Submitted:
16 February 2023
Posted:
01 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
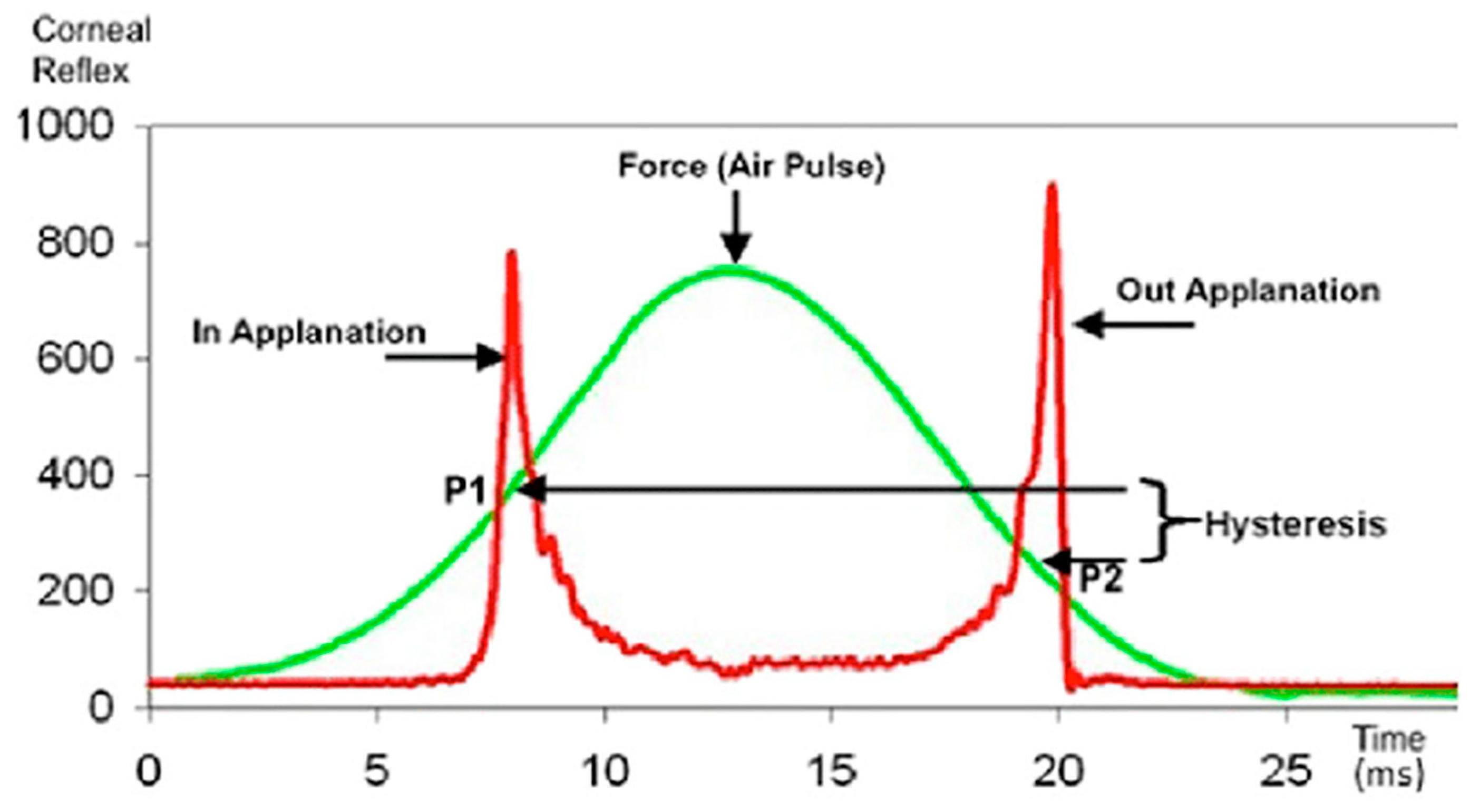
2. The Ocular Response Analyzer
3. The Corvis ST Dynamic Scheimpflug Analyzer
4. Hysteresis and Glaucoma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg 2005;31(1):146-55. [CrossRef]
- Fontes BM, Ambrosio R, Jr., Jardim D, Velarde GC, Nose W. Corneal biomechanical metrics and anterior segment parameters in mild keratoconus. Ophthalmology 2010;117(4):673-9. [CrossRef]
- Luz A, Fontes BM, Lopes B, Ramos I, Schor P, Ambrosio R, Jr. ORA waveform-derived biomechanical parameters to distinguish normal from keratoconic eyes. Arq Bras Oftalmol 2013;76(2):111-7. [CrossRef]
- Ventura BV, Machado AP, Ambrosio R, Jr., et al. Analysis of waveform-derived ORA parameters in early forms of keratoconus and normal corneas. J Refract Surg 2013;29(9):637-43. [CrossRef]
- Silva JASd, Silva RSd, Ambrósio Jr R. Relevância da biomecânica da córnea no glaucoma. Revista Brasileira de Oftalmologia 2012;71.
- Catania F, Morenghi E, Rosetta P, Paolo V, Vinciguerra R. Corneal Biomechanics Assessment with Ultra High Speed Scheimpflug Camera in Primary Open Angle Glaucoma Compared with Healthy Subjects: A meta-analysis of the Literature. Curr Eye Res 2023;48(2):161-171. [CrossRef]
- Qassim A, Mullany S, Abedi F, et al. Corneal Stiffness Parameters Are Predictive of Structural and Functional Progression in Glaucoma Suspect Eyes. Ophthalmology 2021;128(7):993-1004. [CrossRef]
- Salvetat ML, Zeppieri M, Tosoni C, Felletti M, Grasso L, Brusini P. Corneal Deformation Parameters Provided by the Corvis-ST Pachy-Tonometer in Healthy Subjects and Glaucoma Patients. J Glaucoma 2015;24(8):568-74. [CrossRef]
- Lanzagorta-Aresti A, Perez-Lopez M, Palacios-Pozo E, Davo-Cabrera J. Relationship between corneal hysteresis and lamina cribrosa displacement after medical reduction of intraocular pressure. Br J Ophthalmol 2017;101(3):290-294. (In eng). [CrossRef]
- Zeimer RC, Ogura Y. The relation between glaucomatous damage and optic nerve head mechanical compliance. Arch Ophthalmol 1989;107(8):1232-4. (In eng). [CrossRef]
- Kimball EC, Nguyen C, Steinhart MR, et al. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp Eye Res 2014;128:129-40. (In eng). [CrossRef]
- Luce, DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg 2005;31(1):156-62. [CrossRef]
- Brandt, JD. Central corneal thickness, tonometry, and glaucoma risk--a guide for the perplexed. Can J Ophthalmol 2007;42(4):562-6. (https://www.ncbi.nlm.nih.gov/pubmed/17641698).
- Roberts, CJ. Concepts and misconceptions in corneal biomechanics. J Cataract Refract Surg 2014;40(6):862-9. [CrossRef]
- Roberts, CJ. Corneal hysteresis and beyond: Does it involve the sclera? Journal of Cataract & Refractive Surgery 2021;47(4):427-429. [CrossRef]
- Taroni L, Bernabei F, Pellegrini M, et al. Corneal Biomechanical Response Alteration After Scleral Buckling Surgery for Rhegmatogenous Retinal Detachment. Am J Ophthalmol 2020;217:49-54. [CrossRef]
- Kotecha, A. What biomechanical properties of the cornea are relevant for the clinician? Surv Ophthalmol 2007;52 Suppl 2:S109-14. [CrossRef]
- Hager A, Schroeder B, Sadeghi M, Grossherr M, Wiegand W. [The influence of corneal hysteresis and corneal resistance factor on the measurement of intraocular pressure]. Ophthalmologe 2007;104(6):484-9. [CrossRef]
- Zhang H, Sun Z, Li L, Sun R, Zhang H. Comparison of intraocular pressure measured by ocular response analyzer and Goldmann applanation tonometer after corneal refractive surgery: a systematic review and meta-analysis. BMC Ophthalmol 2020;20(1):23. [CrossRef]
- Nguyen BA, Roberts CJ, Reilly MA. Biomechanical Impact of the Sclera on Corneal Deformation Response to an Air-Puff: A Finite-Element Study. Front Bioeng Biotechnol 2018;6:210. (In eng). [CrossRef]
- Yuhas PT, Roberts CJ. Clinical Ocular Biomechanics: Where Are We after 20 Years of Progress? Curr Eye Res 2023;48(2):89-104. [CrossRef]
- Nguyen BA, Reilly MA, Roberts CJ. Biomechanical contribution of the sclera to dynamic corneal response in air-puff induced deformation in human donor eyes. Exp Eye Res 2020;191:107904. (In eng). [CrossRef]
- Metzler KM, Mahmoud AM, Liu J, Roberts CJ. Deformation response of paired donor corneas to an air puff: intact whole globe versus mounted corneoscleral rim. J Cataract Refract Surg 2014;40(6):888-96. [CrossRef]
- Ambrósio Jr RR, I. Luz, A. Faria, F. C. Andreas, S. Krug, M. Belin, M. W. Roberts, C. J. Dynamic ultra-high speed Scheimpflug imaging for assessing corneal biomechanical properties. Rev Bras Oftalmol 2013;72(2). [CrossRef]
- Roberts CJ, Mahmoud AM, Bons JP, et al. Introduction of Two Novel Stiffness Parameters and Interpretation of Air Puff-Induced Biomechanical Deformation Parameters With a Dynamic Scheimpflug Analyzer. J Refract Surg 2017;33(4):266-273. [CrossRef]
- Joda AA, Shervin MM, Kook D, Elsheikh A. Development and validation of a correction equation for Corvis tonometry. Comput Methods Biomech Biomed Engin 2016;19(9):943-53. [CrossRef]
- Vinciguerra R, Ambrosio R, Jr., Elsheikh A, et al. Detection of Keratoconus With a New Biomechanical Index. J Refract Surg 2016;32(12):803-810. [CrossRef]
- Ambrosio R, Jr. , Lopes BT, Faria-Correia F, et al. Integration of Scheimpflug-Based Corneal Tomography and Biomechanical Assessments for Enhancing Ectasia Detection. J Refract Surg 2017;33(7):434-443. [CrossRef]
- Sedaghat MR, Momeni-Moghaddam H, Ambrosio R, Jr., et al. Long-term Evaluation of Corneal Biomechanical Properties After Corneal Cross-linking for Keratoconus: A 4-Year Longitudinal Study. J Refract Surg 2018;34(12):849-856. [CrossRef]
- Eliasy A, Chen KJ, Vinciguerra R, et al. Determination of Corneal Biomechanical Behavior in-vivo for Healthy Eyes Using CorVis ST Tonometry: Stress-Strain Index. Front Bioeng Biotechnol 2019;7:105. [CrossRef]
- Fujishiro T, Matsuura M, Fujino Y, et al. The Relationship Between Corvis ST Tonometry Parameters and Ocular Response Analyzer Corneal Hysteresis. J Glaucoma 2020;29(6):479-484. [CrossRef]
- Wong BJ, Moghimi S, Zangwill LM, et al. Relationship of Corneal Hysteresis and Anterior Lamina Cribrosa Displacement in Glaucoma. Am J Ophthalmol 2020;212:134-143. [CrossRef]
- Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol 2006;141(5):868-75. [CrossRef]
- De Moraes CV, Hill V, Tello C, Liebmann JM, Ritch R. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma 2012;21(4):209-13. [CrossRef]
- Susanna CN, Diniz-Filho A, Daga FB, et al. A Prospective Longitudinal Study to Investigate Corneal Hysteresis as a Risk Factor for Predicting Development of Glaucoma. Am J Ophthalmol 2018;187:148-152. [CrossRef]
- Agarwal DR, Ehrlich JR, Shimmyo M, Radcliffe NM. The relationship between corneal hysteresis and the magnitude of intraocular pressure reduction with topical prostaglandin therapy. Br J Ophthalmol 2012;96(2):254-7. [CrossRef]
- Hirneiss C, Sekura K, Brandlhuber U, Kampik A, Kernt M. Corneal biomechanics predict the outcome of selective laser trabeculoplasty in medically uncontrolled glaucoma. Graefes Arch Clin Exp Ophthalmol 2013;251(10):2383-8. [CrossRef]
- Sun L, Shen M, Wang J, et al. Recovery of corneal hysteresis after reduction of intraocular pressure in chronic primary angle-closure glaucoma. Am J Ophthalmol 2009;147(6):1061-6, 1066 e1-2. [CrossRef]
- Kotecha A, Elsheikh A, Roberts CR, Zhu H, Garway-Heath DF. Corneal thickness- and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Invest Ophthalmol Vis Sci 2006;47(12):5337-47. [CrossRef]
- Brandt JD, Gordon MO, Beiser JA, et al. Changes in central corneal thickness over time: the ocular hypertension treatment study. Ophthalmology 2008;115(9):1550-6, 1556 e1. [CrossRef]
- Sullivan-Mee M, Katiyar S, Pensyl D, Halverson KD, Qualls C. Relative importance of factors affecting corneal hysteresis measurement. Optom Vis Sci 2012;89(5):E803-11. [CrossRef]
- Silva JASd, Silva RSd, Jr RA. Relevância da biomecânica da córnea no glaucoma. Revista Brasileira de Oftalmologia;73(1):37-39. [CrossRef]
- Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci 2005;46(11):4189-99. [CrossRef]
- Sigal IA, Yang H, Roberts MD, et al. IOP-induced lamina cribrosa deformation and scleral canal expansion: independent or related? Invest Ophthalmol Vis Sci 2011;52(12):9023-32. [CrossRef]
- Coudrillier B, Campbell IC, Read AT, et al. Effects of Peripapillary Scleral Stiffening on the Deformation of the Lamina Cribrosa. Invest Ophthalmol Vis Sci 2016;57(6):2666-77. [CrossRef]
- Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R, Group CS. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 2011;118(9):1766-73. [CrossRef]
- Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004;111(9):1627-35. [CrossRef]
- Vinciguerra R, Rehman S, Vallabh NA, et al. Corneal biomechanics and biomechanically corrected intraocular pressure in primary open-angle glaucoma, ocular hypertension and controls. Br J Ophthalmol 2020;104(1):121-126. [CrossRef]
- Anand A, De Moraes CG, Teng CC, Tello C, Liebmann JM, Ritch R. Corneal hysteresis and visual field asymmetry in open angle glaucoma. Invest Ophthalmol Vis Sci 2010;51(12):6514-8. [CrossRef]
- Helmy H, Leila M, Zaki AA. Corneal biomechanics in asymmetrical normal-tension glaucoma. Clin Ophthalmol 2016;10:503-10. [CrossRef]
- Miki A, Yasukura Y, Weinreb RN, et al. Dynamic Scheimpflug Ocular Biomechanical Parameters in Untreated Primary Open Angle Glaucoma Eyes. Invest Ophthalmol Vis Sci 2020;61(4):19. [CrossRef]
- Pradhan ZS, Deshmukh S, Dixit S, et al. A comparison of the corneal biomechanics in pseudoexfoliation glaucoma, primary open-angle glaucoma and healthy controls using Corvis ST. PLoS One 2020;15(10):e0241296. [CrossRef]
- Silva N, Ferreira A, Baptista PM, et al. Corneal Biomechanics for Ocular Hypertension, Primary Open-Angle Glaucoma, and Amyloidotic Glaucoma: A Comparative Study by Corvis ST. Clin Ophthalmol 2022;16:71-83. [CrossRef]
- Schlotzer-Schrehardt U, Zenkel M, Nusing RM. Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Invest Ophthalmol Vis Sci 2002;43(5):1475-87. (https://www.ncbi.nlm.nih.gov/pubmed/11980863).
- Sharif NA, Kelly CR, Crider JY, Williams GW, Xu SX. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther 2003;19(6):501-15. [CrossRef]
- Shen SR, Fleming GP, Jain SG, Roberts CJ. A Review of Corneal Biomechanics and Scleral Stiffness in Topical Prostaglandin Analog Therapy for Glaucoma. Curr Eye Res 2023;48(2):172-181. [CrossRef]
- Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol 2002;47 Suppl 1:S53-64. [CrossRef]
- Kim JW, Lindsey JD, Wang N, Weinreb RN. Increased human scleral permeability with prostaglandin exposure. Invest Ophthalmol Vis Sci 2001;42(7):1514-21. (https://www.ncbi.nlm.nih.gov/pubmed/11381055).
- Lindsey JD, Crowston JG, Tran A, Morris C, Weinreb RN. Direct matrix metalloproteinase enhancement of transscleral permeability. Invest Ophthalmol Vis Sci 2007;48(2):752-5. [CrossRef]
- Scott JA, Roberts CJ, Mahmoud AM, Jain SG. Evaluating the Relationship of Intraocular Pressure and Anterior Chamber Volume With Use of Prostaglandin Analogues. J Glaucoma 2021;30(5):421-427. [CrossRef]
- Aoki S, Murata H, Matsuura M, et al. The Relationship between the Waveform Parameters from the Ocular Response Analyzer and the Progression of Glaucoma. Ophthalmol Glaucoma 2018;1(2):123-131. [CrossRef]
- Faria-Correia F, Ramos I, Valbon B, Luz A, Roberts CJ, Ambrosio R, Jr. Scheimpflug-based tomography and biomechanical assessment in pressure-induced stromal keratopathy. J Refract Surg 2013;29(5):356-8. [CrossRef]
- Goldich Y, Marcovich AL, Barkana Y, et al. Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: results after 2 years of follow-up. Cornea 2012;31(6):609-14. [CrossRef]
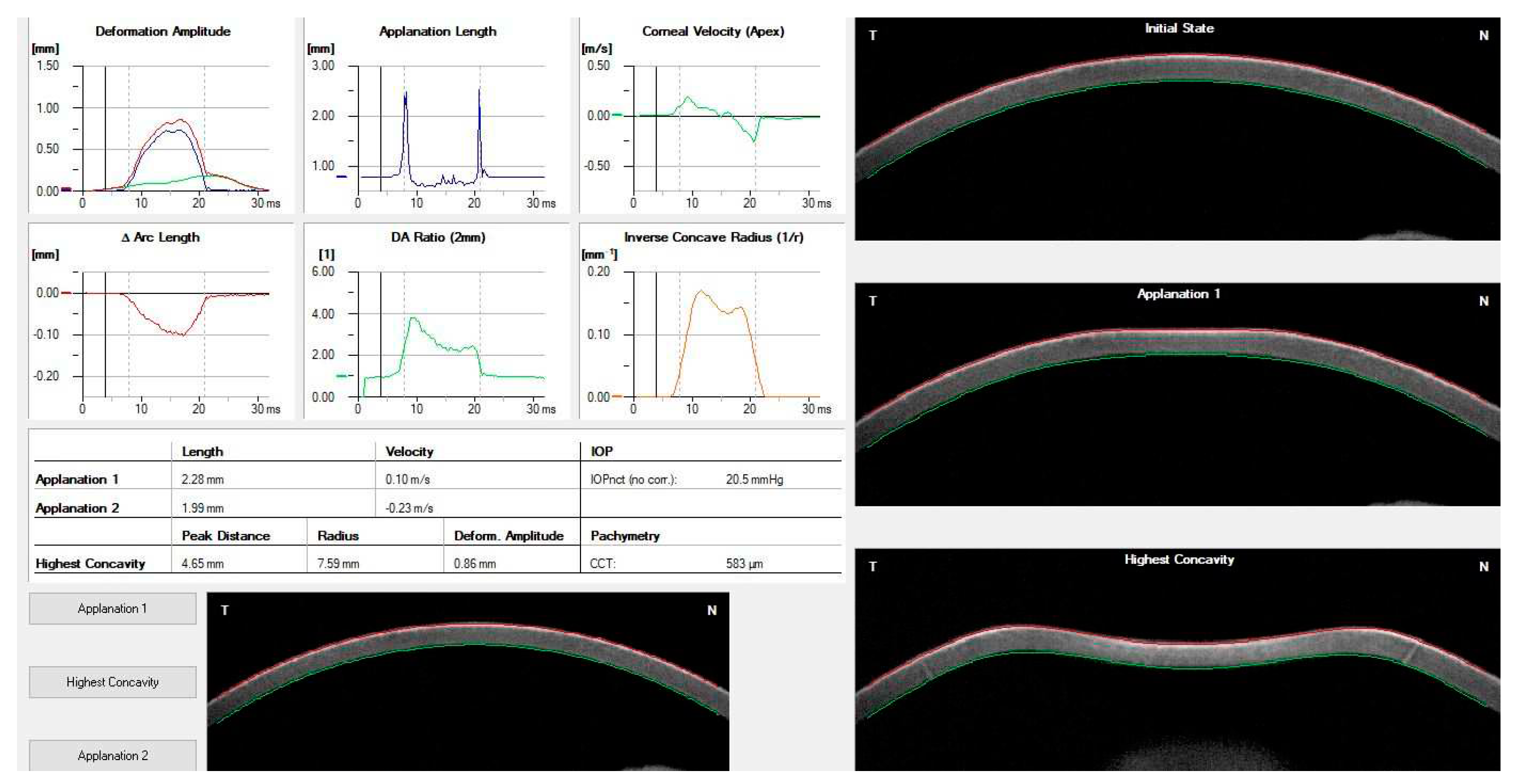
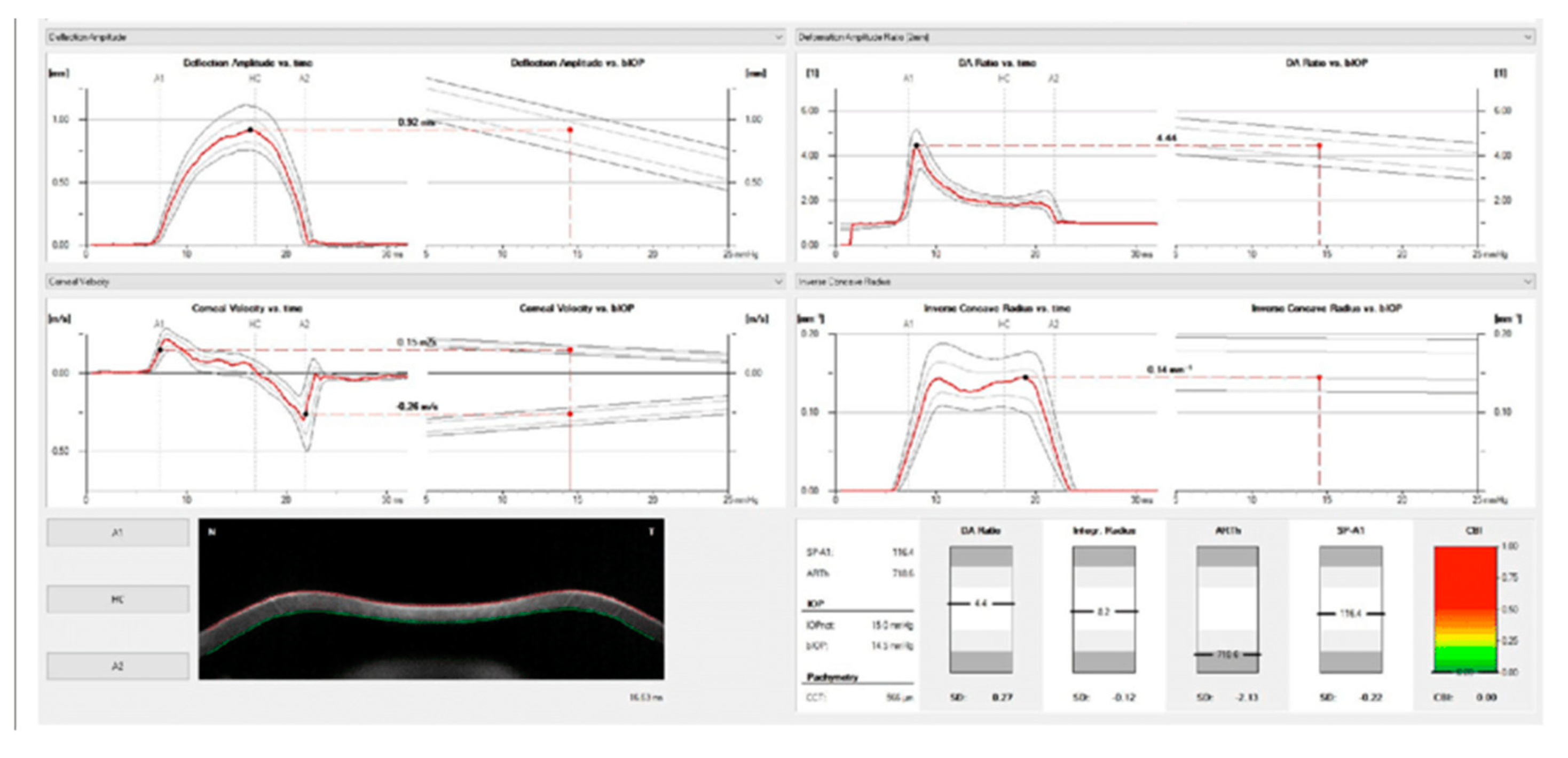
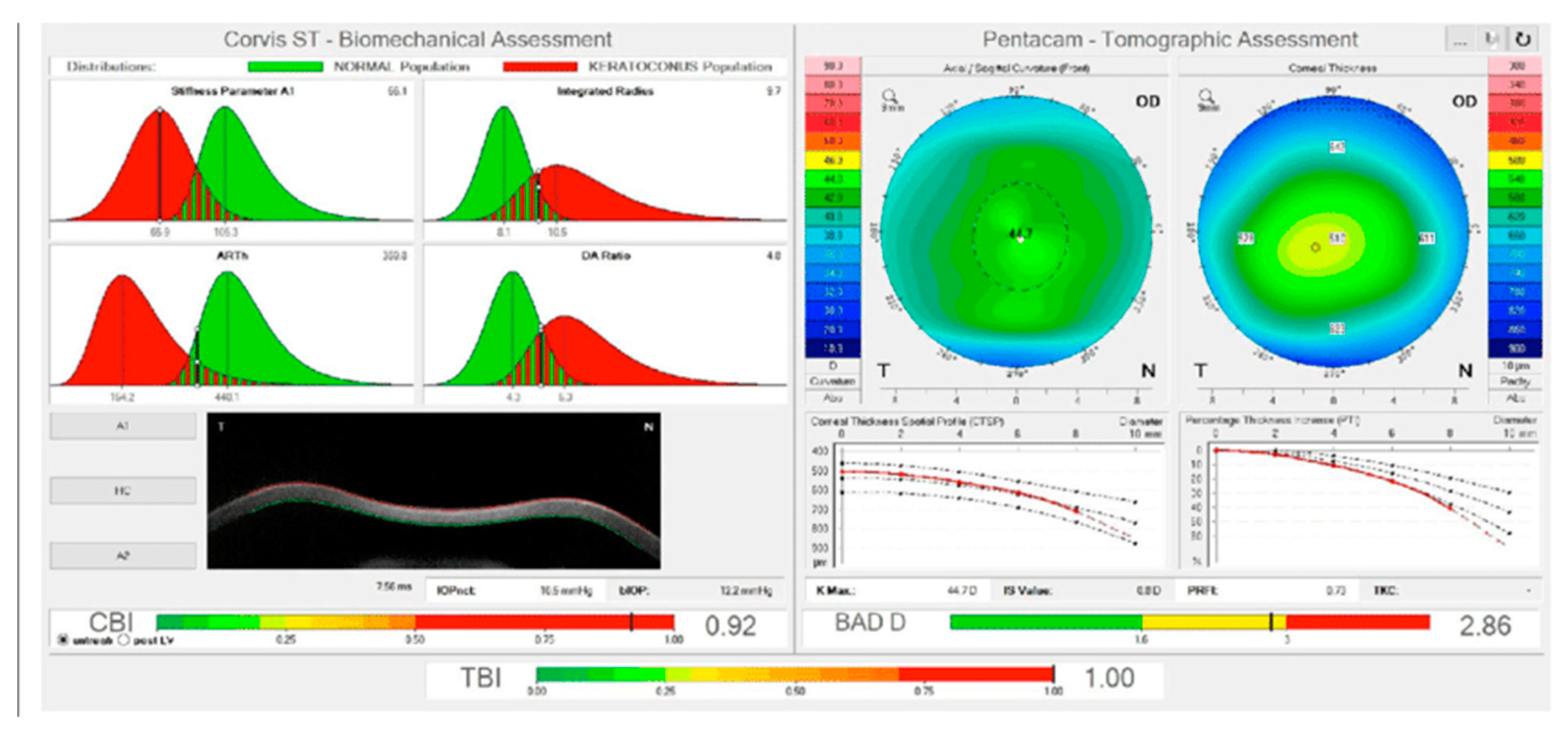
| Corvis ST parameter | Definition |
|---|---|
| 1st Applanation | The first applanation of the cornea during the air puff (in ms). The length of the applanation at this moment appears in parenthesis (in mm). |
| Highest Concavity | The instant that the cornea assumes its maximum concavity during the air puff (in ms). The length of the distance between the two peaks of the cornea at this moment appears in parenthesis (in mm). |
| 2nd Applanation | The second applanation of the cornea during the air puff (in milliseconds). The length of the applanation at this moment appears in parenthesis (in mm). |
| Maximum Deformation | The amount (in mm) of the maximum cornea deformation during the air puff |
| Wing Distance | The length of the distance between the two peaks of the cornea at this instant (in mm) |
| Maximum Velocity (in) | The maximum velocity during the ingoing phase (in m/s) |
| Maximum Velocity | The maximum velocity during the outgoing phase (in m/s) |
| Curvature Radius Normal | The cornea in its natural state radius of curvature (in mm) |
| Curvature Radius HC | The cornea radius of curvature at the time of maximum concavity during the air puff (in mm) |
| Cornea Thickness | Measurement of the corneal thickness (in mm) |
| IOP | Measurement of the intraocular pressure (in mmHg) |
| bIOP | Biomechanically-corrected IOP |
| DA ratio Max (Deformation amplitude ratiomax 2mm) | Ratio between the deformation amplitude at the apex and the average deformation amplitude measured at 2 mm from the center |
| ARth (Ambrósio’s relational thickness to the horizontal profile) | Describes thickness profile in the temporal-nasal direction and defined as corneal thickness thinnest to pachymetric progression |
| SP-A1(Stiffness parameter at A1) | Describes corneal stiffness as defined by resultant pressure (Pr) divided by deflection amplitude at A1 |
| SP-HC | Corneal stiffness at the highest concavity point |
| TBI (Tomographic biomechanical index) | Index that combined tomographic and biomechanical data for keratoconus detection |
| BGF (Biomechanical Glaucoma factor) | Independent risk indicator for normal tension glaucoma |
| SSI (Stress-strain index) | Index that indicates the position of the stress-strain curves. Less dependent on corneal thickness and IOP. |
| CBI (Corvis biomechanical index | Overall biomechanical index for keratoconus detection |
| Whole eye movement (WEM) | The entire globe's movement after the cornea passes its limits during the jet air pulse resisted by the orbital structures. |
| Deformation Amplitude (DA) | The movement of the corneal deformation from apex to highest concavity |
| Deflection amplitude DeflA | The difference between The DA and the WEM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





