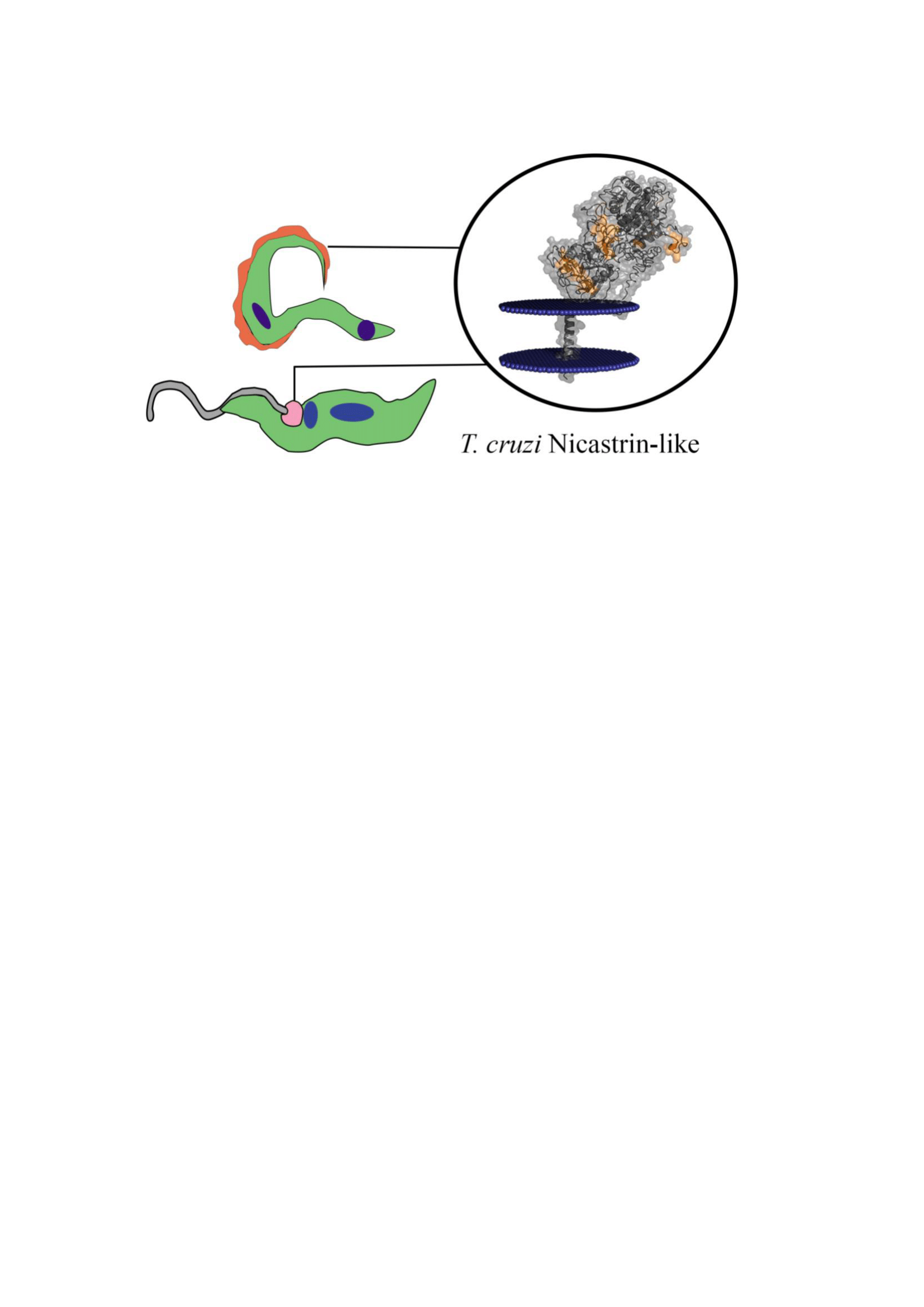
Nicastrin (NICT) is a transmembrane protein physically associated with the polytypical aspartyl protease presenilin that plays a vital role in the correct localization and stabilization of presenilin to the membrane-bound γ-secretase complex. This complex is involved in the regulation of a wide range of cellular events including cell signaling and the regulation of endocytosed membrane proteins for their trafficking and protein processing. Mehtods: In Trypanosoma cruzi, the causal agent of the Chagas disease, an NICT-like protein (Tc/NICT) was identified with a short C-terminus orthologous to the human protein, a large ectodomain (ECD) with numerous glycosylation sites and a single core transmembrane domain containing a putative TM-domain (457GSVGA461) important for the γ-secretase complex activity. Results: Using the Spot-synthesis strategy with Chagasic patient sera, five extracellular epitopes were identified and synthetic forms were used to generate rabbit anti-Tc/NICT polyclonal serum that recognized a ~72-kDa molecule in immunoblots of T. cruzi epimastigote extracts. Confocal microscopy suggests that Tc/NICT is localized in the flagellar pocket, which is consistent with data from our previous studies with a T. cruzi presenilin-like protein. Phylogenetically, Tc/NICT was localized within a subgroup with the T. rangeli protein that are clearly detached from the other Trypanosomatidae such as T. brucei. These results, together with a comparative analysis of the selected peptide sequence regions between the T. cruzi and mammalian proteins suggest a divergence from the human NICT that might be relevant to Chagas disease pathology. As a whole, our data show that an NICT-like protein is expressed in the infective and replicative stages of T. cruzi and may be considered further evidence for a γ-secretase complex in trypanosomatids.