Submitted:
27 February 2023
Posted:
06 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
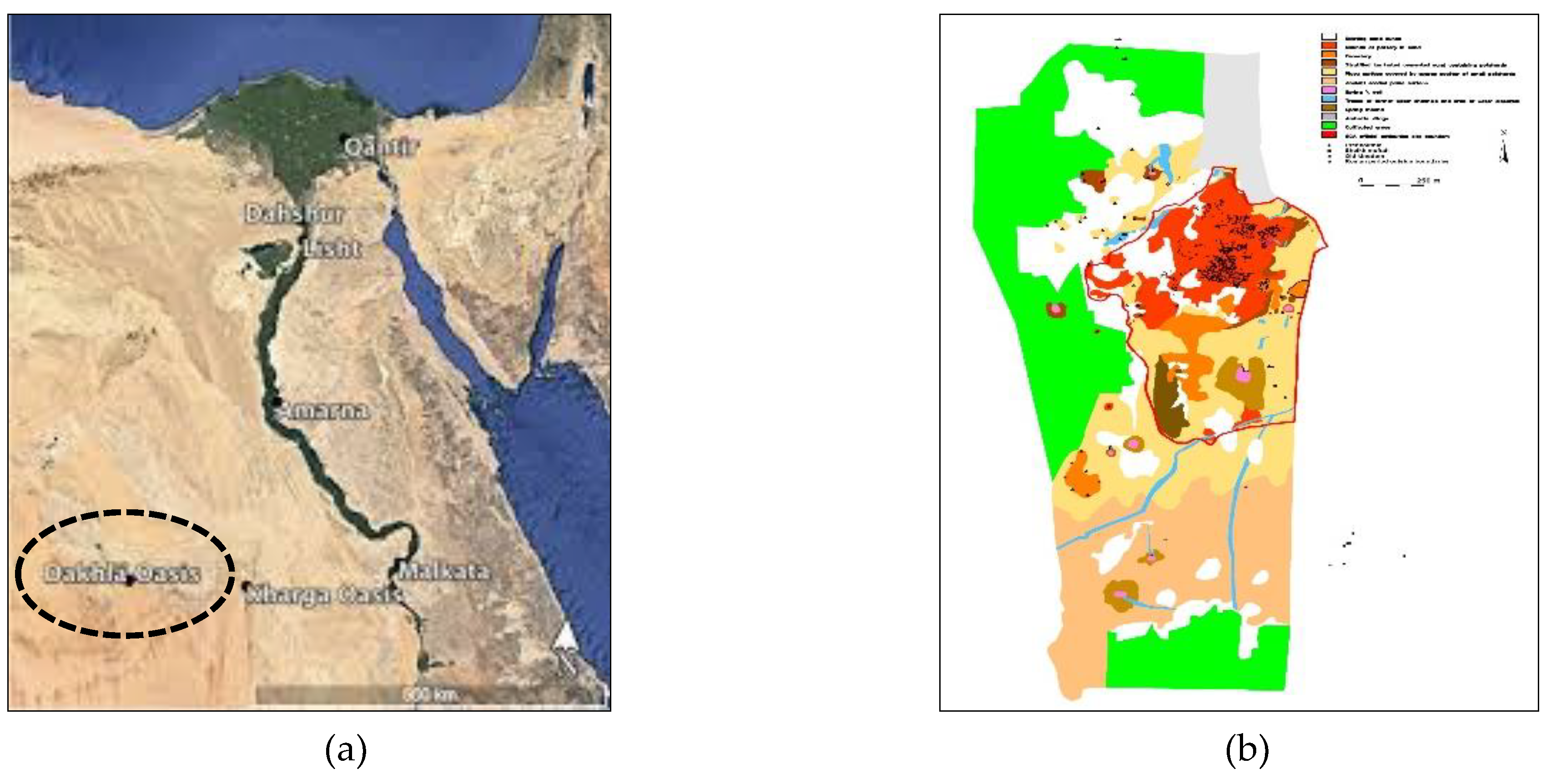
1.1. The study area
1.2. The study objects
2. Materials and Methods
3. Results
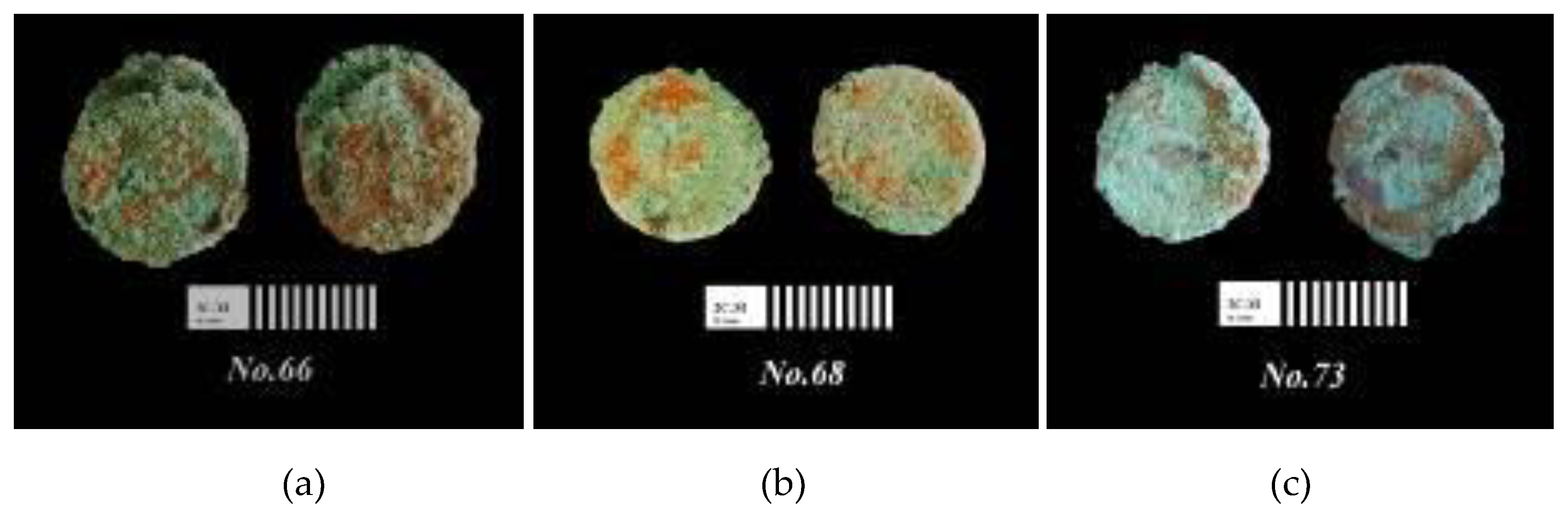
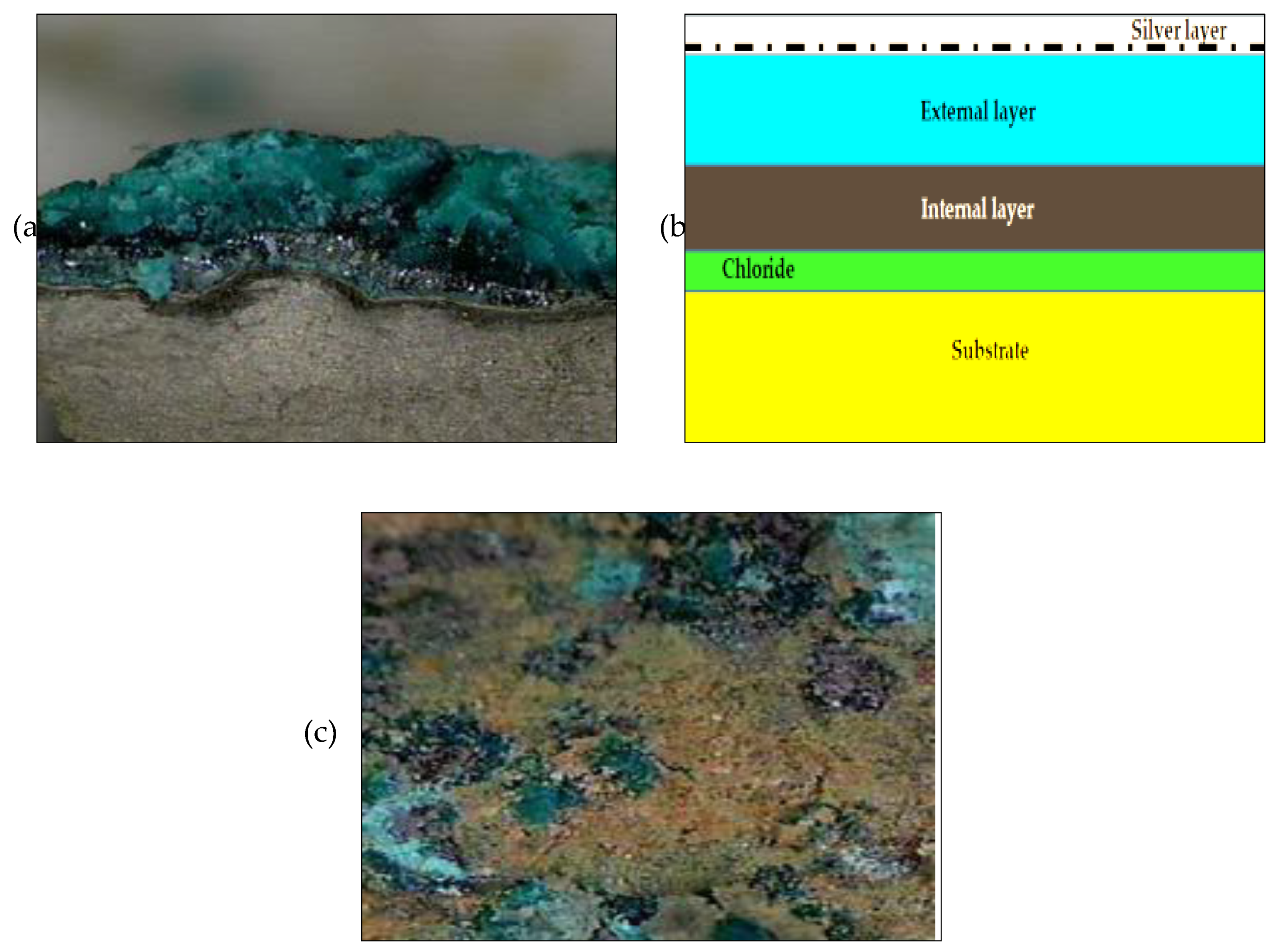
3.1. Optical Microscope
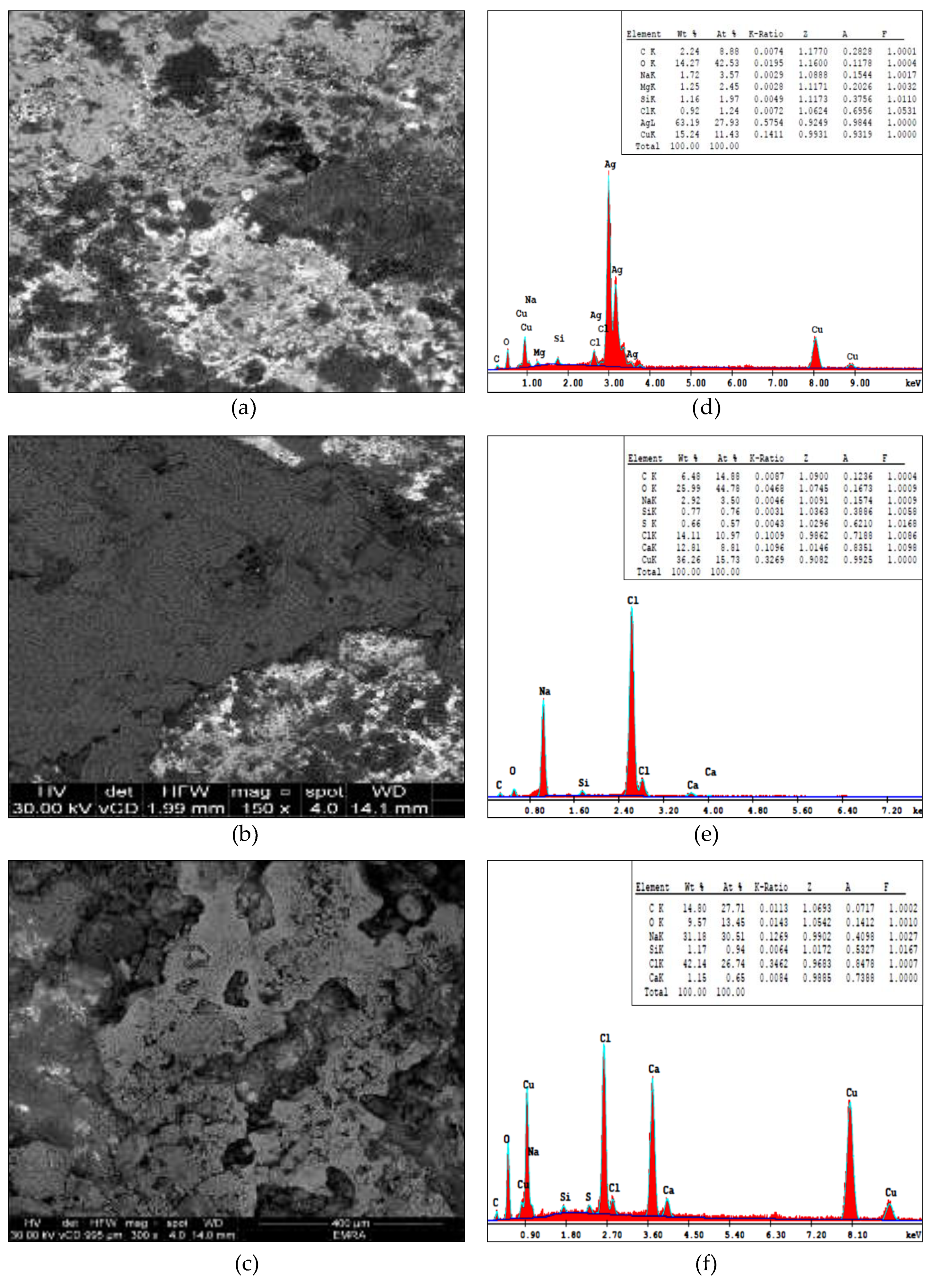
3.2. SEM-EDS
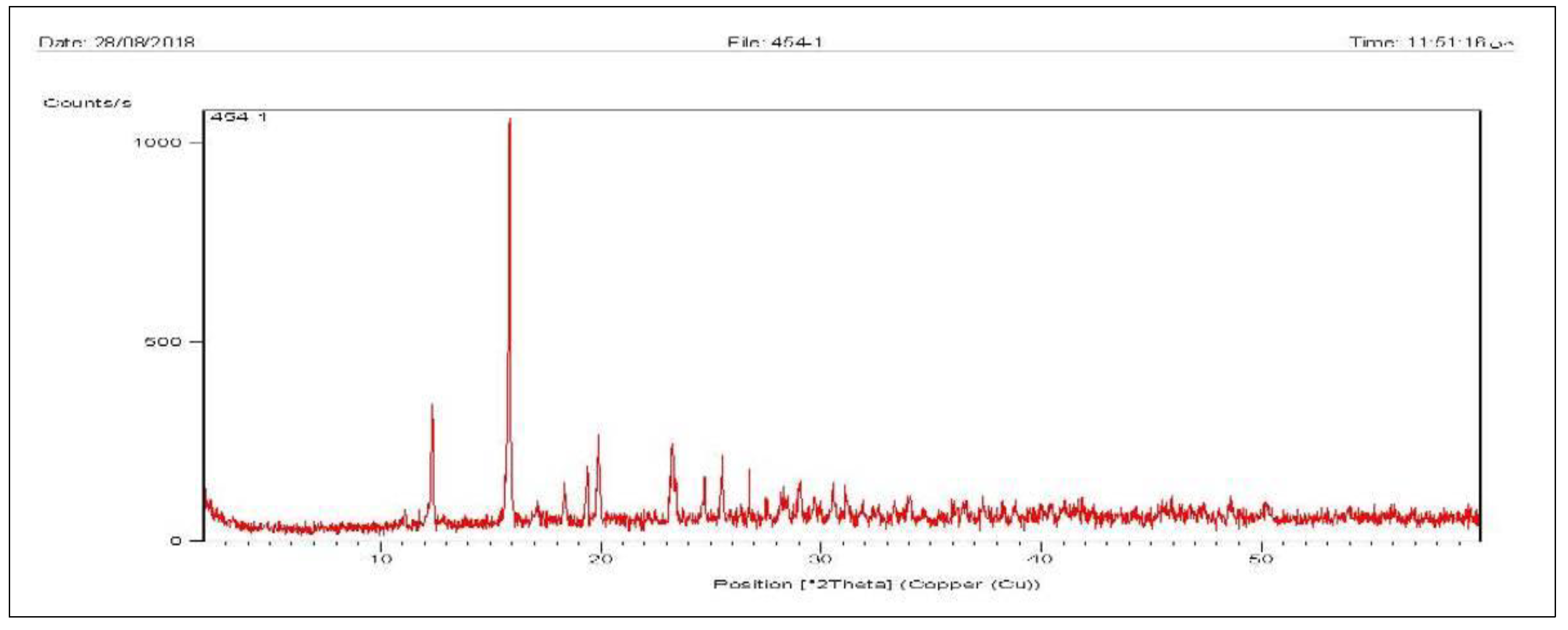
3.3. XRD
4. Discussion
4.1. Optical microscope
4.2. SEM-EDS
4.3. XRD
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abebe, A.; Misganaw, W. Experimental Analysis of Environmental Effects on Corrosion and Degradation of Metals. High Technology Letters 2021, 27, 389–399. [Google Scholar]
- Nord, A.; Mattsson, E.; Tronner, K. Factors Influencing the Long-term Corrosion of Bronze Artefacts in Soil. Protection of Metals 2005, 41, 309–316. [Google Scholar] [CrossRef]
- Akimov, G. Factors Influencing Corrosion. Corrosion 1959, 15, 23–36. [Google Scholar] [CrossRef]
- Gerwin, W.; Baumhauer, R. Effect of Soil Parameters on the Corrosion of Archaeological Metal Finds. Geoderma 2000, 96, 63–80. [Google Scholar] [CrossRef]
- Scharff, W.; Huesmann, L. Accelerated Decay of Metal Soil Finds Due to Soil Pollution: First Report. In Proceedings of the Int. Conf. on Metal Conservation (Metal 95), Semur-en-Auxois, France (25-28/9/1995). [Google Scholar]
- Skogvold, S.; Mikkelsen, Ø.; Billon, G.; Garnier, C.; Lesven, L.; Barthe, J-F. Electrochem-ical Properties of Silver-copper Alloy Microelectrodes for Use in Voltammetric Field Apparatus. Anal Bioanal Chem 2006, 384, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, D.; Vatalis, K. Entrepreneurial Aspects in East Roman Empire-The Imperial Kommerkiarioi as Quasi-entrepreneurs. Procedia Economics and Finance 2013, 5, 552–561. [Google Scholar] [CrossRef]
- Prashanth, M.; Satish, N.; Ajay kumar, B. Effect of Brass and Silver on Mechanical Properties of Copper. Materials Today: Proceedings 2018, 5, 25404–25411. [Google Scholar] [CrossRef]
- Felicia, D., Rochiem, R. & Laia, S. (2018). The Effect of Silver (Ag) Addition to Mechanical and Electrical Properties of Copper Alloy (Cu) Casting Product. In Proceedings of the 3rd Int. Conf. on Materials and Metallurgical Engineering and Technology (ICOMMET 2017): Advancing Innovation in Materials Science, Technology and Applications for Sustainable Future, Surabaya, Indonesia (30-31/10/2017).
- Mattson, E. Localized Corrosion'. Br. Corros. J 1978, 13, 5–12. [Google Scholar] [CrossRef]
- Macleod, I. Formation of Marine Concretions on Copper and Its Alloys'. Int. J. Naut. Arch. 1982, 11, 267–275. [Google Scholar] [CrossRef]
- Selwyn, L. Metals and Corrosion: A Handbook for the Conservation Professional, Canadian Conservation Institute, Ottawa, 2004; p.1.
- Watkinson, D.; Rimmer, M.; Emmerson, N. The Influence of Relative Humidity and Intrinsic Chloride on Post-excavation Corrosion Rates of Archaeological Wrought Iron. Studies in Conservation 2019, 64, 456–471. [Google Scholar] [CrossRef]
- Marchand, G.; Guilminot, E.; Lemoine, S.; Rossetti, L.; Vieau, M.; Stephant, N. Degradation of archaeological horn silver artefacts in burials. Heritage Science 2014, 2. [Google Scholar] [CrossRef]
- Denker, A.; Bohne, W.; Opitz-Coutureau, J.; Rauschenberg, J.; Röhrich, J.; Strub, E. Influence of Corrosion Layers on Quantitative Analysis. Nucl. Instrum. Methods. Phys. Res. B. am Interactions with Materials and Atoms 2005, 239, 65–70. [Google Scholar] [CrossRef]
- Grimmer, A. Dangers of Abrasive Cleaning to Historic Buildings: Preservation Briefs, U.S. Department of the Interior, National Park Service, Cultural Resources, Heritage Preservation Services, Washington, D.C., 1979.
- El-Gohary, M.; Al-Naddaf, M. Characterization of Brick Used in the External Casing of Roman Bath Walls "Gedara-Jordan". MAA 2009, 9, 29–46. [Google Scholar]
- Domingo, R. Corrosion Control for Aircraft: AC No: 43-4B, US. Department of Transportation Federal Aviation Administration Advisory Circular Subject, USA, 2018.
- Hereher, M.; Ismael, H. The Application of Remote Sensing Data to Diagnose Soil Deg-radation in the Dakhla Depression – Western Desert, Egypt. Geocarto Int 2016, 31, 527–543. [Google Scholar] [CrossRef]
- Brookes, I. Geomorphology and Quaternary Geology of the Dakhla Oasis Region, Egypt. Quat Sci Rev 1993, 12, 529–552. [Google Scholar] [CrossRef]
- Kuciewicz, E.; Polkowski, P.; Kobusiewicz, M. Dakhleh Oasis Project, Petroglyph Unit: Seasons 2012 and 2013. Polish Archaeology in the Mediterranean 2015, 24, 275–296. [Google Scholar] [CrossRef]
- Cribiore, R.; Davoli, P. New Literary Texts from Amheida, Ancient Trimithis (Dakhla Oasis, Egypt). Zeitschrift für Papyrologie und Epigraphik 2013, 187, 1–14. [Google Scholar]
- Abdel Maksoud, K.h.; Elfeky, H.; Ruban, D.; Ermolaev, V. A Unique Coincidence of Geom-orphological, Geological, and Geoarchaeological Features in the Valley of Camels (Dakhla Oasis, Western Desert, Egypt). Geoheritage 2020, 12, 81. [Google Scholar] [CrossRef]
- Said, R. The Geology of Egypt; Elsevier: Amsterdam, 1962; pp. 140–146. [Google Scholar]
- El-Desoky, H.; Khalil, A.; Farouk, S.; Fahmy, W. Dakhla–Kharga Iron-rich Paleosols, Western Desert, Egypt: Geology, Geochemistry, and Mineralization. Arab J. Geosci 2017, 10, 74. [Google Scholar] [CrossRef]
- Masoud, A.; El-Horiny, M.; Atwia, M.; Gemail, K.; Koike, K. Assessment of Groundwater and Soil Quality Degradation Using Multivariate and Geostatistical Analyses, Dakhla Oasis, Egypt. Afr. Earth Sci 2018, 142, 64–81. [Google Scholar] [CrossRef]
- Bagnall, R. Documentary Material: Excavations at Amheida, Preliminary Report, New York University. 2012.
- Ismael, H.; Abdel-Motamed, M.; Abbas, W. Environmental and climatic hazards and their impacts on the cultural heritage of El-Kharga oasis, Western desert, Egypt. EJARS 2020, 10, 135–152. [Google Scholar]
- Doménech-Carbó, A. Electrochemical Analysis of Metallic Heritage Artefacts: Voltammetry of Microparticles (VMP). In Corrosion and Conservation of Cultural Heritage Metallic Artefacts, Dillmann, P., Watkinson, D., Angelini, E., Adriaens, A. Eds.; Woodhead Pub. Limted: Oxford, 2013; pp. 165–189. [Google Scholar]
- Tidblad, J. Tidblad, J. & Swerea, A. Atmospheric corrosion of heritage metallic artefacts: Processes and prevention. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts, Dillmann, P., Watkinson, D., Angelini, E., Adriaens, A. Eds.; Woodhead Pub. Limted: Oxford, 2013, pp. 37–52.
- Ioanida, E.; Ioanida, A.; Rusub, D.; Dorofteia, F. Surface Investigation of Some Medieval Silver Coins Cleaned in High-Frequency Cold Plasma, J. of Cultural Heritage 2011, 12, 220–226. [Google Scholar] [CrossRef]
- Constantinides, I.; Gritsch, M.; Adriaens, A.; Hutter, H.; Adams, F. Microstructural Characterisation of Five Simulated Archaeological Copper Alloys Using Light Microscopy, Scanning Electron Microscopy, Energy Dispersive X-Ray Microanalysis and Secondary Ion Mass Spectrometry. Analytica Chimica Acta 2001, 440, 189–198. [Google Scholar] [CrossRef]
- Borges, R.; Alves, L.; Silva, R.; Araujo, M.; Candeias, A.; Corregidor, V.; Valerio, P.; Barrulas, P. Investigation of Surface Silver Enrichment in Ancient High Silver Alloys by PIXE, EDXRF, LA-ICP-MS and SEM-EDS, Microchem. J 2017, 131, 103–111. [Google Scholar]
- Di Turoa, F.; Colettic, F.; De Vitob, C. Investigations on Alloy-Burial Environment Interaction of Archaeological Bronze Coins. Microchemical J 2020, 157. [Google Scholar] [CrossRef]
- Dinnebier, R.; Fischer, A.; Eggert, G.; Runčevski, T.; Wahlberg, N. X-Ray Powder Diffraction in Conservation Science: Towards Routine Crystal Structure Determination of Corrosion Products on Heritage Art Objects. J. of Visualized Experiments 2016, 112, e54109. [Google Scholar] [CrossRef]
- Dowsett, M.; Sabbe, P.-J.; Anjos, J.; Schofield, E.; Walker, D.; Thomas, P.; York, S.; Brown, S.; Wermeille, D.; Adriaens, M. Synchrotron X-ray Diffraction Investigation of the Surface Condition of Artefacts from King Henry VIII’s Warship the Mary Rose. J. Synchrotron Radiation 2020, 27, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.; Herrera, V.; Muñoz, E. Corrosion and Protection of the Metallic Structures in the Petroleum Industry Due to Corrosion and the Techniques for Protection. In Handbook of Materials Failure Analysis With Case Studies from the Construction Industries, Makhlouf, A., Aliofkhazraei, M., Eds. Elsevier: Amsterdam, 2018; pp. 107–122. [Google Scholar]
- Schlamp, G. Noble Metals and Noble Metal Alloys". In Springer Handbook of Materials Data. Warlimont, H., Martienssen, W. Eds.; Springer Cham, Switzerland, 2018; pp. 339–412.
- Bude, R.; Bigelow, E. Nano - CT Evaluation of Totally Corroded Coins a Demonstrations Study to Determine if Might Still Detail be Discernible Despite the Lack of Internal Non –Corroded, Metal. Archaeometry 2020, 62, 1195–1201. [Google Scholar] [CrossRef]
- Wilson, A. The Metal Supply of the Roman Empire. J. of Roman Archaeology 2007, 69, 110–125. [Google Scholar]
- Levard, C.; Hotze, E.; Lowry, G.; Brown, G. Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ. Sci. Technol 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- El-Gohary, M. A Holistic Approach to the Assessment of the Groundwater Destructive Effects on Stone Decay in Edfu Temple Using AAS, SEM-EDX and XRD. Environ Earth Sci 2016, 75, 13. [Google Scholar] [CrossRef]
- Vassiliou, P.; Novakovic, J.-M. Corrosion of Ancient Silver Alloys. In Proceedings of 17th Int. Corrosion Congress: Corrosion Control in the Service of Society, Curran Associates, Inc., NY (6-10/10/2008).
- El-Gohary, M.; Redwan, M. Alteration Parameters Affecting the Luxor Avenue of the Sphinxes-Egypt. Science of the Total Environment 2018, 626, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Dracott, J. Piezoelectric Printing and Pre-corrosion, Electrical Resistance Corrosion Monitors for the Conservation of Heritage iron. PhD, University of Manchester, UK, 2014.
- Merk, L. A Study of Reagents Used in the Stripping of Bronzes. Studies in Conservation 1978, 23, 15–22. [Google Scholar] [CrossRef]
- Agrawal, O. Conservation of Metals—Problems and Prospects. In Conservation of Metals in Humid Climate, Agrawal, O., Ed.; 1CCROM & NRLC: Rome & Lucknow, 1987; pp. 1–17. [Google Scholar]
- Quaranta, M. On the Degradation Mechanisms under the Influence of Pedological Factors Through the Study of Archaeological Bronze Patina. PhD, Alma Mater Studiorum – Università di Bologna, Italy, 2009.
- Schweizer, F. Bronze Objects from Lake Sites: From Patina to Biography. In Ancient & Historic Metals: Conservation and Scientific Research, Scott, D., Podany, J., Considine, B. Eds.; Paul Getty Museum & Getty Conservation Institute: USA, 1991, pp. 33–50.
- Robbiola, L. Characterisation de L’alteration de Bronzes Archeologiques Enfouis a Partir d’un Corpus D’objets de L’age du Bronze: Mechanismes de Corrosion. PhD, Matériaux. Université Pierre et Marie Curie, Paris, 1990.
- Chase, W. Chinese Bronzes: Casting, Finishing, Patination, and Corrosion. In Ancient & Historic Metals: Conservation and Scientific Research, Scott, D.; Podany, J., Considine, B., Eds.; Paul Getty Museum & Getty Conservation Institute: USA, 1991; pp. 95–117. [Google Scholar]
- Argyropoulos, V.; Boyatzis, S.; Giannoulaki, M.; Polikreti, K. The Role of Standards in Conservation Methods for Metals in Cultural Heritage. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts, Dillmann, P., Watkinson, D., Angelini, E., Adriaens, A. Eds.; Woodhead Pub. Limted: Oxford, 2013, pp. 478–517.
- Wang, Q. An Investigation of Deterioration of Archaeological Iron. Studies in Conservation 2007, 52, 125–134. [Google Scholar] [CrossRef]
- Watkinson, D. (2013). Conservation, corrosion science and evidence-based preservation strategies for metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts, Dillmann, P., Watkinson, D., Angelini, E., Adriaens, A. Eds.; Woodhead Pub. Limted: Oxford, 2013, pp. 9–36.
- Matthiesen, H. (2013). Oxygen monitoring in the corrosion and preservation of metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts, Dillmann, P., Watkinson, D., Angelini, E., Adriaens, A. Eds.; Woodhead Pub. Limted: Oxford, 2013, pp. 368–391.
- Organ, R. The Current Status of the Treatment of Corroded Metal Artifacts, Corrosion and Metal Artifacts, Special Pub. 479; National Bureau of Standards: Washington, D.C, 1977; pp. 107–142. [Google Scholar]
- Wanhill, R.; Steijaart, J.; Leenheer, R.; Koens, J. Damage Assessment and Preservation of an Egyptian Silver Vase (300-200 BC). Archaeometry 1998, 40, 123–137. [Google Scholar] [CrossRef]
- Schweizer, F.; Meyers, P. A New Approach to the Authenticity of Ancient Silver Objects: The Discontinuous Precipitation of Copper from a Silver-Copper Alloy. In Proceedings of the 18th Int. Symp. on Archaeometry and Archaeological Prospection, Rheinland– Verlag GmbH, Cologne, (14-17/3/1978).
- Bloeck, M. Aluminium Sheet for Automotive Applications. In Advanced Materials in Automotive Engineering, 1st ed.Rowe, J. Ed. Woodhead Pub.: UK, 2012; pp. 85–108. [Google Scholar]
- Bardal, E. Different Forms of Corrosion Classified on the Basis of Appearance. In Corrosion and Protection. Engineering Materials and Processes, Bardal, E. Ed.; Springer: London, 2004; pp. 89–191. [Google Scholar]
- He, L.; Liang, J.; Zhao, X.; Jiang, B. Corrosion Behavior and Morphological Features of Archeological Bronze Coins from Ancient China. Microchemical J 2011, 99, 203–212. [Google Scholar] [CrossRef]
- Tylecote, R. The effect of soil conditions on the long-term corrosion of buried tin-bronzes and copper. J. of Archaeological Science 1979, 6(4), 345–368. [Google Scholar] [CrossRef]
- Baker, H. Properties of Metals. In Metals Handbook, 2nd ed.; Davis, J. Ed., ASM Int.: Ohio, 1998. [Google Scholar] [CrossRef]
- Gouda, V.; Youssef, G.; Abdel Ghany, N. Characterization of Egyptian Bronze Archaeological Artifacts, Surf. Interface Anal 2012, 44, 1338–1345. [Google Scholar] [CrossRef]
- Mereu, M.; Basilissi, V.; Guida, G.; Vidale, M.; Pia Casaletto, M.; Maria Ingo, G.; Drago, L.; Greco, E. Conservation of Copper Alloys Artefacts from Archaeological Excavation. In YOCOCU: Contribute and Role of Youth in Conservation of Cultural Heritage, Macchia, A.; Greco, E., Chiarandà, B., Barbabietola, N., Eds.; Italian Association of Conservation Scientist: Rome, 2011; pp. 163–175. [Google Scholar]
- Eggert, G.; Sobottka-Braun, U. Black Spots on Bronzes and Elemental Sulphur. In: 12th ICOM-CC Triennial Meeting, Vol. 2, ICOM Eds., James & James: London, 1999; pp. 823–827.
- Cura, J.; Junior, D.; Freitas, V.; Lins, C. Surface Characterization of a Corroded Bronze-leaded Alloy in Salt Sprays Cabinet. Applied Surface Science 2007, 253, 1104–7107. [Google Scholar]
- Young, M.; Casadio, F.; Marvin, J.; Chase, W.; Dunand, D. An Ancient Chinese Bronze Fragment Re-examined after 50 Years: Contributions from Modern and Traditional Techniques. Archaeometry 2010, 52, 1015–1043. [Google Scholar] [CrossRef]
- Smith, J.; Qin, Z.; King, F.; Werme, L.; Shoesmith, D. Sulfide Film Formation on Copper under Electrochemical and Natural Corrosion Conditions. Corrosion Science Section 2007, 63, 135–144. [Google Scholar] [CrossRef]
- Trentelman, K.; Stodulski, L.; Lints, R.; Kim, C. Comparative Study of the Composition and Corrosion of Branches from Eastern Han Dynasty Money Trees. Studies in Conservation 1999, 44, 170–183. [Google Scholar] [CrossRef]
- Syrett, B. Accelerated Corrosion of Copper in Flowing Pure Water Contaminated with Oxygen and Sulfide. Corrosion 1977, 33, 257–262. [Google Scholar] [CrossRef]
- Syrett, B. The Mechanism of Accelerated Corrosion of Copper-Nickel Alloys in Sulphide-Polluted Seawater. Corrosion Science 1981, 21, 187–209. [Google Scholar] [CrossRef]
- Kato, C.; Pickering, H.; Castle, J. Effect of Sulfide on the Corrosion of Cu-9.4 Ni-1.7 Fe Alloy in Aqueous NaCl Solution. J. of the Electrochemical Society 1984, 131, 1225–1229. [Google Scholar] [CrossRef]
- Mor, E.; Beccaria, A. Behaviour of Copper in Artificial Seawater Containing Sulphides. British Corrosion J 1975, 10, 33–38. [Google Scholar] [CrossRef]
- North, N.; MacLeod. I. Corrosion of Metals. In Conservation of Archaeological Objects, Pearson, C. Ed., Butterworths: London, 1987; pp. 669–698.
- Gharib, A. A Scientific Study of the Patina, Corrosion Morphology, and Conservation of Egyptian Brass Object. EJARS 2014, 4, 25–33. [Google Scholar]
- Oudbashi, O.; Emami, S.; Ahmadi, H.; Davami, P. Micro-stratigraphical investigation on corrosion layers in ancient Bronze artefacts by scanning electron microscopy energy dispersive spectrometry and optical microscopy. Heritage Science 2013. [Google Scholar] [CrossRef]
- Scott, D. Copper and Bronze in Art: Corrosion, Colorants, Conservation, Getty Conservation Institute, USA, 2012; p.35.
- Vink, B. Stability Relations of Malachite and Azurite. Mineralogical Magazine 1986, 50, 41–47. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




