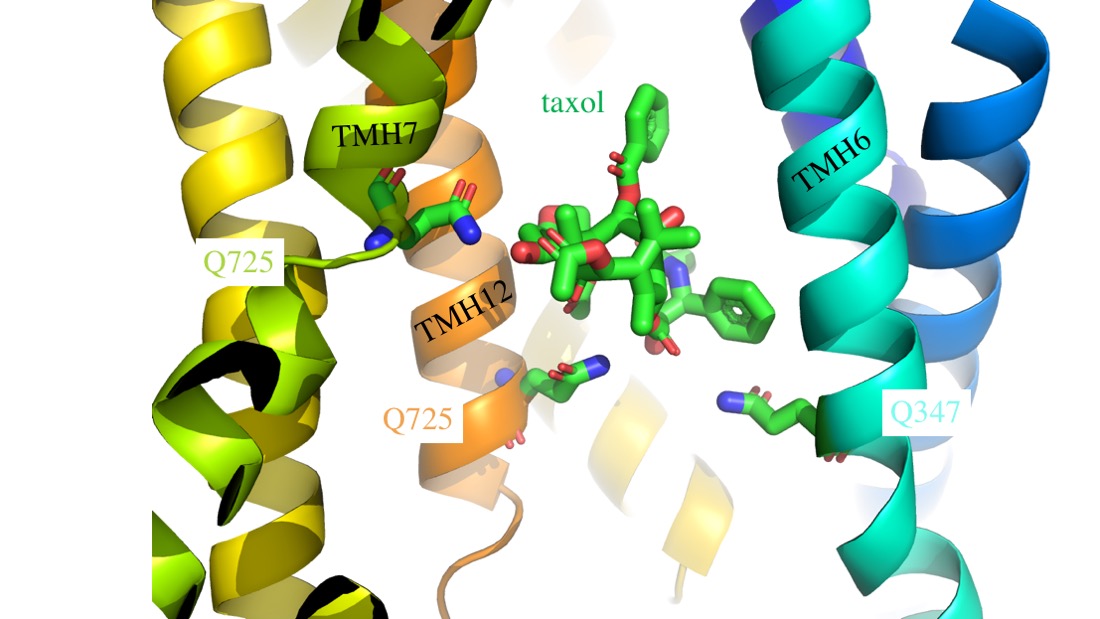
The multidrug efflux transporter ABCB1 is clinically important for drug absorption and distribution and can be a determinant of chemotherapy failure. Recent structure data shows that three glutamines donate hydrogen bonds to co-ordinate taxol in the drug binding pocket. This is consistent with earlier drug structure-activity relationships that implicated the importance of hydrogen bonds in drug recognition by ABCB1. By replacing the glutamines with alanines we have tested whether any or all of Q347, Q725 and Q990 are important for the transport of three different drug classes. Flow cytometric transport assays show that Q347A and Q990A act synergistically to reduce transport of Calcein-AM, BODIPY-verapamil and OREGON GREEN-taxol bisacetate but the magnitude of the effect was dependent on the test drug and no combination of mutations completely abrogated function. Surprisingly, Q725A mutants generally improved transport of Calcein-AM and BODIPY-verapamil, suggesting that engagement of the wild-type Q725 in a hydrogen bond is inhibitory for the transport mechanism. To test transport of unmodified taxol, stable expression of Q347/725A and the triple mutant was engineered and shown to confer equivalent resistance to the drug as the wild-type transporter, further indicating that none of these potential hydrogen bonds between transporter and transport substrate are critical for function of ABCB1. The implications of the data for plasticity of the drug binding pocket are discussed.