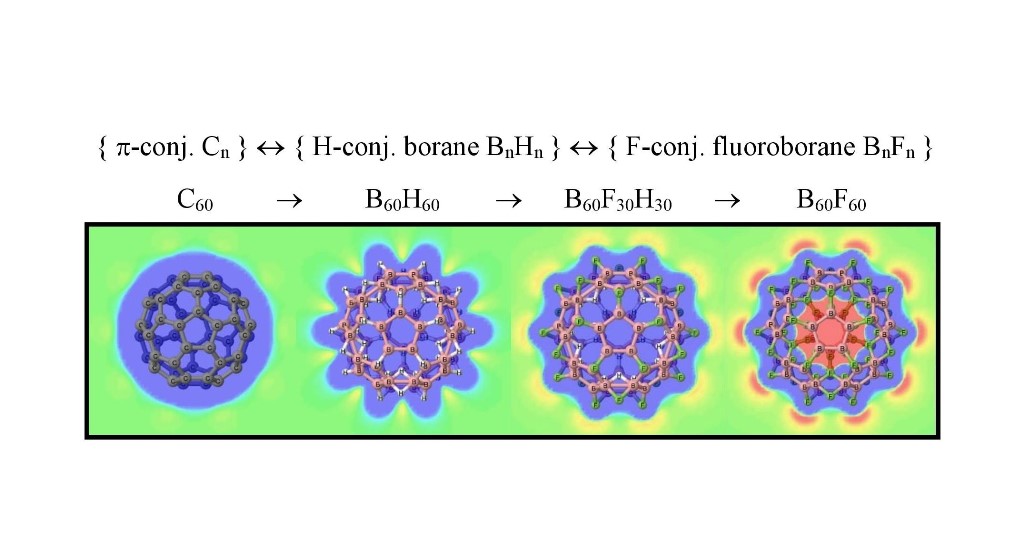
The one-to-one structural correspondence between any conjugated hydrocarbon CnHm and the borane BnHm+n is extended here, with the B3LYP/cc-pVDZ model, to pure conjugated carbon systems with the example of buckminsterfullerene C60 with the corresponding icosahedral isoelectronic system closo-borane B60H60, and the fluorine substituted systems B60F30H30 and B60F60 , all with icosahedral Ih symmetry. All systems correspond to energy minima in the potential energy hypersurface, except for B60(F30)in(H30)out . Selected electronic structure methods are used to characterize all systems: molecular electrostatic potentials (MEP), atomic charges, bond orders, and topological properties of the electron density within quantum theory of atoms-in-molecules (QTAIM) and electron-localization function (ELF) theory. In the particular case of B60H60 we use the recently developed Hückeloid model to characterize this system. The stability of the energy minimum icosahedral structure B60F60 could have an origin in F···F attractive interactions of the inner fluorine atoms of the cage.