechanisms affected by IL-13. Proc Natl Acad Sci USA. 2022 Apr 19;119(16):e2119680119. [CrossRef]
- Takeda, K.; Sakakibara, S.; Yamashita, K.; Motooka, D.; Nakamura, S.; El Hussien, M.A.; Katayama, J.; Maeda, Y.; Nakata, M.; Hamada, S.; et al. Allergic conversion of protective mucosal immunity against nasal bacteria in patients with chronic rhinosinusitis with nasal polyposis. J. Allergy Clin. Immunol. 2019, 143, 1163–1175.e15. [CrossRef]
- Ahn JH, Kim J, Hong SP, Choi SY, Yang MJ, Ju YS, et al. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J Clin Invest. 2021 Jul 1;131(13):e148517, 148517. [CrossRef]
- Robinot R, Hubert M, de Melo GD, Lazarini F, Bruel T, Smith N, et al. SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance. Nat Commun. 2021 Jul 16;12(1):4354. [CrossRef]
- Wahl A, Gralinski L, Johnson C, Yao W, Kovarova M, Dinnon K, et al. Acute SARS-CoV-2 Infection is Highly Cytopathic, Elicits a Robust Innate Immune Response and is Efficiently Prevented by EIDD-2801. Res Sq. 2020 Sep 24;rs.3.rs-80404. [CrossRef]
- Huang B. Mucins produced by type II pneumocyte: culprits in SARS-CoV-2 pathogenesis. Cell Mol Immunol. 2021 Jul;18(7):1823–5. [CrossRef]
- Hu, G.; Christman, J.W. Editorial: Alveolar Macrophages in Lung Inflammation and Resolution. Front. Immunol. 2019, 10, 2275. [CrossRef]
- Keidar, S.; Gamliel-Lazarovich, A.; Kaplan, M.; Pavlotzky, E.; Hamoud, S.; Hayek, T.; Karry, R.; Abassi, Z.; M, G.; K, W.; et al. Mineralocorticoid Receptor Blocker Increases Angiotensin-Converting Enzyme 2 Activity in Congestive Heart Failure Patients. Circ. Res. 2005, 97, 946–953. [CrossRef]
- Gagnon H, Refaie S, Gagnon S, Desjardins R, Salzet M, Day R. Proprotein convertase 1/3 (PC1/3) in the rat alveolar macrophage cell line NR8383: localization, trafficking and effects on cytokine secretion. PLoS One. 2013;8(4):e61557. [CrossRef]
- Boumaza, A.; Gay, L.; Mezouar, S.; Bestion, E.; Diallo, A.B.; Michel, M.; Desnues, B.; Raoult, D.; La Scola, B.; Halfon, P.; et al. Monocytes and Macrophages, Targets of Severe Acute Respiratory Syndrome Coronavirus 2: The Clue for Coronavirus Disease 2019 Immunoparalysis. J. Infect. Dis. 2021, 224, 395–406. [CrossRef]
- Zheng, J.; Wang, Y.; Li, K.; Meyerholz, D.K.; Allamargot, C.; Perlman, S. Severe Acute Respiratory Syndrome Coronavirus 2–Induced Immune Activation and Death of Monocyte-Derived Human Macrophages and Dendritic Cells. J. Infect. Dis. 2020, 223, 785–795. [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [CrossRef]
- Niles MA, Gogesch P, Kronhart S, Ortega Iannazzo S, Kochs G, Waibler Z, et al. Macrophages and Dendritic Cells Are Not the Major Source of Pro-Inflammatory Cytokines Upon SARS-CoV-2 Infection. Front Immunol. 2021;12:647824. [CrossRef]
- Carfì A, Bernabei R, Landi F, Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020 Aug 11;324(6):603–5. [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; de Noordhout, C.M.; Jong, C.P.-D.; Cleemput, I.; Heede, K.V.D. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann. Med. 2022, 54, 1473–1487. [CrossRef]
- Bernard I, Limonta D, Mahal L, Hobman T. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses. 2020 Dec 26;13(1):29. [CrossRef]
- Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell. 2020 Nov;183(4):1043-1057.e15. [CrossRef]
- Robson B. Bioinformatics studies on a function of the SARS-CoV-2 spike glycoprotein as the binding of host sialic acid glycans. Computers in Biology and Medicine. 2020 Jul;122:103849. [CrossRef]
- Lim S, Zhang M, Chang TL. ACE2-Independent Alternative Receptors for SARS-CoV-2. Viruses. 2022 Nov 16;14(11):2535. [CrossRef]
- Nader D, Fletcher N, Curley GF, Kerrigan SW. SARS-CoV-2 uses major endothelial integrin αvβ3 to cause vascular dysregulation in-vitro during COVID-19. PLoS One. 2021;16(6):e0253347. [CrossRef]
- Schimmel L, Chew KY, Stocks CJ, Yordanov TE, Essebier P, Kulasinghe A, et al. Endothelial cells are not productively infected by SARS-CoV-2. Clin Transl Immunology. 2021;10(10):e1350. [CrossRef]
- Henry, B.M.; Vikse, J.; Benoit, S.; Favaloro, E.J.; Lippi, G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin. Chim. Acta 2020, 507, 167–173. [CrossRef]
- Costa TJ, Potje SR, Fraga-Silva TFC, da Silva-Neto JA, Barros PR, Rodrigues D, et al. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vascul Pharmacol. 2022 Feb;142:106946. [CrossRef]
- Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE2. bioRxiv. 2020 Dec 4;2020.12.04.409144. [CrossRef]
- Italia, L.; Tomasoni, D.; Bisegna, S.; Pancaldi, E.; Stretti, L.; Adamo, M.; Metra, M. COVID-19 and Heart Failure: From Epidemiology During the Pandemic to Myocardial Injury, Myocarditis, and Heart Failure Sequelae. Front. Cardiovasc. Med. 2021, 8, 713560. [CrossRef]
- Tudoran C, Tudoran M, Elena Lazureanu V, Raluca Marinescu A, Novacescu D, Georgiana Cut T. Impairment of the Cardiovascular System during SARS-CoV-2 Infection. In: Shah Y, editor. RNA Viruses Infection [Internet]. IntechOpen; 2022 [cited 2023 Mar 5]. Available from: https://www.intechopen. 8173.
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [CrossRef]
- Dixit NM, Churchill A, Nsair A, Hsu JJ. Post-Acute COVID-19 Syndrome and the cardiovascular system: What is known? American Heart Journal Plus: Cardiology Research and Practice. 2021 May;5:100025. [CrossRef]
- DePace, N.L.; Colombo, J. Long-COVID Syndrome and the Cardiovascular System: A Review of Neurocardiologic Effects on Multiple Systems. Curr. Cardiol. Rep. 2022, 24, 1711–1726. [CrossRef]
- Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–50. [CrossRef]
- Farshidfar, F.; Koleini, N.; Ardehali, H. Cardiovascular complications of COVID-19. J. Clin. Investig. 2021, 6. [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [CrossRef]
- Ramakrishnan, R.K.; Kashour, T.; Hamid, Q.; Halwani, R.; Tleyjeh, I.M. Unraveling the Mystery Surrounding Post-Acute Sequelae of COVID-19. Front. Immunol. 2021, 12, 686029. [CrossRef]
- Maghool F, Valiani A, Safari T, Emami MH, Mohammadzadeh S. Gastrointestinal and renal complications in SARS-CoV-2-infected patients: Role of immune system. Scand J Immunol [Internet]. 2021 Apr [cited 2023 Mar 5];93(4). Available from: https://onlinelibrary.wiley.com/doi/10.1111/sji.12999. [CrossRef]
- de Oliveira, P.; Cunha, K.; Neves, P.; Muniz, M.; Gatto, G.; Filho, N.S.; Guedes, F.; Silva, G. Renal Morphology in Coronavirus Disease: A Literature Review. Medicina 2021, 57, 258. [CrossRef]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intensiv. Care Med. 2020, 46, 1339–1348. [CrossRef]
- Werion A, Belkhir L, Perrot M, Schmit G, Aydin S, Chen Z, et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020 Nov;98(5):1296–307. [CrossRef]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 28;395(10229):1054-1062. [CrossRef]
- Bowe, B.; Xie, Y.; Xu, E.; Al-Aly, Z. Kidney Outcomes in Long COVID. J. Am. Soc. Nephrol. 2021, 32, 2851–2862. [CrossRef]
- Yende, S.; Parikh, C.R. Long COVID and kidney disease. Nat. Rev. Nephrol. 2021, 17, 792–793. [CrossRef]
- Svetitsky, S.; Shuaib, R.; McAdoo, S.; Thomas, D.C. Long-term effects of Covid-19 on the kidney. Qjm: Int. J. Med. 2021, 114, 621–622. [CrossRef]
- Ahmadian, E.; Khatibi, S.M.H.; Soofiyani, S.R.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Vahed, S.Z. Covid-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med Virol. 2021, 31, e2176. [CrossRef]
- Carriazo S, Aparicio-Madre MI, Tornero-Molina F, Fernández-Lucas M, Paraiso-Cuevas V, González-Parra E, et al. Impact of different COVID-19 waves on kidney replacement therapy epidemiology and mortality: REMER 2020. Nephrol Dial Transplant. 2022 Oct 19;37(11):2253–63. [CrossRef]
- Basic-Jukic, N.; Racki, S.; Tolj, I.; Aleckovic, M.; Babovic, B.; Juric, I.; Furic-Cunko, V.; Katalinic, L.; Mihaljevic, D.; Vujic, S.; et al. Hospitalization and death after recovery from acute COVID-19 among renal transplant recipients. Clin. Transplant. 2022, 36, e14572. [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; de Noordhout, C.M.; Jong, C.P.-D.; Cleemput, I.; Heede, K.V.D. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann. Med. 2022, 54, 1473–1487. [CrossRef]
- Stanifer ML, Kee C, Cortese M, Zumaran CM, Triana S, Mukenhirn M, et al. Critical Role of Type III Interferon in Controlling SARS-CoV-2 Infection in Human Intestinal Epithelial Cells. Cell Rep. 2020 Jul 7;32(1):107863. [CrossRef]
- Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020 ;5(47):eabc3582. [CrossRef]
- Vodnar DC, Mitrea L, Teleky BE, Szabo K, Călinoiu LF, Nemeş SA, et al. Coronavirus Disease (COVID-19) Caused by (SARS-CoV-2) Infections: A Real Challenge for Human Gut Microbiota. Front Cell Infect Microbiol. 2020 Dec 9;10:575559. [CrossRef]
- Bogariu, A.M.; Dumitrascu, D.L. Digestive involvement in Long- COVID syndrome. Med. Pharm. Rep. 2022, 95, 5–10. [CrossRef]
- Weng, J.; Li, Y.; Li, J.; Shen, L.; Zhu, L.; Liang, Y.; Lin, X.; Jiao, N.; Cheng, S.; Huang, Y.; et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol. Hepatol. 2021, 6, 344–346. [CrossRef]
- Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021 Mar 25;591(7851):639–44. [CrossRef]
- Baig AM. Deleterious Outcomes in Long-Hauler COVID-19: The Effects of SARS-CoV-2 on the CNS in Chronic COVID Syndrome. ACS Chem Neurosci. 2020 Dec 16;11(24):4017–20. [CrossRef]
- Villadiego J, García-Arriaza J, Ramírez-Lorca R, García-Swinburn R, Cabello-Rivera D, Rosales-Nieves AE, et al. Full protection from SARS-CoV-2 brain infection and damage in susceptible transgenic mice conferred by MVA-CoV2-S vaccine candidate. Nat Neurosci [Internet]. 2023 Jan 9 [cited 2023 Jan 31]; Available from: https://www.nature. 4159. [CrossRef]
- Banks, W.A.; Kastin, A.J.; Akerstrom, V. HIV-1 protein gp120 crosses the blood-brain barrier: Role of adsorptive endocytosis. Life Sci. 1997, 61, PL119–PL125. [CrossRef]
- Achar, A.; Ghosh, C. COVID-19-Associated Neurological Disorders: The Potential Route of CNS Invasion and Blood-Brain Barrier Relevance. Cells 2020, 9, 2360. [CrossRef]
- Baig, A.M. Counting the neurological cost of COVID-19. Nat. Rev. Neurol. 2022, 18, 5–6. [CrossRef]
- Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020 Jul 31;6(31):eabc5801. [CrossRef]
- Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020 Jun 4;382(23):2268–70. [CrossRef]
- Benameur, K.; Agarwal, A.; Auld, S.C.; Butters, M.P.; Webster, A.S.; Ozturk, T.; Howell, J.C.; Bassit, L.C.; Velasquez, A.; Schinazi, R.F.; et al. Encephalopathy and Encephalitis Associated with Cerebrospinal Fluid Cytokine Alterations and Coronavirus Disease, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 2020, 26, 2016–2021. [CrossRef]
- Xia, H.; Lazartigues, E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J. Neurochem. 2008, 107, 1482–1494. [CrossRef]
- Davies J, Randeva HS, Chatha K, Hall M, Spandidos DA, Karteris E, et al. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol Med Rep. 2020 Nov;22(5):4221–6. [CrossRef]
- Solomon T. Neurological infection with SARS-CoV-2—the story so far. Nat Rev Neurol. 2021 Feb;17(2):65–6. [CrossRef]
- Al-Sarraj, S.; Troakes, C.; Hanley, B.; Osborn, M.; Richardson, M.P.; Hotopf, M.; Bullmore, E.; Everall, I.P. Invited Review: The spectrum of neuropathology in COVID-19. Neuropathol. Appl. Neurobiol. 2020, 47, 3–16. [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Physiol. 2022, 322, C1–C11. [CrossRef]
- Visco, V.; Vitale, C.; Rispoli, A.; Izzo, C.; Virtuoso, N.; Ferruzzi, G.J.; Santopietro, M.; Melfi, A.; Rusciano, M.R.; Maglio, A.; et al. Post-COVID-19 Syndrome: Involvement and Interactions between Respiratory, Cardiovascular and Nervous Systems. J. Clin. Med. 2022, 11, 524. [CrossRef]
- Hugon, J.; Msika, E.-F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 2022, 269, 44–46. [CrossRef]
- Backman, L.; Möller, M.C.; Thelin, E.P.; Dahlgren, D.; Deboussard, C.; Östlund, G.; Lindau, M. Monthlong Intubated Patient with Life-Threatening COVID-19 and Cerebral Microbleeds Suffers Only Mild Cognitive Sequelae at 8-Month Follow-up: A Case Report. Arch. Clin. Neuropsychol. 2022, 37, 531–543. [CrossRef]
- Nau R, Soto A, Bruck W. Apoptosis of Neurons in the Dentate Gyrus in Humans Suffering from Bacterial Meningitis: Journal of Neuropathology and Experimental Neurology. 1999 Mar;58(3):265–74. [CrossRef]
- Wenzel J, Lampe J, Müller-Fielitz H, Schuster R, Zille M, Müller K, et al. The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci. 2021 Nov;24(11):1522–33. [CrossRef]
- Salzano, C.; Saracino, G.; Cardillo, G. Possible Adrenal Involvement in Long COVID Syndrome. Medicina 2021, 57, 1087. [CrossRef]
- Qin, Y.; Wu, J.; Chen, T.; Li, J.; Zhang, G.; Wu, D.; Zhou, Y.; Zheng, N.; Cai, A.; Ning, Q.; et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J. Clin. Investig. 2021, 131. [CrossRef]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. J. Bone Jt. Surg. Am. 2020, 102, 1197–1204. [CrossRef]
- Versace, V.; Sebastianelli, L.; Ferrazzoli, D.; Romanello, R.; Ortelli, P.; Saltuari, L.; D’Acunto, A.; Porrazzini, F.; Ajello, V.; Oliviero, A.; et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin. Neurophysiol. 2021, 132, 1138–1143. [CrossRef]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [CrossRef]
- Ng, W.; Gong, C.; Yan, X.; Si, G.; Fang, C.; Wang, L.; Zhu, X.; Xu, Z.; Yao, C.; Zhu, S. Targeting CD155 by rediocide-A overcomes tumour immuno-resistance to natural killer cells. Pharm. Biol. 2021, 59, 47–53. [CrossRef]
- Zhang Y, Chen Y, Li Y, Huang F, Luo B, Yuan Y, et al. The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-Ι. Proc Natl Acad Sci U S A. 2021 Jun 8;118(23):e2024202118. [CrossRef]
- Masselli E, Vaccarezza M, Carubbi C, Pozzi G, Presta V, Mirandola P, et al. NK cells: A double edge sword against SARS-CoV-2. Adv Biol Regul. 2020 Aug;77:100737. [CrossRef]
- Li JY, Liao CH, Wang Q, Tan YJ, Luo R, Qiu Y, et al. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 2020 Sep;286:198074. [CrossRef]
- Andrade, B.S.; Siqueira, S.; Soares, W.R.d.A.; Rangel, F.d.S.; Santos, N.O.; Freitas, A.d.S.; da Silveira, P.R.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [CrossRef]
- Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020 Apr;42(2):505–14. [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [CrossRef]
- Xiao, N.; Nie, M.; Pang, H.; Wang, B.; Hu, J.; Meng, X.; Li, K.; Ran, X.; Long, Q.; Deng, H.; et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021, 12, 1618. [CrossRef]
- Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021 Mar;19(3):141–54. [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [CrossRef]
- Peluso MJ, Deitchman AN, Torres L, Iyer NS, Munter SE, Nixon CC, et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Reports. 2021 Aug;36(6):109518. [CrossRef]
- Wiech M, Chroscicki P, Swatler J, Stepnik D, De Biasi S, Hampel M, et al. Remodeling of T Cell Dynamics During Long COVID Is Dependent on Severity of SARS-CoV-2 Infection. Front Immunol. 2022 Jun 10;13:886431. [CrossRef]
- Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021 Apr;27(4):626–31. [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Hear. J. 2022, 43, 1157–1172. [CrossRef]
- Bechmann, N.; Barthel, A.; Schedl, A.; Herzig, S.; Varga, Z.; Gebhard, C.; Mayr, M.; Hantel, C.; Beuschlein, F.; Wolfrum, C.; et al. Sexual dimorphism in COVID-19: potential clinical and public health implications. Lancet Diabetes Endocrinol. 2022, 10, 221–230. [CrossRef]
- Laha S, Chakraborty J, Das S, Manna SK, Biswas S, Chatterjee R. Characterizations of SARS-CoV-2 mutational profile, spike protein stability and viral transmission. Infection, Genetics and Evolution. 2020 Nov;85:104445. [CrossRef]
- Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. The Lancet. 2022 Jun;399(10343):2263–4. [CrossRef]
- Niemi, M.E.K.; Daly, M.J.; Ganna, A. The human genetic epidemiology of COVID-19. Nat. Rev. Genet. 2022, 23, 533–546. [CrossRef]
- Caron P. Thyroid disorders and SARS-CoV-2 infection: From pathophysiological mechanism to patient management. Annales d’Endocrinologie. 2020 Oct;81(5):507–10. [CrossRef]
- Gentile S, Strollo F, Mambro A, Ceriello A. COVID -19, ketoacidosis and new-onset diabetes: Are there possible cause and effect relationships among them? Diabetes Obes Metab. 2020 Dec;22(12):2507–8.
- Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021 Jul 26;n1648. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




