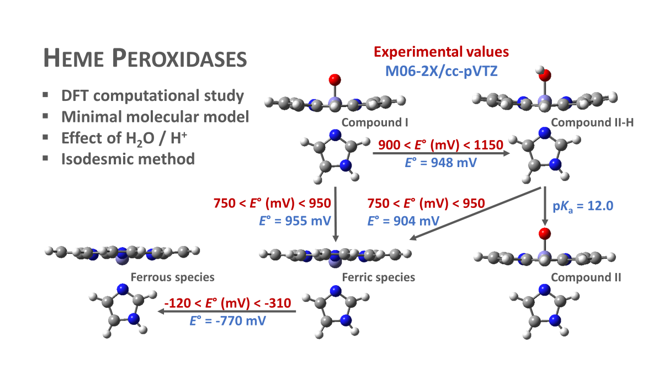
Electronic structure calculations have been carried out to examine the effect of protonation and water molecules on the heme group of peroxidases in different redox (ferric, ferrous, compounds I and II) and spin states. Shared geometries, spectroscopic properties at Soret region, and thermodynamics of peroxidases are discussed. B3LYP and M06-2X density functionals with different basis sets were employed on a common molecular model of the active site (Fe-centred porphine and proximal imidazole). Computed Gibbs free energies indicate that the corresponding aquo complexes are not thermodynamically stable, supporting the five-coordinate Fe(III) centre in native ferric peroxidases, with a water molecule located at a non-bonding distance. Protonation of the ferryl oxygen of compound II is discussed in terms of thermodynamics, Fe–O bond distances, and redox properties. It is demonstrated that this protonation is necessary to account for the experimental data and computed Gibbs free energies that reveal pKa values of compound II about 8.5 – 9.0. Computation suggests that the origin of the general oxidative properties of peroxidase species, as well as their reactivity towards water and protons and Soret spectral properties, mostly resides on the iron porphyrin and its proximal ligand histidine.