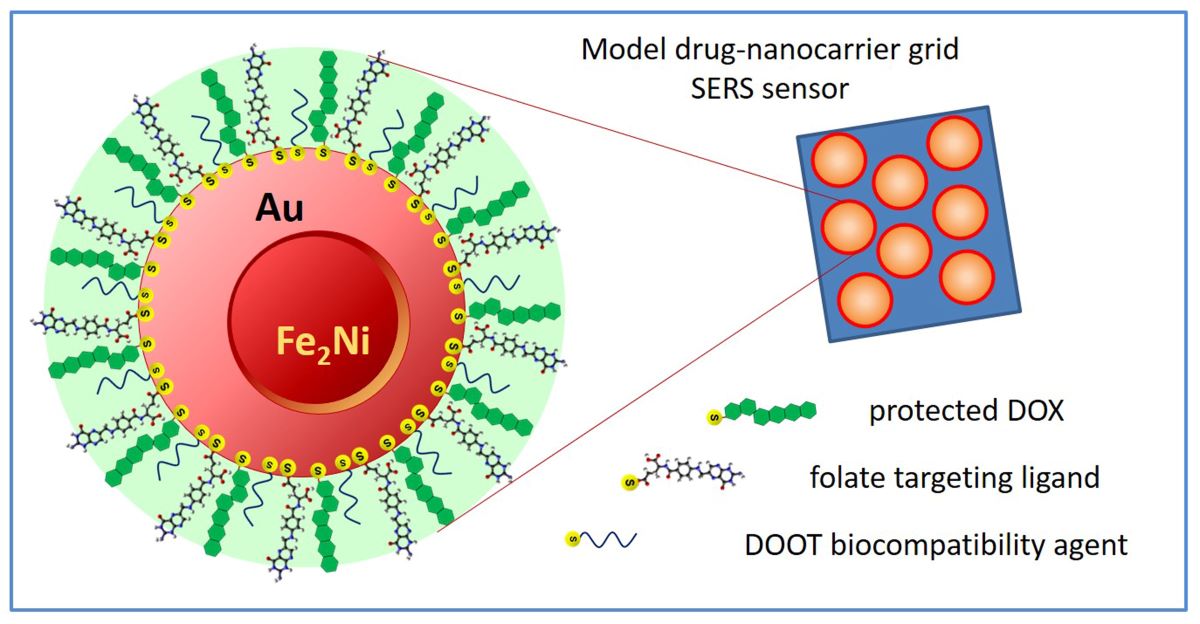
Safe administration of highly cytotoxic chemotherapeutic drugs is a challenging problem in cancer treatment due to the adverse side effects and collateral damage to non-tumorigenic cells. To mitigate these problems, new promising approaches, based on the paradigm of controlled targeted drug delivery (TDD), utilizing drug nanocarriers with biorecognition ability to selectively target neoplastic cells, are being considered in cancer therapy. Herein, we report on the design and testing of a nanoparticle-grid based biosensing platform to aid in the development of new targeted drug nanocarriers. The proposed sensor grid consists of superparamagnetic gold-coated core-shell Fe2Ni@Au nanoparticles, further functionalized with folic acid targeting ligand, model thiolated chemotherapeutic drug doxorubicin (DOX), and a biocompatibility agent, 3,6,-dioxa-octanethiol (DOOT). The employed dual transduction based on electrochemical and enhanced Raman scattering detection have enabled efficient monitoring of the drug loading onto the nanocarriers, attached to the sensor surface, as well as the drug release under simulated intracellular conditions. The grid’s nanoparticles serve here as the model nanocarriers for new TDD systems under design and optimization. The superparamagnetic properties of the Fe2Ni@Au NPs aid in nanoparticles’ handling and constructing a dense sensor grid with high plasmonic enhancement of the Raman signals due to the minimal interparticle distance.