Table 1.
Glossary of key terms.
Table 1.
Glossary of key terms.
1. Introduction
A functional national immunisation programme is integral to enhance public health and pandemic preparedness. A key priority for each immunisation programme should be vaccine pharmacovigilance and in particular the surveillance of adverse events following immunisation (AEFI). Passive post-marketing AEFI surveillance is recommended globally. However, there are both inherent limitations and significant challenges in implementing this method. First, the e-Health technology, which mobile health (m-Health) is part of, is now applied globally to improve health surveillance and outcomes. A noteworthy area is the application of digital tools to improve immunisation programmes including AEFI surveillance. Secondly, the m-Health for active vaccine safety surveillance (AVSS) is eliciting growing interest because of its ability to engage consumers directly to elicit AEFI reports, which could overcome some of the limitations inherent in passive reporting by health professionals(3-5). The m-Health active participant-centred (MAPC) AEFI surveillance could be defined as a data collection system that seeks to ascertain as completely as possible the number of AEFIs in a defined population via a continuous organised process(1).
The m-Health approaches are showing promise as a tool to improve AEFI detection in many high-income countries (HICs). This approach directly prompts vaccinees or their caregivers to report an AEFI quickly and seek care (3-6). A Canadian scoping review of MAPC AEFI surveillance suggests that mHealth could be the method for collecting self-reported subjective symptoms from vaccinees which could complement existing AEFI surveillance systems (5). The study recommends the evaluation of digital solutions to improve vaccine surveillance systems for contemporary and future public health needs (5). The performance of these m-Health approaches in LMIC settings is poorly described with only Cambodia (7) and Sierra Leone (8) reporting on their experiences for active m-Health adverse drug reaction (ADRs) but not on AEFIs. Moreover, the underperformance of the m-Health system in aboriginal communities in Australia highlights the need to assess the feasibility and performance of such systems in culturally diverse populations with poor socioeconomic groups (9).
A comprehensive evaluation of SMS-based AEFI surveillance is required prior to diverting scarce public health resources towards widespread implementation (4). The Australian stimulated telephone assisted rapid safety surveillance (Au-STARSS) is one of two published randomised controlled trials (RCT) which has compared the AEFI detection rate in an active (SMS surveillance) and control (passive surveillance) groups. The study evaluated the feasibility and acceptability of SMS based surveillance using a two-step process with an initial SMS being the entry point for detection whilst a subsequent digital interaction elicited information to determine the nature of the event (4). The outcome of the Au-STARSS study showed a 13-fold greater AEFI detection rate in the SMS group (Pearson’ 𝜒2 test =76.0, p<0.0001) compared to the control group (passive surveillance) (4).
Established in 1997, the Zimbabwe national AEFI surveillance system has continued to grow, but the burden of reporting AEFIs by overwhelmed health workers (HCWs) has remained a key barrier to the timely detection of serious or severe AEFIs (10, 11, 12). Zm-STARSS RCT is a proof-of-concept study evaluating SMS based surveillance for AEFI detection in a LMIC using an adapted Au-STARSS mHealth platform. The objective was to ascertain if STARSS could be adapted, implemented, and evaluated in Zimbabwe for the detection of AEFI.
2. Aim and hypothesis.
The primary aim was to determine if Zm-STARSS was more effective in detecting an AEFI than routine passive reporting of AEFIs. The primary hypothesis was that the proportion of people in which an AEFI is detected is greater in the SMS intervention group compared to a comparison group (passive surveillance). The secondary aim was to provide a narrative description covering the challenges of establishing a Zm-STARSS platform.
3. Methods and Materials
3.1. Study population, sample size and inclusion criteria
A total of 4560 individuals presenting for vaccination at CITIMED and Chitungwiza hospitals in peri-urban Chitungwiza near Harare, Zimbabwe, were eligible for enrolment from November 2020 to August 2021. Participants were eligible if they understood English (Zimbabwe’s official language), were adult vaccinees, or were parents or guardians of vaccinated children under the age of 18 years, had access to a functional mobile phone and consented to being part of the study.
Exclusion criteria: Vaccinees who were unwell before vaccination, child vaccinees associated with multiple births (e.g., a twin child) and children with a parent/guardian ≤18 years were not eligible for enrolment.
3.2. Consent process
Opt-in, informed consent was sought. Participants were informed that the study was examining diverse ways of monitoring vaccine ‘side-effects’ and that they could receive a follow-up SMS to ascertain any medical event requiring attention, together with an invitation to provide further details (by CATI) of their ‘experience’ during the post-vaccination period. Consent was obtained for receipt of SMSs, participation in a CATI interview and access to any passive AEFI reports submitted to Zimbabwe Expanded Programme on Immunisation (ZEPI) and Medicines Control Authority of Zimbabwe (MCAZ) through a database search for performance of a causality assessment by the national AEFI Committee.
3.3. Study design and randomisation
The Zm-STARSS software was customised to include all national ZEPI antigens including COVID-19 vaccines. Zm-STARSS study design included only two randomised groups (SMS and control) and excluded the web-based component which was in the Au-STARSS, because of the high cost and limited internet access to health care workers (HCWs) and vaccinees. The Zm-STARSS trial was a multi-centre, single-blinded and active-controlled parallel two-grouped RCT with a repeated measures design to collect responses to SMS’s sent on days 0-2 and 14 post-immunisations to ascertain whether the participant had experienced a medical event following immunisation (MEFI). Participants were enrolled at both CITIMED and Chitungwiza hospitals vaccination sites. Site selection was based on availability of reliable internet services to enable the platform to function. Randomisation was implemented using an algorithm residing in the study server which allowed automatic allocation in a 1:1 ratio in permuted sequence to the SMS intervention or the control group. The participant was the unit of allocation, which occurred on receipt of the vaccinee’s immunisation and demographic data into the study portal. Research healthcare workers (RHCWs) involved with the enrolment of participants and immunisation providers were blinded to allocation.
3.4. SMS dispatch and response
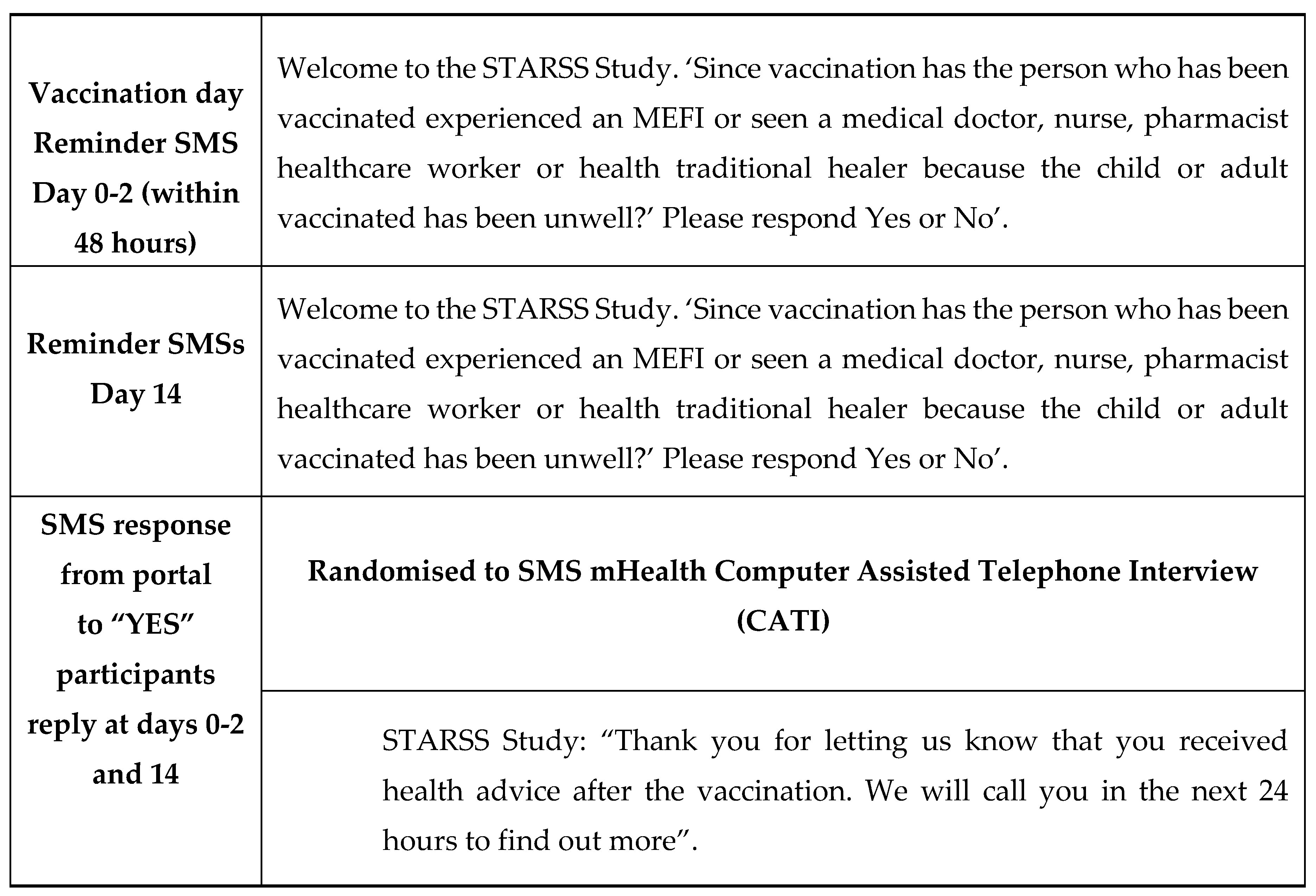
Extraction of participant and vaccinee data from a completed enrolment form was entered manually by RHCWs, via a secure web portal, and within 48 hours of vaccination. Upon receipt of this data an information technology (IT) application, located on the MCAZ server, automated SMSs to be dispatched without daily restriction (with a curfew between 8 PM and 8AM) and managed the flow of outgoing and incoming SMSs which included customised SMS replies to these responses. On receipt of the enrolment data participants were reminded via SMS that they should expect follow-up messages on days 0-2 and 14 post-immunisations. These time intervals were consistent with the expected temporal onset of reactions for the vaccines administered to the participants in the study. Each prompt SMS message aimed to determine if a MEFI had occurred by asking the question: “Since vaccination has the person who has been vaccinated seen a medical doctor, nurse, pharmacist, healthcare worker or health traditional healer because the child or adult vaccinated has been unwell?’ Please respond “Yes” or “No.” Those who responded “Yes” were contacted telephonically to complete an AEFI report which took the form of a CATI administered by a RHCW. The purpose of the CATI was to obtain details about the MEFI so that it could be determined if this was consistent with the WHO, AEFI definition(12). The CATI interview questionnaire included most of the 25 AEFI core variables as recommended by WHO(12). The “No” SMS responders were sent an automated SMS acknowledgement. A final SMS prompt was sent on day14 post-vaccination. The specific wording of the SMSs is detailed in Table 2.
Table 2.
Details of the wording of the SMS’s generated following enrolment and randomisation to the SMS-CATI intervention group.
Table 2.
Details of the wording of the SMS’s generated following enrolment and randomisation to the SMS-CATI intervention group.
The CATI sought to verify the following details on day 0-2 and 14 post vaccination if participants responded Yes to the SMS prompts: 1) vaccine(s) administered and batch numbers already prefilled at enrolment on the online Zm-STARSS by the nurses and also available on participant’s hard copy vaccination card; 2) type of medical attention sought; 3) adverse symptom(s); 4) other details including hospitalisation and recovery. An AEFI report was regarded as confirmed if the reported vaccination details were the same as those documented through the Zm-STARSS online recruitment portal, if medical attention/attendance following the immunisation was confirmed and one or more symptom(s) were reported. No follow-up occurred for the participants randomised to the control and the occurrence of an AEFI was determined from the ZEPI and MCAZ AEFI database/AEFI line listing.
3.5. Zm-STARSS-information technology (IT) platform
The Zm-STARSS IT platform was centralised, with encrypted data transmitted securely from the two study sites to a secure MCAZ server. The IT platform provided a single site for the collation of participant demographics, immunisation data, and SMS messages including repository for all data collected from CATI reports. The platform automatically dispatched and received SMS messages. The vaccinee’s immunisation details (vaccine antigen(s), brand, batch number, vaccine administration site(s), date, and time together with demographic information (vaccinee’s name, date of birth (DOB), sex, address, and mobile number) were collected at enrolment. Participant reports of a MEFI automatically triggered an email alert to RHCWs. Access to the site was password protected and RHCWs had access to the global dashboard. All portals offered a real-time view of the MEFI and AEFI reports, and all data was added to a single structured query language (SQL) database stored in the study server, which allowed export of the data (CSV format) and circumvented the need for unsecure data transmission. The Zm-STARSS surveillance system was developed with assistance from Econet Wireless Zimbabwe Ltd (Econet), ( a company with 65% mobile phone market share) and in line with the Postal and Telecommunications Regulatory Authority of Zimbabwe(POTRAZ) requirements for SMS mobile phone test codes. A major challenge was that participants would have insufficient telephone credit, to respond to the automatic SMS messages. This could introduce a significant bias in the perceived responsiveness of participants, and the reported/unreported symptoms. To address this issue, Econet created a test channel pre-paid by MCAZ , where participants’ SMSs were not charged, and hence successful transmission could be achieved despite insufficient telephone credit.
3.6. AEFI outcome measures and definitions
For the SMS-CATI group a MEFI notifier was defined as a participant who replied “Yes” to the SMS prompt on at least one of the time points (0-2 or 14 days). A participant was deemed to not have had a MEFI if a “No” response was received at any of the time points. A MEFI was defined as completed when all four CATI verification steps had occurred. An event was classified as an AEFI if it met the WHO case definition. Zimbabwe’s national AEFI expert committee trained by WHO performed the causality assessment on all reported cases according to WHO AEFI causality assessment algorithm (2019)(13). Some participants however might experience more than one AEFI, e.g., abscess and convulsions.
The number of AEFI cases in each group (SMS vs control) formed the basis for the analysis of the primary outcome. Since multiple MEFI/AEFI reports may have been completed for the same participant, the primary outcome was based on a single individual. For the control group all AEFI reports for the duration of the RCT, were matched to the vaccinee’s details (name, date of birth (DOB), etc.) to determine if a report had been received within 21 days of vaccination. Any submitted report was counted as an AEFI for the control group.
3.7. Secondary outcomes
SMS compliance was assessed by the number and percentage of participants who responded with a “Yes” or “No” response to the surveillance SMS. Fully compliant responders were defined as responders at the three points (0-2 and 14 days), partial compliers were those responding at 0-2 or 14-days’ time points and non-compliers never responded at all. MEFI completion was assessed by the number and percentage of participants, in the SMS-CATI group, who notified an MEFI and whose report was completed and verified by the RHCWs. The time for detection of an AEFI was defined as the time (in hours) from medical attendance/attention to completion of the MEFI report for the SMS-CATI group or the time from healthcare attendance to receipt of an AEFI report by ZEPI or MCAZ for the control group.
3.8. Statistical analysis plan
The primary analyses were performed on the intention-to-treat (ITT) population, with participants analysed per their allocated pools. Given the low AEFI cases expected in the control group, two-tailed Fisher’s exact tests (α=0.025) were done to compare the events in the SMS group to those in the control group. Pearson’ 𝜒2 tests were carried out for the different demographic characteristics, such as gender, age, sex, index of socio-economic advantage and disadvantage as well as the vaccine characteristics to determine any significant differences in the SMS group and control groups, including those participants who responded “Yes” or “No” to the SMS or the non-responders to any SMS. Median and 95% confidence intervals based on the binomial distribution were used to describe the median time in hours of the various time lags from enrolment to AEFI detection.
4. Results
4.1. Eligibility, enrolment, randomisation, and intention to treat numbers.
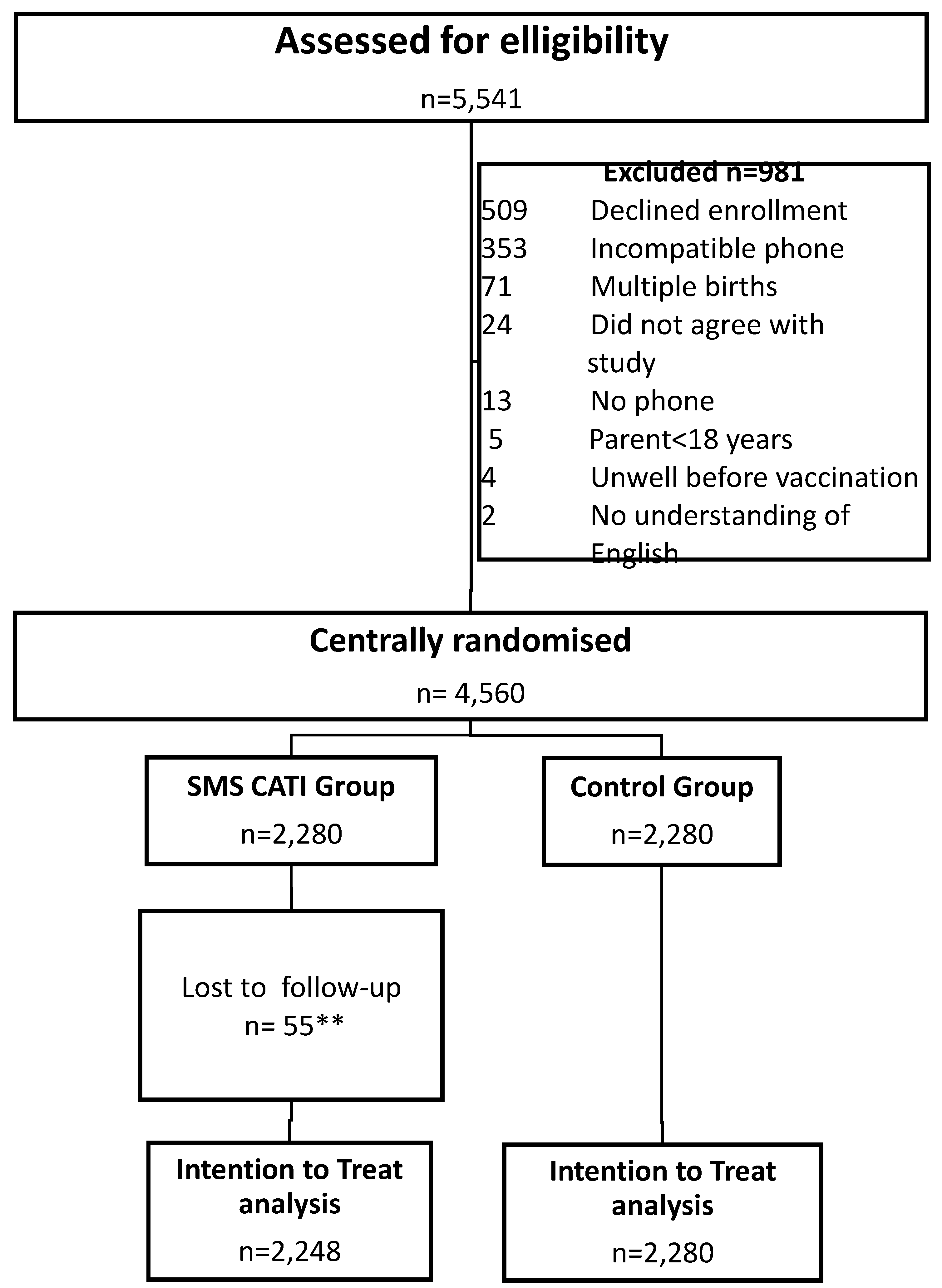
Between November 2020 and August 2021, a total of 5,541 eligible adults and children attending the two immunisation sites were approached and screened for enrolment. A total of 4,560 who met inclusion criteria and signed the consent forms were enrolled and randomly assigned (1:1) equally to the SMS or the control groups, and 981 subjects were excluded with details documented in the consort diagram (
Figure 1).
4.2. Participant demographic and vaccine characteristics.
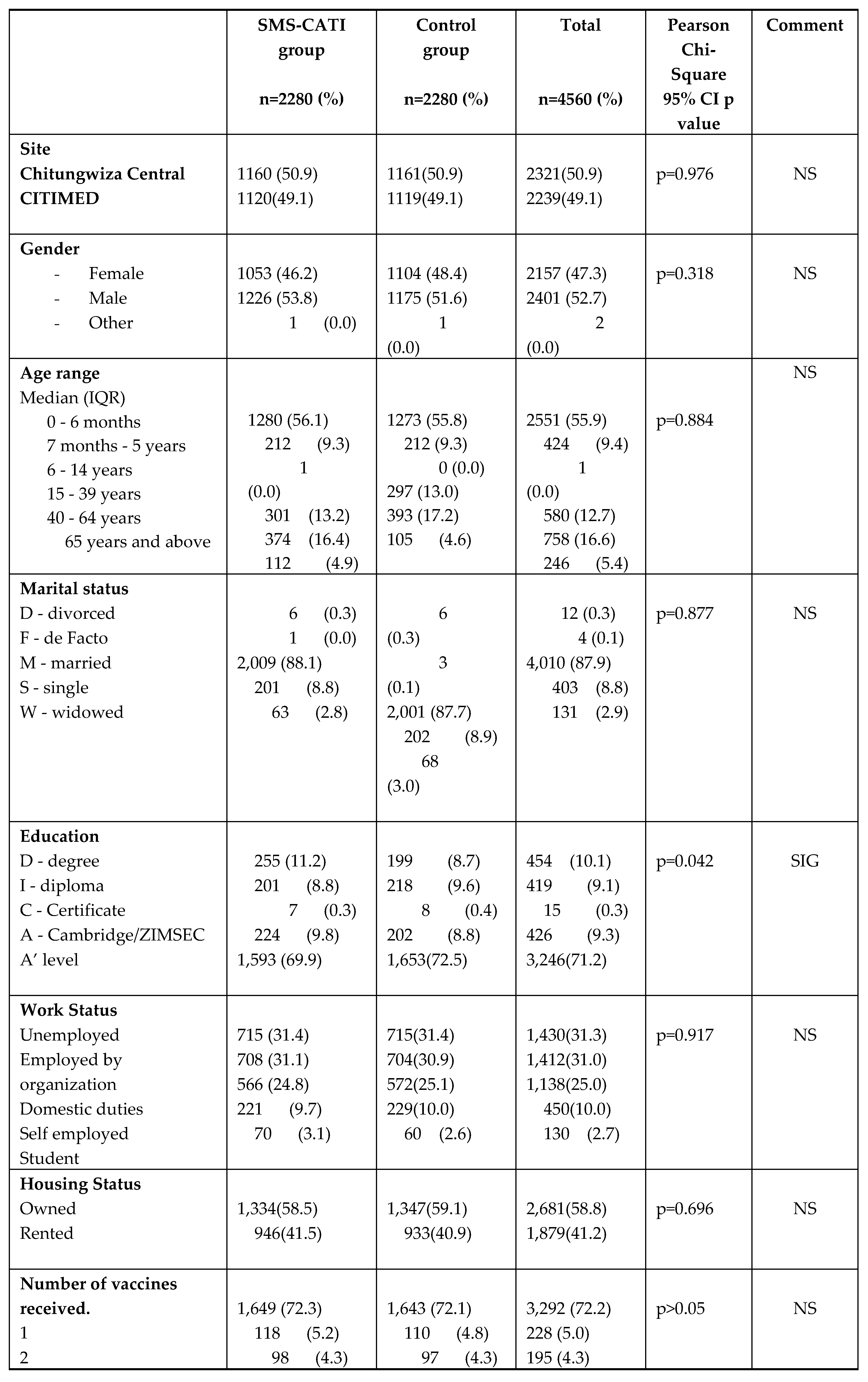
The demographic and vaccine characteristics of enrolled participants are detailed in
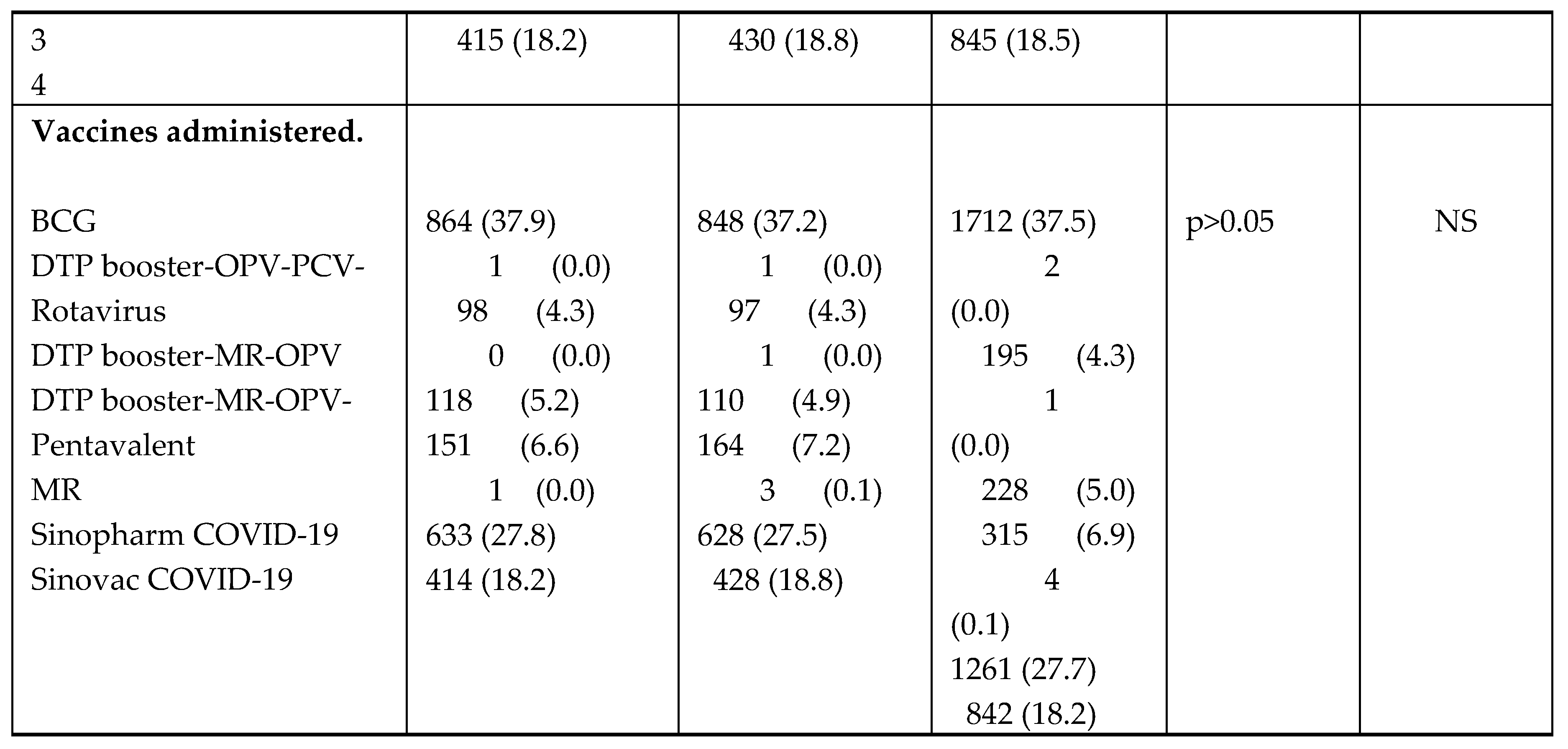
Table 3. Whilst participants were distributed across a wide age range (birth to 65 and above years), 2,551 (55.9%) were aged from birth to 6 months (or with almost equal gender balance (females 2,157 or 47.3% and males 2,401 or 52.7%). A total of 11 different vaccines were administered to 4,560 participants, with the following combinations being administered; Bacille Calmette-Guerin BCG (37.5%) was the most frequently administered followed by Sinopharm COVID-19 vero cell vaccine (27.7%), Sinovac COVID-19 vero cell vaccine (18.2%), oral polio vaccine (OPV) - Pneumococcal-Pentavalent (DTP-Hib-HepB)-Rotavirus (6.9%), Diphtheria Tetanus Pertussis (DTP) booster Measles Rubella (MR) (5.0%), Pneumococcal Rotavirus (4.3%) PCV and Sputnik V COVID-19 vaccine (0.1%).
Table 3 shows that the Pearson 𝜒
2 tests have no significant differences in any of the demographic or vaccine characteristics between the SMS intervention or control groups except for the education status p=0.042 that is statistically significant, with marginally more individuals in the SMS-CATI group having a degree or A level (Cambridge/ZIMSEC).
4.3. Primary outcome determination of MEFI notification, verification, report completion and AEFI causality classification.
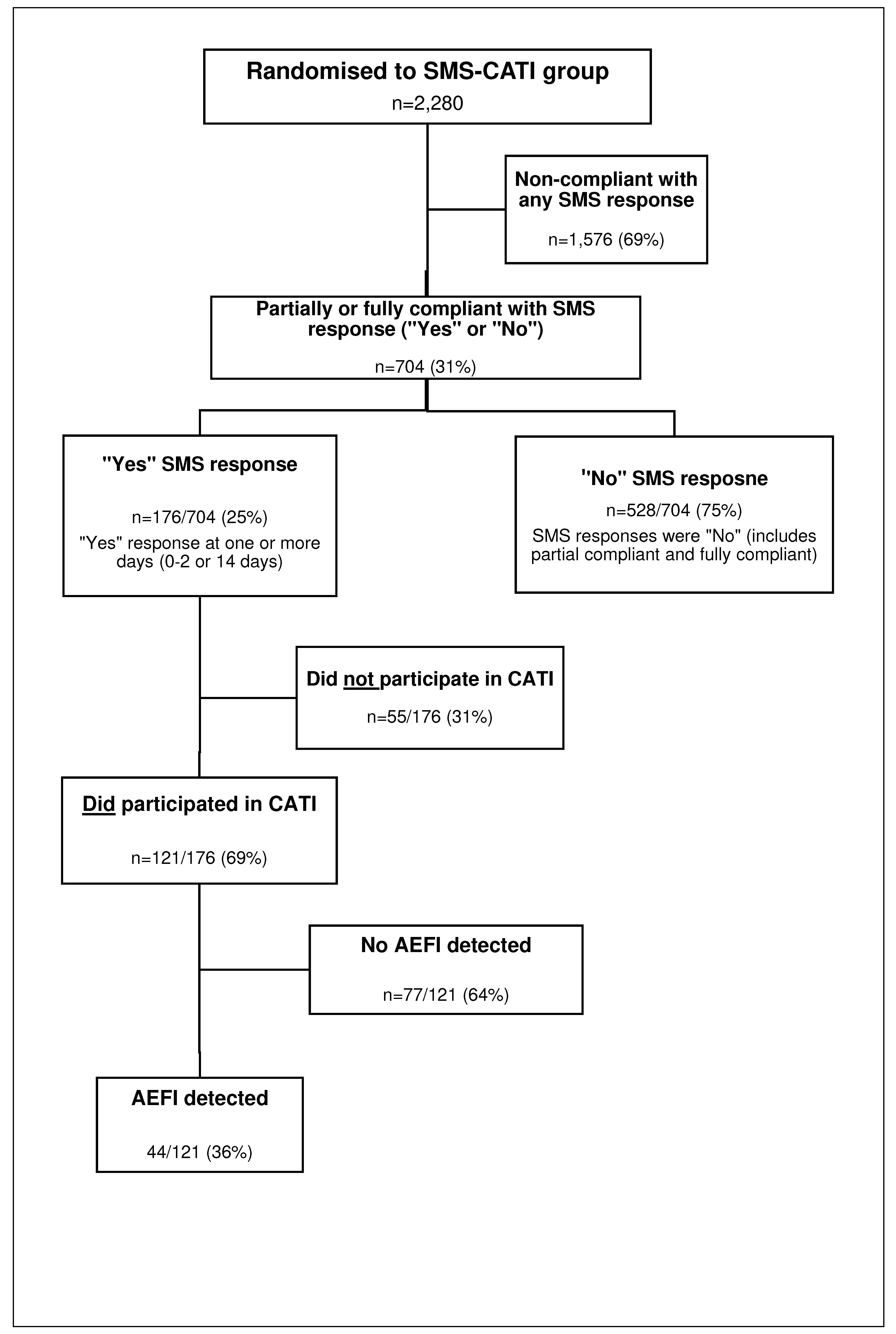
The SMS responses, MEFI completion and final AEFI classification for the participants randomised to the SMS-CATI group are detailed in Figure 2. A total of 6,840 SMSs were dispatched to the participants within 0-2 and 14-days post-vaccination. Overall, 69% (1,576/2,280) of participants, in the SMS intervention groups, were classified as non-compliant. Of the 704 SMS (31% of 2280) responses received, 75% (528/704) indicated that “No” MEFI had been experienced whilst 25% (176/704) responded “Yes” to experiencing an MEFI,( Figure 2) at one or more of the time points (day 0-2 and/or 14). In this group of 176 participants, 81 were partial compliers (responded to one SMS) and 95 were complete compliers (responded to all SMS). Of the 176 who were “Yes” respondents 31% (55/176) could not be contacted for a CATI despite at least 3 separate attempts. The remaining 69% (121/176) were contacted successfully by RHCWs who completed their CATI and 36% (44/121) of these were assessed as they met the WHO AEFI criteria. The remainder 64% (77/121) did not experience any AEFI. No passive AEFI reports were identified in the control group after verification of the ZEPI and MCAZ AEFI databases.
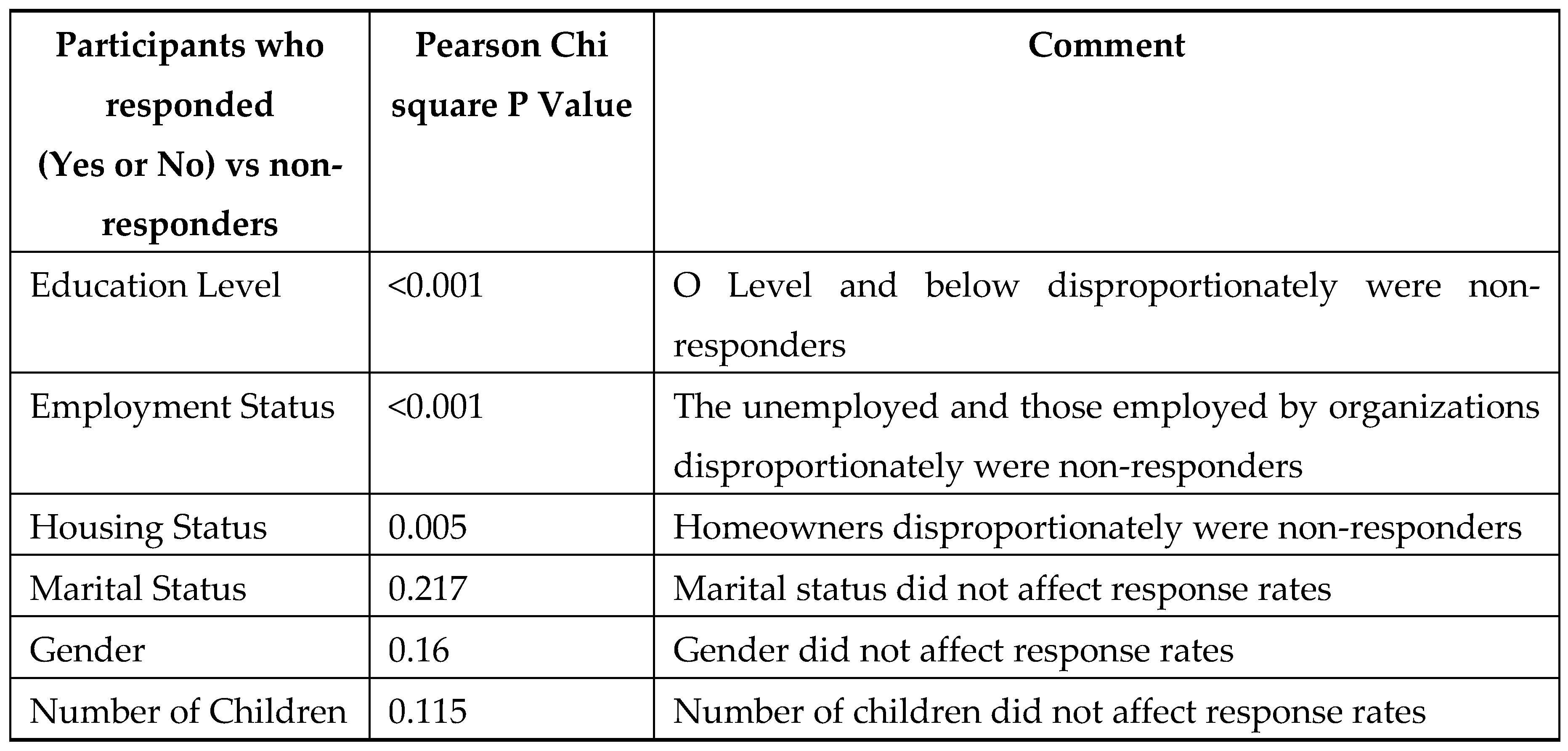
The overall health care attended AEFI in the SMS intervention group was 2% (44/2,248) with no AEFI detection in the control group. The AEFI detection rate was 2% greater in the SMS group compared to the control group (0%) which was significantly higher Pearson’s Х2 = 44.19 for AEFI detection at p<0.0001. Pearson’s , p<0.0001; Fisher’s exact test (2-sided), p<0.0001) which is statistically significant. The non-response rates to SMS prompts did not differ significantly for the day 0-2 and 14 post vaccination SMS prompts. However, those participants who responded to the SMS (“Yes” or “No”) compared with the non-responders demonstrated Pearson Х2 significant differences for education level, employment, and housing status but not for marital status, gender, and number of children, as shown in Table 5.
Table 5.
Participants randomised to SMS-CATI who responded (“Yes” or “No”) compared with non-responders to day 0-2 and 14 SMS according to demographic and socioeconomic status.
Table 5.
Participants randomised to SMS-CATI who responded (“Yes” or “No”) compared with non-responders to day 0-2 and 14 SMS according to demographic and socioeconomic status.
Figure 2.
SMS-CATI group participants – SMS response, MEFI report notification, verification of AEFI report completion.
Figure 2.
SMS-CATI group participants – SMS response, MEFI report notification, verification of AEFI report completion.
4.4. Reported AEFIs and causality assessment classifications in the SMS group.
The adverse symptom and causality assessment classifications of those in the SMS group are shown in
Table 6 including the Medical Dictionary for Regulatory Activities (MedDRA) term reactions system organ classifications (SOCs) and preferred terms (PTs), suspected vaccines, and causality assessment outcomes done by the National AEFI Committee using the WHO AEFI causality assessment algorithm (2019)(13). Fever was reported with an overall rate of 0.9% and all other symptoms occurred with a frequency of less than 0.5%. In the ITT analysis 2% (44/2,248) of all SMS participants experienced an AEFI and reported seeking health advice from HCWs. Of these, 25% (11/44) were hospitalised including one fatality (
Table 6). The reported rate of hospitalisation was 0.5% (11/2,280). Participants sought advice mostly from a community health advisor (57%) followed by a pharmacist (18%), GP (13.6%) or nurse (11.4%).
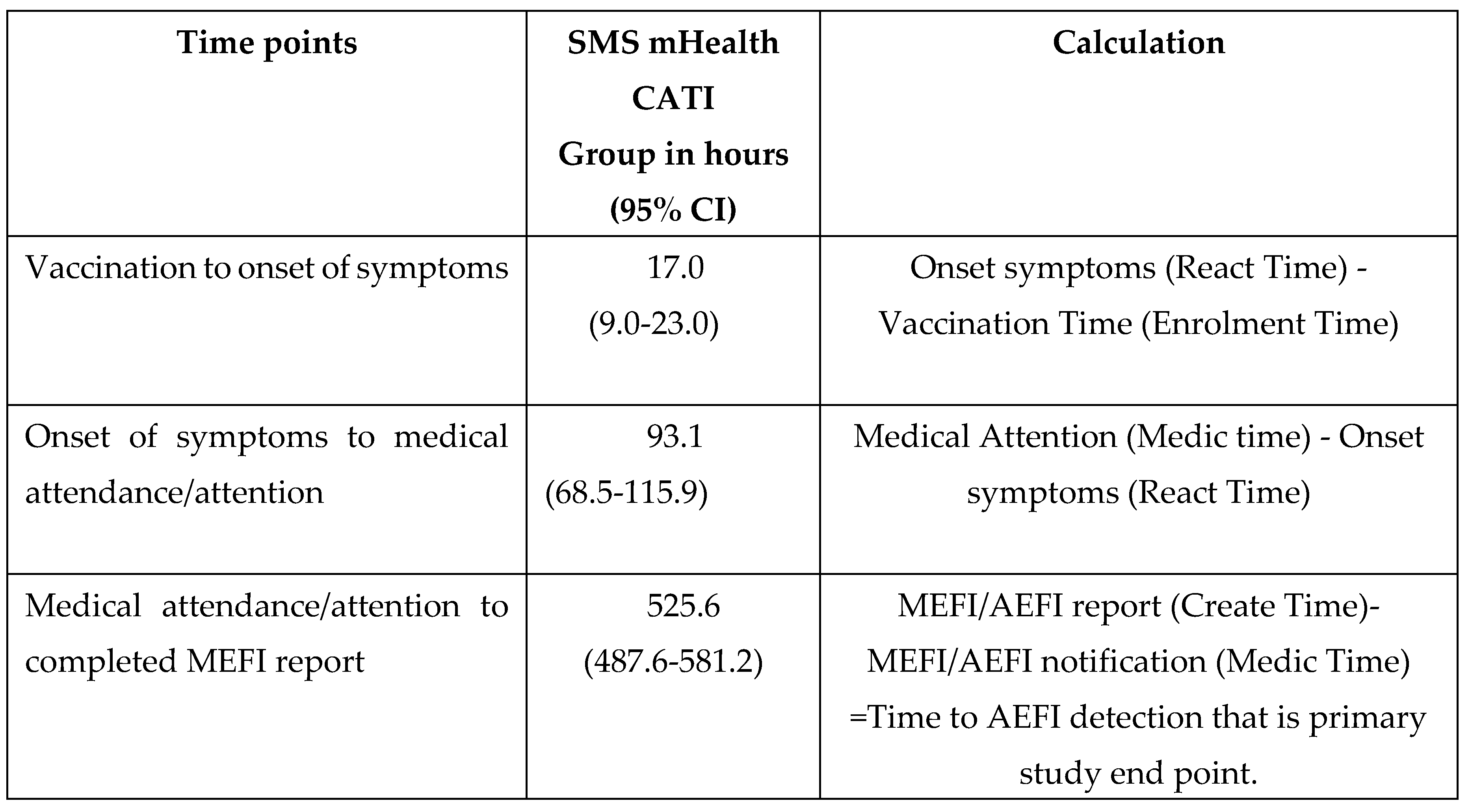
4.5. Time to detection of an AEFI.
For those SMS participants who had a confirmed MEFI the time to detection of an AEFI was determined (Table 7). For the time periods of vaccination to symptom onset and vaccination to MEFI notification there was a wide distribution as expected because participants received vaccines with a variable reactogenicity profile. However, the median time was 17.0 hours (CI 95%: 9.0-23.0) from onset of symptoms to presenting for medical attention, regardless of when this occurred following immunisation. The median time from medical attendance to Zm-STARSS completion of an AEFI report was 525.6 hours (CI 95%: 487.6-581.2).
Table 7.
Time to AEFI detection; Surveillance time points (in hours) in participants with confirmed AEFIs for Zm-STARSS, RCT mHealth SMS CATI intervention group.
Table 7.
Time to AEFI detection; Surveillance time points (in hours) in participants with confirmed AEFIs for Zm-STARSS, RCT mHealth SMS CATI intervention group.
5. Discussion
The STARSS platform was designed to address some of the deficiencies inherent in passive surveillance with low rates of AEFI detection. If an AEFI occurred and not be reported, this was due to barriers in the AEFI reporting cycle, confusion among HCWs of what type of events constitute a reportable AEFI and unsatisfactory reporting processes (15-17). Targeting consumers is a strategy to improve AEFI reporting (3-5, 16). Our SMS surveillance prompts were designed to ascertain if a serious or severe event, which prompted health care attention or attendance, had occurred. We designed the first SMS questionnaire to include a broad range of health care and community health care advisors with the aim of capturing as many potential AEFI as possible. Using the Zm-STARSS we demonstrated that the detection rate of health care attention and/or attended AEFI using SMS-based surveillance exceeded reporting by passive surveillance. This occurred despite high non-compliance rates to SMS responses. Contrasting the Au-STARSS with the Zm-STARSS provides some valuable insights into the implementation of SMS based surveillance in a LMIC. The SMS-based AEFI detection rates in Zm-STARSS were 2%, which compares with a rate of 5.3% in the Au-STARSS-CATI group. For AEFI passive surveillance the rates were 0% and 0.3% (respectively, for Zm and Au-STARSS). Overall, hospitalisation rates, following immunisation, were 6-fold higher for Zm-STARSS (0.5%) compared with Au-STARSS rate (0.08%), which is likely to reflect co-incidental diseases, including SARS Cov-2 infection. This underlies the importance of performing causality assessment for serious AEFI reports, using the WHO methodology(13) to differentiate adverse vaccine reactions from co-incidental events, which occurred in Zm-STARSS but not in the Au-STARSS. Collectively these trials have demonstrated the utility of the STARSS platform in both HIC and LMIC settings.
The SMS non-compliance rate in Zm-STARSS was 69% compared with 9.7 % in the Au-STARSS, although the latter had an additional time point for a solicited response (Day 7). SMS non-compliance (response) rates have varied between different SMS AEFI surveillance studies and a comparison has limitations because of the variability in the timing, number of and content of the SMSs, different populations, cultures, mHealth services cost in LMICs, and study settings(4). In general, studies with opt-in consent show non-compliance rates which vary between 10-30% (4, 19, 20). There are several likely reasons for non-compliance rates in Zm-STARSS. First, it was noted that a low education level and unemployment was associated with a higher rate of non-response both factors which need further interrogation. Second, the study was conducted during the peak of the COVID-19 pandemic with inherent challenges in implementation of the study with some HCWs and community members succumbing to COVID-19 and some of the RHCWs testing COVID-19 positive required quarantine and prolonged absence from work from time to time. Furthermore, access to phone credit in a LMIC setting is likely to be a significant barrier to SMS responses. We conclude that SMS in LMICs could be ineffective where mobile phone plan pricing structures often encourage data-only plans (without an allocated phone number) and where phones are often used with Wi-Fi communications only. Future studies should investigate the use of online/digital messaging services such as WhatsApp, Viber, Meta messenger and Sasai. The preferred platforms would obviously depend on local popularity, and support for multiple pathways may be required for best coverage. The IT platform could connect to the API gateways of these services and send instant messages through their networks instead of the telephone network. These options are likely to introduce their own issues around privacy and confidentiality in capturing and recording recipient health information and around adverse treatment of messages by spam filters. However, digital services do not rely on a formal mobile number, they rather offer the promise of broader access to the local population than SMS and avoid the issue around transmission of replies being prevented due to lack of credit on the participant phone. In LMICs, SMS platforms on cheaper mobile phones are more accessible than online/digital messaging. If a user is offline, there is no communication. Furthermore, the user requires smartphone/tablet/computer to go online/digital, which is more expensive than a flip phone. We may therefore conclude that using online/digital messaging, in addition to SMS could be more effective in LMICs if resources permitted.
In the Zm-STARSS, 69% of the “Yes” respondents completed a CATI survey compared with 83% in the Au-STARSS-CATI group. As noted above this is likely a reflection of the context of the Zm-STARSS during the COVID-19 pandemic and the limitations of availability of telephone credit and connectivity in a LMIC. Of the 64% (77/121) SMS “Yes” respondents who did not actually experience an AEFI it might be due to cultural reasons as most Shona speaking people who were most of the study participants usually regard “Yes” as to imply they are alright or in good health. Similarly, due to cultural factors, Shona people do not always RSVP to wedding/birthday invitations, funerals etc. and hence the high non-response rates to the post-vaccination SMS prompts.
The median time of about 17.0 hours (CI 95%:9.0-23.0) from onset of symptoms to presenting for medical attention, regardless of when this occurred following immunisation, were similar for Zm-STARSS and Au-STARSS. However, the median time from medical attendance to completion of an AEFI report in Zm-STARSS [525.6 hours (CI 95%:(487.6-581.2)] was longer than the Au-STARSS [74.8 hours (CI 95%:(54.3-96.1)], for the CATI arm. This is likely to reflect the difficulties for RHCWs in implementing the Zm-STARSS trial particularly during the COVID-19 pandemic. MAPC web-based reporting (WBR) has the potential advantage of a shortened time to AEFI detection as demonstrated in the Au-STARSS. However, this is currently difficult in a LMIC because of limited internet connectivity and expensive online/digital tools. Further studies are required to using appropriately and timely communication methods to ascertain the barriers to obtaining information about health care attended events following immunisation for vaccinees or their guardians.
The Zm-STARSS has demonstrated the utility of an SMS-based surveillance platform to enhance AEFI reporting as shown in Au-STARSS(4). This has been demonstrated despite higher non-compliance and non-CATI completion rates in a LMIC setting. The researchers were so far not aware of any publications evaluating MAPC AEFI surveillance in Africa. The relative costs and benefits of implementing active SMS-based AEFI surveillance in addition to passive surveillance remains to be determined and should be considered using evidence-based cost-effective holistic approaches of integration with other existing or future m-Health, e-Health including digital initiatives in resource limited settings.
6. Conclusion
SMS-based AEFI surveillance can improve AEFI detection in an LMIC setting and should be considered as an approach to augment passive surveillance in these settings for both COVID-19 vaccines and childhood vaccines although the challenges of using SMS mentioned in the discussion ought to be addressed. The findings of Zm-STARSS should inform the wider use of SMS-based AEFI surveillance which is particularly relevant at this time since establishing robust and novel pharmacovigilance systems to monitor existing and novel pandemic vaccines. The utility of SMS-based surveillance in AEFI signal detection is another useful risk minimisation factor amongst other considerations for evidence-based integration with other e-Health, digital health, and m-Health systems in resource-limited settings.
7. Limitations, confounding factors and/or bias.
The limitations of the inherent resource and technology limited challenges of Zm-STARSS resulted in a different study design of only 3 SMS prompts with no WBR component unlike Au- STARSS. The use of a Zm-STARSS test code meant that some participants could not be enrolled if they only subscribed only to the other two mobile phone operators. We are uncertain if the participants who had responded “Yes” to day 0-2 and 14 SMS prompts but did not respond to CATI surveys by the RHCWs could have sought medical attention. Confounding factors and bias were minimised by the RCT study design. Additionally, further studies are required to investigate the reasons for the high SMS non-response rates and to identify other factors that may predict response rates in LMIC settings. The study sites included the largest vaccination clinics in a peri-urban setting in Chitungwiza hence the results might not be representative of a rural population.
Author Contributions
Author Contributions. PPMN designed the study and wrote the main manuscript as the lead author. PPMN, LC, RM, TN, and ENZ conducted the study. MSG and SC provided Zm-STARSS Technical IT support, and copy rights based on AU-STARSS Australia, University of Adelaide innovation. MSG, SC, and UM supervised the study, data cleaning and reviewed the manuscript. PPMN analysed data and performed major analysis including tables and figures. All authors read and approved the final manuscript.
Funding
The MCAZ funded the study in line with its mandate of the Zimbabwe National Pharmacovigilance Centre. However, additional funding was obtained from unrestricted research grants from the WHO, and Au-STARSS Project, University of Adelaide, Australia.
Acknowledgments
The authors acknowledge the two study sites management and staff Chitungwiza Central Hospital and CITIMED Private Hospital vaccination clinics, Zimbabwe Expanded Program on Immunisation (ZEPI-MoHCC), MCAZ management and NPC staff, HCWs, and all participants who participated in the Zm-STARSS study, and the national AEFI Committee experts who did the AEFI causality assessment.
Ethics approval and consent to participate
The Zm-STARSS (II) RCT (ACTRN12614001046695) was approved by the Medical Research Council of Zimbabwe (MRCZ) ethical approval referenced MRCZ/A/2268 and MRCZ ethical exemption (reference E/148) from consenting participants for passive AEFI surveillance. Ethical approvals were also obtained from the Director of Health Services City of Chitungwiza Municipality Department and the University of Cape Town (UCT) Human Research Ethics Committee (HREC 184/2020) for the Zm-STARSS study. The data was published in deidentified anonymised format.
Consent for publication
The authors obtained consent for publication of the manuscript from the MCAZ National Pharmacovigilance Centre the custodian of the safety data since this is a work-related study.
Competing of interest
The authors declare no conflict of interest nor potential competing interest.
References
- Heininger U, Holm K, Caplanusi I, Bailey S, Abdoellah SA, Arellano F, et al. Guide to active vaccine safety surveillance: Report of CIOMS working group on vaccine safety–executive summary. Vaccine. 2017;35(32):3917-21. [CrossRef]
- Ross J, Stevenson F, Lau R, Murray E. Factors that influence the implementation of e-health: a systematic review of systematic reviews (an update). Implementation science. 2016;11(1):1-12. [CrossRef]
- Cashman P, Macartney K, Khandaker G, King C, Gold M, Durrheim DN. Participant-centred active surveillance of adverse events following immunisation: a narrative review. Int Health. 2017;9(3):164-76. [CrossRef]
- Gold M, Lincoln G, Cashman P, Braunack-Mayer A, Stocks N. Efficacy of m-Health for the detection of adverse events following immunization–The stimulated telephone assisted rapid safety surveillance (STARSS) randomised control trial. Vaccine. 2021;39(2):332-42. [CrossRef]
- Psihogios A, Bota AB, Mithani SS, Greyson D, Zhu DT, Fung SG, et al. A scoping review of active, participant-centred, digital adverse events following immunization (AEFI) surveillance: A Canadian immunization research network study. Vaccine 2022;40;4065-4080. [CrossRef]
- Cashman P, Moberley S, Chee K, Stephenson J, Chaverot S, Martinelli J, et al. Participant centred safety surveillance of health care workers receiving influenza vaccination. Vaccine. 2019;37(18):2427-9. [CrossRef]
- Baron S, Goutard F, Nguon K, Tarantola A. Use of a text message-based pharmacovigilance tool in Cambodia: pilot study. Journal of medical Internet research. 2013;15(4):e68. [CrossRef]
- Tsafack M, Ateudjieu J. Improving community based AEFI (Adverse Events Following Immunization) reporting rate through telephone" beep" in a Cameroon health district: a randomized field trial. Pan African Medical Journal. 2015;22(1). [CrossRef]
- Cashman P, Munnoch S-A, Clark K, Allan N, Clarke S, Macartney K, et al. The Aboriginal gap in online active vaccine safety surveillance. Australian Indigenous HealthBulletin. 2020;1(1):3. [CrossRef]
- Masuka JT, Khoza S. Adverse events following immunisation (AEFI) reports from the Zimbabwe expanded programme on immunisation (ZEPI): an analysis of spontaneous reports in Vigibase® from 1997 to 2017. BMC Public Health. 2019;19(1):1166-. [CrossRef]
- Shaum A, Mujuru HA, Takamiya M, Ticklay I, Nathoo K, Sreenivasan N, et al. Enhanced surveillance for adverse events following immunization during the 2019 typhoid conjugate vaccine campaign in Harare, Zimbabwe. Vaccine 2022;40(26); 3573-3580. [CrossRef]
- Nyambayo PP, Manyevere R, Chirinda L, Zifamba EN, Marekera SF. Descriptive Research Study of the Adverse Events Following Immunization (AEFIs) Surveillance System in Zimbabwe. Clinical Case Reports and Studies. 2023 Volume 2, Issue 1 https://www.doi.org/brs/2023/ccrs/0023. [CrossRef]
-
https://www.who.int/publications/i/item/9789241516990 (accessed 24/3/23).
- Bohn-Goldbaum E, Cross T, Leeb A, Peters I, Booy R, Edwards KM. Adverse events following influenza immunization: understanding the role of age and sex interactions. Expert Review of Vaccines. 2022;21(3):415-22. [CrossRef]
- Gidudu JF, Shaum A, Dodoo A, Bosomprah S, Bonsu G, Amponsa-Achiano K, et al. Barriers to healthcare workers reporting adverse events following immunization in four regions of Ghana. Vaccine. 2020;38(5):1009-14. [CrossRef]
- Clothier HJ, Selvaraj G, Easton ML, Lewis G, Crawford NW, Buttery JP. Consumer reporting of adverse events following immunization. Hum Vaccin Immunother. 2014;10(12):3726-30. [CrossRef]
- Dey A, Wang H, Quinn H, Hiam R, Wood N, Beard F, et al. Surveillance of adverse events following immunisation in Australia annual report, 2017. Commun Dis Intell. 2019;43:1-28. [CrossRef]
- Jacoby P, Glover C, Damon C, Fathima P, Pillsbury A, Durrheim D, et al. Timeliness of signal detection for adverse events following influenza vaccination in young children: a simulation case study. BMJ open. 2020;10(2):e031851. [CrossRef]
- Munnoch S-A, Cashman P, Peel R, Attia J, Hure A, Durrheim DN. Participant-Centered online active surveillance for adverse events following vaccination in a large clinical trial: feasibility and usability study. Journal of Medical Internet Research. 2019;21(10):e14791. [CrossRef]
- Stuurman AL, Verstraeten T, De Schryver A. Rapid assessment of the reactogenicity of a 2016-2017 seasonal influenza vaccine: results from a feasibility study. Expert review of vaccines. 2017;16(2):187-91. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).